Abstract
Aim
The plant species reported here are traditionally used in Northern Peru to treat bacterial infections, often addressed by the local healers as “inflammation”. The aim of this study was to evaluate the Minimum Inhibitory Concentration (MIC) of their antibacterial properties against Gram-positive and Gram-negative bacteria.
Materials and methods
The antimicrobial activity of ethanolic and water extracts of 141 plant species was determined using a deep-well broth microdilution method on commercially available bacterial strains.
Results
The ethanolic extracts of 51 species inhibited Escherichia coli, and 114 ethanolic extracts inhibited Staphylococcus aureus. In contrast, only 30 aqueous extracts showed activity against E. coli and 38 extracts against S. aureus. The MIC concentrations were mostly very high and ranged from 0.008 to 256mg/ml, with only 36 species showing inhibitory concentrations of <4mg/ml. The ethanolic extracts exhibited stronger activity and a much broader spectrum of action than the aqueous extracts. Hypericum laricifolium, Hura crepitans, Caesalpinia paipai, Cassia fistula, Hyptis sidifolia, Salvia sp., Banisteriopsis caapi, Miconia salicifolia and Polygonum hydropiperoides showed the lowest MIC values and would be interesting candidates for future research.
Conclusions
The presence of antibacterial activity could be confirmed in most species used in traditional medicine in Peru which were assayed in this study. However, the MIC for the species employed showed a very large range, and were mostly very high. Nevertheless, traditional knowledge might provide some leads to elucidate potential candidates for future development of new antibiotic agents.
Keywords: South America, Medicinal Plants, ethnobotany, Minimum Inhibitory Concentration (MIC), antibacterial, Escherichia coli, Staphylococcus aureus
1. Introduction
In developing countries, Traditional Medicine (TM) is often the only accessible and affordable treatment available. In Latin America, the World Health Organization (WHO) Regional Office for the Americas (AMRO/PAHO) reports that 71% of the population in Chile and 40% of the population in Colombia have used TM. In many Asian countries TM is widely used, even though Western Medicine is often readily available. In the US the number of visits to providers of Complementary Alternative Medicine (CAM) now exceeds by far the number of visits to all primary care physicians (WHO 1999a,b; 2002), and CAM is becoming increasingly popular in many developed countries (WHO 1998), and a US survey reported the use of at least one of 16 alternative therapies increased from 34% in 1990 to 42% in 1997 (UNCCD 2000).
The expense for the use of TM and CAM is growing exponentially in many parts of the world. The 1997 out-of-pocket CAM expenditure was estimated at $ 2.7 billion in the USA. The world market for herbal medicines based on traditional knowledge is now estimated at US$ 60 billion (Breevort 1998).
Peru is a country rich in biodiversity. For millennia, traditional healers have used the rich flora to cure ailments. The same plants are still being used today. TM continues to be very popular since a large part of the population has either no access to, or no resources to afford Western Medicine. Bacterial infections and inflammation are among the ailments treated by traditional healers. Northern Peru is believed to be the center of the Central Andean Health Axis (Camino 1992), and TM practices in this region remain an important component of everyday life (Bussmann and Sharon 2006; De Feo 1992; Joralemon and Sharon 1993; Polia 1988; Revene et al. 2008; Sharon 1978, 1980, 1994, 2000; Sharon and Bussmann 2006). TM is also gaining more and more respect by national governments and health providers. Peru’s National Program in Complementary Medicine and the Pan American Health Organization recently compared Complementary Medicine to allopathic medicine in clinics and hospitals operating within the Peruvian Social Security System (EsSalud 2000). The WHO has expressed high interest in TM. It is important to demonstrate scientifically that the remedies employed in folk medicine are indeed therapeutically active (Baker et al. 1995; Cox and Balick 1994; Elisabetsky and Castilhos 1990; Farnsworth et al. 1985; Muñoz and Sauvain 2002).
Plants with potential medicinal activity have recently come to the attention of Western scientists, and studies have reported that some are bioactive (e.g. Perumal Samy and Ignacimuthu 2000). Potentially active compounds have been isolated from a few of the plants tested (D’Agostino et al. 1995a,b; Okuyama et al. 1994; Rodriguez et al. 1994; Umana and Castro 1990). Plant species from the Cordillera Blanca in Peru, demonstrated antimicrobial, anti-cancer, and wound-healing activities (Bussmann et al. 2008, 2009a,b; Hammond et al. 1998;, Lee at al. 1999; Neto et al. 2002; Villegas et al. 1997). However, despite the fact that the center of healing traditions in Northern Peru is located in the Trujillo/Chiclayo coastal region, no in-depth studies had been undertaken.
In this communication we report on antibacterial assays for 141 plant species with a wide range of traditional uses.
2. Materials and Methods
2.1. Plant Material
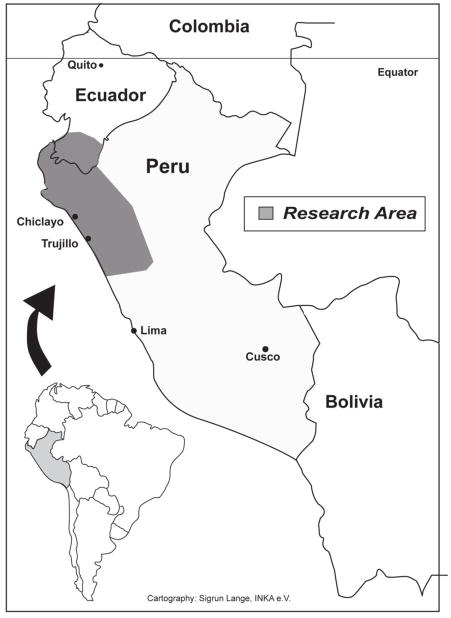
Plants were collected in Northern Peru (Fig. 1) in the field, in markets, and at the homes of traditional healers (curanderos) during August-September 2001, July-August 2002, July-August 2003, June-August 2004, July-August 2005, July-August 2006, June-August 2007, June-August 2008, March-April 2009 and June-August 2009. The specimens are registered under the collection series “JULS,” “ISA,” “GER,” “EHCHL,” “RBU/PL,” “ACR,” “KMM,” and AKT,” depending on the year of fieldwork and collection location. Surveys were conducted in Spanish by fluent speakers. Surveyors would approach healers, collectors and market vendors and explain the premise for the study, including the goal of conservation of medicinal plants in the area. All were asked to participate, but due to expected resistance information could not be recorded from everybody. From those who gave their prior informed consent, information was collected regarding their knowledge and inventory of medicinal plants.
