Abstract
This paper describes the application of best practice recommendations for using accelerometers in a physical activity (PA) intervention trial, and the concordance of different methods for measuring PA. A subsample (n=63; 26%) of the 239 healthy, sedentary adults participating in a PA trial (mean age=47.5; 82% women) wore the ActiGraph monitor at all 3 assessment time points. ActiGraph data were compared with self-report (i.e., PA weekly recall and monthly log) and fitness variables. Correlations between the PA recall and ActiGraph for moderate intensity activity ranged from 0.16–0.48 and from 0.28–0.42 for vigorous intensity activity. ActiGraph and fitness [estimated VO2(ml/kg/min)] had correlations of 0.15–0.45. The ActiGraph and weekly self-report were significantly correlated at all time points (correlations ranged from 0.23–0.44). In terms of detecting intervention effects, intervention groups recorded more minutes of at least moderate-intensity PA on the ActiGraph than the control group at 6 months (min=46.47, 95% CI=14.36–78.58), but not at 12 months. Limitations of the study include a small sample size and only 3 days of ActiGraph monitoring. To obtain optimal results with accelerometers in clinical trials, the authors recommend following best practice recommendations: detailed protocols for monitor use, calibration of monitors and validation of data quality, and use of validated equations for analysis. The ActiGraph has modest concordance with other assessment tools and is sensitive to change over time. However, until more information validating the use of accelerometry in clinical trials becomes available, properly administered self-report measures of PA should remain part of the assessment battery.
Keywords: exercise, objective monitoring, best practice recommendations, ActiGraph
Introduction
Although there are positive physical and psychological benefits of physical activity, a majority of the population does not participate in physical activity at the recommended levels [1, 2]. One challenge in clinical trials is obtaining accurate measures of behavior [3, 4] while balancing participant and staff burden. In physical activity clinical trials, there are a number of considerations when measuring an outcome, such as study goals, participant burden, project budget and accuracy/precision [5]. Self-reported assessments, while having the benefit of being a relatively cost-effective method for assessing activity, may be subject to bias given error recall, social desirability and cognitive capacity [6, 7]. Given the variability in different measures, and that one technique cannot measure all aspects of physical activity in free-living situations, complementary methods of measurement have been recommended [5]. It also is important to demonstrate the validity of accelerometry and different methods in intervention trials [7, 8].
Complementary techniques of measurement include self-report, interviewer-based and objective forms. The use of objective measures of physical activity and fitness include direct observation [9–11], doubly labeled water [4], heart rate monitoring [10], and fitness testing [12], all of which can be prohibitive due to cost and the level of staff expertise needed. Accelerometry is an objective way to measure physical activity that has been extensively evaluated for validity and reliability [5, 13–24]. Currently available accelerometers have been found to be effective at detecting bouts of activity, including both continuous and intermittent [15, 22, 25], and have been validated against other clinic-based [26] and self-report methods of physical activity [6, 25, 27, 28]. However, the concordance between these measures varies greatly, depending on the type of accelerometer, complementary measure used, and the study methodology (e.g., laboratory or more controlled setting, free-living environment).
Best practice criteria have been recommended for accelerometry use in research studies [7], specifically: 1) thoughtful decision making regarding monitor selection, including quality of data collected, and dependability; 2) adequate monitor use protocols (e.g., defining wearing days, monitor placement); 3) proper monitor calibration; 4) effective analysis of accelerometer data (e.g., defining a day, handling spurious data); 5) integration of other data sources. These are important factors to consider when implementing accelerometry for use in clinical trials.
Our research team published results from a randomized clinical trial that examined two strategies (telephone-based and print-based) to improve physical activity in adults, of which a subsample wore an ActiGraph monitor at all assessment time points [29]. The current study will examine factors relating to implementing an accelerometer (i.e., the ActiGraph [30]) in an intervention trial and to examine the change in ActiGraph data over time. This study also aims to examine the concordance of the ActiGraph with other methods of physical activity measurement (i.e., the interviewer-administered PAR [31, 32], an exercise stress test, and self-reported data) in an intervention trial. The authors hypothesize that the ActiGraph will be shown to be a useful physical activity measure when compared to other methods of physical activity measurement, and as a reliable outcome measure for implementation in clinical trials.
Material and Methods
Study Sample
The participants in this study were a subsample of a larger PA intervention trial [29]. Participants for the parent intervention trial were recruited through newspaper advertisements, radio advertisements, email notices and postings on a worksite website. The primary inclusion criteria included being healthy, aged 18–65, and sedentary (i.e., participating in moderate or vigorous physical activity for less than 90 minutes per week). Individuals had to be willing to be randomly assigned to any of the three treatment groups, described below. Additional recruitment information is described elsewhere [29]. All participants provided written informed consent and the study protocol was approved by the Institutional Review Board at the Miriam Hospital.
Treatment Groups
Participants were randomly assigned to one of three year-long treatment groups: 1) telephone-based, individualized motivationally-tailored feedback; 2) print-based, individualized motivationally-tailored feedback; or 3) contact control, delayed treatment. The interventions contained information based on the Stages of Motivational Readiness model and Social Cognitive Theory [33–35] intervention content was delivered to participants 14 times over the course of 1 year. Participants in the treatment groups were instructed that their goal was to meet or exceed the CDC/ACSM recommendation (engage in any form of moderate-intensity physical activity at least 5 days per week for a total of at least 30 minutes each time [36]. No supervised exercise sessions were conducted. Participants in the contact control/delayed treatment group received health and wellness materials and were able to choose to receive either the print or the telephone-based intervention after 12 months. More descriptive information regarding the treatment groups is presented elsewhere [29].
Assessments
In-person assessments occurred at baseline, 6 and 12 months, which included the 7-day PAR, anthropometric assessments, an exercise fitness test, as well as a battery of psychosocial questionnaires. Primary outcomes measured by the PAR showed that both the print and telephone-based intervention groups reported a significantly greater increase in physical activity than the contact control group at 6 months, and the print-based intervention group reported significantly greater increase in physical activity at 12 months, but not the telephone-based group. More details on these and other results have been presented elsewhere [29].
