Abstract
An Electronic Safety Reporting (ESR) module was developed and integrated into a home-grown web-based Clinical Trial Management System (CTMS) to enhance the efficiency, completeness and consistency of reporting and reviewing serious adverse events, monitoring safety, and submitting safety reports to regulatory authorities for a large multicenter clinical trial. The architecture of this integrated module provided many advantages. First, the ESR module was developed based on a comprehensive procedure which incorporated both computer logic processing steps and human intervention steps in order to deal with the complex and unexpected situations where pre-programmed computer logic may fail. Secondly, safety and efficacy data were managed within the same relational database. Relevant data captured on efficacy case report forms, such as demographics, medical history, lab data and concomitant medications, were directly retrievable for MedWatch report composition without requiring redundant data entry. Finally, the ESR module shared the same generic user interfaces and data processing functions with other modules in the CTMS. These generic components include data editing, data retrieving, data reporting, dictionary-based automatic and interactive coding, event driven and calendar driven automatic email notifications, and user privilege management. This integrated ESR module was implemented in the Albumin in Acute Stroke (ALIAS) Trial - Part 1. A total of 397 serious adverse event reports were processed and 33 FDA MedWatch reports, 28 initial reports, and 5 follow-up reports were submitted to FDA and Health Canada using this system. Experiences and lessons learned from the development and implementation of this system are presented in this paper.
Keywords: electronic safety reporting, clinical trial management system, MedWatch, web-based, system integration
1. Introduction
As part of the US Food and Drug Administration’s (FDA) mandate to protect human subjects involved in clinical investigations, requirements and procedures for the accurate and timely reporting of serious adverse events (SAEs) in Investigational New Drug (IND) trials are specified in the Code of Federal Regulations (CFR) Title 21 Part 312.1 The FDA has released a proposal for mandatory electronic safety reporting in order to improve the Agency’s ability to obtain safety information more quickly, which would lead to faster identification of potential safety problems. Additionally, the International Conference on Harmonisation has developed guidelines for definitions and standards for expedited reporting (e2a)2, data elements for the transmission of individual case safety reports (e2b) 3, and development of safety update reports (e2f) 4, 5. To ensure compliance with these guidelines and regulations in large multicenter trials, the trial’s sponsor, the participating clinical site Principal Investigators (PI) and Study Coordinators (SC) must have the proper tools to collect, process, and review safety information, as well as to coordinate the submission of reports to regulatory agencies. Additionally, trial sponsors must ensure that the information included in safety reports is consistent, accurate, and complete. Typically, clinical trial medical safety data have been managed outside of the database in which subject Case Report Form (CRF) data are managed. The principal reason for this separation is due to the differences in management procedures between safety and efficacy data. The downfall of stand-alone safety reporting systems is the potential for redundant data entry, and discrepancies between safety and efficacy data. Modern web-based information management technology allows for the combination of safety data management and efficacy data management into an integrated Clinical Trial Management System (CTMS).
2. Background
2.1 ALIAS study
The Albumin in Acute Stroke Trial (ALIAS, ClinicalTrials.gov identifier: NCT00235495) Part 1 was a National Institute of Neurological Disorder and Stroke-(NINDS-)funded, randomized, double blinded, placebo controlled, multicenter phase III trial designed to ascertain whether high-dose human albumin (ALB) therapy increases the proportion of acute ischemic stroke patients with favourable outcome compared to placebo therapy. Favourable outcome was defined as either a National Institutes of Health Stroke Scale (NIHSS) score of 0 or 1 or a modified Rankin Scale score (mRS) of 0 or 1, or both, measured at 3 months from randomization. Eligible subjects were randomized in a 1:1 ratio to either ALB or placebo (saline solution). Subjects are followed up to 12 months after treatment. A total of 434 subjects were enrolled from 49 United States sites and 13 Canadian sites.
