Abstract
Anti-Müllerian hormone (AMH) is produced by granulosa cells in primary to small antral follicles of the adult ovary and helps maintain primordial follicles in a dormant state. The industrial chemical, 4-vinylcyclohexene diepoxide (VCD) causes specific ovotoxicity in primordial and small primary follicles of mice and rats. Previous studies suggest that this ovotoxicity involves acceleration of primordial to primary follicle recruitment via interactions with the Kit/Kit ligand signaling pathway. Because of its accepted role in inhibiting primordial follicle recruitment, the present study was designed to investigate a possible interaction between AMH and VCD-induced ovotoxicity. Protein distribution of AMH was compared in neonatal and adult F344 rat ovaries. AMH protein was visualized by immunofluorescence microscopy in large primary and secondary follicles of the adult ovary, but in small primary follicles in neonatal rat ovaries. In cultured postnatal day (PND) 4 F344 rat ovaries, VCD exposure (30 μM, 2-8d) decreased (P<0.05) AMH mRNA (d4-8) and protein (d6-8). Recombinant AMH (100-400 mg/ml) in PND4 ovaries cultured 8d ± VCD (30 μM) caused an increase (P<0.05) in primordial, and a decrease (P<0.05) in small primary follicles, supporting that AMH retarded primordial follicle recruitment. However, no concentration of AMH had an effect on VCD-induced ovotoxicity. Whereas, VCD caused a reduction in expression of AMH (d4-d8), it followed previously reported initial disruptions in Kit signaling induced by VCD (d2). Thus, collectively, these results do not support a mechanism whereby VCD causes ovotoxicity via generalized activation of primordial follicle recruitment, but instead provide further support for the specificity of other intracellular mechanisms involved in VCD-induced ovotoxicity.
Keywords: anti-Müllerian hormone, Müllerian inhibiting substance, 4-vinylcyclohexene diepoxide, follicle, ovary
Introduction
Female mammals are born with a finite pool of immature ovarian primordial follicles (Hirshfield, 1991). Primordial follicles are selected for activation, however, only a few (<1%) of the oocytes in these follicles will fully develop and be ovulated. Instead, the vast majority become activated and degrade by a process of atresia. Individual follicle activation can occur relatively early or much later in the life span of a female (Hirshfield, 1991). Factors coordinating this activation/recruitment are not clearly understood. It is thought that the rate of primordial follicle activation/recruitment is influenced in part by signaling from larger growing follicles (Adhikari and Liu, 2009).
Anti-Müllerian hormone (AMH), also known as Müllerian inhibiting substance (MIS), is a member of the transforming growth factor β (TGFβ) superfamily and uses a bone morphogenic protein-like pathway for signal transduction. AMH was first recognized for its role in causing regression of Müllerian ducts during fetal development in males (Sinisi et al., 2003). In females, AMH is expressed postnatally by granulosa cells of ovarian growing pre-antral and early antral follicles in which it decreases the responsiveness of growing follicles to FSH (Broekmans et al., 2008). Additionally, AMH inhibits the recruitment of primordial follicles into the pool of growing follicles (Durlinger et al., 2001, 2002a, 2002b). It is unclear, however, whether AMH has a direct effect on primordial follicles or provides the signaling indirectly. Previous studies in adult rats have shown that AMH is not expressed in primordial or small primary follicles, but is first expressed in granulosa cells of maturing large primary follicles and is at its maximal expression in the small preantral follicles (Baarends et al., 1995). At the large antral follicle stage, AMH expression diminishes and ultimately becomes undetectable once FSH-dependent follicular growth is initiated (Durlinger et al., 2001). Thus, it appears that AMH provides a mechanism by which larger follicles can indirectly influence and suppress primordial follicle development by having a direct effect on small follicles. However, the mechanisms involved are not clearly understood since the two receptors for AMH are located in the granulosa cells of secondary and antral follicles (Durlinger et al., 2002b). As a result, the mechanism by which AMH inhibits primordial follicle recruitment is unknown. Further, AMH is not the only important factor for regulating follicle recruitment because AMH knockout mice still retain some primordial follicles at 13 months of age (Durlinger, et al., 1999).
4-Vinylcyclohexene diepoxide (VCD) is a by-product of the chemical synthesis of rubber tires, insecticides, flame retardants, and plasticizers (Rappaport and Fraser, 1977). VCD selectively targets small pre-antral (primordial and small primary) follicles in the ovaries of rats and mice (Springer et al., 1996a; 1996b). Extensive repeated exposure of rats and mice to VCD results in depletion of the follicular pool and premature ovarian failure (Mayer et al., 2002; Devine and Hoyer, 2005). Thus, exposure of women to VCD is viewed as a potential health risk.
The neonatal rat ovarian culture system is an ideal model for studying VCD-induced ovotoxicity because those ovaries predominantly contain the earliest follicle stages, which are selectively targeted by VCD. Prior studies have characterized this model and demonstrated that ovaries cultured for up to 15 days remained healthy, and follicle development progressed to the secondary stage over time (Devine et al., 2002). Further, incubation of cultured neonatal rat ovaries with 30 μM VCD for 8d caused a significant loss of primordial and small primary follicles. However, VCD treatment for 8d did not induce visible signs of necrosis nor was follicle development or morphology grossly altered relative to controls.