Implementation of Best Practice Recommendations for Accelerometry
1. Monitor selection and data quality
The ActiGraph was selected for this study, as it has been shown to have acceptable reliability and is the most generalizable when compared with three other commercially available accelerometers (Intra-class Correlations of at least 0.80 [25, 37]. The ActiGraph Model used in this study was # 7164 (previously denoted as a CSA monitor; Computer Science and Applications, Inc.). It is a lightweight (1.5 ounces), small (2.0 × 1.6 × 0.6 inches) lithium battery-powered single axis accelerometer. The unit used for this trial also recorded and stored acceleration and deceleration of movement in time intervals for up to 22 consecutive days, which made it possible for the monitor to be given to participants in advance and track the behavior necessary. Since the project consisted of rolling admission, intervention, and assessment, 32 monitors were used throughout the study.
2) Monitor use protocols
Since at least 3–4 days of monitoring are needed to achieve 80% reliability in the variance of activity [38], all participants wore monitors for three days at each time point. To minimize participant burden, participants were asked to wear the monitor on two weekdays and one weekend day; this selection of days is also confirmed by an accelerometer-based study in which patterns of activity were found to be different on weekday versus weekend days [19]. Participants were prompted to follow the same day sequence at all assessment time points during the study.
Participants were given verbal and pictorial descriptions as to the correct monitor placement and were called the day prior to wearing the monitor as a reminder. Additionally, participants completed a log documenting the times that they wore the ActiGraph. This log was returned in person, along with the monitor, and was reviewed with the participant.
3) Calibration and data checking
The monitors were calibrated before and after each use, and were set to collect data at epoch lengths of one minute. Upon receipt of each monitor, the data were checked by a research assistant to validate what was recorded. If the data did not match the assigned wear days or the days recorded on the log, the participant was called to verify accuracy. If no data were recorded or if the data appeared inaccurate (i.e., not registering a certain count threshold), the data were not included as complete data. Every ActiGraph also had the battery life tracked and checked as this was an indicator of unintended resets in the field. If a field-battery reset was suspected, those data also were not used as the accuracy could not be validated. As long as the above criteria were met, we did not include criterion that the monitor had to be worn for a certain number of hours each day.
4) Analysis of accelerometer data
Participants were instructed to put on the monitor at the beginning of the day and take it off before bedtime (excluding shower, and water activities). The Freedson equation [39] was used to compute the cut-points for light, moderate, and vigorous activities; this equation has been shown to have good agreement with time spent in different intensity categories (r = 0.43–0.94 [27]).
5) Integration of other data sources
As recommended by Schutz et al [5], we included other physical activity assessments to investigate the concordance the data collected via the ActiGraph as follows: Physical Activity Recall (PAR) [31, 40].
A physical activity recall (PAR) was conducted via an interviewer-based procedure, with participants being prompted to recall bouts of moderate intensity physical activity that were at least 10 minutes in duration [41–43]. Each participant walked on a treadmill for one minute prior to the PAR interview at moderate intensity. The interviewer conducting the PAR was trained directly by the Cooper Institute for Aerobics Research in Dallas, TX and was audio taped with periodic review of to ensure consistent administration of the PAR. At baseline, due to logistical constraints to determine participant eligibility, a separate 3-day PAR was administered on the three days that the participant wore the monitor. At 6 and 12 months, a 7-day PAR was administered that included the three days that the monitor was worn. If for some reason at the 6-month and 12-month assessment, the seven days did not include the days the participant wore the monitor, a separate 3-day PAR was administered. Thus, all correlations between the PAR and the ActiGraph used the same three days of assessment. For the purposes of the current paper, we were interested in better understanding the precision and the concordance between PAR and ActiGraph at different intensities of activity [25], particularly those of at least moderate intensity above, corresponding to the current public health recommendations [44]; thus, the correlations examined herein will be examining moderate intensity activity and vigorous activity.
Self-report data
Questionnaire assessment
Self-report data in this study included two questions to assess activity over the previous 7 days: 1) On how many days over the last week were you physically active?; 2) Approximately how many minutes did you participate in physical activity on each of those days?[45]
Monthly log
Participants were asked to return a physical activity log each month in the form of a monthly calendar. Participants were asked to write the number of minutes each day that they participated in moderate intensity physical activity for at least 10 continuous minutes. The log was not completed at baseline.
Fitness data
At baseline, 6, and 12 months, participants completed a graded sub-maximal exercise fitness test. A Balke protocol was followed, with 2-minute stages beginning at 3 mph and 2.5% grade [46]. The participant was stopped within 20 seconds after reaching 85% of age predicted maximum heart rate (i.e., 220-age); the test was terminated early if the participant experienced any of the ACSM absolute or relative indications for termination of an exercise test (e.g., angina, serious arrhythmias, unusual or severe shortness of breath [12]. Data from the sub-maximal exercise treadmill test included functional capacity, as expressed by estimated VO2 (ml/kg/min) at 85% of age predicted maximum heart rate. The change in fitness from this trial has been presented in the main outcomes paper [29]. For the purposes of this paper, it will be used for comparison with the ActiGraph.
Analysis Plan
Spearman’s rank correlations were conducted to examine the relationship between the ActiGraph, 3-day PAR (corresponding to the days the participant wore the ActiGraph), estimated VO2(ml/kg/min), and the two self-report measures. We chose Spearman’s correlation because it is less sensitive to departures from normality and the presence of outliers. Additionally, bivariate scatterplots of the ActiGraph with the 3-day PAR were examined at each time point, as correlation coefficients do not adequately capture nonlinear relationships.
Change on the ActiGraph data over time were analyzed via a normal linear regression model. The ActiGraph data were converted to change scores by subtracting baseline values from 6-month and 12-month outcomes. These data were analyzed using main-effects models. Preplanned, single-degree-of-freedom contrasts were used to compare a) the two active treatment groups (Print, Telephone) to each other, and b) the overall intervention group to the contact control, delayed treatment group.
Results
Sample Description
A subsample of 63 subjects, 26% of the total study sample, participated in the assessment of physical activity using accelerometry. The purpose of including the ActiGraph data in the parent trial was to provide a validity check on the 7-day Physical Activity Recall (PAR), which is an interviewer-administered assessment of physical activity behavior. In the outcomes paper for the main trial, change over time on the ActiGraph and comparison of intensity of activity were not reported. The sample from the parent trial (n=239) was mostly Caucasian (90.3%), female (82.0%), and middle-aged (M = 44.5 years). Of the 63 participants who were randomly assigned to wear a monitor, 22 were in the Print arm, 20 were in the Telephone arm, and 21 were in the Contact control/delayed treatment arm. One participant (in the telephone group) had missing ActiGraph data at baseline. Table 2 shows sample size (and resulting missing data) by treatment group at each of the two follow-up time points. On average, participants wore the monitors for 14.87 hours each day. Participants who wore a monitor did not significantly differ from participants who did not on the demographic variables presented in Table 1.