2.2 Safety reporting requirements in ALIAS
According to the definitions included in Code of Federal Regulations (CFR) Title 21 Part 3121, and International Conference on Harmonization (ICH) Topic E2B3, an adverse event (AE) is defined as any untoward medical occurrence in a subject that does not necessarily have a causal relationship with the study treatment. A serious adverse event (SAE) is an AE that results in death; is life-threatening; requires inpatient hospitalization or prolongation of existing hospitalization, results in persistent or significant disability/incapacity, or is a congenital anomaly/birth defect. The ALIAS trial was operated under a FDA IND application. As a result, expedited reporting of safety events was required. These reporting requirements were mirrored by Health Canada, the corresponding national authority in Canada. The ALIAS study protocol, approved by the NINDS-appointed Data and Safety Monitoring Board (DSMB), the FDA, and subsequently by the Institutional Review Boards (IRBs) of all participating clinical sites, required documentation and reporting of all AEs that occurred from a subject’s randomization through the 3-month follow-up visit and all SAEs from the subject’s randomization through the End of Study (EOS) visit. Non-serious AEs were required to be reported with 5 days of first knowledge of the event, while SAEs were required to be reported within 24 hours of first knowledge of the event. Site PIs were required to review all SAE reports from their site prior to submission. All SAE reports were reviewed by two independent, blinded Medical Safety Monitors (MSMs) who submitted their opinions within 72 hours of notification as to whether the AE was serious, unexpected, and/or related to the study drug. The SAE review process was coordinated by the Project Manager (PM) for procedural issues and escorted by the Internal Medical Monitor (IMM) for clinical issues. Based on a pre-specified logic, if the opinions of the site PI and one or both of the MSMs concluded that the AE was serious, unexpected and related to the study drug, expedited reporting procedures were followed. The site had 48 hours to complete the MedWatch report. If the SAE was fatal or life-threatening, the PM at the Coordinating Center submitted the MedWatch report to the FDA within 7 days of first knowledge of the event. SAEs that were not fatal or life-threatening, but which required expedited reporting, were submitted to the FDA within 15 days of first knowledge of the event.
2.3 Challenges in ALIAS safety reporting
The implementation of the SAE report-processing procedures outlined above identified a number of issues, primarily due to human factors, that interfered with the efficient flow of SAE reporting and review. The following are some of the principal issues:
Variability in proficiency levels – Extreme unevenness in proficiency levels in identifying and reporting SAEs were evidenced among study personnel across the participating sites. It was essential to reduce these variations to ensure that the information contained in site submitted SAE reports was complete and accurate.
Training requirements – Training sessions on AE reporting were conducted prior to the start of the trial and periodically throughout the trial. Despite this training, there was a significant demand on an ‘as-needed-basis’ for individualized PM assistance.
Moving targets – In the ALIAS Trial, clinical site personnel found it difficult to collect all of the information required for MSM review within the required timeframes. This created a need to allow MSM review to proceed concurrently as additional AE data were gathered or pre-existing data were changed at the site. For example, while an AE report was being reviewed by the PM, the IMM, and the MSMs, the site might need to submit follow-up information or data corrections. This created a moving target for the SAE review and MedWatch preparation activities and caused problems for the coordination of the review activities.
3. Method
3.1 AE processing procedure development
A detailed and flexible AE processing procedure was necessary to meet the safety reporting requirements of regulatory agencies, to fit the ALIAS Trial’s operational practices, and to address the challenges listed above.
To streamline line AE reporting, SAE review and MedWatch report filing procedures, three different types of AE reports were defined: initial AE reports, AE follow-up reports, and AE data correction reports. Initial reports were used for new AEs. Clinical sites were required to report new AEs within the protocol specified time lines based on the most accurate and complete data available at time of the report. Follow-up reports were used when new or additional information for a previously reported AE became available (e.g., the outcome at the time of the original report was “Continuing” and later it was learned that the AE was “Resolved”). Data correction reports were used to edit incorrect data submitted on previous AE reports (e.g., the previous AE report indicated that the date of onset was Jan. 1, 2008, however, the date of onset was actually Jan. 2, 2008).
When a new AE was reported, site PI or designee was required to provide the following information: AE name, onset date and time, severity, outcome, date of resolution, seriousness, relationship to stroke, relationship to tissue plasminogen activator (tPA) therapy (if given), relationship to study drug, and actions taken. If the AE was serious, additional information was required, including whether the SAE was expected and a detailed narrative about the event. All SAEs were reviewed by two independent, blinded MSMs. The two MSMs were charged with voting on three criteria: serious (yes/no), expected (yes/no) and related to study drug (yes/no). When AE Follow-up reports or AE correction reports were submitted, the MSMs’ reviews were based on the most recent report. If the MSMs’ review resulted in the need for expedited reporting, the clinical, site study coordinator (SC) and PI were notified via an automated email generated by the computer system indicating that a MedWatch report must be completed for that SAE.