Previous mechanistic studies using the ovary culture system demonstrated that VCD interacts with the Kit/Kit ligand signaling pathway. VCD exposure caused a decrease (P<0.05) in Kit mRNA expression in rat ovaries by both in vivo and in vitro exposure (Fernandez et al., 2008). A time course of postnatal day (PND) 4 rat ovaries exposed in vitro to VCD (d2-8) observed a decrease in Kit mRNA on d4, prior to the observed follicle loss on d6 (Fernandez et al., 2008; Keating et al., 2009a). Additionally, exogenous Kit ligand was able to attenuate VCD-induced follicle loss (Fernandez et al., 2008). These findings provided a possible mechanism by which the Kit/Kit ligand pathway is targeted in VCD-induced ovotoxicity.
Previous observations investigated an involvement of PI3kinase as a signaling molecule downstream of Kit which directs primordial follicle activation and recruitment (Liu et al., 2006). LY294002, a competitive inhibitor of PI3kinase, was used to prevent the recruitment of primordial follicles into the primary follicle pool. Interestingly, this inhibition also prevented VCD-induced ovotoxicity in primordial follicles, but increased follicle loss in small primary follicles (Keating et al., 2009a). AMH signaling has also been shown to play a significant role in primordial follicle recruitment; however, its potential ability to interact with VCD-induced ovotoxicity is unknown. Therefore, the present study was designed to further investigate the selectivity of VCD for the Kit/PI3K signaling pathway by determining whether inhibition of primordial follicle recruitment using exogenous AMH could also impact VCD-induced loss of primordial and/or small primary follicles. In addition, AMH mRNA and protein expression patterns are compared in neonatal and adult rat ovaries.
Materials and Methods
Reagents
4-vinylcyclohexene diepoxide (VCD; CAS # 106-87-6; >99% purity), bovine serum albumin (BSA), ascorbic acid (Vitamin C), and transferrin were purchased from Sigma-Aldrich Inc. (St Louis, MO). Dulbecco’s Modified Eagle Medium: nutrient mixture F-12 (Ham) 1X (DMEM/Ham’s F12), Albumax, penicillin/streptomycin (5000U/ml, 5000μg/ml, respectively), Hanks’ Balanced Salt Solution (without CaCl2, MgCl2, or MgSO4) were obtained from Invitrogen Co. (Carlsbad, CA). Millicell-CM filter inserts were purchased from Millipore (Bedford, MA), and 48-well cell culture plates were obtained from Corning Inc. (Corning, NY). Anti-AMH antibody and donkey anti-goat secondary antibody were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). BCA protein quantification kits were obtained from Pierce Biotechnology (Rockford, IL). Cy-5-streptavidin was obtained from Vector (Burlingame, CA). YOYO-1 was purchased from Molecular Probes (Eugene, OR). Recombinant human AMH was purchased from R&D Systems (Minneapolis, MN).
Animals
A breeding colony was established from Fischer 344 rats that were originally purchased from Harlan Laboratories (Indianapolis, IN) to use as a source of PND4 female rat pup ovaries for culture. All pregnant animals were housed singly in plastic cages, and maintained in a controlled environment (22 ± 2°C; 12h light/ 12h dark cycles). The animals were provided a standard diet with ad libidum access to food and water, and allowed to give birth. All animal experiments were approved by the University of Arizona’s Institutional Animal Care and Use Committee.
Rat ovary collection
Ovaries were collected from sixteen week old adult cycling female F344 rats (n=3) in the breeding colony. Ovaries were collected on the day of estrus (determined by vaginal cytology). Ovaries were also collected from PND4 and PND6 rats. Rats were euthanized by CO2 inhalation and the ovaries were removed. The oviduct and excess tissue was trimmed from the ovaries, which were snap frozen in liquid nitrogen, and stored at −80°C until processing for histological evaluation or for protein isolation.
In vitro ovarian culture
Ovaries from PND4 F344 rats were cultured as described by Devine et al. (2002). Briefly, PND4 female F344 rats were euthanized by CO2 inhalation followed by decapitation. Each ovary was removed, and after oviduct and excess tissue were trimmed, it was placed on a piece of Millicell-CM membrane floating on 250 μl of DMEM/Ham’s F12 medium containing 1 mg/ml BSA, 1 mg/ml Albumax, 50 μg/ml ascorbic acid, 5 U/ml penicillin/5 μg/ml streptomycin, and 27.5 μg/ml transferrin per well in a 48-well plate previously equilibrated to 37°C. Using fine forceps, a drop of medium was placed to cover the top of the ovary to prevent drying. Plates containing ovaries were cultured at 37°C and 5% CO2 in air. For those cultures lasting more than 2d, media were removed and fresh medium ± treatment were replaced every 2d. Ovaries were treated with vehicle control medium (1% DMSO), or VCD (30 μM) ± AMH (100, 200, 400 ng/ml) for 8d. The concentrations and conditions of VCD exposures were previously determined to cause follicle loss in vitro (Fernandez et al., 2008). All cultured ovaries remained viable up through 8 days in culture; consistent with a previous report (Devine et al., 2002).