Table 2.
Unadjusted minutes of activity recorded by the ActiGraph.
| Phone | Delayed Treatment | All | Delayed Treatment | Intervention | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean ± SD | N | Mean ± SD | N | Mean ± SD | N | Mean ± SD | N | Mean ± SD | N | Mean ± SD | |
| Baseline | 22 | 61.5 ± 48.4 | 19 | 40.4 ± 36.8 | 21 | 57.4 ± 42.5 | 62 | 53.6 ± 43.3 | 21 | 57.4 ± 42.5 | 41 | 51.7 ± 44.2 |
| Month 6 | 17 | 91.4 ± 68.0* | 18 | 74.1 ± 46.1* | 20 | 45.0 ± 35.2 | 55 | 68.9 ± 53.5 | 20 | 45.0 ± 35.2** | 35 | 82.5 ± 57.6 |
| Month 12 | 18 | 77.9 ± 65.4 | 16 | 56.3 ± 52.1 | 19 | 55.0 ± 44.6 | 53 | 63.2 ± 54.6 | 19 | 55.0 ± 44.6 | 34 | 67.8 ± 59.6 |
p<0.05 (print v. delayed treatment and phone v. delayed treatment)
p<0.01
Table 1.
Baseline descriptive statistics.
| Variable | Full Sample* (n=239) | ActiGraph Sample (n=63) | p value |
|---|---|---|---|
| Age (years) | 44.55 (9.6) | 42.98 (10.1) | 0.53 |
| Gender (% Female) | 82% | 82.50% | 0.90 |
| Body Mass Index | 28.55 (5.6) | 27.72 (4.7) | 0.05 |
| Percentage body fat | 32.17 (9.3) | 29.48 (8.4) | 0.26 |
| Physical Activity (minutes per week) | 19.64 (25.0) | 20.95 (25.5) | 0.80 |
| Estimated VO2 @ 85% of predicted max (ml/kg/min) | 25.59 (6.3) | 26.18 (5.8) | 0.25 |
Notes: Standard deviations are in parenthesis
Data on the full sample have been previously published (see [29])
Comparing the ActiGraph to Other Methods
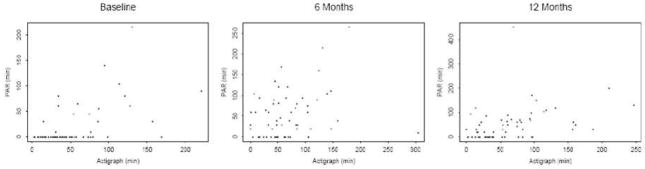
Bivariate scatterplots of the ActiGraph with the 3-day PAR were examined at each time point (See Figure 1), which indicate a substantial amount of underreporting of activity on the PAR, in comparison to the data captured by the ActiGraph. At baseline, there are 46 participants who reported no physical activity on the PAR, but whose activity via the ActiGraph ranged from 3 to 169 minutes of moderate intensity. The proportion of those with discrepant data declined in month 6 and month 12 (21 at Month 6 and 20 at Month 12).
Figure 1.
Bivariate Scatterplots of ActiGraph with 3-day PAR at baseline, 6 months, and 12 months.
The Spearman correlation between the ActiGraph and the PAR, self-reported activity and estimated VO2 (ml/kg/min) are presented in Table 3. Significant correlations were found between moderate intensity activity on the PAR compared with the ActiGraph at baseline, and month 12, but not at month 6; vigorous intensity activity on the PAR compared with the ActiGraph was found to have significant correlations at all time points. Correlations between the monthly log data and the ActiGraph were significant at month 6 and the self-report questionnaire was significant at baseline, month 6, but not at 12 months. Spearman correlations with estimated VO2 (ml/kg/min) were significant at baseline and 12 months, but not at 6 months.
Table 3.
Spearman’s Rank Correlations between ActiGraph and Complementary Measures.
| Baseline | 6 months | 12 months | |
|---|---|---|---|
| ActiGraph—PAR (moderate mins) | 0.48, p <0.0001 | 0.16, p=0.23 | 0.47, p <0.001 |
| ActiGraph—PAR (hard/very hard mins) | 0.28, p <0.05 | 0.29, p <0.05 | 0.42, p <0.01 |
| ActiGraph—Estimated VO2 (ml/kg/min) | 0.30, p <0.05 | 0.15, p =0.30 | 0.48, p <0.0001 |
| ActiGraph—Self-report days/mins | 0.34, p <0.01 | 0.44, p <0.05 | 0.19, p =0.19 |
| ActiGraph— Monthly log | Did not complete | 0.44, p <0. 01 | 0.23, p =0.19 |
Physical activity participation measured by the ActiGraph
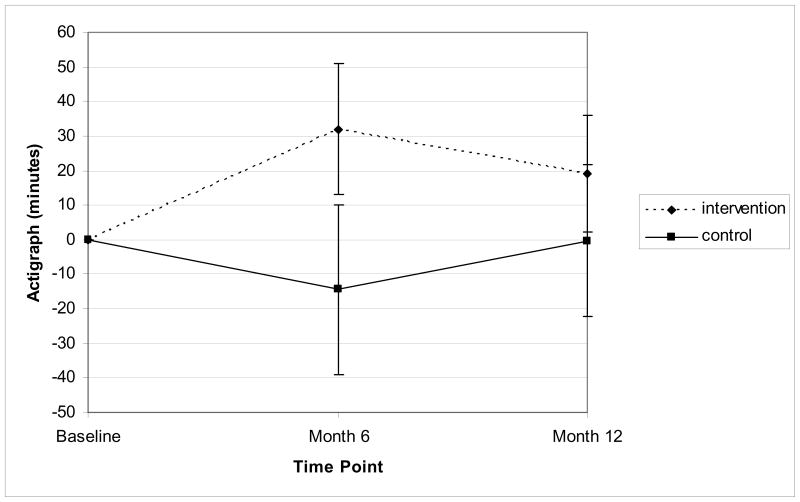
Table 2 presents the unadjusted ActiGraph means by study group. The print and phone groups did not differ from one another in terms of minutes of at least moderate intensity physical activity recorded on the ActiGraph at either 6 months (p = 0.96) or 12 months (p = 0 .98). However, in comparison to the delayed treatment group, both print and phone groups had higher recorded amounts of activity on the ActiGraph at 6 months (p < 0.05), but not at 12 months (p =NS). When the two intervention groups are combined (and baseline values of the ActiGraph are controlled for), the overall intervention effect was statistically significant at the 5% level with the intervention group recording more minutes of at least moderate intensity physical activity than the delayed treatment control group at 6 months (min = 46.47, 95% CI = 14.36 – 78.58) but not at 12 months (p =0 .18). Figure 2 presents the change in ActiGraph minutes by study group.