To assist the clinical site SC and PI, and the MSMs in their SAE review, an Internal Medical Monitor (IMM) function was included in the ALIAS SAE review process. The IMM’s primary role was: 1) to use his clinical expertise in reviewing the narrative information submitted by the clinical site PI and SC; 2) to provide a review based on his clinical experience and interact with clinical site PIs and SCs in order to reduce variations in safety reporting practices among different clinical sites; and, 3) to ensure that adequate information was available for the MSMs’ review. The IMM accomplished this by verifying the seriousness of reported SAEs and ensuring SAE narratives were complete prior to MSM review. The IMM also was available for consultation regarding whether follow-up reports of SAEs previously reviewed by the MSMs required subsequent review by MSMs.
The Project Manager (PM) was the central coordinator of ALIAS safety reporting. The PM provided professional support in regulatory compliance, safety reporting procedure, coordination and communication among clinical site PIs and SCs, MSMs, IMMs, and government agencies. The PM also provided procedural and technical assistance to clinical site PIs/SCs, MSMs, and IMMs to ensure that each properly completed his/her work in a timely manner. The PM was responsible for submitting MedWatch reports to FDA and Health Canada, and for notifying the Data and Safety Monitoring Board (DSMB) and all participating clinical site PIs and SCs of MedWatch events.
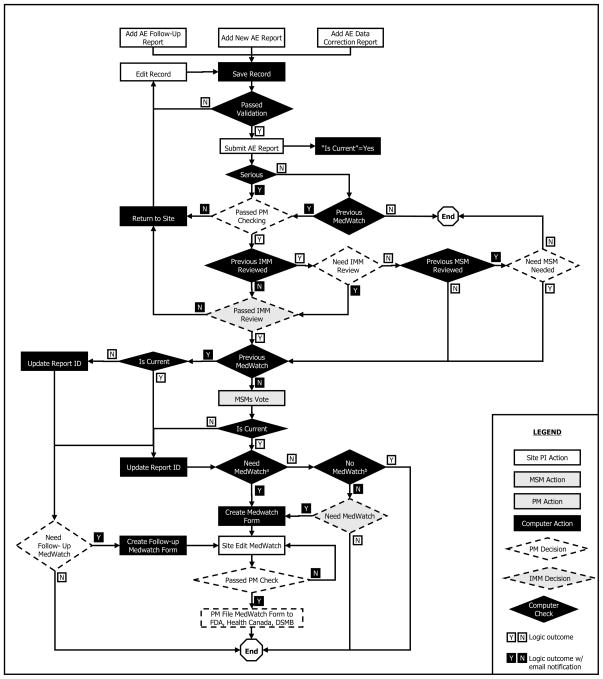
Based on the considerations discussed above, a comprehensive AE report-processing procedure was developed as shown in Figure 1.
Figure 1.
Comprehensive adverse event report processing procedure
a. b.: The algorithm for the necessity of MedWatch has three results: “Yes”, “No” and “No conclusion” based on scores obtained from Table 1.
This procedure was designed to allow for the processing of initial AE reports, AE follow-up reports and AE data correction reports. An indicator (“Is Current”) was used to flag the most current report for the AE. After an AE CRF was submitted, the following processing actions were applied to the current report: The PM checked the SAE report for completeness and accuracy; the IMM checked to ensure that the AE was serious and that the narrative of the event was complete and clinically relevant; AE reports that failed the PM and/or IMM review were returned to the clinical site for correction. The site SC and/or PI were required to address the concerns of the PM and/or IMM, and re-submit the AE CRF; all SAEs that passed the PM and IMM reviews were forwarded to the two MSMs in parallel; the MSMs reviewed the SAE to determine if the AE was serious, unexpected, and related to the study drug. Determination of whether a SAE required expedited reporting (a MedWatch report) was based upon the independently cast votes of the clinical site PI and the two MSMs. The algorithm is specified in Table 1.
Table 1.