Figure 1.
Study area: Peruvian Departments of Amazonas, Piura, Lambayeque, La Libertad, Cajamarca, San Martin, and the Ecuadorian Province of Loja.
Vouchers of all specimens were deposited at the Herbarium Truxillense (HUT, Universidad Nacional de Trujillo), and Herbario Antenor Orrego (HAO, Universidad Privada Antenor Orrego, Trujillo). In recognition of Peru’s rights under the Convention on Biological Diversity, most notably with regard to the conservation of genetic resources in the framework of a study treating medicinal plants, the identification of the plant material was conducted entirely in Peru. No plant material was exported in any form whatsoever.
2.2. Nomenclature
The nomenclature of plant families, genera, and species follows the Catalogue of the Flowering Plants and Gymnosperms of Peru (Brako and Zarucchi 1993) and the Catalogue of the Vascular Plants of Ecuador (Jørgensen and León-Yanez 1999). The nomenclature was compared to the TROPICOS database (Tropicos, 2010). Species were identified using the available volumes of the Flora of Peru (McBride 1936–1981), as well as Jørgensen and Ulloa Ulloa (1994), Pestalozzi (1998) and Ulloa Ulloa and Jørgensen (1993), and the available volumes of the Flora of Ecuador (Sparre and Harling 1978–2009), and reference material in the herbaria HUT, HAO, QCA, LOJA and QCNE.
2.3. Preparation of Extracts
For each species tested, above ground material (in case of trees leaves or bark as indicated by the collaborating healers) was collected, and the entire material used for extract preparation. This corroborates with the traditional preparation (Bussmann and Sharon 2006). Plant material was dried at 35°C for three days. After drying, the material was ground with an industrial grinder, and 2 samples of 5g. of plant material each were weighted out. One sample was submerged in 100ml of 96% ethanol and left to macerate for 7 days, while another sample was submerged in 100ml of boiling distilled water and left to macerate for 24h. After maceration the plant material was filtered and 100 ml 96% ethanol was added to the water extracts to allow faster solvent removal. The solvent was then evaporated to complete dryness using a standard Buchi rotary-evaporator. The resulting dry extracts were re-suspended in 5 ml distilled water. In order to determine the real concentration of each extract, 1ml of previous homogenization of the respective extracts was removed and again completely oven-dried and then weighed to determine amount of extract per ml of final solution. The remaining extract was used for MIC assays.
2.4. Antimicrobial assays
2.4.1. Bacteria and culture media
Staphylococcus aureus ATCC 25923 (Gram-positive) and Escherichia coli ATCC 25922 (Gram-negative) were used for the current study. Bacterial cultures were grown on 5% sheep red blood agar (SBA). Following the initial incubation, organisms were suspended in 10ml of physiological saline solution and optical density readings were compared to a 0.5 McFarland standard. For the MIC determination bacterial solutions of 5×105 colony-forming units (cfu) ml were employed.
2.4.2. Minimal inhibitory concentration (MIC) determination
The antibacterial activity of the plant extracts was determined using sterile 2ml 96-well plates (Wiegand et al. 2008). The 12 wells of each row were filled with 0.5 ml sterilized Mueller Hinton agar. Sequentially, wells 2–11 received an additional 0.5 ml of a mixture of culture medium and plant extract serially diluted to create a concentration sequence from 0.512 ml to 0.008 ml. Well 1 served as growth control, well 12 as antibiotic control. Tetracycline Hydrochloride (0.1mg/ml) and Amoxicillin (0.1mg/ml) were used as controls for the S. aureus and E. coli assays respectively. The respective antibiotics were chosen because they are often employed as first line antibiotics in the respective bacterial infections. The MIC of Tetracycline Hydrochloride (for S. aureus assays) was 0.25 μg/ml and the MIC of Amoxicillin (for E. coli assays) was 8 μg/ml. The deep-wells were incubated for 24h at 37°C. The resulting turbidity was observed, and after 24h MIC was determined to be where growth was no longer visible by assessment of turbidity by optical density readings at 600nm with a Beckman DU-70 UV-Vis Spectrophotometer. At least three repetitions were run for each assay. Strong activity was defined as MIC < 5 mg/ml.
3. Results and Discussion
The selection of plant material for this study was based on ethnobotanical data on the traditional use of the plants in treatment of bacterial diseases, and conditions classified by the traditional healers as “infection” and “inflammation,” the latter characterized by reddening (e.g. in wounds), or internal afflictions causing gastric discomfort (Table 1). The plant species were initially tested in simple agar-bioassays, which included plants that are used or other purposes by the local population (Bussmann et al. 2007, 2008, 2009a,b). The initial testing yielded 141 species with antibacterial activities which were chosen for this study to establish their MIC values. Because many traditional preparations are prepared by maceration in ethanol or water, we tried to use both extraction methods to prepare the starting extracts for this study.
Table 1.
