Abstract
A simple and rapid method with high performance liquid chromatography/tandem mass spectrometry is described for the quantitation of the kinase inhibitor sorafenib and its active metabolite sorafenib N-oxide in human plasma. A protein precipitation extraction procedure was applied to 50 µL of plasma. Chromatographic separation of the two analytes, and the internal standard [2H3 13C]-sorafenib, was achieved on a C18 analytical column and isocratic flow at 0.3 mL/min for 4 min. Mean within-run and between-run precision for all analytes were <6.9 % and accuracy was <5.3%. Calibration curves were linear over the concentration range of 50 to 10,000 ng/mL for sorafenib and 10 to 2,500 ng/mL for sorafenib N-oxide. This method allows a specific, sensitive, and reliable determination of the kinase inhibitor sorafenib and its active metabolite sorafenib N-oxide in human plasma in a single analytical run.
1. Introduction
Sorafenib (4- pyridine 2 - carboxylic acid methylamide 4-methylbenzenesulfonate) (Fig. 1) is an orally administered kinase inhibitor that exhibits antiangiogenic and antitumor activity [1]. Sorafenib is an inhibitor of C-RAF, B-RAF, c-KIT, FLT-3, platelet-derived growth factor receptor-β (PDGFR-β), and vascular endothelial growth factor receptor (VEGFR) 1, 2, and 3, and is approved for the treatment of advanced renal cell carcinoma and hepatocellular carcinoma [1]. Sorafenib is currently being investigated for the treatment of other solid tumor malignancies [1] and acute myelogenous leukemia [2,3].
Figure 1.
Chemical structures of sorafenib and sorafenib N-oxide.
After oral administration of [14C]-sorafenib to healthy volunteers, approximately 77% of the dose is recovered in feces as unchanged drug and metabolites, and only 19% of the dose is excreted in the urine mainly as glucuronide conjugates of parent drug and metabolites [4]. Sorafenib undergoes oxidative metabolism by CYP3A4 and glucuronidation by UGT1A9 to 8 metabolites, in vitro and in vivo [5]. Sorafenib N-oxide, which is formed by CYP3A4-mediated metabolism, is the main circulating metabolite in human plasma and has similar in vitro potency to that of sorafenib [5]. In several clinical trials, sorafenib accounted for approximately 70 to 85% of circulating analytes in plasma and sorafenib N-oxide accounted for approximately 9 to 17% [5].
Wide intersubject variation in liver CYP3A4 activity has been observed in cancer patients (50-fold) which has been attributed to many factors including disease stage, multiple co-medications leading to drug interactions, and environmental factors [6]. To comprehensively characterize CYP3A4-mediated metabolism of sorafenib in cancer patients and the contribution of the principal metabolite sorafenib N-oxide to drug toxicity and efficacy, the quantitation of both analytes in human plasma was necessary. Several validated analytical assays have been described in detail for sorafenib. These methods are based on high performance liquid chromatography with ultraviolet detection [7] or tandem mass spectrometric detection (LC-MS/MS) [8–10]. In a study by Lathia et al. [4], concentrations of sorafenib and the metabolite sorafenib N-oxide were reported, but the analytical methods were not described in detail. Based on our previous analytical method for sorafenib [10], we established and validated a rapid, specific and reproducible method for the assessment of sorafenib and its active metabolite sorafenib N-oxide in human plasma in a single analytical run using LC-MS/MS. The method was successfully applied to pharmacokinetic monitoring of both analytes after a single sorafenib dose and at steady-state in children and adults with cancer.
2. Experimental
2.1. Chemical and reagents
Sorafenib was obtained from Chemie Tek (>99% purity, Indianapolis, IN) and sorafenib N-oxide was obtained from Toronto Research Chemicals Inc. (>99% purity, North York, ON, Canada). The internal standard [2H3,15N]-sorafenib was obtained from Alsachimie Inc. (>99% purity, Strasbourg, France). Methanol and acetonitrile were obtained from B&J Company (Muskegon, MI). Deionized water was generated from a Milli-Q-UF system (Millipore, Milford, MA). Normal donor human plasma was obtained from the St. Jude Children’s Research Hospital Blood Bank (Memphis, TN).
