Abstract
Medicinal leeches (Hirudo spp.) swim using a metachronal, front-to-back undulation. The behavior is generated by central pattern generators (CPGs) distributed along the animal’s midbody ganglia and is coordinated by both central and peripheral mechanisms. Here we report that a component of the venom of Conus imperialis, α-conotoxin ImI, known to block nicotinic acetylcholine receptors in other species, disrupts swimming. Leeches injected with the toxin swam in circles with exaggerated dorsoventral bends and reduced forward velocity. Fictive swimming in isolated nerve cords was even more strongly disrupted, indicating that the toxin targets the CPGs and central coordination, while peripheral coordination partially rescues the behavior in intact animals.
Keywords: Swimming, Central pattern generator, Intersegmental coordination, Medicinal leech, Hirudo verbana
Introduction
In studying the neuronal basis of behaviors, repetitive and rhythmic behaviors have received much attention [1]. Locomotion in many species depends on waves of contraction sweeping along the body under the control of a chain of semi-autonomous segmental central pattern generators (CPGs) with intersegmental connections. Well-studied examples include the swimmerets of crayfish [2, 3] and the sinuous swimming of lampreys [4, 5]. Swimming behavior in medicinal leeches (Hirudo spp.) provides a particularly successful focus of study, because their nervous systems are relatively simple, their bodies sturdy, and their behaviors readily quantifiable [6]. Accordingly, Hirudo’s nervous system, and in particular its swimming circuit, is the best-understood among annelids.
Leech swimming requires coordination of muscles along the entire body. The basic swim pattern is generated by core CPGs that are repeated in each segment. Connections among core CPGs are spread among several adjacent ganglia in the ventral nerve cord; muscle contraction and relaxation in each segment is controlled by motor neurons in that segment’s ganglion. Whole-body coordination of swimming is achieved by two mechanisms: central interganglionic connections through the ventral nerve cord, and peripheral sensory feedback through stretch receptors embedded in the dorsal and ventral longitudinal muscles [7, 8]. The central connections are sufficient to produce the motor pattern: electrically stimulating a nerve causes an isolated nerve cord to generate coordinated motor neuron output in the absence of body wall [9]. Remarkably, the peripheral mechanism is also sufficient to impose coordination: after transecting the nerve cord between two midbody ganglia, the anterior and posterior parts of the animal still produce swimming movements, and the phase relationship between swim strokes in the two parts remains largely unaltered [8].
To tease apart central and peripheral mechanisms, we screened venoms from cone snails. All previously characterized components of these venoms target neurons or muscles. We found that venom from the annelid-hunting snail Conus imperialis produced recognizable, but reliably abnormal, swimming. Chemical fractionation (by the laboratory of Dr. Baldomero Olivera, University of Utah) revealed that the active fraction was α-conotoxin ImI, which blocks nicotinic acetylcholine receptors in several vertebrates [10, 11, 12]. We treated intact leeches or isolated nerve cords with ImI and compared the results to ask whether the apparent redundancy between central and peripheral control makes the system less vulnerable to external disruptions.
Methods
Behavior
Leeches (Hirudo verbana, Carolina Biological Supply, Burlington NC) were maintained at 15 °C in artificial pond water (36 mg/L Instant Ocean salts; Aquarium Systems, Mentor OH). Leeches were fed cow blood semiannually (no feeding occurred within 1 month of experiments) and weighed about 1 gram at the time of experiments unless otherwise noted. ImI (from Dr. Baldomero Olivera, University of Utah or Peptides International, Louisville KY) was injected intramuscularly in the dorsal body wall near midbody segment M15 at a concentration of 10 nanomoles/gram body mass in 25 μL HEPES-buffered leech saline [13]. For ImI concentrations between 6 nmol/g and 50 nmol/g, the latency to behavioral onset decreased from 8 to 3.5 minutes, but the response itself did not qualitatively change. Effects persisted from days to weeks, but all leeches eventually returned to normal behavior. Control animals were injected with 25 μL saline. All behavioral observations were done blinded.
Following injection, each leech was placed individually in shallow (2 cm deep) water, which forced it to swim on its side rather than in the normal dorsal-up posture. Swimming in this unusual orientation facilitated videotaping from above, but was otherwise indistinguishable from normal swimming in deeper water. When necessary, leeches were gently prodded to encourage swimming.
Analysis of Behavior
Video clips of swimming leeches were recorded at 30 frames/s and digitized using standard equipment. The location and body shape of the leech were identified in each frame using the “Wormfinder” algorithm [14], which yields a curve along the midline of the animal from head to tail (Supplemental Figure 1a). Sections of this curve where the ventral longitudinal muscles contracted (causing the dorsal surface to be convex) were termed peaks, whereas sections where the ventral longitudinal muscles contracted (causing the dorsal surface to be concave) were termed troughs.