Histological evaluation of follicle numbers
Following incubation, ovaries were placed in Bouin’s fixative for 1.5h, transferred to 70% ethanol, embedded in paraffin, serially sectioned (5 μM thick), and every 6th section was mounted. All ovarian sections were stained with hematoxylin and eosin. Healthy oocyte-containing follicles were classified and counted in every 12th section. Unhealthy follicles were distinguished from healthy follicles by granulosa cell content of pyknotic bodies and intense eosinophilic staining of oocytes (Devine et al., 2002). Follicle population classification was according to the procedure of Flaws et al. (1994) with modifications. Briefly, primordial follicles contained the oocyte surrounded by a single layer of squamous-shaped granulosa cells, small primary follicles contained the oocyte surrounded by three to twenty cuboidal-shaped granulosa cells, large primary follicles contained the oocyte surrounded by greater than twenty cuboidal-shaped granulosa cells and secondary follicles contained the oocyte surrounded by multiple layers of granulosa cells. In cultured neonatal rat ovaries, no follicle development beyond the secondary stage was observed. Histological images were captured with an Olympus IX-70 inverted microscope.
Immunofluorescence staining and confocal microscopy
Following in vitro culture or collection from 16 week old adult rats, ovaries were fixed in 4% buffered formalin for 2-4 h, transferred to 70% ethanol, embedded in paraffin, serially sectioned, and every 10th section was mounted. Sections were deparaffinized (approximately 10 sections/ovary) and incubated with primary antibody directed against AMH (1:200 dilution) 4°C overnight. Secondary biotinylated antibody was applied for 1 h, followed by CY-5-streptavidin (1 h; 1:100 dilution). Sections were treated with Ribonuclease A (100μg/ml) for 1 h, followed by staining with YOYO-1 (10 min; 5nM). Slides were repeatedly rinsed with phosphate buffered saline (PBS), cover-slipped, and stored in the dark (4°C) until visualization. Primary antibody was not added to immuno-negative ovarian sections. Immunofluorescence was visualized on a Zeiss (LSM 510 NLO-Meta) confocal microscope with an argon and helium-neon laser projected through the tissue into a photomultiplier at λ = 488 and 633 nm for YOYO-1 (green) and CY-5 (red), respectively. All images were captured using a 40 X objective lens. Multiple sections were imaged and analyzed throughout each ovary.
RNA isolation
Following in vitro culture, ovaries treated with vehicle control or VCD (30 μM) were stored in RNAlater at −80°C. Total RNA was isolated (n=3; 10 ovaries per pool) using an RNeasy Mini kit. Briefly, ovaries were lysed and homogenized using a motor pestle followed by applying the mixture onto a QIAshredder column. The QIAshredder column containing ovarian tissue sample was then centrifuged at 14,000 rpm for 2 min. The resulting flow-through was applied to an RNeasy mini column, allowing RNA to bind to the filter cartridge. Following washing, RNA was eluted from the filter, and concentrated using an RNeasy MinElute kit. Briefly, isolated RNA was applied to an RNeasy MinElute spin column, and after washing, RNA was eluted using 14μL of RNase-free water. RNA concentration was determined using an ND-1000 Spectrophotometer (λ = 260/280nm; NanoDrop technologies, Inc., Wilmington, DE).
First strand cDNA synthesis and real-time polymerase chain reaction (PCR)
Total RNA (0.5μg) was reverse transcribed into cDNA utilizing the Superscript III One-Step RT-PCR System. cDNA was diluted (1:25) in RNase-free water. Two microliters of diluted cDNA were amplified on a Rotor-Gene 3000 using Quantitect™ SYBR Green PCR kit and custom designed primers for AMH (forward primer: 5′ GGA GAC CTA CCA AGC CAA CA 3′; reverse primer: 5′ CAT TTT TAG CAG CAG CCA CCA; NCBI Genbank accession number NM_007445) and β-actin (forward primer: 5′ TCT ATC CTG GCC TCA CTG TC 3′; reverse primer: 5′ ACG CAG CTC AGT AAC AGT CC 3′; NCBI Genbank accession number NM_031144). The cycling program consisted of a 15 min hold at 95°C and 45 cycles of: denaturing at 95°C for 15s, annealing at 58°C for 15s, and extension at 72°C for 20s at which point data were acquired. Product melt conditions were determined using a temperature gradient from 72°C to 99°C with a 1°C increase at each step. There was no difference in β-actin mRNA between vehicle control and VCD-treated ovaries. Therefore, each sample was normalized to β-actin before quantification.
Protein Isolation
Pools of whole ovarian protein homogenates (cultured ovaries, 10 per pool; 16 week old ovaries, 1 per pool) were prepared from cultured ovaries via homogenization in tissue lysis buffer as previously described (Thompson et al., 2005). Briefly, homogenized samples were placed on ice for 30 min, followed by two rounds of centrifugation at 10,000 rpm for 15 min. Supernatant was aliquoted and stored at −80°C until further use. Protein was quantified using a standard BCA protocol on a 96-well assay plate. Emission absorbance values were detected with a λ= 540nm excitation on a Synergy™ HT Multi-Detection Microplate Reader using KC4™ software (Bio-Tek® Instruments Inc., Winooski, VT). Protein concentrations were calculated from a BSA protein standard curve.