2.2. Stock solution, calibration and quality control samples
The sorafenib and sorafenib N-oxide stock solutions were prepared by dissolving 10 mg and 5 mg, respectively, with methanol in a 10 ml volume flask. Stock solutions were stored at −20°C. The working solutions of sorafenib and sorafenib N-oxide were prepared by diluting the stock solutions with 50% aqueous acetonitrile. The working solutions were diluted in blank human plasma to prepare calibration standards at concentrations of 50 to 10,000 ng/mL for sorafenib and 10 to 2500 ng/mL for sorafenib N-oxide. Quality control (QC) samples were prepared independently in blank plasma at four different concentrations (lower limit of quantitation [LOQ], low, medium and high concentrations) for sorafenib and sorafenib N-oxide. An additional dilutional QC was prepared at 80,000 ng/mL or 20,000 ng/mL of sorafenib or sorafenib N-oxide, respectively, and diluted 1:10 (v/v) in pooled human plasma for quantitation. The stock solution for the internal standard was prepared at a concentration of 1 mg/mL in methanol and were stored at −20°C.
2.3. Sample preparation
Frozen samples were thawed at room temperature. A 50 µL aliquot of standard, QC or patient sample was spiked into a polypropylene microcentrifuge tube and 250 µL of acetonitrile:methanol (1:1, v/v) solution containing the internal standard at a concentration of 150 ng/mL was added. The tube was vortex-mixed for 10 sec, followed by centrifugation at 10,000 rpm for 8 min at 4°C. The supernatant was transferred to an autosampler vial and 20 µL was injected for analysis.
2.4.HPLC and mass spectrometry conditions
Quantitation of analytes was carried out with a Waters 2692 separation system (Milford, MA) and Micromass Quattro LC triple-quadrupole system (Beverly, MA). Separation was achieved on a Waters X-Terra MS C18 column (3.5 µm, 50 × 2.1 mm) using a column heater operating at 30°C with a Waters X-Terra RP18 guard column (3.5 µm, 10 × 2.1 mm). The mobile phase was composed of 10 mM ammonium acetate (pH3.8 adjusted with formic acid)-0.1% formic acid in acetonitrile (35:65, v/v). The flow rate was 0.3 ml/min and the isocratic separation was completed within 4 min. The instrument was equipped with an electrospray interface, and was controlled by Masslynx 4.0 software (Micromass, UK). The analysis was performed in MRM mode: m/z 465.1>252.0 for sorafenib; m/z 481.0>286.0 for sorafenib N-oxide and m/z 469.0>256 for the internal standard. The MS/MS conditions were as follows: capillary voltage: 3kV; cone voltage: 50 v; source temperature: 130°C; desolvation temperature: 350 °C; desolvation gas flow: 600 l/h; and collision energy: 33 for sorafenib, 27 for sorafenib N-oxide and 35 for the internal standard.
2.5. Method validation
2.5.1. Specificity and selectivity
Interferences from endogenous compounds were investigated by analysis of six different lots of human blank plasma. The peak area needed to be less than 10% than the peak area for the lower limit of quantitation (LOQ) for both sorafenib and sorafenib N-oxide in plasma.
2.5.2. Calibration, accuracy and precision
Calibration curves of sorafenib and sorafenib N-oxide were created by plotting the peak area ratios of analyte to the internal standard against the analyte concentrations in the spiked plasma. Seven concentration points were used to generate the calibration curves. The back-calculated concentration for each standard was to be less than 15 % of the nominal concentration except the LOQ which was to be less than 20%. The intra-day and inter-day accuracy and precision were determined by assaying QC samples (LOQ, low, medium, and high) in triplicate at three different concentrations for four days. The estimates of the precision and accuracy were calculated as previously described [11].