Minimum Inhibitory Concentration (MIC) for plants tested
| Family | Scientific name | Common name | MIC E. coli (ethanol extract) mg/ml | MIC S. aureus (ethanol extract) mg/ml | MIC E. coli (H2O extract) mg/ml | MIC S. aureus (H2O extract) mg/ml | Traditional antibacterial use? | Collection # |
|---|---|---|---|---|---|---|---|---|
| Adiantaceae | Adiantum concinnum Willd. | Culantrillo | 8 | Blood purification | ACR91 | |||
| Amaranthaceae | Alternanthera porrigens (Jacq.) Kuntze | Moradillo Blanco | 16 | Inflammation | ACR149 | |||
| Amaranthaceae | Gomphrena globosa L. | Siempre viva (corta) | 16 | Inflammation | ACR191 | |||
| Amaranthaceae | Iresine herbstii Hook. | Colores | 256 | Inflammation | ACR162 | |||
| Amaryllidaceae | Eustephia coccinea Cav. | Pumapara | 32 | Inflammation | ACR119 | |||
| Annonaceae | Annona muricataL. | Guanabana | 128 | Inflammation | ACR81 | |||
| Apiaceae | Apium graveolens L. | Apio del Campo | 32 | 256 | Inflammation | KMM439 | ||
| Apocynaceae | Nerium oleander L. | Laurel | 128 | 64 | Wounds | ACR34 | ||
| Apocynaceae | Vallesia glabra (Cav.) Link | Cuncuno | 64 | 16 | 32 | Snake bite | ACR192 | |
| Aquifoliaceae | Ilex guayusa Loes. | Gauyusa | 16 | 128 | Inflammation | KMM513 | ||
| Asteraceae | Achyrocline alata (Kunth) DC | Hierba de Ishpingo | 8 | 32 | 32 | Spiritual cleansing | AKT1199 | |
| Asteraceae | Baccharis vaccinioides Kunth | Sigueme Sigueme | 64 | 64 | Spiritual cleansing | KMM565 | ||
| Asteraceae | Baccharis sp | Chilca Chica | 2 | 4 | 8 | 8 | Inflammation | KMM562 |
| Asteraceae | Bidens pilosa L. | Amor seco | 16 | 32 | Inflammation kidneys | KMM427 | ||
| Asteraceae | Diplostephium sagasteguii Cuatrec. | Gato Cimuro | 8 | 8 | 8 | 8 | Spiritual cleansing | AKT1192 |
| Asteraceae | Eupatorium cf. gayanum Wedd. | Asma chilca | 32 | 32 | Bronchitis | KMM555 | ||
| Asteraceae | Gamochaeta sp. | Lechuguilla | 8 | Spiritual cleansing | ACR41 | |||
| Asteraceae | Matricaria recutita L. | Manzanilla | 16 | 32 | Wounds | ACR6 | ||
| Asteraceae | Munnozia lyrata (A. Gray) Rob. & Brett. | Caniahuanga | 16 | Spiritual cleansing | KMM519 | |||
| Asteraceae | Munnozia sp. | Salvia Blanca | 128 | Spiritual cleansing | ACR148 | |||
| Asteraceae | Porophyllum ruderale Less. | Hierba de Gallinazo | 4 | Spiritual Cleansing | KMM515 | |||
| Asteraceae | Pseudogynoxys cordifolia (Cass.) Cabrera | San Juan | 32 | 16 | Spiritual Cleansing | AKT1168 | ||
| Asteraceae | Schkuhria pinnata (Lam.) Kuntze | Encanchallacha | 64 | 128 | Inflammation kidneys | ACR17 | ||
| Asteraceae | Senecio cf. tephrosioides Turcz. | Huamanripa | 64 | 64 | 32 | 32 | Bronchitis | ACR65 |
| Asteraceae | Senecio sp. | Huamanripa | 64 | Inflammation | KMM449 | |||
| Asteraceae | Senecio sp. | Ornamo | 8 | 2 | 64 | Inflammation | KMM480 | |
| Asteraceae | Tagetes erecta L. | Flor de Muerto | 16 | 16 | 64 | 64 | Inflammation | JULS156 |
| Asteraceae | Tagetes filifolia Lag. | Anis | 16 | 16 | Diarrhea | KMM524 | ||
| Asteraceae cf. | Chunguez | Inflammation | KMM405 | |||||
| Asteraceae | Hierba del Amor | 8 | Inflammation | KMM522 | ||||
| Balanophoraceae | Corynaea crassa Hook.f. | Huanarpo | 2 | Impotence | KMM474 | |||
| Berberidaceae | Berberis buceronis J.F. Macbr. | Palo Amarillo | 16 | Hepatitis | KMM573 | |||
| Bignoniaceae | Jacaranda acutifolia Humb. & Bonpl. | Arabisco | 32 | 16 | Bronchitis | ACR89 | ||
| Bombacaceae | Ochroma pyramidale (Cav. ex Lam.) Urb. | Balsa | 1 | ACR206 | ||||
| Boraginaceae | Borago officinalis L. | Borraja | 16 | 8 | 32 | 8 | Bronchitis | ACR9 |
| Brassicaceae | Rorippa nasturtium-aquaticum (L.) Hayek | Berros | 64 | 64 | Bronchitis | AKT1163 | ||
| Bromeliaceae | Tillandsia cacticola L.B. Sm. | Siempre viva | 16 | Spiritual cleansing | ACR183 | |||
| Cactaceae | Opuntia ficus-indica (L.) Mill. | Tuna | 128 | Diabetes | AKT1220 | |||
| Campanulaceae cf. | Centropogon sp. | Trinoso | 16 | 16 | 8 | 4 | Liver | KMM545 |
| Capparidaceae | Capparis scabrida Kunth | Zapote | 16 | 8 | 64 | Inflammation | KMM554 | |
| Caprifoliaceae | Sambucus peruviana Kunth | Flor de Novia | 32 | 32 | 32 | Bronchitis | KMM539 | |
| Chenopodiaceae | Chenopodium ambrosioides L. | Paico | 8 | Parasites | ACR31 | |||
| Chloranthaceae | Hedyosmum racemosum (Ruiz. & Pav.) G. Don. | Asarsito | 8 | Bronchitis | KMM505 | |||
| Clethraceae | Clethra castaneifolia Meiss. | Hierba del Olvido | 16 | Spiritual cleansing | KMM549 | |||
| Clethraceae | Clethra castaneifolia cf. Meiss. | Olvido | 32 | 64 | Spiritual cleansing | ACR109 | ||
| Clusiaceae | Hypericum laricifolium Juss. | Pachuli | 0.16 | 16 | Spiritual cleansing | AKT1172 | ||
| Cucurbitaceae | Sicana odorifera Naudin | Sicana | 128 | Spiritual cleansing | ACR96 | |||
| Dioscoreaceae | Dioscorea tambillensis Kunth | Papa Semitona | 16 | Inflammation | KMM583 | |||
| Dioscoreaceae | Dioscorea trifida L.f. | Papa Madre | 4 | Wounds | KMM503 | |||
| Dipsaceae | Scabiosa atropurpurea L. | Ambarina | 32 | Bronchitis | ACR158 | |||
| Ephedraceae | Ephedra americana Engl. | Diego Lopez | 64 | 32 | 32 | 32 | Wounds | KMM511 |