Western Blot Analysis
SDS-PAGE (10%) was used to separate proteins in homogenates (25μg; n=3) and subsequently transferred onto nitrocellulose membranes as previously described (Thompson et al., 2005). Briefly, membranes were blocked for 1 h with shaking at 4°C in 5% milk in Tris-buffered saline with Tween-20 (TTBS). Membranes were incubated with primary antibody in 5% milk in TTBS overnight at 4°C. The antibody dilution used was AMH (1:200). Membranes were washed three times for 10 min each with TTBS. HRP-conjugated secondary antibody (1:2000 dilution) was added for 1 h at room temperature. Membranes were washed three times for 10 min each in TTBS, followed by a single wash for 10 min in Tris Buffered Saline (TBS). Western blots were detected by chemiluminescence (using ECL plus chemiluminescence detection substrate) and exposed to X-ray film. Densitometry of the appropriate bands was performed using LabWorks™ software from a UVP Bioimaging system (UVP Inc., Upland, CA). There was no difference in β-actin protein between vehicle control and VCD-treated ovaries. Therefore, each sample was normalized to β-actin before quantification and expressed as the relative protein intensity.
Statistical analysis
For Western blot data (Figure 3), a 2-way ANOVA with a Bonferroni post test was performed. All other data were analyzed using ANOVA with a Tukey post test.
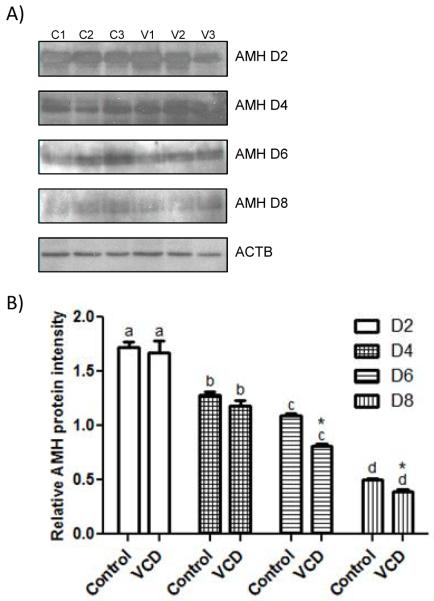
Figure 3.
Effect of VCD on expression of AMH protein. Ovaries from PND4 F344 rats were cultured with control medium ± VCD (30 μM) for 2-8 days. (labeled D2-8) (A) Representative Western blots for AMH and β-actin (ACTB) expression in cultured ovaries in control medium (C1, C2, C3) or medium containing VCD (V1, V2, V3). One representative ACTB blot from d2 is shown, however, ACTB expression was measured at each time point. (B) AMH protein intensity was normalized to ACTB protein and expressed as mean ± SEM; n = 3; 10 ovaries/pool for cultured ovary samples; different superscript letters indicate a differences between time points (P < 0.05) difference; *P < 0.05, different from control.
Analysis was performed using GraphPad Prism version 5.02 (GraphPad Software, San Diego, CA). The assigned level of significance for all tests was P < 0.05.
Results
Localization of AMH protein in cultured neonatal and adult ovary
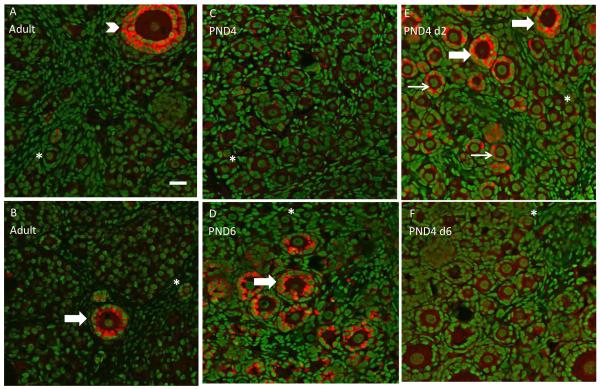
A comparison of AMH protein distribution was investigated in adult and neonatal F344 rat ovaries. Protein was observed in PND4 ovaries cultured for 2d or 6d to confirm AMH protein expression patterns at time points chosen for later experiments. In adult ovaries, immunofluorescence staining with confocal microscopy demonstrates AMH protein in cytoplasm of granulosa cells in large primary and secondary follicles, but not in primordial or small primary follicles (Figure 1A, 1B). Also, no AMH staining can be observed in theca cells, interstitium, or corpora lutea (data not shown). In PND4 ovaries there is weak staining for AMH protein (Figure 1C), however, AMH staining increases after 2d of culture (Figure 1E). In PND4 ovaries cultured for 2d, AMH staining is seen in cytoplasm of granulosa cells in small and large primary follicles (Figure 1E). Cultured ovaries exposed to control media for 6d demonstrate a decrease in AMH protein staining compared to d2 of culture (Figure 1E, 1F). AMH staining intensity was similar between PND6 ovaries and PND4 ovaries cultured for 2d (analogous time points; Figure 1D, 1E). All samples of in vivo and in vitro ovaries appeared healthy, with no signs of apoptosis or necrosis.
Figure 1.