2.5.3. Recovery and matrix effect
Recovery of sorafenib and sorafenib N-oxide from matrix was assessed by comparing the peak area of analyte spiked in the human plasma that underwent the extraction procedure with the peak area of analyte in neat solution. Three concentrations (high, medium, low) for each analyte were tested in triplicate. Matrix effect was evaluated by injecting blank human plasma extracts with continuous post-column infusion of the analytes.
2.5.4. Stability
Sorafenib and sorafenib N-oxide QC samples at three different concentrations were subjected to three freeze-thaw cycles at −70°C. Frozen samples were allowed to thaw at room temperature and were subsequently refrozen for at least 12 h. Short-term bench top stability was assessed for both analytes at room temperature at 0, 1, 2, 4 and 6 h.
2.6. Cross-validation
Low, medium, and high QC samples (4 replicates each), which contained sorafenib and sorafenib N-oxide, and 115 patient samples from 7 children receiving sorafenib were analyzed using the analytical method described in this manuscript in the laboratory at St. Jude Children’s Research Hospital, Memphis, TN and the laboratory at the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Baltimore, MD. Minor modifications to the method implemented at Johns Hopkins included: 1) 100 µL plasma aliquot; 2) the extraction solution consisted of 100% acentonitrile; 3) the flow rate was 0.2 mL/min; and 4) the run time was 6 min.
2.7. Application of method to clinical samples
Three children participating in a phase I trial at St. Jude Children’s Research Hospital received sorafenib 90 mg/m2 orally twice daily concurrently with oral cyclophosphamide once daily and intravenous bevacizumab once every 3 weeks. Blood samples were collected in heparin-containing tubes before drug administration and at 0.5, 2, 4.5, 6, 7.5, 24, and 48 h after administration of the first sorafenib dose. On day 3, twice daily administration was resumed and additional pre-treatment samples were collected prior to dose administration on days 7, 13 and 21 of cycle 1. Three adults participating in a phase I trial at Johns Hopkins received sorafenib 400 mg twice daily. Blood samples were collected in heparin-containing tubes before drug administration and at 0.25, 0.5, 1, 2, 4, 6, and 8 h after administration of the first sorafenib dose. Additional pre-treatment samples were collected prior to dose administration on days 2, 3, 8 and 15 of cycle 1. Blood samples were processed by centrifugation for 10 min at 1000 × g at 4 °C. Plasma supernatant was stored at −70 °C until subsequent analysis within 60 days for sorafenib and sorafenib N-oxide. All patients provided written informed consent and the clinical protocols were approved by the Institutional Review Board at each respective institution.