Figure 2.
Change in ActiGraph minutes per week by study group.
Note.
Means at Month 6 and Month 12 adjusted for baseline value. Standard error bars represent 95% confidence limits.
At 6 months: Intervention > Contact Control, p < .05
At 12 months: Intervention = Contact Control, p = 0.18
Discussion
In the publication of physical activity clinical studies, more detail is necessary for researchers and practitioners to evaluate the methods used in data collection (i.e., the implementation of best practice recommendations). This paper provides information about integrating an accelerometer into the methodology of a physical activity clinical trial. This study demonstrates the application of best practice recommendations for accelerometry in an intervention study. Specifically, studies employing the use of accelerometry need to follow and report on the following best practice recommendations in order to ensure the integrity of the objective data: 1) appropriate monitor selection and data quality; 2) detailed protocols for monitor use; 3) consistent calibration of monitors and checking of data quality; 4) use of validated equations for analysis; and 5) integration of other sources of physical activity measurement.
Based on the best practice recommendations cited above, the ActiGraph did detect improvements in physical activity observed by other measures, specifically the PAR. Also, the pattern of ActiGraph results from baseline to month 6 is consistent with the results from the PAR, indicating that the ActiGraph is sensitive to detecting physical activity changes in an intervention study. These results also confirm the findings of the main trial at 6 months [29], with both treatment arms outperforming the control arm on physical activity behavior. The results were not consistent at 12 months, as the data from the ActiGraph show no differences between treatment and control, while in the main trial, the print arm outperformed the control arm. It is notable that the ActiGraph, even on a 26% sample and for only 3 days detected similar effects at 6 months. However, given that the sample was small and only captured 3 days of monitoring, it is not surprising the pattern did not remain at month 12.
In this study, the correlations between the ActiGraph and the interviewer-administered PAR were small-to-medium [47], ranging from 0.28–0.48. These results are comparable with other studies in which correlations on the PAR range from 0.06–0.90 [25, 48]. These findings show that both the ActiGraph and the PAR, while not perfectly correlated, have enough overlap to indicate the ActiGraph can be used as an appropriate measurement of activity.
When self-report of physical activity, rather than the interviewer-based method, is used as the comparison, the ActiGraph and weekly self-report were significantly correlated at baseline and month 6, but not at month 12, with correlations ranging from 0.19 to 0.44; the monthly log was found to have a significant correlation at month 6, but not a month 12. These values are similar to those from other studies, which found correlations ranging from 0.15– 0.50 [26, 49]. These small-to-moderate sized correlations show that while there remain some improvements necessary for precision, the measures can be used as complementary methods in physical activity trials.
ActiGraph and fitness [VO2(ml/kg/min)] were significantly correlated at baseline, again with range in the small-to-moderate category. These correlations are similar to those found in another study with ranges of 0.49–0.54 for men and 0.14–0.47 for women [48]. However, even with best practices, there is error inherent in comparing fitness to activity behavior due to recall biases in self-report, cut point errors with the accelerometer, and that physical activity behavior is not a proxy for fitness.
At baseline and month 12, there appears to be a more consistent relationship between the ActiGraph and other complementary self-report and interviewer-based measures. Post-hoc tests were undertaken to examine the reasons the relationships were not significant at Month 6. Specifically, we examined whether there were group differences based on treatment assignment. Interestingly at Month 6, there was a correlation of −0.20 between the PAR and ActiGraph for the delayed treatment/contact control group. This suggests that participants in the delayed treatment group, who received no physical activity advice or behavior change information, may have inaccurately reported their behavior due to social desirability or simply due to a lack of experiential awareness as to what constitutes moderate intensity activity.
The relationship for vigorous intensity activity became more consistent over time. Research has found that individuals have difficulty differentiating between activities of moderate and vigorous intensities [50], which highlights a gap between the current exercise prescription and the understanding of general public [50]. Vigorous activities tend to be well-defined (e.g., discrete events such as sports, jogging); thus these may result in more accurate recall [48]. It may be that any effort associated is due to the type of activity and less accurate recall of moderate to light activities. In this study, our correlations ranged from 0.28–0.42 for vigorous intensity, and showed consistent relationships when compared at each time point. However, based on the PAR, only 9–17% of the activities recorded were of vigorous intensity. These low rates of vigorous intensity activity make it difficult to fully assess this supposition. It is also possible that improved self-report of physical activity at month 6 and month 12 may exaggerate the gains from participating in the intervention, irrespective of treatment group. This should be explored further in future studies [22].
When the scatterplots presented in Figure 1 are examined, they demonstrate that there are participants who report no physical activity on the PAR at, yet register between 3 to 169 minutes of moderate intensity physical activity according to the ActiGraph. This shows a large degree of underreporting, which declines over time but is still present. There are a few hypothesized reasons for this finding. First, it is possible that as participants interacted more with the research team, they became better aware of what constituted moderate-intensity physical activity. Second, much of the activity that was recorded as light activity on the ActiGraph at baseline may have been actually misclassified. For data processing of the ActiGraph information, the Freedson equation was used [39]. However, some research has shown that the Freedson cut-points overestimates resting/light activity by 14% and underestimates moderate intensity by 60% [22]. Third, there may have been a response effect to being in a physical activity trial. For the purposes of the parent trial, the interviewer administered 7-day PAR was used as the primary screening tool for physical activity behavior, and was administered prior to the ActiGraph monitor assessment. More research is needed to determine if an ActiGraph should be used as entry criteria for study eligibility.