Score summary algorithm of SAE votes for MedWatch
| Voter | Serious Yes=1, No=0 | Unexpected Yes=1, No=0 | Related Yes=1, No=0 | Total Score |
|---|---|---|---|---|
| MSM #1 | S11 | S12 | S13 | S1T=S11+S12+S13 |
| MSM #2 | S21 | S22 | S23 | S2T=S21+S22+S23 |
| Site PI | S31 | S32 | S33 | S3T=S31+S32+S33 |
| Sum | S= S1T+S2T+S3T | |||
If at least two individuals voted “Yes” to all three criteria, the SAE required a MedWatch report. If the total score sum was less than 6, the SAE did not require a MedWatch report. If no conclusion was made, the PM in consultation with the IMM was asked to make the final decision. This situation happened when the total score of the three voters was 3-2-2, 3-2-1, or 2-2-2. When an AE follow-up report or AE data correction report was submitted for a SAE that had previously been reported on a MedWatch report, the PM, in consultation with the IMM, determined whether a follow-up MedWatch report was required. When a follow-up MedWatch report was required, the PM notified the clinical site SC and PI that they needed to complete a follow-up MedWatch report and resubmit it. With finalization of the follow-up MedWatch report, the PM followed the same process outlined above for submission of the follow-up MedWatch report to FDA, Health Canada, the DSMB, and the PIs/SCs of all participating clinical sites.
3.2 Integration ESR module with CTMS
The ALIAS safety reporting module included data items in AE reports, AE report processing procedures, AE coding procedures and MedWatch reports, as shown in Table 2 and Table 3.
Table 2.
Data items for AE reporting and processing
| 1. AE Reporta | 2. AE Report Processingb | 3. AE Codingc |
|---|---|---|
| AE Report ID | PM: AE report completeness check | ALIAS AE Code |
| AE Report Type | PM: Need IMM review again? | ALIAS Coded By/On |
| Site | PM: Need MSM review again? | Modified AE name |
| Site PI | PM: MedWatch report completeness check | MedDRA low level term code |
| Subject ID | PM: Is MedWatch report needed? | MedDRA preferred term code |
| Subject Stratum | PM: Is Follow-up MedWatch needed? | MedDRA high level term code |
| Initial Report Time | IMM: SAE report check | MedDRA high level group code |
| Study Phase | MSM #1: Is AE Serious | MedDRA system organ class code |
| AE Name | MSM #1: Is AE Related to Study Drug | MedDRA preferred term coding path |
| Severity | MSM #1: Is AE Unexpected | MedDRA high level coding path |
| Serious | MSM #1: Review Notes | MedDRA high level group coding path |
| Expected | MSM #1: Voted By/On | MedDRA SOC coding path |
| Data/Time of Onset | MSM #2: Is AE Serious | MedDRA coded By/On |
| Outcome | MSM #2: Is AE Related to Study Drug | |
| Date of Resolution | MSM #2: Is AE Unexpected | |
| Related to Stroke | MSM #2: Review Notes | |
| Related to Study Drug | MSM #2: Voted By/On | |
| Related to tPA | ||
| Action Taken | ||
| SAE narrative |
data items included on the AE case report form.
data items generated during the review and processing of AE reports.
data items generated in the process of AE coding.
Table 3.
Data Items for MedWatch report
| Section | Data Item | Data Source |
|---|---|---|
| A. Patient Information | A1: Subject ID | [AE] |
| A2: Age at Time of AE | [AE] + [Subject] | |
| A3: Sex | [Subject] | |
| A4: Weight | [Baseline Vital] (editable) | |
| B. Adverse Event | B1: AE/Product Problem | =”AE” |
| B2: Outcome Attributed to AE | [AE] | |
| B2a: Date of death | [AE] | |
| B3: Date of event | [AE] | |
| B4: Date of this report | =System Date | |
| B5: Describe Event | [AE] | |
| B6: Relevant Tests/lab Data | [Lab Data] | |
| B7: Other Relevant History | [Medical History] | |
| C. Suspect Product | C1: Suspect Medication Name | =”Albumin 25% or Saline” |
| C2: Dose, Frequency & Route Used | [Treatment] | |
| C3: Therapy Date | [Treatment] | |
| C4: Diagnosis for Use | [Enrolment] | |
| C5: Event Abated after Use Stopped or Dose Reduced | Not Apply | |
| C6: Lot# | [Study Drug] | |
| C7: Exp. Date | [Study Drug] | |
| C8: Event Reappeared after Reintroduction | Not Apply | |
| C9: NDC# or Unique ID | =”7002” | |
| C10: Concomitant Medication | [Concomitant Meds] | |
| E. Initial Reporter | E1: Initial Reporter (Name, Address, Email) | [Site], [People] |
| E2: Initial Report Health Profession | =”Yes” | |
| E3: Initial Report Occupation | =”Neurologist” | |
| E4: Initial Reporter Also Sent Report to FDA | =”No” | |
| G. All Manufacturers | G1: Contact Office (Name, Address) | [Project] |
| G2: Contact Office Phone | [Project] | |
| G3: Report Source | =”Study” | |
| G4: Date Received by Manufacturer | NAa | |
| G5: IND Number | =”12817” | |
| G6: Protocol Number | =”BB-IND 12817” | |
| G7: Type of Report | [AE] | |
| G8: Adverse Event Term | [AE] | |
| G9: MedWatch Number | System |
Requirement for this item was waived by the regulatory agency.