3. Results and discussion
3.1. HPLC-MS spectrometry and specificity
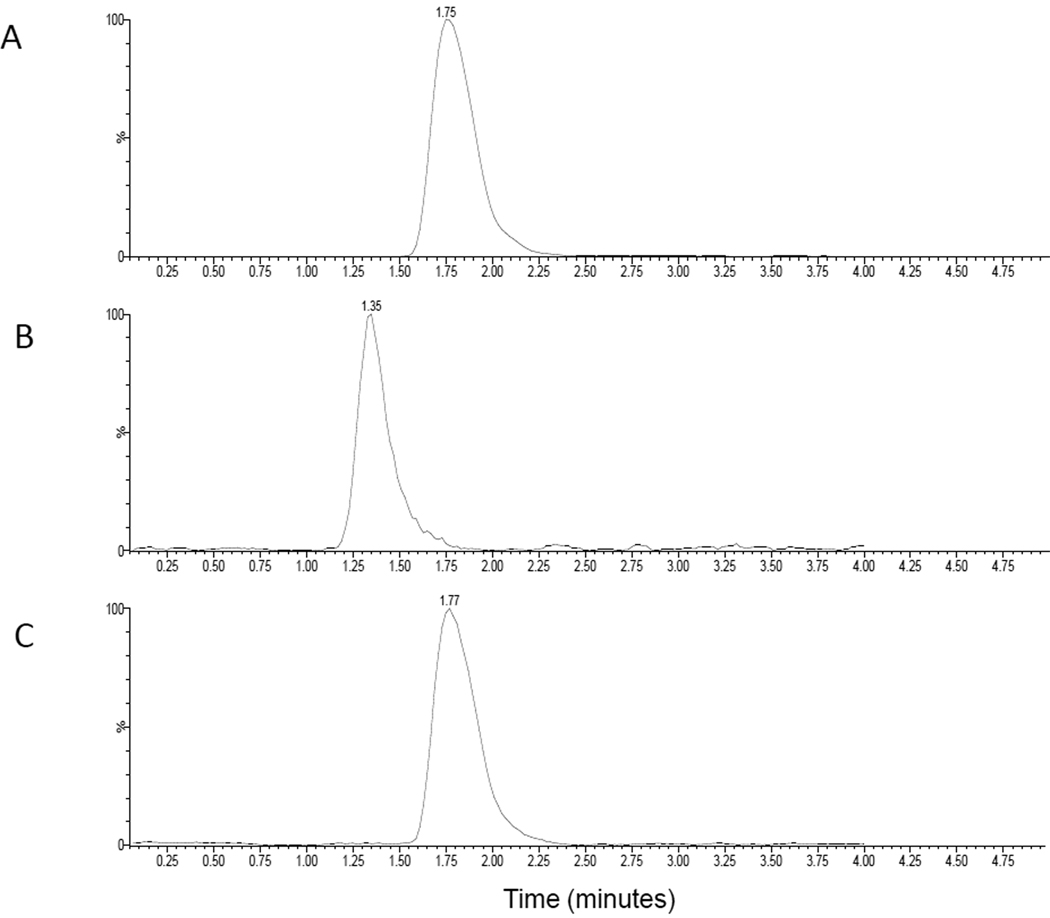
Electrospray ionization operated in positive ion mode was used for the LC-MS/MS analysis. The mass spectrums of sorafenib, sorafenib N-oxide and sorafenib isotope showed protonated molecular ions [M+H] + at 465.1, 481.0 and 469, respectively. The major products ions for sorafenib, sorafenib N-oxide and internal standard were at m/z 252.0, 286.0 and 256.0, respectively. Under the optimal HPLC conditions, sorafenib, sorafenib N-oxide and internal standard eluted at 1.77, 1.36 and 1.75 min. The total run time was within 4 min. Representative chromatograms of human plasma spiked with sorafenib (50 ng/ml), sorafenib N-oxide (10ng/ml) and internal standard (150 ng/ml) are shown in Fig. 2. Carryover was not obvious in blank matrices (less 10% of LOQ). Blank plasma samples from six different lots of human plasma showed no interference for the two analytes and internal standard.
Figure 2.
Representative chromatograms of human plasma spiked with A) internal standard 150 ng/mL, B) sorafenib N-oxide 10 ng/mL, and C) sorafenib 50 ng/ml.
3.2. Linearity and LOQ
The calibration curve for sorafenib was linear over the range of 50 to 10,000 ng/mL with a least-squares linear-regression correlation coefficient (R2) of ≥ 0.98 in all analytical runs. A weighting factor, which was inversely proportional to the variance at the given concentration (1/x2), was used. This weighting factor was compared to uniform weighting and then selected after evaluation of goodness-of-fit which included the following parameters: R2 value closest to a value of 1.0; intercept value closest to a zero value; the best agreement between back-calculated standard concentrations and the nominal value; and minimization of residuals. The mean signal to noise ratio for sorafenib 50 ng/mL was 49.4 (range, 26.4 to 115.8). The calibration curve for sorafenib N-oxide was linear between 10 to 2,500 ng/ml with a R2 of ≥ 0.995 in all analytical runs. The mean signal to noise ratio for sorafenib N-oxide 10 ng/mL was 37.7 (range, 15.8 to 52.1). The back-calculated concentrations for each point on the calibration curve for both compounds were always within 15% of nominal concentration except for the LOQ which was within 20 %.