Additionally, research has found that accelerometer counts were higher during track locomotion versus treadmill locomotion [51]. The Freedson values [39] were originally established for treadmill walking versus walking in free-living environment; therefore, there may have been misclassification from using it to establish the cutoff values for moderate intensity and above physical activity. Also, studies have found that the relationships between ActiGraph counts and actual activities were r=0.77 for walking and only r=0.59 for activities combined [13]. This may indicate a problem with using accelerometers in free-living situations. If a participant was doing a different type of activity other than walking, the monitor may not have captured this activity. Furthermore, accelerometers are known to be unable to detect upper body movement and the energy cost associated with that and with changes in surface or terrain [13]. Given the potential limitations of using accelerometers in free-living situations (which is the method used in home-based physical activity interventions), data reported in physical activity trials may contain inaccurate estimates and should be interpreted with caution.
There are other factors that investigators need to consider when incorporating accelerometers for use in clinical trials. First is the selection of the device itself. The ActiGraph is a uniaxial monitor that has been used in a number of research trials [e.g., 52–54]; other monitors that are triaxial (e.g., Tritrac) should also be considered depending the needs of the study. Additionally, the number of monitors to be purchased is a consideration. Factors such as how compliant participants would be with returning the monitors, as well as the length of time for the data download, and charging/recalibrating process are important. For the current study, the Investigative team chose to have a pool of 32 monitors as we felt it was prudent to have an excess of monitors rather than miss an assessment window due to lack of available equipment. This was particularly important in light of the rolling admission for this trial, at times we were conducting ActiGraph assessments for baseline, Month 6 and Month 12 time points simultaneously. A metric (e.g., 1 monitor for every 3 participants) should be developed by each investigative team to ensure adequate device coverage for the study needs.
In terms of limitations, the sample size was small (n=63). Additionally, to minimize burden, participants were asked to wear the monitor for only three days, given research has found that three to four days of monitoring is needed to achieve 80% reliability in the variance of activity [38]. Therefore, we were not able to capture the entire weekly pattern of participants’ activity. This limits the ability to fully compare the complementary measures (i.e., monthly physical activity logs, weekly self-report data) from the main trial to this one. Future studies should have all participants wear a monitor as well as consider seven days versus three days of monitoring. We would recommend monitoring for a seven day period, as this would best capture the patterns and duration of activity over a complete recording period.
In this trial, moderate intensity physical activity was demonstrated for participants prior to each administration of the PAR by having them walk on a treadmill for one minute at moderate intensity. While we felt this one minute demonstration would be effective for reminding participants of what moderate intensity “felt like”, further training of participants may be necessary to further improve the validity of self-report, particularly for participants who are in a control or delayed treatment condition. Other methods for teaching participants to recognize how their body feels when exercising at moderate intensity and above include taking the participant on a one minute walk in a free-living situation (e.g., hallway, sidewalk) [55, 56] and longer simulated walks on a treadmill (i.e., 10 minutes) [e.g., 57]. These longer 10 minute walks demonstrate the minimum duration necessary to be considered a bout of activity [44].
An interesting methodological question is whether the mere act of wearing an accelerometer influences participants’ behavior and reporting. The mean physical activity level obtained from the PAR for the participants who wore the monitor did not differ from the participants who did not wear the monitor. Additionally, there were no differences on change in the PAR at 6 or 12 months between those who wore a monitor and those who did not. This indicates that the addition of an accelerometer did not unduly influence the reporting of behavior and/or amount of activity.
The current study used a more traditional form of data capture, examining cut-points of activity for light, moderate and vigorous intensity activity [39]. However, more recent studies have recommended more sophisticated methodology for data processing to distinguish types of activity for specific behaviors previously difficult to capture (such as vacuuming, walking uphill) [59]. For example, Crouter, Clowers, and Bassett [60] suggested using the coefficient of variation in activity to identify between walking/running and lifestyle activities, and then applying the corresponding regression model equations. The authors found that their approach was better at predicting energy expenditure than previous one-regression models. Unfortunately, these methods could not be used for the current study because the ActiGraph data collected for the parent study specified certain data collection parameters which preclude a reanalysis using the Crouter method [60]. It is possible that as the technology improves, the sensitivity of these methods for detecting bouts and types of activity also will improve.
Conclusions
The results indicate that the ActiGraph can be effectively implemented in physical activity intervention trials following best practice recommendations, and can be used successfully to capture data in a longitudinal fashion. Although the ActiGraph is an effective tool for objectively measuring activity and complementing self-report methodology, it’s use needs to be balanced with study demands. The benefits of using an accelerometer are that it provides objective data on physical activity behavior without the significant staff/participant time associated with interviewer-administered questionnaire and fitness testing. Alternatively, using ActiGraphs in an intervention study require an initial investment in the devices, appropriate data management support, and proper oversight to monitor the integrity of the data. The ActiGraph also may be limited by current equations that over or underestimate data, although some of the newer equations may remedy this problem (e.g., Crouter et al., 2006 [60]).
Objective measurement of physical activity behavior is a critical missing component in physical activity clinical trials. Some trials are using the ActiGraph as the only outcome measure for physical activity (e.g., [53]. The data from the present clinical trial suggest, however, that it is premature to recommend the exclusive use of accelerometry as the primary outcome measure in clinical trials. Until more precise equations for processing ActiGraph data are identified and further research confirms their validity [61], physical activity clinical trials should continue to evaluate the use of accelerometers in conjunction with other assessments of physical activity behavior. Thus, for the assessment of physical activity in clinical trials, we recommend a combination of ActiGraph monitoring for seven days and properly administered interviewer-based measures incorporating the same period. This combination of assessments will allow for further evaluation of the metrics used in quantifying ActiGraph data while also giving the richness of the type and nature of activities performed by individuals to better inform future interventions.