| Equisetaceae | Equisetum bogotense Kunth | Cola de Caballo | 64 | Wounds | ACR1 | |||
| Ericaceae | Bejaria aestuans L. | Hierba de la Postema | 16 | Inflammation | KMM527 | |||
| Ericaceae | Gaultheria reticulata Vent. | Maique | 128 | Spiritual cleansing | KMM531 | |||
| Euphorbiaceae | Croton lechleri Müll. Arg. | Sangre de Grado | 4 | 2 | 4 | Wounds | KMM546 | |
| Euphorbiaceae | Hura crepitans L. | Habilla | 1 | Wounds | AKT1225 | |||
| Euphorbiaceae | Jatropha macrantha L. | Hoja de piñon | 32 | Wounds | KMM487 | |||
| Fabaceae | Caesalpinia paipai Ruiz. & Pav. | Pai Pai | 1 | Wounds | KMM581 | |||
| Fabaceae | Caesalpinia spinosa (Molina) Kuntze | Tara | 64 | 16 | Wounds | ACR111 | ||
| Fabaceae | Cassia fistula L. | Caña fistula | 1 | Epilepsy | ACR88 | |||
| Fabaceae | Medicago sativa L. | Trebol de aqua | 64 | Bronchitis | KMM463 | |||
| Fabaceae | Senna bicapsularis (L.) Roxb. | Alcaparilla | 0.016 | 256 | 16 | Blood purification | ACR194 | |
| Fabaceae | Senna monilifera H.S. Irwin & Barnaby | Sen | 4 | Cleansing stomach | KMM470 | |||
| Fabaceae | Spartium junceumL. | Ratania | 4 | Blood purification | AKT1222 | |||
| Gentianaceae | Gentianella bicolor (Wedd.) J.S. Pringle | Corpus Way | 8 | 8 | 16 | Blood purification | KMM526 | |
| Gentianaceae | Gentianella dianthoides (Kunth) Fabris ex J.S. Pringle | Chagape | 64 | Blood purification | ACR155 | |||
| Geraniaceae | Erodium cicutarium (L.) L’Her. | Agujilla | 64 | 16 | 4 | Inflammation | AKT1171 | |
| Geraniaceae | Geranium sessiliflorum Cavanilles | Pasuchaca | 64 | 8 | Inflammation | ACR38 | ||
| Geraniaceae | Geranium sessiliflorum Cavanilles | Pasuchaca | 32 | Inflammation | KMM400 | |||
| Geraniaceae | Pelargonium odoratissimum Soland. cf. | Malva de Olor | 2 | Inflammation womb | ACR26 | |||
| Krameriaceae | Krameria lappacea (Dombey) H.M. Burdet & B.B. Simpson | Ratamia | 128 | Inflammation | ACR48 | |||
| Lamiaceae | Hyptis sidifolia (L’Her.) Briq. | Pedorera | 1 | Gastritis | ACR69 | |||
| Lamiaceae | Hyptis sp. | Albaca Serrana | 256 | Gastritis | ACR18 | |||
| Lamiaceae | Mentha x piperita L. | Poleo | 16 | 64 | Inflammation | ACR68 | ||
| Lamiaceae | Mentha spicata L. | Menta | 32 | Parasites | KMM453 | |||
| Lamiaceae | Minthostachys mollis (Kunth) Griseb. | Chancas del muerto | 16 | Inflammation | AKT1142 | |||
| Lamiaceae | Ocimum basilicum L. | Albaca | 16 | Inflammation | KMM437 | |||
| Lamiaceae | Origanum vulgare L. | Oregano | 128 | Inflammation | KMM509 | |||
| Lamiaceae | Otholobium mexicanum (L.f.) Grimes | Culen | 8 | Diarrhea | ACR67 | |||
| Lamiaceae | Salvia sp. | Alamo Silvestre | 8 | 16 | 8 | Inflammation | ACR184 | |
| Lamiaceae | Salvia sp. | Paja Amargoza | 1 | Inflammation | KMM567 | |||
| Lamiaceae | Satureja pulchella (Kunth.) Briq. | Panizara | 16 | 2 | 32 | Bronchitis | KMM543 | |
| Lamiaceae | Satureja sericea (C. Presl. & Benth.) Briq. | Romerillo | 16 | 16 | 8 | Inflammation | KMM397 | |
| Lauraceae | Cinnamomum verum J. Presl. | Canela | Bronchitis | KMM575 | ||||
| Lauraceae | Persea americana Mill. | Palta | 16 | Diarrhea | AKT1120 | |||
| Lycopodiaceae | Huperzia sp. | Condor Misha | 16 | Spiritual cleansing | KMM479 | |||
| Lycopodiaceae | Lycopodium sp. | Guamingo | 64 | Spiritual cleansing | AKT1206 | |||
| Lythraceae | Cuphea sp. | Hierba del Toro | 32 | Blood purification | AKT1102 | |||
| Lythraceae | Cuphea sp. | Hierba del Toro | 8 | 8 | 4 | Blood purification | KMM448 | |
| Malvaceae | Malva parviflora L. | Malva Rosa | 2 | Wounds | AKT1200 | |||
| Malvaceae | Malva cf. sylvestris L. | Malva Blanca | 16 | 8 | Wounds | ACR8 | ||
| Malpighiaceae | Banisteriopsis caapi (Spruce ex Grieseb.) Morton | Ayahuasca | 0.0625 | 1 | Hallucinogen | ACR135 | ||
| Maranthaceae | Monotagma plurispicatum (Koern.) Schum. | Patiquina | ACR114 | |||||
| Melastomataceae | Brachyotum naudinii Triana | Carcilleja | 16 | 8 | 16 | 8 | Blood circulation | ACR140 |
| Melastomataceae | Micronia salicifolia (Bonpl. ex Naud.) Naud. | Porontillo | 2 | 0.0625 | 16 | 8 | Blood purification | KMM544 |
| Menispermaceae | Abuta grandifolia (Mard.) Sandwith. | Abuta | 8 | Inflammation | ACR136 | |||
| Monimiaceae | Peumus boldus Molina cf. | Boldo | Inflammation kidneys | AKT1132 | ||||
| Moraceae | Brosimum rubescens Taub. | Palo Sangre | 4 | 2 | Inflammation | KMM570 | ||
| Myrtaceae | Eucalyptus globulus Labill | Eucalipto | 8 | Bronchitis | AKT1110 | |||
| Myrtaceae | Eugenia obtusifolia Cambess. | Limoncillo/Arra yan | 16 | 2 | 2 | 2 | Inflammation | ACR19 |
| Myrtaceae | Eugenia obtusifolia Cambess. | Limoncillo/Arra yan | 8 | 4 | 32 | 0.008 | Inflammation | ACR180 |
| Myrtaceae | Psidium guajava L. | Guanabana | 16 | Liver | KMM399 | |||
| Myrtaceae | Scutia spicata Weberb. in J.F. Macbr. | Pus | 512 | Spiritual cleansing | ACR207 | |||
| Myrtaceae | Syzygium aromaticum (L) Merr. & L.M. Perry | Clavo de olor | 8 | 2 | 32 | Inflammation | ACR188 | |
| Myrtaceae | Syzygium jambos (L.) Alston | Poma Rosa | 8 | Diarrhea | ACR174 | |||