Instead of creating an independent Electronic Safety Report (ESR) system to manage all of this information, we chose to integrate the ESR system into our existing home-grown CTMS as a module based on the following considerations:
The process for AE data collection and cleaning is similar to that of other Case Report Form (CRF) data. Treating the AE report as a CRF allowed all the data management tools developed within the CTMS to be used for AE reporting; this included CRF scheduling, data entry, rule-based data validation, data clarification query and response handling, data monitoring, data listing, data filtering and searching, triggering of event-driven email notifications, etc.
While reviewing an AE report, the PM, IMM, and MSMs often needed information beyond that included in the AE report, and, in most cases, collected on other ALIAS CRFs (e.g., medical history, prior medications, concomitant medications, lab data, study treatment data, etc.). Maintaining the AE data and other CRF data in the same database system increased the efficiency of AE review activities.
Many data items required for the MedWatch report could be retrieved from the CRFs, as shown in Table 3. When a new MedWatch report was needed, the system was able to pull much of the required information from the CRFs in the central database to pre-populate the “draft” MedWatch report which would subsequently be completed by clinical site personnel. Pre-populating the MedWatch report eliminated the duplication of data entry and reduced the risk of data discrepancies between the efficacy and safety data.
The tools needed for managing AEs, SAEs, and MedWatch reports were similar to the tools that were already developed in the CTMS for other project management tasks. These tools included user-account and user-privilege management, data-record adding, editing, saving, viewing, listing, and audit record checking, event-driven database actions and email notifications. By integrating the safety reporting system into the previously validated functions of the CTMS, the implementation of all safety reporting processes became easier, more efficient, and more reliable.
3.3 AE coding
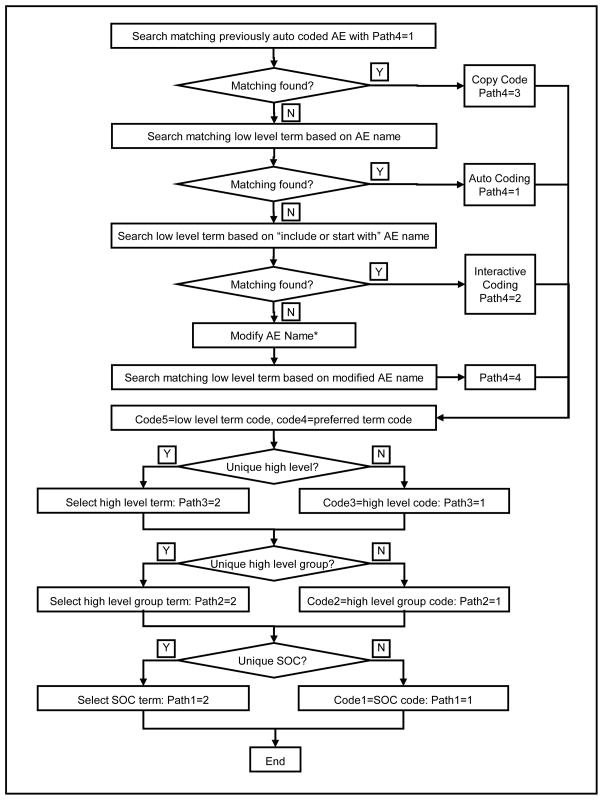
The Medical Dictionary for Regulatory Activities Terminology (MedDRA) was developed to facilitate the coding of “regulatory data” in biopharmaceutical development, clinical trials, and the reporting of adverse events.6 All adverse events were independently and centrally coded using MedDRA Version 12.0 based on the verbatim AE name entered into the AE CRF. The MedDRA system includes terms and codes at five levels: low-level term, preferred term, high-level term, high-level group term and system/organ-class term. Relationships between the terms of two consecutive levels are divided into two categories, primary and non-primary. With primary relationships each term at the current level links to only one term in the next level. Therefore, when an AE matches one term at the low level, the low-level code, the preferred-level code, the high-level code, the high-level group code, and the system/organ-class code can all be automatically coded based upon the primary relationship. Non-primary relationships apply to 6153 (out of 18483) preferred terms, 20 (out of 1699) high-level terms and 17 (out of 333) high-level group terms. In ALIAS AE MedDRA coding, we considered both primary and non-primary relationships. Figure 2 illustrates the MedDRA coding process. Specific data items, listed in column 3 of Table 2, were used to document the AE MedDRA coding process.