Acknowledgments
The authors would like to acknowledge the contributions of Linda Christian, Robin Cram, Lisa Cronkite, Santina Horowitz, Maureen Hamel, Kenny McParlin, Hazel Ouellette, Yunxia Sui, Susan Pinheiro, Regina Traficante, and Kate Williams. The authors also would like to thank Chi Chan for her assistance in manuscript preparation. This research was supported by grant HL64342 to Dr. Marcus from the National Heart, Lung, and Blood Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Melissa A. Napolitano, Email: napolita@temple.edu, Temple University, Center for Obesity Research and Education, 3223 N. Broad St. Suite 175, Philadelphia, PA 19140, T: 215-707-8639 F: 215-707-6475
Kelley E. Borradaile, Email: borradak@temple.edu, Temple University, Center for Obesity Research and Education, 3223 N. Broad St. Suite 175, Philadelphia, PA 19140
Beth A. Lewis, Email: blewis@umn.edu, University of Minnesota, 209 Cooke Hall, 1900 University Ave SE., Minneapolis, MN, 55455
Jessica A. Whiteley, Email: jessica.whiteley@umb.edu, University of Massachusetts, Boston, 100 Morrissey Boulevard, Boston, MA, 02125
Jaime L. Longval, Email: jlongval@lifespan.org, The Miriam Hospital, Coro Building, Suite 500, One Hoppin, Street, Providence, Rhode Island 02903
Alfred F. Parisi, The Miriam Hospital, 164 Summit Avenue, Providence, Rhode, Island 02906
Anna E. Albrecht, Email: aalbrecht@lifespan.org, The Miriam Hospital, Centers for Behavioral and Preventive Medicine, Coro West Suite 500, 1 Hoppin Street, Providence, RI 02903
Christopher N. Sciamanna, Email: cns10@psu.edu, Penn State Hershey Medical Center, M.C. HU15, PO Box 850, Hershey, PA, 17033
John M. Jakicic, Email: jjakicic@pitt.edu, University of Pittsburgh, Suite 600, Birmingham Towers, 2100, Wharton Street, Pittsburgh, PA 15203
George D. Papandonatos, Email: Gdp@stat.brown.edu, Brown University, Center for Statistical Sciences, Brown University, Box G-S121, 121 South Main St., 7th Floor, Providence, RI 02912
Bess H. Marcus, Email: Bess_Marcus@brown.edu, The Miriam Hospital, Centers for Behavioral and Preventive Medicine, Brown University Program in Public Health, One Hoppin Street, Coro West, Suite 500, Providence, RI 02903
References
- 1.Centers for Disease Control and Prevention (CDC) Prevalence of regular physical activity among adults--United States, 2001 and 2005. MMWR Morb Mortal Wkly Rep. 2007;56:1209–12. [PubMed] [Google Scholar]
- 2.Centers for Disease Control. Physical activity trends--United States, 1990–1998. Morbidity and Mortality Weekly Report. 2001;50:166–8. [PubMed] [Google Scholar]
- 3.Dollman J, Okely AD, Hardy L, Timperio A, Salmon J, Hills AP. A hitchhiker’s guide to assessing young people’s physical activity: Deciding what method to use. J Sci Med Sport. 2009;12:518–25. doi: 10.1016/j.jsams.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 4.Prince SA, Adamo KB, Hamel ME, Hardt J, Gorber SC, Tremblay M. A comparison of direct versus self-report measures for assessing physical activity in adults: A systematic review. Int J Behav Nutr Phys Act. 2008;5:56. doi: 10.1186/1479-5868-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schutz Y, Weinsier RL, Hunter GR. Assessment of free-living physical activity in humans: An overview of currently available and proposed new measures. Obes Res. 2001;9:368–79. doi: 10.1038/oby.2001.48. [DOI] [PubMed] [Google Scholar]
- 6.Melanson EL, Freedson PS. Validity of the computer science and applications, inc. (CSA) activity monitor. Medicine & Science in Sports & Exercise. 1995;27:934–40. [PubMed] [Google Scholar]
- 7.Ward DS, Evenson KR, Vaughn A, Rodgers AB, Troiano RP. Accelerometer use in physical activity: Best practices and research recommendations. Med Sci Sports Exerc. 2005;37(11 Suppl):S582–8. doi: 10.1249/01.mss.0000185292.71933.91. [DOI] [PubMed] [Google Scholar]
- 8.Murphy SL. Review of physical activity measurement using accelerometers in older adults: Considerations for research design and conduct. Prev Med. 2009;48:108–14. doi: 10.1016/j.ypmed.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adamo KB, Prince SA, Tricco AC, Connor-Gorber S, Tremblay M. A comparison of indirect versus direct measures for assessing physical activity in the pediatric population: A systematic review. Int J Pediatr Obes. 2009;4:2–27. doi: 10.1080/17477160802315010. [DOI] [PubMed] [Google Scholar]
- 10.Dollman J, Okely AD, Hardy L, Timperio A, Salmon J, Hills AP. A hitchhiker’s guide to assessing young people’s physical activity: Deciding what method to use. J Sci Med Sport. 2009;12:518–25. doi: 10.1016/j.jsams.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 11.Sallis JF. Measuring physical activity environments: A brief history. Am J Prev Med. 2009;36:S86–92. doi: 10.1016/j.amepre.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.American College of Sports Medicine. ACSM’s guidelines for exercise testing and prescription. 7. Philadelphia, PA: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 13.Hendelman D, Miller K, Baggett C, Debold E, Freedson P. Validity of accelerometry for the assessment of moderate intensity physical activity in the field. Medicine & Science in Sports & Exercise. 2000;32:S442–9. doi: 10.1097/00005768-200009001-00002. [DOI] [PubMed] [Google Scholar]
- 14.Leenders NY, Sherman WM, Nagaraja HN. Energy expenditure estimated by accelerometry and doubly labeled water: Do they agree? Med Sci Sports Exerc. 2006 Dec;38:2165–72. doi: 10.1249/01.mss.0000235883.94357.95. [DOI] [PubMed] [Google Scholar]