| Olacaceae | Heisteria acuminata (Humb. & Bonpl.) Engl. | Chuchu Wasi | 32 | Cough | KMM507 | |||
| Onagraceae | Fuchsia sp. | Añasquero | 8 | 64 | Colds | AKT1187 | ||
| Orchidaceae | Epidendrum sp. | Hierba de la Espada | 64 | Spiritual cleansing | AKT1177 | |||
| Passifloraceae | Passiflora punctata L. | Norgo | 16 | 16 | Inflammation | KMM510 | ||
| Piperaceae | Piper aduncum L. | Matico | 16 | 32 | Wounds | ACR12 | ||
| Plantaginaceae | Plantago sericea Ruiz. & Pav.var. lanuginosa Grieseb. | Paja Blanca | 16 | Vaginal discharge | AKT1182 | |||
| Poaceae | Arundo donax L. | Carrizo | 16 | Hemorrhoids | KMM389 | |||
| Polygonaceae | Polygonum hydropiperoides Michaux cf. | Pica Pica | 1 | Wounds | ACR80 | |||
| Polypodiaceae | Cheilanthes myriophylla Desv. | Cuti Cuti | 32 | Spiritual cleansing | KMM461 | |||
| Polypodiaceae | Cheilanthes myriophylla Desv. | Cuti Cuti | 32 | 32 | Spiritual cleansing | AKT1108 | ||
| Proteaceae | Oreocallis grandiflora R. Br. | Chucharilla | 2 | Inflammation uterus | ACR176 | |||
| Ranunculaceae | Laccopetalum giganteum (Wedd.) Ulbrich | Pacra | 16 | 32 | 64 | 32 | Bronchitis | AKT1119 |
| Rosaceae | Cydonia oblonga Mill. | Membrillo | 15 | Heart problems | ACR56 | |||
| Rosaceae | Margyricarpus pinnatus (Lam.) Kuntze | China Linda | ACR146 | |||||
| Rosaceae | Polylepis racemosa Ruiz. & Pav. | Quinal | 8 | After birth | ACR3 | |||
| Rosaceae | Rubus robustus C. Presl. | Zarzamora | 32 | Wounds | ACR70 | |||
| Rosaceae | Sanguisorba minor Scop. | Pimpinella | 4 | Blood purification | ACR23 | |||
| Rubiaceae | Cinchona officinalis L. | Cascarilla | 16 | Cough | ACR123 | |||
| Rubiaceae | Morinda citrifolia L. | Noni | 32 | 32 | 64 | Inflammation | ACR160 | |
| Rutaceae | Citrus limetta Risso | Lima | 2 | Inflammation | KMM425 | |||
| Scrophulariaceae | Capraria peruviana Bentham | Te de Indio | 32 | Inflammation of kidneys | KMM574 | |||
| Scrophulariaceae | Chiciricoma | 32 | KMM440 | |||||
| Smilacaceae | Smilax sp. | Palo China | 32 | Cancer | KMM516 | |||
| Solanaceae | Cestrum auriculatum L’Her. | Hierba Santa | 32 | 32 | Typhoid | ACR36 | ||
| Solanaceae | Cestrum sp. | Agrasejo | 64 | Inflammation | AKT1121 | |||
| Solanaceae | Solanum americanum Mill. | Hierba Mora | 128 | Flu | ACR37 | |||
| Valerianaceae | Valeriana cf. bonplandiana Wedd. | Fortuna | 8 | 32 | Spiritual cleansing | ACR181 | ||
| Valeriana | Valeriana plantaginea Kunth | Ornamo Caballero | 32 | Purgative | ACR120 | |||
| Valerianaceae | Valeriana sp. cf. | Ornamo | 8 | Spiritual cleansing | AKT1141 | |||
| Verbenaceae | Verbena litoralis Kunth. | Bervena | 64 | Inflammation | ACR13 | |||
| Verbenaceae | Verbesine sp. | Sabadilla | 8 | 2 | 64 | 4 | Spiritual cleansing | ACR154 |
| Viscaceae | Phoradendron cf. | Suelda con suelda | Inflammation | ACR189 | ||||
| Ajo Caspi | 128 | 32 | ACR133 | |||||
| Arnica | 128 | Inflammation | ACR193 | |||||
| Beldaco | 2 | 32 | 2 | KMM501 | ||||
| Huarate | 2 | Diabetes | AKT1209 |
Table 1 shows the antibacterial activity of Northern Peruvian medicinal plants against Gram-positive and Gram-negative bacteria. The extracts were subjected to the determination of MIC values. The ethanolic extracts of 51 species inhibited Escherichia coli, and 114 ethanolic extracts inhibited Staphylococcus aureus. In contrast, only 30 water extracts showed activity against E. coli and 38 extracts against S. aureus. The MIC concentrations ranged from 0.008 to 256mg/ml. The very high values in many species indicate only a very limited antibacterial efficacy. The ethanolic extracts exhibited stronger activity and a much broader spectrum of action than the water extracts. The most interesting activity on E. coli was obtained from ethanolic extracts of Baccaris sp., Ochroma pyramidale, Croton lechleri, Banisteriopsis caapii, Miconia salicifolia, and Eugenia obtusifolia. Only the latter species also showed strong activity in the aqueous extract. A much wider range of species, including most species active against E. coli showed inhibition of S. aureus. Poropohyllum ruderale, Senecio sp., Corynaeae crassa, Dioscorea trifida, Senna monilifera, Spartium junceum, Pelargonium odoratissimum, Satureja pulchella, Cuphea sp., Malva parviflora, Brosmium rufescens, Syzygium aromaticum, Sanguisorba minor, Citrus limetta, Verbesine sp. and 2 unidentified species all showed MIC values between 1–4mg/ml. Most of them however did not portray any efficacy in aqueous extract. Hypericum laricifolium, Hura crepitans, Caesalpinia paipai, Cassia fistula, Hyptis sidifolia, Salvia sp., Banisteriopsis caapi, Miconia salicifolia and Polygonum hydropiperoides showed the lowest MIC values and would be interesting candidates for future research. Most MIC values reported in this work were largely higher than those obtained for South American species (Bastos et al. 2009; Jimenez et al. 2001; Meléndez et al. 2006; Zampini et al. 2009) and African studies (Kirira et al. 2006). However, they were in range or lower than concentrations reported by Kloucek et al. (2007), Nascimento et al. (2000) and Psewu et al. (2008).