3.3. Accuracy, precision, recovery and matrix effect
The results of the accuracy and precision are presented in Table 1. Assessment of between-run variation occurred over 4 days, and within-run variability was assessed over 2.5 hours each day. The within- and between-run precisions for sorafenib and sorafenib N-oxide were less than 6.9% for both analytes and the mean measured concentrations (accuracy) were all less than 5.3% of the nominal value for both analytes. Recovery of sorafenib and sorafenib N-oxide were from 80.5% to 95.3% over the analytical range. The recovery of internal standard from human plasma was 89%. No obvious matrix effect on sorafenib and sorafenib N-oxide was observed through the post-column infusion process.
Table 1.
Precision and accuracy of sorafenib and sorafenib N-oxide in human plasma.
| Sorafenib (ng/mL) | Sorafenib N-oxide (ng/mL) | |||||||
|---|---|---|---|---|---|---|---|---|
| 150 | 800 | 8000 | 80000 (1:10 dilution) |
30 | 200 | 2000 | 20000 (1:10 dilution) |
|
| Accuracy | ||||||||
| Mean (ng/mL) | 147 | 771 | 8088 | 80161 | 28.5 | 190 | 2103 | 20929 |
| SDa | 2.2 | 8.9 | 145 | 2509 | 1.8 | 12.5 | 157 | 1367 |
| DEVb (%) | −2.0 | −3.6 | 1.1 | 0.2 | −5.1 | −5.3 | 5.1 | 4.6 |
| Precision | ||||||||
| Within-run (%) | 1.2 | 0.9 | 1.6 | 0.2 | 6.5 | 6.9 | 6.2 | 0.5 |
| Between-run (%) | 1.1 | 0.8 | 1.0 | 0.3 | * | * | 4.6 | 0.5 |
SD: standard deviation;
DEV: deviation from the nominal value;
No significant variation was observed as a result of performing the assay in different runs.
3.4. Stability
Both sorafenib and sorafenib N-oxide are stable at room temperature up to 6 h with less or equal to 15% deviation from initial concentrations, except for the sorafenib N-oxide high QCs which were Less than 18% at 1 h and 4 h. Both compounds are stable through 3 freeze-thaw cycles with less than 15% deviation from initial concentrations. Sorafenib is stable in human plasma at −70°C for 204 days (last time-point tested). Sorafenib N-oxide is stable at −70°C for 85 days.
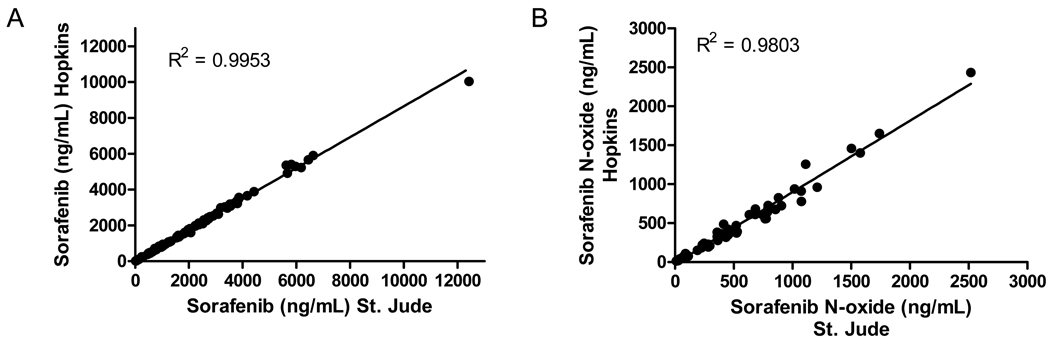
3.5. Cross validation
Low, medium, and high QC samples that were analyzed for sorafenib and sorafenib N-oxide at 2 separate institutions were all within 15% of nominal concentration. Plasma samples from children receiving sorafenib were also analyzed for sorafenib and sorafenib N-oxide at the 2 institutions. The agreement in analyte concentrations in patient samples are shown graphically in Fig. 3. Both analytical methods resulted in similar concentrations with variation less than 17%.