Figure 2.
AE MedDRA Coding Process
*Modified name is for AE MedDRA coding purpose only. Original AE name stays in database and will be used for reporting
If an AE name matched a low-level term, the low-level code and the preferred-level code were accepted, and the coding path 4 was set to 1 (auto coding). If the preferred-level term linked to only one high-level term, the high-level code was accepted, and coding path 3 was set to 1. This auto-coding process continued until the system/organ-class code was obtained or multiple next level terms occurred. In the situation in which multiple terms were provided, the central coder would pick the appropriate code based upon additional information included in the AE report. In this case, the coding path was set to 2 (interactive coding). After an interactive coding, if further coding levels existed, the system would attempt auto-coding first. If an AE name was auto-coded at the System Organ Class level, the MedDRA codes were propagated to other AE records with the same AE name. All coding paths for these AE records were set to 3 (copy previously auto-coded results). If no matching low-level term was found, the central coder was able to search the MedDRA low-level term table based on “starting from” or “including” the original AE name or a modified AE name. In this case, the coding path 4 was set to 4. If a modified AE name was used for MedDRA coding, it was saved in the database. This will not change the original AE name entered by the site. This modified AE name is used for MedDRA coding purpose only. Site will not be notified nor required to confirm this AE coding name modification. The original AE name will be used for safety reporting.
4. Results
ALIAS Trial Part 1 enrolled 434 subjects from 62 clinical sites. The AE CRF was posted as a required and repeatable CRF for each subject visit starting from the randomization until the 12-month follow-up visit. If there was no AE to be reported at a specific subject visit, the site was requested to submit a “No Data” AE CRF to confirm that there were no AEs to report. If more than one AE required reporting at a subject visit, the site was able to add more AE CRFs as needed.
4.1 AE reports summary
During the entire course of the trial, a total of 5,510 AE reports were submitted, including 4,719 initial AE reports, 472 AE follow-up reports, and 319 AE data correction reports. Follow-up and data-correction reports were submitted for 691 (14.6%) of the 4,719 initial reports. Of the 4,719 initial reports, 1,743 (36.9%) reports indicated “No Data”, and 2,976 (63.1%) reports confirmed occurrences of AEs, including 2,579 (86.7%) non-SAE reports and 397 (13.3%) SAE reports. More than half of the 397 SAEs occurred after hospital discharge through the 12 month follow-up period. Causes for these 397 SAE (in terms of MedDRA preferred term) include cerebrovascular accident, haemorrhagic transformation stroke, pneumonia, brain oedema, pneumonia aspiration, pulmonary oedema, myocardial infarction, cardiac failure congestive, etc. The MSMs reviewed a total of 415 SAE reports, including 376 initial AE reports, 15 follow-up reports and 24 data-correction reports. These 415 reports involved 392 individual adverse events; four were finalized as non-serious AEs and 388 were SAEs. Based on the MSMs’ reviews, 28 initial MedWatch reports and 5 follow-up MedWatch reports were filed with the FDA and Health Canada.
4.2 Timeliness of initial AE reporting
The median times between onset date and initial report date were 25 days and 11 days for non-serious AEs and SAEs, respectively. More detailed information presented by visit is provided in Table 4.