Most species effective against S. aureus are traditionally used to treat wound infection, throat infections, serious inflammations, or are post partum infections. Interestingly many species used in cleansing baths also showed high activity against this bacterium. Many of these species are either employed topically, or in synergistic mixtures, so that possible toxicity seems not to be an issue. The species effective against E. coli were mostly employed in indications that traditional healers identified as “inflammation”.
Most of the plants used by the healers have antibacterial activity, but only 8 of the 141 plants (5.6%) examined in this study show any MIC values of 200 or less mg/ml of extract. Of these 8 plants 5 are used to treat diseases believed to be in bacterial origin by TM, one is a disease not believed to be caused by bacteria and one is used for undefined treatment purposes.
Nine out of 141 plants (6.3%) tested that were not used for diseases believed to be bacterial in origin by TM, 5 showed high antibacterial activity with MIC values below 16 mg/ml. Four of these were among the most potent plants tested with MIC values of 2 or less mg/ml including the hallucinogen and extracts used to treat diabetes and epilepsy. Diseases such as diabetes often compromise the health of the individual and antibacterial treatments can be warranted for secondary complications of the disease. In addition, TM does determine sometimes that diseases not originally believed to be bacterial in origin, such as ulcers, are actually caused by bacteria. Currently TM is seriously looking the role of inflammation (which can certainly be bacterial in origin) in heart disease.
The results presented in this paper demonstrate that most of the plants used by the healers in Peru to treat disease of bacterial origin do show limited antibacterial activity and that some treatments for diseases not currently believed to be of bacterial origin also show antibacterial activity. These facts support the medicinal value of traditional Peruvian remedies and suggest that those plants widely used by the curanderos could be new sources of antibacterial therapies.
4. Conclusions
The antibacterial activity of 141 ethanolic and aqueous extracts belonging to 140 plant species used in TM in Northern Peru was demonstrated. Most species tested showed limited antibacterial activity. It is important to note however, that most species are not employed as single plant extracts in the traditional context. The results indicate that the often very elaborate traditional knowledge can serve as guideline to provide leads for further testing of potentially interesting plants that can serve for further studies that would allow the clinical validation of the traditional uses and the application of the species in modern treatment forms. Further studies on the toxicity of the species employed, as well as their application in often complex traditional mixtures would allow to elucidate possible candidates for future development of antimicrobial agents.
Acknowledgments
The present study was financed through MHIRT (Minority Health Disparity International Research and Training, Fund: 54112B MHIRT Program, Grant: G0000613), coordinated by San Diego State University (SDSU) in cooperation with the San Diego Museum of Man (SDMM), the P.A. Hearst Museum of Anthropology at the University of California Berkeley (PAHMA-UCB), the Missouri Botanical Garden, USA, and the Universidad Privada Antenor Orrego (UPAO, Herbarium HAO), the Universidad Nacional de Trujillo (UNT, Herbarium HUT and Instituto de Medicina Tropical) and the Clínica Anticona Trujillo (CAT) in Peru.
None of the work would have been possible without the invaluable collaboration of our Peruvian colleagues, curanderas Julia Calderón, Isabel Chinguel, and Olinda Pintado, curandero Germán Santisteban, and herbalists Manuel Bejarano, Elmer Cruz, and Iván Cruz. With regard to ritual and therapeutic practices we especially want to thank the above curanderas, as well as curandero Leoncio Carrión.