- 15.Masse LC, Fulton JE, Watson KL, Heesch KC, Kohl HW, 3rd, Blair SN, et al. Detecting bouts of physical activity in a field setting. Res Q Exerc Sport. 1999;70:212–9. doi: 10.1080/02701367.1999.10608041. [DOI] [PubMed] [Google Scholar]
- 16.Masse LC, Fuemmeler BF, Anderson CB, Matthews CE, Trost SG, Catellier DJ, et al. Accelerometer data reduction: A comparison of four reduction algorithms on select outcome variables. Med Sci Sports Exerc. 2005;37:S544–54. doi: 10.1249/01.mss.0000185674.09066.8a. [DOI] [PubMed] [Google Scholar]
- 17.Matthews CE. Physical activity in the united states measured by accelerometer: Comment. Med Sci Sports Exerc. 2008;40:1188. doi: 10.1249/MSS.0b013e31817057da. [DOI] [PubMed] [Google Scholar]
- 18.McClain JJ, Craig CL, Sisson SB, Tudor-Locke C. Comparison of lifecorder EX and ActiGraph accelerometers under free-living conditions. Appl Physiol Nutr Metab. 2007;32:753–61. doi: 10.1139/H07-060. [DOI] [PubMed] [Google Scholar]
- 19.Metzger JS, Catellier DJ, Evenson KR, Treuth MS, Rosamond WD, Siega-Riz AM. Patterns of objectively measured physical activity in the united states. Med Sci Sports Exerc. 2008;40:630–8. doi: 10.1249/MSS.0b013e3181620ebc. [DOI] [PubMed] [Google Scholar]
- 20.Rothney MP, Apker GA, Song Y, Chen KY. Comparing the performance of three generations of ActiGraph accelerometers. J Appl Physiol. 2008;105:1091–7. doi: 10.1152/japplphysiol.90641.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sirard JR, Melanson EL, Li L, Freedson PS. Field evaluation of the computer science and applications, inc. physical activity monitor. Medicine & Science in Sports & Exercise. 2000;32:695–700. doi: 10.1097/00005768-200003000-00022. [DOI] [PubMed] [Google Scholar]
- 22.Strath SJ, Holleman RG, Ronis DL, Swartz AM, Richardson CR. Objective physical activity accumulation in bouts and nonbouts and relation to markers of obesity in US adults. Prev Chronic Dis. 2008;5:A131. [PMC free article] [PubMed] [Google Scholar]
- 23.Strath SJ, Brage S, Ekelund U. Integration of physiological and accelerometer data to improve physical activity assessment. Med Sci Sports Exerc. 2005;37:S563–71. doi: 10.1249/01.mss.0000185650.68232.3f. [DOI] [PubMed] [Google Scholar]
- 24.Wood AC, Kuntsi J, Asherson P, Saudino KJ. Actigraph data are reliable, with functional reliability increasing with aggregation. Behav Res Methods. 2008;40:873–8. doi: 10.3758/brm.40.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leenders NYJM, Sherman WM, Nagaraja HN. Comparisons of four methods of estimating physical activity in adult women. Med Sci Sports Exerc. 2000;32:1320–6. doi: 10.1097/00005768-200007000-00021. [DOI] [PubMed] [Google Scholar]
- 26.Philippaerts RM, Westerterp KR, Lefevre J. Comparison of two questionnaires with a tri-axial accelerometer to assess physical activity patterns. Int J Sports Med. 2001;22:34–9. doi: 10.1055/s-2001-11359. [DOI] [PubMed] [Google Scholar]
- 27.Ainsworth BE, Bassett DR, Jr, Strath SJ, Swartz AM, O’Brien WL, Thompson RW, et al. Comparison of three methods for measuring the time spent in physical activity. Med Sci Sports Exerc. 2000;32:S457–64. doi: 10.1097/00005768-200009001-00004. [DOI] [PubMed] [Google Scholar]
- 28.Alhassan S, Robinson TN. Objectively measured physical activity and cardiovascular disease risk factors in african american girls. Ethn Dis. 2008;18(4):421–6. [PMC free article] [PubMed] [Google Scholar]
- 29.Marcus BH, Napolitano MA, King AC, Lewis BA, Whiteley JA, Albrecht A, et al. Telephone versus print delivery of an individualized motivationally tailored physical activity intervention: Project STRIDE. Health Psychology. 2007 Jul;26:401–9. doi: 10.1037/0278-6133.26.4.401. [DOI] [PubMed] [Google Scholar]
- 30.Actigraph. [homepage on the Internet] Pensacola (FL): Available from: www.theactigraph.com. [Google Scholar]
- 31.Blair SN, Haskell WL, Ho P, Paffenbarger RS, Jr, Vranizan KM, Farquhar JW, et al. Assessment of habitual physical activity by seven-day recall in a community survey and controlled experiments. American Journal of Epidemiology. 1985;122:794–804. doi: 10.1093/oxfordjournals.aje.a114163. [DOI] [PubMed] [Google Scholar]
- 32.Sallis JF, Haskell WL, Wood PD, Fortmann SP, Rogers T, Blair SN, et al. Physical activity assessment methodology in the five-city project. American Journal of Epidemiology. 1985 Jan;121(1):91–106. doi: 10.1093/oxfordjournals.aje.a113987. [DOI] [PubMed] [Google Scholar]
- 33.Bandura A. Social cognitive theory: An agentic perspective. Asian Journal of Social Psychology. 1999;2:21–41. doi: 10.1146/annurev.psych.52.1.1. [DOI] [PubMed] [Google Scholar]
- 34.Bandura A. Self-efficacy: The excercise of control. New York: W. H. Freeman and Co; 1997. [Google Scholar]
- 35.Marcus BH, Forsyth LA. Motivating people to be physically active. Champaign, IL: Human Kinetics; 2003. [Google Scholar]
- 36.Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, et al. Physical activity and public health: Updated recommendation for adults from the american college of sports medicine and the american heart association. Circulation. 2007;116:1081–93. doi: 10.1161/CIRCULATIONAHA.107.185649. [DOI] [PubMed] [Google Scholar]
- 37.Welk G, Schaben J, Morrow J. Reliability of accelerometry-based activity monitors: A generalizability study. Medicine And Science In Sports And Exercise. 2004;36:1637–45. [PubMed] [Google Scholar]
- 38.Trost SG, McIver KL, Pate RR. Conducting accelerometer-based activity assessments in field-based research. Med Sci Sports Exerc. 2005;37:S531–43. doi: 10.1249/01.mss.0000185657.86065.98. [DOI] [PubMed] [Google Scholar]