Table 4.
Number of days between AE onset date and initial reporting date
| Study Visit | No-serious AE | Serious AE | ||||
|---|---|---|---|---|---|---|
| N | Median | Mean | N | Median | Mean | |
| Treatment | 921 | 14.0 | 33.9 | 122 | 3.0 | 8.0 |
| 7 Day/Discharge | 690 | 23.0 | 55.9 | 64 | 9.5 | 15.0 |
| 1 Month Follow-up | 469 | 30.0 | 48.6 | 51 | 19.2 | 36.9 |
| 3 Month Follow-up | 273 | 49.0 | 62.9 | 38 | 39.5 | 44.9 |
| 6 Month Follow-up | 73 | 46.0 | 67.1 | 32 | 22.5 | 41.2 |
| 9 Month Follow-up | 68 | 54.0 | 59.2 | 42 | 32.5 | 39.5 |
| 12 Month Follow-up | 77 | 85.0 | 133.6 | 43 | 62.0 | 81.0 |
| Total | 2571 | 25.0 | 50.2 | 392 | 11.0 | 30.6 |
In many situations, the clinical site PI and/or SC did not become aware of an AE until days, weeks, or months after the onset date. This was especially true for AEs occurring after hospital discharge. Additionally, some AEs were not identified until the data monitor visited the sites and reviewed the medical record. Typically, site monitoring visits were conducted bi-annually. Thus the time delays between AE onset date and initial reporting date could be quite lengthy.
4.3 Medical Safety Monitor review
Two MSMs were involved in the independent review of SAEs. They determined whether the adverse event was serious (yes/no), unexpected (yes/no) and related to the study drug (yes/no). A total of 415 SAE reports were reviewed by the MSMs. Table 5 summarizes agreement between the clinical site PIs and the two MSMs on these 3 elements.
Table 5.
Agreement between clinical site PI and Medical Safety Monitors
| Serious | Unexpected | Related to Study Drug | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PI | MSM1 | MSM2 | N | % | PI | MSM1 MSM2 | N | % | PI | MSM1 MSM2 | N | % | ||
| Yes | Yes | Yes | 384 | 92.5 | Yes | Yes | Yes | 190 | 45.8 | Yes | Yes | Yes | 23 | 5.5 |
| Yes | Yes | No | 10 | 2.4 | Yes | Yes | No | 105 | 25.3 | Yes | Yes | No | 1 | 0.2 |
| Yes | Yes | --- | 4 | 1.0 | Yes | Yes | --- | 3 | 0.7 | Yes | Yes | --- | 2 | 0.5 |
| Yes | No | Yes | 11 | 2.7 | Yes | No | Yes | 5 | 1.2 | Yes | No | Yes | 5 | 1.2 |
| Yes | --- | Yes | 1 | 0.2 | Yes | No | No | 12 | 2.9 | Yes | No | No | 2 | 0.5 |
| Yes | --- | --- | 2 | 0.5 | Yes | --- | Yes | 1 | 0.2 | No | No | No | 344 | 82.9 |
| No | Yes | Yes | 1 | 0.2 | No | No | No | 32 | 7.7 | No | Yes | Yes | 1 | 0.2 |
| No | Yes | No | 1 | 0.2 | No | No | Yes | 11 | 2.7 | No | Yes | No | 2 | 0.5 |
| No | Yes | No | 27 | 6.5 | No | No | Yes | 29 | 7.0 | |||||
| No | Yes | Yes | 25 | 6.0 | No | No | --- | 2 | 0.5 | |||||
| No | Yes | --- | 1 | 0.2 | No | --- | No | 2 | 0.5 | |||||
| No | --- | No | 1 | 0.2 | No | --- | --- | 2 | 0.5 | |||||
| No | 2 | 0.5 | ||||||||||||
4.4 MedWatch report generation
Twenty-eight initial MedWatch reports and 5 Follow-up MedWatch reports were generated and submitted to FDA and Health Canada during the August 2006 to November 2007 period of the ALIAS Trial Part 1. The time to process an entire SAE (including site report, MSM review, MedWatch report generation, and site completion of the MedWatch report) took a median number of 21 days.