Figure 3.
Plot showing agreement between sorafenib (A) and sorafenib N-oxide (B) concentrations in patient samples analyzed at two institutions. The line is the fit of linear regression analysis to the data.
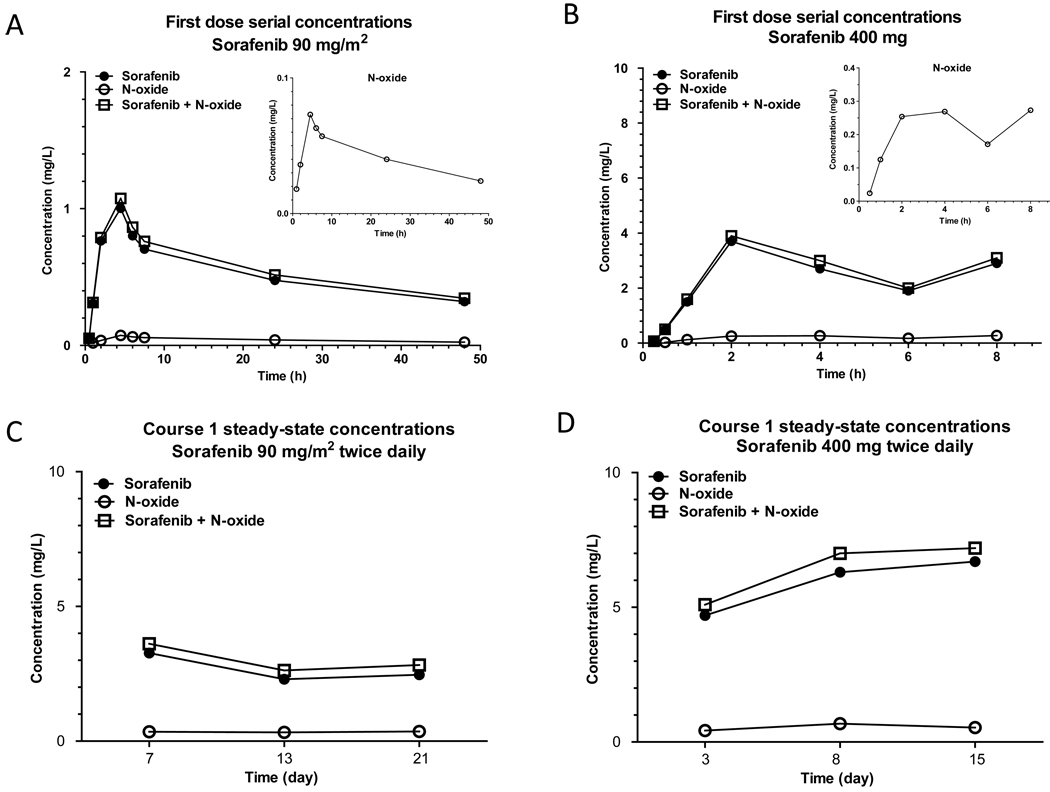
3.6. Clinical Application
The LC-MS/MS method was applied to the quantitation of sorafenib in plasma samples from 3 children and 3 adults receiving sorafenib. Fig. 4 shows mean plasma concentrations of sorafenib, sorafenib N-oxide, and the combination of the 2 analytes after the first dose (panel A) and in pre-treatment samples at steady-state (panel C) in children receiving sorafenib 90 mg/m2 twice daily. Plasma concentrations for adults receiving sorafenib 400 mg twice daily are also shown (panels B and D). In children, mean percentage N-oxide:sorafenib metabolic ratios at steady-state on days 7, 13, and 21 were 14% (range, 7% to 24%). In adults, mean percentage N-oxide: sorafenib metabolic ratios on days 8 and 15, were 8% (range, 4% to 13%).
Figure 4.