- 39.Freedson PS, Melanson E, Sirard J. Calibration of the computer science and applications, inc. accelerometer. Med Sci Sports Exerc. 1998;30:777–81. doi: 10.1097/00005768-199805000-00021. [DOI] [PubMed] [Google Scholar]
- 40.Blair SN, Haskell WL, Ho P, Paffenbarger RS, Jr, Vranizan KM, Farquhar JW, et al. Assessment of habitual physical activity by seven-day recall in a community survey and controlled experiments. American Journal of Epidemiology. 1985;122:794–804. doi: 10.1093/oxfordjournals.aje.a114163. [DOI] [PubMed] [Google Scholar]
- 41.Dunn AL, Andersen RE, Jakicic JM. Lifestyle physical activity interventions. history, short- and long-term effects, and recommendations. American Journal of Preventive Medicine. 1998;15:398–412. doi: 10.1016/s0749-3797(98)00084-1. [DOI] [PubMed] [Google Scholar]
- 42.Dunn AL, Marcus BH, Kampert JB, Garcia ME, Kohl HW, 3rd, Blair SN. Comparison of lifestyle and structured interventions to increase physical activity and cardiorespiratory fitness: A randomized trial. Journal of the American Medical Association. 1999;281:327–34. doi: 10.1001/jama.281.4.327. [DOI] [PubMed] [Google Scholar]
- 43.King AC, Friedman R, Marcus B, Castro C, Napolitano M, Ahn D, et al. Ongoing physical activity advice by humans versus computers: The community health advice by telephone (CHAT) trial. Health Psychol. 2007;26:718–27. doi: 10.1037/0278-6133.26.6.718. [DOI] [PubMed] [Google Scholar]
- 44.Haskell WL, Lee IM, Pate RR, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116:1081–1093. doi: 10.1161/CIRCULATIONAHA.107.185649. [DOI] [PubMed] [Google Scholar]
- 45.Marcus BH, Rossi JS, Selby VC, Niaura RS, Abrams DB. The stages and processes of exercise adoption and maintenance in a worksite sample. Health Psychology. 1992;11:386–395. doi: 10.1037//0278-6133.11.6.386. [DOI] [PubMed] [Google Scholar]
- 46.Howley ET, Franks BD. Health fitness instructor’s handbook. Champaign, IL: Human Kinetics Books; 1992. Cardiorespiratory fitness; pp. 153–77. [Google Scholar]
- 47.Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillside, NJ: Lawrence Erlbaum; 1988. [Google Scholar]
- 48.Richardson MT, Ainsworth BE, Jacobs DR, Leon AS. Validation of the Stanford 7-day recall to assess habitual physical activity. Ann Epidemiol. 2001 Feb;11:145–53. doi: 10.1016/s1047-2797(00)00190-3. [DOI] [PubMed] [Google Scholar]
- 49.Schmidt MD, Cleland VJ, Thomson RJ, Dwyer T, Venn AJ. A comparison of subjective and objective measures of physical activity and fitness in identifying associations with cardiometabolic risk factors. Ann Epidemiol. 2008;18:378–86. doi: 10.1016/j.annepidem.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 50.Rosenberg DE, Bull FC, Marshall AL, Sallis JF, Bauman AE. Assessment of sedentary behavior with the international physical activity questionnaire. J Phys Act Health. 2008;5:S30–44. doi: 10.1123/jpah.5.s1.s30. [DOI] [PubMed] [Google Scholar]
- 51.Yngve A, Nilsson A, Sjostrom M, Ekelund U. Effect of monitor placement and of activity setting on the MTI accelerometer output. Med Sci Sports Exerc. 2003;35:320–6. doi: 10.1249/01.MSS.0000048829.75758.A0. [DOI] [PubMed] [Google Scholar]
- 52.Kirk A, Barnett J, Leese G, Mutrie N. randomized trial investigating the 12-month changes in physical activity and health outcomes following a physical activity consultation delivered by a person or in written form in Type 2 diabetes: Time2Act. Diab Med. 2009;26:293–301. doi: 10.1111/j.1464-5491.2009.02675.x. [DOI] [PubMed] [Google Scholar]
- 53.Keyserling TC, Samuel Hodge CD, Jilcott SB, Johnston LF, Garcia BA, Gizlice Z, et al. Randomized trial of a clinic-based, community-supported, lifestyle intervention to improve physical activity and diet: The North Carolina enhanced WISEWOMAN project. Prev Med. 2008;46:499–510. doi: 10.1016/j.ypmed.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 54.Webber LS, Catellier DJ, Lytle LA, Murray DM, Pratt CA, Young DR, et al. Promoting activity in middle school girls: Trial of Activity for Adolescent Girls. Am J Prev Med. 2008;34:173–184. doi: 10.1016/j.amepre.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marcus BH, Lewis BA, Williams DM, et al. Step Into Motion: A randomized trial examining the relative efficacy of internet vs. print-based physical activity interventions. Contemp Clin Trials. 2007;28:737–47. doi: 10.1016/j.cct.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 56.Marcus BH, Lewis BA, Williams DM, et al. A comparison of Internet and print-based physical activity interventions. Arch Intern Med. 2007;167:944–949. doi: 10.1001/archinte.167.9.944. [DOI] [PubMed] [Google Scholar]
- 58.Williams DM, Papandonatos G, Jennings E, Napolitano MA, Lewis BA, Whiteley JA, Bock BC, Albrecht A, Dunsiger S, Parisi A, King AC, Marcus B. Does Tailoring on Additional Theoretical Constructs Enhance the Efficacy Print-based Physical Activity Promotion Intervention? Manuscript Under Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pober DM, Staudenmayer J, Raphael C, Freedson PS. Development of novel techniques to classify physical activity mode using accelerometers. Med Sci Sports Exerc. 2006;38:1626–34. doi: 10.1249/01.mss.0000227542.43669.45. [DOI] [PubMed] [Google Scholar]
- 60.Crouter SE, Clowers KG, Bassett DR., Jr A novel method for using accelerometer data to predict energy expenditure. J Appl Physiol. 2006;100:1324–31. doi: 10.1152/japplphysiol.00818.2005. [DOI] [PubMed] [Google Scholar]
- 61.Rothney MP, Schaefer EV, Neumann MM, Choi L, Chen KY. Validity of physical activity intensity predictions by ActiGraph, actical, and RT3 accelerometers. Obesity (Silver Spring) 2008;16:1946–52. doi: 10.1038/oby.2008.279. [DOI] [PMC free article] [PubMed] [Google Scholar]