Ovarian AMH protein staining. In vivo ovaries were collected from adult F344 rats (16 weeks; A and B), PND4 (C) or PND6 (D) rats. PND4 ovaries were cultured in vitro with control medium for 2 (E) or 6 days (F). All ovaries were processed for confocal microscopy as described in materials and methods. All panels are a combined overlay of AMH (Cy-5 red stain) and genomic DNA (green YOYO stain) at 40X magnification. Thin arrow, small primary follicle; thick arrow, large primary follicle; arrowhead, secondary follicle; asterisk, primordial follicle. Scale-bar equal to 25 μM.
Effect of VCD on AMH mRNA and protein levels
The effect of VCD (30 μM) on ovarian Amh mRNA was measured using real-time RT-PCR. The relative abundance of Amh mRNA on d 2-8 following VCD (30 μM) exposure is shown in Figure 2. Relative to control, Amh mRNA was decreased (P<0.05) beginning on d4 (4d: 0.48±0.15, VCD/control; 6d: 0.52±0.11 VCD/control; 8d: 0.73±0.07 VCD/control).
Figure 2.
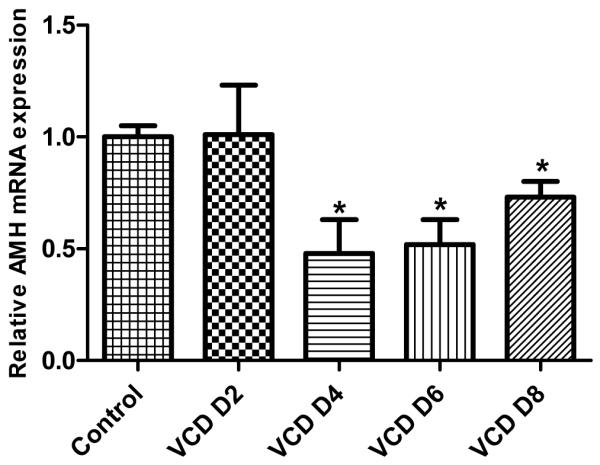
Effect of VCD on expression of mRNA encoding Amh. Ovaries from PND4 F344 rats were cultured with control medium ± VCD (30 μM) for 2-8 days. (labeled VCD D2-8). Total RNA was extracted, and relative amounts of mRNA encoding Amh were determined using quantitative real-time PCR as described in Materials and Methods. Values indicate the mean fold change (normalized to β-actin) ± SEM; n = 3; 10 ovaries/pool; *P < 0.05, different from control.
The effect of VCD on relative amounts of total ovarian AMH protein in cultured neonatal ovaries was also measured using Western blotting followed by densitometric analysis. Ovaries were collected from F344 PND4 rats and cultured for 2-8 days ± VCD (30 μM, Fig 3A). AMH protein levels declined (P<0.05) over the time course of culture (d2-8), while β-actin protein levels remained consistent among samples. Thus, β-actin was used to normalize the intensity of AMH in each sample. Relative to the time-matched control, VCD exposure caused a decrease (P<0.05) in AMH protein on 6d and 8d of culture (Figure 3B).
Effect of exogenous AMH on VCD-induced follicle loss
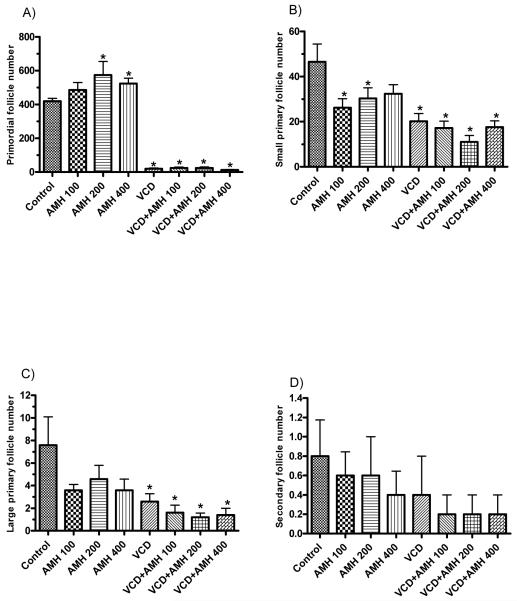
The effect of recombinant AMH on specific ovarian follicle populations on d8 of culture was investigated (Figure 4). At 200 and 400 ng/ml of AMH, there was an increase (P<0.05) in primordial follicle numbers relative to controls (Figure 4A). Furthermore, at 100 and 200 ng/ml of AMH, there was a decrease (P<0.05) in small primary follicles (P < 0.05; Figure 4B). Relative to control, there was no effect of exogenous AMH alone on large primary follicles or secondary follicles (Figure 4C, 4D). Follicle loss (P<0.05) occured on d8 with 30 μM VCD in culture for primordial, and small and large primary follicles, relative to control incubations, with no effect (P<0.05) of VCD on secondary follicles. Exogenous AMH did not affect (P<0.05) VCD-induced ovotoxicity at any concentration measured (Figure 4A-D).
Figure 4.
Effect of exogenous AMH on VCD-induced follicle loss. PND4 F344 rat ovaries were cultured in culture medium or medium containing VCD (30 μM) with or without recombinant human AMH (100, 200, 400 ng/ml; labeled AMH 100, AMH 200 or AMH 400, respectively) for 8 days. Ovaries were collected and processed for histological evaluation as described in Materials and Methods. Healthy follicles in every 12th section were counted. Values indicate the mean number of healthy (A) primordial follicles, (B) small primary follicles, (C) large primary follicles, (D) secondary follicles counted per ovary ± SEM; n = 5 ovaries per treatment group (A-D); *P < 0.05 different from control.