Thanks also go to Eric Rodriguez (Herbarium Truxillense, HUT) and Abundio Sagastegui, Segundo Leiva, and Mario Zapata (Herbario Antenor Orrego, HAO) for the use of their facilities and their assistance in plant identification
Most of all, we want to express our sincere gratitude to the people of Northern Peru for sharing their ethnobotanical knowledge.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baker J, Borris R, Carte B, Cordell G, Soejarto D, Cragg G, Gupta M, Iwo M, Madulid D, Tyler V. Natural Product Discovery and Development: New Perspectives on International Collaboration. Journal of Natural Products. 1995;58:1325–1357. doi: 10.1021/np50123a003. [DOI] [PubMed] [Google Scholar]
- Bastos MLA, Lima MRF, Conserva LM, Andrade VS, Roche EMM, Lemos RPL. Studies on the antimicrobial activity and brine shrimp toxicity of Zeyheria tuberculosa (Vell.) Bur. (Bignoniaceae) extracts and their main constituents. Annals of Clinical Microbiology and Antimicrobials. 2009;8:16. doi: 10.1186/1476-0711-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brako L, Zarucchi JL. Catalogue of the Flowering Plants and Gymnosperms of Peru. Missouri Botanical Garden; Saint Louis, MO: 1993. [Google Scholar]
- Breevort P. The Booming U. S. Botanical Market: A New Overview. HerbalGram. 1998;44:33–46. [Google Scholar]
- Bussmann RW, Sharon D, Lopez A. Blending Traditional and Western Medicine: Medicinal Plant Use Among Patients at Clinica Anticona in El Porvenir, Peru. Ethnobotany Research and Applications. 2007:5. [Google Scholar]
- Bussmann RW, Sharon D. Traditional plant use in Northern Peru, Tracking two thousand years of health culture. Journal of Ethnobiology and Ethnomedicine. 2006;2:47. doi: 10.1186/1746-4269-2-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussmann RW, Sharon D, Diaz D, Cardenas R, Chait G, Castro M, Regalado S, Del Toro CR, Malca GG, Perez AF, Glenn A. Antibacterial activity of medicinal plant species in Northern Peru. Arnaldoa. 2009a;16:93–103. [Google Scholar]
- Bussmann RW, Sharon D, Castro M, Cardenas R, Chait G, Regalado S, Del Toro CR, Malca GG, Perez AF, Glenn A. Phyto-Chemical Analysis of Peruvian Medicinal Plants. Arnaldoa. 2009b;16:105–110. [Google Scholar]
- Bussmann RW, Sharon D, Perez F, Díaz D, Ford T, Rasheed T, Silva R. Antibacterial activity of Northern-Peruvian Medicinal Plants - a low cost laboratory approach to assess biological activity. Arnaldoa. 2008;15:127–148. [Google Scholar]
- Camino L. Cerros, plantas y lagunas poderosas, la medicina al norte de Perú. Lima: Lluvia Editores; 1992. [Google Scholar]
- Cox P, Balick M. The Ethnobotanical Approach to Drug Discovery. Scientific American. 1994;270:82–87. [PubMed] [Google Scholar]
- D’Agostino M, De Simone F, Tomais N, Pizza C. Constituents of Culcitiumcanescens. Fitoterapia. 1995a;66:550–551. [Google Scholar]
- D’Agostino M, Pizza C, De Simona F. Flavone and flavonal glycosides from Desmodium molliculum. Fitoterapia. 1995b;66:384–385. [Google Scholar]
- De Feo V. Medicinal and magical plants on northern Peruvian Andes. Fitoterapia. 1992;63:417–440. [Google Scholar]
- Elisabetsky E, Castilhos C. Plants used as analgesics by Amazonian caboclos as a basis for selecting plants for investigation. International Journal of Crude Drug Research. 1990;28:309–320. [Google Scholar]
- EsSalud/Organización Panamericana de Salud. Seguro Social de EsSalud (Study of Cost-Effectiveness: National Program in Complementary Medicine. Social Security of EsSalud) Lima: EsSalud/Organización Panamericana de Salud (Pan American Health Organization); 2000. Estudio Costo-Efectividad: Programa Nacional de Medicina Complementaria. [Google Scholar]
- Farnsworth N, Akerele O, Bingel A, Soejarto D, Guo Z. Medicinal Plants in therapy. Bulletin of the World Health Organization. 1985;63:965–981. [PMC free article] [PubMed] [Google Scholar]
- Hammond GB, Fernandez ID, Villegas L, Vaisberg AJ. A survey of traditional medicinal plants from the Callejon de Huaylas, Department of Ancash, Peru. Journal of Ethnopharmacology. 1998;61:17–30. doi: 10.1016/s0378-8741(98)00009-9. [DOI] [PubMed] [Google Scholar]
- Jiménez G, Hasegawa M, Rodríguez M, Estrada O, Méndez J, Castillo A, Gonzalez-Mujica F, Motta N, Vázquez J, Romero-Vecchione E. Biological screening of plants from the Venezuelan Amazon. Journal of Ethnopharmacology. 2001;77:77–83. doi: 10.1016/s0378-8741(01)00271-9. [DOI] [PubMed] [Google Scholar]
- Joralemon D, Sharon D, editors. Sorcery and Shamanism, Curanderos and Clients in Northern Peru. University of Utah Press; Salt Lake City: 1993. [Google Scholar]
- Jørgensen PM, León-Yanez S. Catalogue of the vascular plants of Ecuador. Monographs in Systematic Botany from the Missouri Botanical Garden. 1999:75. [Google Scholar]
- Jørgensen PM, Ulloa Ulloa C. Seed plants of the High Andes of Ecuador - a Checklist. AAU Reports. 1994;34:1–443. [Google Scholar]
- Kirira PG, Rukunga GM, Wanyonyi AW, Gathirwa JW, Mathaura CN, Omar SA, Tolo F, Mungai GM, Ndiege IO. Anti-plasmodial activity and toxicity of extracts of plants used in traditional malaria therapy in Meru and Kilifi Districts in Kenya. Journal of Ethnopharmacology. 2006;106:403–407. doi: 10.1016/j.jep.2006.01.017. [DOI] [PubMed] [Google Scholar]
- Kloucek P, Svoboda B, Polesny Z, Langrova I, Smrcek S, Kokoska L. Antimicrobial activity of some medicinal barks used in Peruvian Amazon. Journal of Ethnopharmacology. 2007;111:427–429. doi: 10.1016/j.jep.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Lee KK, Zhou BN, Kingston DGI, Vaisberg AJ, Hammond GB. Bioactiveindole alkaloids from the bark of Uncaria guianensis. Planta Medica. 1999;65:750–760. doi: 10.1055/s-2006-960860. [DOI] [PubMed] [Google Scholar]
- McBride JF, editor. Fieldiana, Botany. Field Museum of Natural History; Chicago: 1936–1981. Flora of Peru. [Google Scholar]