The forward velocity of a swimming leech was calculated as the velocity of its center of mass after low-pass filtering of the trajectory to exclude the undulatory motion perpendicular to the swimming direction.
Electrophysiology of the Isolated Cord
The central nervous system of the leech was isolated as previously described [9]. Briefly, leeches were anesthetized in ice-cold saline, pinned down on wax, and opened along the dorsal midline. The entire nerve cord minus the head brain was extracted and placed in a Petri dish. (Removal of the head brain promotes fictive swimming [15, 16].) Dorsal posterior (DP) nerves and the first branch (B1) of anterior anterior (AA) nerves were exposed in selected segments to allow recording of motor neuron activity.
Data Acquisition
Suction electrodes where placed on DP and AA nerves (Supplemental Figure 1b). Signals were amplified 10,000x and bandpass filtered (300 Hz–5 kHz) using a differential AC amplifier (A-M Systems, Sequim WA) and were digitized at 10 kHz (DigiData 1320A, Axon Instruments, Sunnyvale CA). Fictive swimming was elicited by electrical stimulation of one of the DP nerves from ganglia M10–12 using a Grass (West War-wick RI) S88 stimulator. Stimuli consisted of trains of 5–10 pulses (1–2 V, 1 ms long) at 10 Hz. We initially recorded 5–10 evoked swim episodes in normal saline, at 1–3 minute intervals (to avoid habituation). We then replaced the saline with 10–20 μM ImI (in fresh saline) and continued the same stimulation protocol. Recordings made within 10 minutes of ImI application were discarded to avoid the transition period.
Analysis of Electrophysiological Data
Several motor neurons project through the DP nerves. Based on spike amplitude, we isolated action potentials attributable to motor neuron DE-3, which provides excitatory drive to the dorsal longitudinal muscles and plays a major role in swimming behavior [6]. Spikes from cell VE-108, which excites ventral longitudinal muscles, were similarly isolated from AA-B1 recordings [17]. Bursts were defined as groups of at least 2 spikes separated from other groups by at least 100 ms. The duration of a burst was the time between the first and last spikes in the burst. The period of bursting was the time between the middle spikes in consecutive bursts. Bursts shorter than 750 ms were classified as swim-like if either or both of the preceding and following burst periods were shorter than 1500 ms. A fictive swim episode was defined as a stretch of time during which at least one ganglion fired a sequence of at least three contiguous swim-like bursts. To quantify burst propagation along the cord, bursts recorded from adjacent pairs of electrodes were matched up, and interganglionic delays were calculated as the latency between the middle spikes in matching bursts, divided by the number of segments separating the electrodes. For this calculation, we included only bursts that occurred while at least one ganglion produced recognizable fictive swimming.
Results
Behavior
Treatment with ImI profoundly disrupted the kinematics of swimming. While saline-injected control leeches swam with a nearly symmetric dorsoventral stroke that produced effective forward motion (Figure 1a), ImI-injected animals craned their heads backward during part of their stroke and exaggerated the magnitude of troughs (Figure 1b1). As a result, they typically changed direction about 90° per stroke (Figure 1b2). This pattern of swimming was quite distinct from normal cornering behavior as an untreated leech swims along the wall of its tank: it gently curves its body and does not crane its head (data not shown).
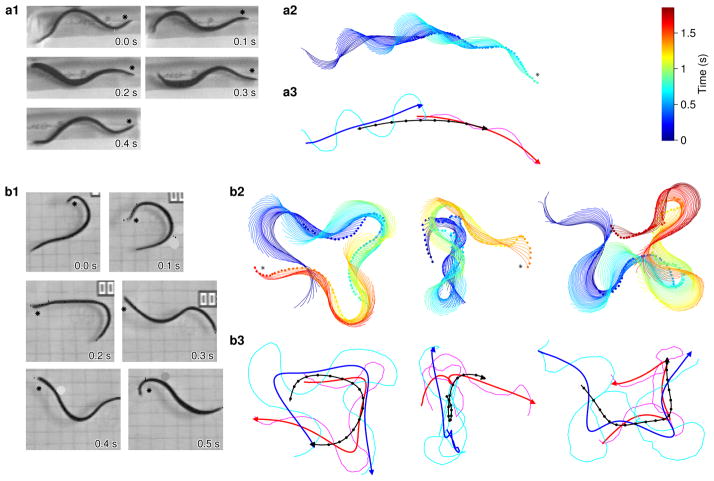
Figure 1.