Discussion
The present study was designed to characterize a potential involvement of AMH in primordial and primary follicle loss caused by VCD in the neonatal rat ovarian culture system. In order to more fully understand ovarian AMH expression, the distribution of AMH protein was compared in adult vs. neonatal rat ovaries. In an in situ study by Baarends et al., AMH mRNA expression was in granulosa cells of healthy preantral and small antral follicles of adult (30-day-old) rat ovaries, with little or no expression in oocytes, theca cells, and interstitial cells (Baarends et al., 1995). AMH staining was also not present in primordial, small primary, and large antral follicles, or corpora lutea. Consistent with that study, the present study in ovaries from adult cycling rats identified AMH protein localization in large primary and secondary follicles and not in primordial or small primary follicles, with its highest expression in the largest preantral follicles. In contrast to adult ovaries, PND4 and PND6 ovaries and PND4 ovaries cultured for either 2d or 6d showed a different distribution of AMH protein. There was weak staining for AMH protein in PND4 ovaries. However, in PND4 ovaries following 2 days of culture as well as in PND6 (analogous time points) ovaries, there was similar prominent staining intensity for AMH protein in small and large primary follicles, follicle types not stained in adult ovaries. Further, AMH staining of these follicles in cultured PND4 ovaries diminished after 6 days as compared with d2 in vitro. Interestingly, the distribution of AMH staining in primordial and small primary follicles became reduced as time in culture progressed to more closely resemble its distribution in the adult ovary. In addition, overall AMH protein levels were decreased with time in culture. This may reflect a biological response in which the AMH signaling system shifts in pattern of expression to larger follicles as the ovary becomes more functionally established. Although cultured ovaries remained fully viable throughout the culture period in all experiments (8d), this may reflect selective effects in response to culturing or nonphysical changes in the culture that make the ovaries different from those that develop in vivo.
Conversely, decreased ovarian AMH with time in culture may reflect that the age of the rat plays an important part in determining the ovarian distribution of AMH expression. AMH has been shown to play a vital role in primordial follicle activation and recruitment by maintaining primordial follicles in a dormant state, thus inhibiting their recruitment (Visser and Themmen, 2005). It is tempting to speculate that during fetal development and neonatal primordial follicle formation in the rodent, AMH serves a critical role in preventing activation of those small follicles as they are being formed. Based on studies in genetically altered mice (Amh −/−, FOXO3−/−, Akt−/−), it is known that uncoordinated global activation and recruitment of primordial follicles results in premature ovarian failure and reduced reproductive lifespan (Visser et al., 2007; Castrillon, et al., 2003; Brown, et al., 2010). Thus, tightly regulated maintenance of newly formed primordial follicles in a dormant state could serve as a protective measure against premature ovarian senescence. Subsequently, once the ovary has become fully populated with primordial follicles, expression of AMH and its role in regulating follicle recruitment may shift to larger preantral and early antral follicles. This hypothesis is difficult to address directly in the ovarian culture system because larger follicles do not develop under the conditions used here.
The Kit/Kit ligand pathway has been shown to be targeted by VCD to mediate its ovotoxic effects. When PND4 rat ovaries were exposed to VCD, relative to controls there was a decrease in mRNA encoding the pro-survival gene Kit on d4 and an increase in mRNA encoding its ligand, Kit ligand (Kitl) on d6 (Fernandez et al., 2008). When exogenous Kit ligand was added to cultured PND4 ovaries, VCD-induced ovotoxicity on d8 was attenuated. Importantly, other exogenous survival growth factors, including growth and differentiation factor 9 (GDF9) and bone morphogenic protein 4 (BMP4), had no effect on follicle loss caused by VCD (Fernandez et al., 2008). This observation provided additional support for the specificity of the Kit/Kit ligand pathway as a target for VCD.
Since the prevention of primordial follicle recruitment by inhibition of PI3kinase has been shown to be important for protection from VCD-ovotoxicity, and AMH has also been shown to prevent the recruitment of small primary follicles from the primordial follicle pool, one goal of the present study was to determine if exogenous AMH could also protect against VCD-induced ovotoxicity. The increase in primordial and decrease in small primary follicle numbers following culture of ovaries with exogenous AMH supported a previously proposed role for AMH in retarding primordial follicle activation and recruitment (Nilsson et al., 2007). However, AMH did not prevent VCD-induced ovotoxicity at any concentration. This finding provides further support for the specificity of VCD targeting the Kit/PI3kinase pathway. Because VCD caused earlier decreases in signaling in the Kit/Kit ligand pathway (pAkt; d2 in culture; Keating et al., 2009b) it is likely that the reduced AMH expression caused by VCD is the result of, rather than the initiator of VCD-induced ovotoxicity.