Mean serial plasma concentrations of sorafenib, sorafenib N-oxide and the total of both analytes after administration of the first dose of sorafenib 90 mg/m2 to three children (A) and the first dose of sorafenib 400 mg to three adults (B). Mean steady-state (pre-dose) plasma concentrations during cycle 1 in children (C) and adults (D).
4. Conclusion
A simple and specific LC-MS/MS method has been developed and validated for simultaneous quantitation of sorafenib and sorafenib N-oxide in human plasma. The straightforward sample preparation method and short analysis time allow potential high throughput sample analysis. This method has been successfully applied to the monitoring of sorafenib and its metabolite sorafenib N-oxide during daily continuous administration of sorafenib to adults and children over a range of doses and will be utilized to characterize the pharmacokinetics of these compounds in different patient populations receiving sorafenib administered as a single-agent and in combination with chemotherapy and other concurrent medications.
Acknowledgements
This work was supported by the United States Public Health Service Cancer Center Support Grants P30 CA021765 and P30 CA069773, the American Lebanese Syrian Associated Charities (ALSAC), and the National Institutes of Health grants UL1 RR025005 and U01 CA70095.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wilhelm S, Carter C, Lynch M, Lowinger T, Dumas J, Smith RA, Schwartz B, Simantov R, Kelley S. Nat Rev Drug Discov. 2006;5:835. doi: 10.1038/nrd2130. [DOI] [PubMed] [Google Scholar]
- 2.Mori S, Cortes J, Kantarjian H, Zhang W, Andreef M, Ravandi F. Leuk Lymphoma. 2008;49:2246. doi: 10.1080/10428190802510349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ravandi F, Cortes JE, Jones D, Faderl S, Garcia-Manero G, Konopleva MY, O'Brien S, Estrov Z, Borthakur G, Thomas D, Pierce SR, Brandt M, Byrd A, Bekele BN, Pratz K, Luthra R, Levis M, Andreeff M, Kantarjian HM. J Clin Oncol [Google Scholar]
- 4.Lathia C, Lettieri J, Cihon F, Gallentine M, Radtke M, Sundaresan P. Cancer Chemother Pharmacol. 2006;57:685. doi: 10.1007/s00280-005-0068-6. [DOI] [PubMed] [Google Scholar]
- 5.Keating GM, Santoro A. Drugs. 2009;69:223. doi: 10.2165/00003495-200969020-00006. [DOI] [PubMed] [Google Scholar]
- 6.Baker SD, van Schaik RH, Rivory LP, Ten Tije AJ, Dinh K, Graveland WJ, Schenk PW, Charles KA, Clarke SJ, Carducci MA, McGuire WP, Dawkins F, Gelderblom H, Verweij J, Sparreboom A. Clin Cancer Res. 2004;10:8341. doi: 10.1158/1078-0432.CCR-04-1371. [DOI] [PubMed] [Google Scholar]
- 7.Blanchet B, Billemont B, Cramard J, Benichou AS, Chhun S, Harcouet L, Ropert S, Dauphin A, Goldwasser F, Tod M. J Pharm Biomed Anal. 2009;49:1109. doi: 10.1016/j.jpba.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 8.Haouala A, Zanolari B, Rochat B, Montemurro M, Zaman K, Duchosal MA, Ris HB, Leyvraz S, Widmer N, Decosterd LA. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:1982. doi: 10.1016/j.jchromb.2009.04.045. [DOI] [PubMed] [Google Scholar]
- 9.Jain L, Gardner ER, Venitz J, Dahut W, Figg WD. J Pharm Biomed Anal. 2008;46:362. doi: 10.1016/j.jpba.2007.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao M, Rudek MA, He P, Hafner FT, Radtke M, Wright JJ, Smith BD, Messersmith WA, Hidalgo M, Baker SD. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;846:1. doi: 10.1016/j.jchromb.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Rosing H, Man WY, Doyle E, Bult A, Beijnen JH. J Liq Chromatogr Relat Technol. 2000;23:329. [Google Scholar]