- Meléndez PA, Capriles VA. Antibacterial properties of tropical plants from Puerto Rico. Phytomedicine. 2006;13:272–276. doi: 10.1016/j.phymed.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Muñoz V, Sauvain M. A search for natural bioactive compounds in Bolivia through a multidisciplinary approach: Part I. Evaluation of the antimalarial activity of plants used by the Chacobo Indians. Journal of Ethnopharmacology. 2002;69:127–137. doi: 10.1016/s0378-8741(99)00148-8. [DOI] [PubMed] [Google Scholar]
- Nascimento GF, Locatelli J, Freitas PC, Silva GL. Antibacterial activity of plant extracts and phytochemicals on antibiotic resistet bacteria. Brazilian Journal of Microbiology. 2000;31:247–256. [Google Scholar]
- Neto C, Owens C, Langfield R, Comeau A, Onge J, Hammond G, Vaisberg A. Antibacterial Activity of Some Peruvian Medicinal Plants from Callejon de Huaylas. Journal of Ethnopharmacology. 2002;79:133–138. doi: 10.1016/s0378-8741(01)00398-1. [DOI] [PubMed] [Google Scholar]
- Okuyama E, Umeyama K, Ohmori S, Yamazaki M, Satake M. Pharmacologically active components from a Peruvian medicinal plant, Huira-Huira (Culcitium canescens H. and B.) Chemical and Pharmaceutical Bulletin. 1994;42:2183–2186. doi: 10.1248/cpb.42.2183. [DOI] [PubMed] [Google Scholar]
- Perumal Samy R, Ignacimuthu S. Antibacterial activity in some medicinal plants used by tribes in Western Ghats, India. Journal of Ethnopharmacology. 2000;69:63–71. doi: 10.1016/s0378-8741(98)00156-1. [DOI] [PubMed] [Google Scholar]
- Pesewu GA, Cutler RR, Humber DP. Antibacterial activity of plants used in traditional medicines of Ghana with particular reference to MRSA. Journal of Ethnopharmacology. 2008;116:102–111. doi: 10.1016/j.jep.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Pestalozzi HU. Flora ilustrada altoandina. Herbario Nacional de Bolivia and Herbario Forestal Nacional Martín Cardenas; Cochabamba: 1998. [Google Scholar]
- Polia M. Las Lagunas de los Encantos – Medicina Tradicional Andina en el Peru septentrional. Lima: CePeSer; 1988. [Google Scholar]
- Revene Z, Bussmann RW, Sharon D. From Sierra to Coast: Tracing the Supply of Medicinal Plants in Northern Peru – a plant collector’s tale. Journal of Ethnobotany Research and Application. 2008;6:15–22. [Google Scholar]
- Rodriguez J, Pacheco P, Razmilic I, Loyola JI, Schmeda-Hirschmann G, Theoduloz C. Hypotensive and diuretic effects of Equisetum bogotense and Fuchsia magellanica and micropropagation of E. bogotense. Phytotherapy Research. 1994;8:157–160. [Google Scholar]
- Sharon D. Wizard of the Four Winds, A Shaman’s Story. Free Press; New York: 1978. [Google Scholar]
- Sharon D. El Chamán de los Cuatro Vientos. Siglo veintiuno editores; México, D.F: 1980. [Google Scholar]
- Sharon D. Tuno y sus colegas, notas comparativas. In: Millones L, Lemlij M, editors. En el Nombre del Señor, Shamanes, demonios y curanderos del norte del Perú. Australis S.A; Lima: 1994. pp. 128–147. [Google Scholar]
- Sharon D. Shamanismo y el Cacto Sagrado - Shamanism and the Sacred Cactus. San Diego Museum Papers; 2000. p. 37. [Google Scholar]
- Sharon D, Bussmann RW. Plantas Medicinales en la Obra del Obispo Don Baltasar Jaime Martínez Compañon (Siglo XVIII) In: Millones L, Kato T, editors. Desde el exterior, El Perú y sus estudios. Tercer Congreso Internacional de Peruanistas, Nagoya, UNMSM, FEFCS; Lima: 2006. pp. 147–165. [Google Scholar]
- Sparre G, Harling B. Flora of Ecuador (various authors) Council for Nordic Publications in Botany; 1978–2009. [Google Scholar]
- Tropicos. Missouri Botanical Garden plant database. 2010 www.tropicos.org.
- Ulloa Ulloa C, Jørgensen PM. Arboles y arbustos de los Andes del Ecuador. AAU Reports. 1993;30:1–263. [Google Scholar]
- Umana E, Castro O. Chemical constituents of Verbena littoralis. International Journal of Crude Drug Research. 1990;28:175–17. [Google Scholar]
- United Nations Conference on Trade and Development. Background Note by the UNCTAD Secretariat. Geneva: United Nations Conference on Trade and Development; 2000. Systems and National Experiences for Protecting Traditional Knowledge, Innovations and Practices. (document reference TD/B/COM.1/EM.13/2) [Google Scholar]
- Villegas L, Fernandez I, Maldonado H, Torres R, Zavaleta A, Vaisberg A. Evaluation of the Wound-Healing Activity of Selected Traditional Medicinal Plants. Journal of Ethnopharmacology. 1997;55:193–200. doi: 10.1016/s0378-8741(96)01500-0. [DOI] [PubMed] [Google Scholar]
- Wiegand I, Hilpert K, Hancock REW. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nature Protocols. 2008;3(2):163–175. doi: 10.1038/nprot.2007.521. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Report, Technical Briefing on Traditional Medicine. Forty-ninth Regional Committee Meeting; Manila, Philippines. 18 September 1998; Manila: WHO Regional Office for the Western Pacific; 1998. [Google Scholar]
- World Health Organization. Consultation Meeting on Traditional Medicine and Modern Medicine, Harmonizing the Two Approaches. Geneva: World Health Organization; 1999a. (document reference (WP)TM/ICP/TM/001/RB/98– RS/99/GE/32(CHN)) [Google Scholar]
- World Health Organization. Traditional, Complementary and Alternative Medicines and Therapies. Washington DC: WHO Regional Office for the Americas/Pan American Health Organization (Working group OPS/OMS); 1999b. [Google Scholar]
- World Health Organization. WHO Traditional Medicine Strategy 2002–2005. World Health Organization; Geneva: 2002. [Google Scholar]
- Zampini IC, Cuello S, Alberto MR, Ordoñez RM, Alameida RD, Solorzano E, Isla MI. Antimicrobial activity of selected plant species from the “Argentine Puna” against sensitive and multi-resistant bacteria. Journal of Ethnopharmacology. 2009;124:499–505. doi: 10.1016/j.jep.2009.05.011. [DOI] [PubMed] [Google Scholar]