The effect of VCD on ovarian expression of AMH mRNA and protein was also investigated using a time course in the PND4 ovary culture. VCD specifically targets small pre-antral follicles (primordial and small primary) in ovaries of rats and mice by enhancing the natural process of atresia (Springer et al., 1996a). Further, VCD-induced ovotoxicity has been proposed to result from the activation of primordial follicle recruitment (Keating et al., 2009a). In the present study VCD exposure caused a reduction in Amh mRNA on d4-8 and a decrease in AMH protein on d6 and 8 of culture. Because AMH is thought to inhibit primordial follicle recruitment, a VCD-induced decrease in AMH expression (d4, mRNA; d6, protein) might serve to facilitate the increase in primordial follicle recruitment being caused by VCD.
In summary, results of the present study demonstrate that the ovarian distribution of AMH expression may shift from small pre-antral to large pre-antral/early antral follicles as the neonatal ovary matures. Additionally, whereas, AMH-mediated inhibition of primordial follicle recruitment had no effect on VCD-induced ovotoxicity, reduced AMH expression that follows VCD exposure may accelerate primordial follicle recruitment thought to be caused by VCD. Finally, these results provide further evidence of the Kit/Kit ligand pathway as the specific target of VCD for induction of ovotoxicity. The specificity of that mechanism is currently under further investigation.
Summary sentence.
Expression of AMH in neonatal rat ovaries and its lack of involvement in VCD-induced ovotoxicity provides further evidence for direct VCD effects on the Kit/Kit ligand signaling pathway.
Acknowledgements
The authors wish to thank Andrea Grantham and Doug Cromey from the Histology Core Facility for all of their technical assistance.
Support: This research was supported by training grant ES007091, grant R01 ES09246, and Center grant 06694.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest:
The authors declare that there are no conflicts of interest.
References
- Adhikari D, Liu K. Molecular mechanisms underlying the activation of mammalian primordial follicles. 2009;30(5):438–464. doi: 10.1210/er.2008-0048. [DOI] [PubMed] [Google Scholar]
- Andersen CY, Byskov AG. Estradiol and regulation of anti-Müllerian hormone, inhibin-A, inhibin-B secretion: analysis of small antral and preovulatory human follicles’ fluid. J. Clin. Endocrinol. Metab. 2006;91:4064–4069. doi: 10.1210/jc.2006-1066. [DOI] [PubMed] [Google Scholar]
- Baarends WM, Uilenbroek JT, Kramer P, Hoogerbrugge JW, van Leeuwen EC, Themmen AP, Grootegoed JA. Anti-Müllerian hormone and anti-Müllerian hormone type II receptor messenger ribonucleic acid expression in rat ovaries during postnatal development, the estrous cycle, and gonadotropin-induced follicle growth. Endocrinol. 1995;136:4951–4962. doi: 10.1210/endo.136.11.7588229. [DOI] [PubMed] [Google Scholar]
- Broekmans FJ, Knauff EAH, Velde ER, Macklon NS, Fauser BC. Female reproductive ageing: current knowledge and future trends. Trends Endcrinol. Metabol. 2007;18(2):58–65. doi: 10.1016/j.tem.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Broekmans FJ, Visser JA, Laven JSE, Borer SL, Themmen APN, Fauser BC. Anti-Müllerian hormone and ovarian dysfunction. Trends in Endocrinol. Metabol. 2008;19(9):340–347. doi: 10.1016/j.tem.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Brown C, LaRocca J, Pietruska J, Ota M, Anderson L, Smith SD, Weston P, Rasoulpour T, Hixon ML. Subfertility caused by altered follicular development and oocyte growth in female mice lacking PKBalpha/Akt1. Biol. Reprod. 2010;82:246–256. doi: 10.1095/biolreprod.109.077925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castrillion DH, Miao L, Kollipara R, Horner JW, DePinho RA. Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science. 2003;301:215–218. doi: 10.1126/science.1086336. [DOI] [PubMed] [Google Scholar]
- Devine PJ, Sipes IG, Skinner MK, Hoyer PB. Characterization of a rat in vitro ovarian culture system to study the ovarian toxicant 4-vinylcyclohexene diepoxide. Toxicol. Appl. Pharmacol. 2002;184:107–115. [PubMed] [Google Scholar]
- Devine PJ, Hoyer PB. Ovotoxic environmental chemicals: indirect endocrine disruptors. In: Nog R, editor. Endocrine disruptors: effects on male and female reproductive system. CRC Press; Boca Raton, Fl: 2005. pp. 67–100. [Google Scholar]
- Durlinger AL, Kramer P, Karels B, De Jong FH, Uilenbroek THJ, Grootegoed JA, Themmen A. Control of primordial follicle recruitment by anti-Müllerian hormone in the mouse ovary. Endocrinol. 1999;140:5789–5796. doi: 10.1210/endo.140.12.7204. [DOI] [PubMed] [Google Scholar]