4.5 MedDRA coding
A total of 3,239 AE reports were coded using MedDRA, based upon the coding algorithm described in Figure 3. Auto-coding (a direct match between the original AE name and the MedDRA low-level term) was done for 1,602 (49.5%) records. Interactive coding based on the original AE name was applied to 154 (4.8%) records. The remaining 1,483 AEs were renamed by the AE central coder, so that the AE name would be a legitimate verbatim medical diagnosis. Of the renamed AEs, 906 passed auto coding, and 577 were coded interactively.
5. Discussion
In the ALIAS Trial Part 1, the implementation of the safety-reporting module integrated into the CTMS benefitted the entire study community, site PIs/SCs, MSMs, the PM, and data managers. The development and the use of this new system, however, created some unexpected challenges along the way.
Depending upon the nature of the disease studied and the organizational structure of the trial, the SAE reporting and review process might differ significantly among different trials. Even within a single trial, this process could be modified through the course of the study. Thus, it is beneficial to develop an ESR system with a generic structure, which is sufficiently flexible to be configured for different SAE processing procedures. The ESR module developed for ALIAS Trial part 1 used generic user interfaces for the AE CRF data capturing and processing. However, the underlying database table structure and the data-processing work-flow were customized specifically for the ALIAS Trial.
Due to the innate variability in the flow of SAE processing, not all steps can be adequately managed by pre-programmed computer logic. Proper placement of human intervention steps is necessary. The Project Manager in the ALIAS Trial Part 1 not only played a central coordinating role for the site SC/PI, MSMs and IMM, but also garnered the first-hand knowledge which was applied towards system improvement and enhancement.
Users are the key to the success of a computerized information management system. Unlike full-time data managers and project managers working in the trial’s coordination center, clinical site PIs and SCs often have only a small portion of their daily effort covered by the trial. They may use the ESR system only on an as-needed basis. A complex system is not adequate for this type of low-frequency user. Therefore, it is important to set up a system with simple structure and intuitive logic. During the ALIAS Trial Part1, users often had a hard time distinguishing an AE follow-up report and an AE data-correction report. It is better to allow the PM to make this distinction.
Acknowledgments
This work was supported by funding through the NIH grant (U01 NS054630) and (U01 NS40406) from National Institute of Neurological Disorders and Stroke (NINDS). The findings reported here are those of the authors and do not necessarily represent the views of NINDS. The authors would like to thank Drs. Stephan Mayer (Columbia University), Andrew Naidech (North Western University), and Alejandro Rabinstein (Mayo Clinic) for acting as the Medical Safety Monitors and Dr. Diego Tamariz for acting as the Central Coder for the ALIAS-Part 1 Trial. The authors would also like to thank the following ALIAS Executive Committee members for their guidance through the course of the trial: Dr. Claudia Moy (National Institute of Neurological Disorders and Stroke), Dr. Renee Martin (Medical University of South Carolina), Dr. Sharon Yeatts (Medical University of South Carolina), Mr. Richard Leinster (Medical University of South Carolina), Ms. Karla Ryckborst (University of Calgary), and Ms. Isa Mendez (University of Miami). Finally, the authors would like to thank the ALIAS- Part 1 Clinical Investigators and subjects without whom this manuscript would not have been possible.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Code of Federal Regulations, Title 21, Part 312: Investigational new drug application, US FDA, HHS, April 1, 2007.
- 2.ICH Harmonised Tripartite Guideline. Clinical Safety Data Management: Definitions and Standards for Expedited Reporting E2A, Current Step 4, version. Oct 27, 1994. [Google Scholar]
- 3.ICH Harmonised Tripartite Guideline. Maintenance of the ICH guideline on clinical safety data management: Data elements for transmission of individual case safety reports E2B(R2) Feb, 2001. [Google Scholar]
- 4.ICH Topic E2F. Development Safety Update Report, Step 3, Note for Guidance on Development Safety Update Report. European Medicines Agency; Jun, 2008. [Google Scholar]
- 5.The Development Safety Update Report (DSUR) Harmonizing the Format and Content for Periodic Safety Reporting During Clinical Trials: Report of CIOMS Working Group VII. Geneva: 2007. [Google Scholar]
- 6.Merrill Gary H. The MedDRA Paradox. AMIA Annu Symp Proc. 2008:470–474. [PMC free article] [PubMed] [Google Scholar]