- Durlinger AL, Gruijters MJ, Kramer P, Karels B, Kumar TR, Matzuk MM, Rose UM, de Jong FH, Uilenbroek JT, Grootegoed JA, Themmen AP. Anti-Müllerian hormone attenuates the effects of FSH on follicle development in the mouse ovary. Endocrinol. 2001;142:4891–4899. doi: 10.1210/endo.142.11.8486. [DOI] [PubMed] [Google Scholar]
- Durlinger AL, Gruijters MJ, Kramer P, Karels B, Ingraham HA, Nachtigal MW, Uilenbroek JT, Grootegoed JA, Themmen AP. Anti-Müllerian hormone inhibits initiation of primordial follicle growth in the mouse ovary. Endocrinol. 2002a;143:1076–1084. doi: 10.1210/endo.143.3.8691. [DOI] [PubMed] [Google Scholar]
- Durlinger AL, Visser JA, Themmen AP. Regulation of ovarian function: the role of anti-Müllerian hormone. Reprod. 2002b;124:601–609. doi: 10.1530/rep.0.1240601. [DOI] [PubMed] [Google Scholar]
- Fernandez SM, Keating AF, Christian PJ, Sen N, Hoying JB, Brooks HL, Hoyer PB. Involvement of the Kit/kit ligand signaling pathway in 4-vinylcyclohexene diepoxide-induced ovarian follicle loss in rats. Biol. Reprod. 2008;79:318–327. doi: 10.1095/biolreprod.108.067744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaws JA, Doerr JK, Sipes IG, Hoyer PB. Destruction of preantral follicles in adult rats by 4-vinyl-1-cyclohexene diepoxide. Reprod. Toxicol. 1994;8:509–514. doi: 10.1016/0890-6238(94)90033-7. [DOI] [PubMed] [Google Scholar]
- Hirshfield AN. Development of follicles in the mammalian ovary. Inter. Rev. Cytol. 1991;124:43–101. doi: 10.1016/s0074-7696(08)61524-7. [DOI] [PubMed] [Google Scholar]
- Keating AF, Mark CJ, Sen N, Sipes IG, Hoyer PB. Effect of phosphatidylinositol-3 kinase inhibition on ovotoxicity caused by 4-vinylcyclohexene diepoxide and 7, 12-dimethylbenz[a]anthracene in neonatal rat ovaries. Toxicol. Appl. Pharmacol. 2009a;241:127–134. doi: 10.1016/j.taap.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating AF, Sen N, Sipes IG, Hoyer PB. 1-4-vinylcyclohexene diepoxide exposure alters expression of Kit signaling pathway members to induce ovotoxicity. Biol. Reprod. 2009b doi: 10.1095/biolreprod.110.087650. Special Issue #94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K, Rajareddy S, Liu L, Jagarlamundi K, Boman K, Selstam G, Reddy P. Control of mammalian oocyte growth and early follicular development by the oocyte PI3 kinase pathway: new roles for an old timer. Dev. Biol. 2006;299:1–11. doi: 10.1016/j.ydbio.2006.07.038. [DOI] [PubMed] [Google Scholar]
- Mayer LP, Pearsall NA, Christian PJ, Devine PJ, Payne CM, McCuskey MK, Marion SL, Sipes IG, Hoyer PB. Long-term effects of ovarian follicular depletion in rats by 4-vinylcyclohexene diepoxide. Reprod. Toxicol. 2002;16:775–781. doi: 10.1016/s0890-6238(02)00048-5. [DOI] [PubMed] [Google Scholar]
- Nilsson E, Rogers N, Skinner MK. Actions of anti-Müllerian hormone on the ovarian transcriptome to inhibit primordial to primary follicle transition. Reproduct. 2007;134:209–211. doi: 10.1530/REP-07-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappaport SM, Fraser DA. Air sampling and analysis in rubber vulcanization area. Am. Ind. Hyg. Assoc. J. 1977;38:205–209. doi: 10.1080/0002889778507601. [DOI] [PubMed] [Google Scholar]
- Sinisi AA, Pasquali D, Notaro A, Bellastella A. Sexual differentiation. J. Endocrinol. Invest. 2003;26:23–28. [PubMed] [Google Scholar]
- Springer LN, McAsey ME, Flaws JA, Tilly JL, Sipes IG, Hoyer PB. Involvement of apoptosis in 4-vinylcyclohexene diepoxide-induced ovotoxicity in rats. Toxicol. Appl. Pharmacol. 1996a;139:394–401. doi: 10.1006/taap.1996.0180. [DOI] [PubMed] [Google Scholar]
- Springer LN, Tilly JL, Sipes IG, Hoyer PB. Enhanced expression of bax in small preantral follicles during 4-vinylcyclohexene diepoxide-induced ovotoxicity in the rat. Toxicol. Appl. Pharmacol. 1996b;139:402–410. doi: 10.1006/taap.1996.0181. [DOI] [PubMed] [Google Scholar]
- Thompson KE, Bourguet SM, Christian PJ, Benedict JC, Sipes IG, Flaws JA, Hoyer PB. Differences between rats and mice in the involvement of the aryl hydrocarbon receptor in 4-vinylcyclohexene diepoxide-induced ovarian follicle loss. Toxicol. Appl. Pharmacol. 2005;203:114–123. doi: 10.1016/j.taap.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Visser JA, Durlinger ALL, Peters IJJ, van den Heuvel ER, Rose UM, Kramer P, de Jong FH, Themmen APN. Increased oocyte degeneration and follicular atresia during the estrous cycle in anti-Müllerian hormone null mice. Endocrinol. 2007;148(5):2301–2308. doi: 10.1210/en.2006-1265. [DOI] [PubMed] [Google Scholar]
- Visser JA, Themmen APN. Anti-Müllerian hormone and folliculogenesis. Mol. Cell. Endocrinol. 2005;234:81–86. doi: 10.1016/j.mce.2004.09.008. [DOI] [PubMed] [Google Scholar]