Swimming in intact leeches. a. Free swimming in control (saline-injected) leeches. a1. Video frames of a typical swimming leech (at 0.1-s intervals). Dots indicate the dorsal side of the animal’s head. a2. Path followed by the control leech shown in a1, as extracted by the “Wormfinder” algorithm. The animal’s location and shape in sequential frames (16.7 ms intervals) are plotted as colored lines with small dots marking the head. Color indicates time; the color bar applies to b2 and c2 as well. The head of the leech at the end of the clip is indicated by a black star. a3. Abstraction of the path shown in a2. Lines show the actual trajectory of the head (magenta) and the tail (cyan), as well as the same data after filtering out the undulations of the head (red), tail (blue), and center (black). Dots along the black curve indicate 0.1-s intervals; arrowheads indicate the direction of locomotion. b. After injection with ImI. b1. Video frames. b2–b3. Examples of paths followed by three ImI-injected leeches, using the same conventions as in (a).
In control animals, peaks and troughs propagated linearly from head to tail (Figure 2a). After ImI injection, propagation became less coordinated, and a stationary trough (corresponding to head craning) typically appeared at the anterior end of the body (Figure 2b). In control animals, propagating peaks and troughs lasted equally long, and a full cycle lasted 368 ± 48 ms (mean ± SSD, N = 3, Figure 2c). After ImI injection, the durations of both peaks and troughs increased about 30%–50%, and a full cycle lasted 568 ± 58 ms (N = 3, p < .001, t-test). Although the cycle period was longer following ImI, the rate of wave propagation from head to tail was not significantly different from controls (Figure 2d).
Figure 2.
Propagation of dorsoventral waves along the bodies of swimming leeches. a. Peaks (gray) and troughs (black) in a normally swimming leech progressed from head to tail. When the leech turned toward its ventral side (middle of the graph), the wave temporarily lacked a trough. b. Propagation of peaks and troughs in an ImI-injected animal. Note the stationary trough (black dots) near the middle of the graph. c. Cycle periods (i.e., the time separating frames when peaks or troughs passed an animal’s midpoint) for ImI-injected animals (black, N = 3) compared with controls (gray, N = 4). d. Propagation delays (expressed as time per percent body length) of swim waves passing through the body of ImI-injected animals (black) and controls (gray). *,p < .05. e. Forward velocities of ImI-injected animals (black), straight-swimming control animals (gray) and corner-turning control animals (white, N = 3). *, p < .05; **, p < .01; ***, p < .001; n.s., not significant; unpaired t-tests.. All error bars represent sample standard deviation (SSD).
ImI-injected leeches made significantly slower forward progress than control animals (Figure 2e): they also never got very far away from their starting points because they failed to swim in a straight line. For instance, the distance between the initial and final positions of the center of one ImI-injected leech (Figure 1b3, left) was 3.3 cm, traversed in 1.6 s. A control leech (Figure 1a3) covered 9.6 cm in 0.8 s, making the ImI-injected leech’s swimming nearly 6 times less effective.
Electrophysiology
We used extracellular electrodes to record simultaneously from dorsal excitor motor neurons DE-3 of up to eight ganglia in isolated nerve cords. In each cord, recordings were made first in saline, then in ImI. The effect of ImI was not removed by 1 hr of saline washing (data not shown), but the behavioral experiments established that the effects of the toxin took hours to days to reverse.
In saline, nerve cords readily produced fictive swimming in response to electrical nerve stimulation (Figure 3a, top). The period and the propagation of fictive swim bursts were very regular, although somewhat slower than in intact animals (see Figure 2), as previously reported [6]. ImI altered the activity of isolated nerve cords dramatically. Although stimulation still readily elicited patterned motor neuron output, the pattern of bursting became much less regular (Figure 3a, bottom). At times, a semblance of a swim-like pattern remained (solid lines below the rasters), but the swim bursts were less regular, especially toward the posterior end of the cord (M14–16). Output also commonly included extended periods (dotted lines) of relatively unpatterned motor neuron activity devoid of swim-like bursts, which was extremely rare in controls. Whereas ImI did not change the total duration of responses to identical stimuli (Figure 3b), responses contained far fewer recognizable swim cycles (Figure 3c), especially in posterior ganglia. Interestingly, the cycle period during those portions of the behavior that resembled fictive swimming changed only slightly (Figure 3d). To quantify regularity of bursts, we calculated histograms of interspike intervals (ISIs; Figure 3i). In saline, ISIs within bursts were much shorter than between bursts, resulting in two distinct peaks in the histogram. ImI changed ISIs only slightly on average, but they became more variable, and interburst intervals became so irregular that they no longer produced a distinct peak in the histogram.
Figure 3.
ImI disrupted fictive swimming in isolated nerve cords. a. Spike trains recorded simultaneously from motor neurons DE-3 in several ganglia extending from anterior segment M3 to posterior segment M16 along an isolated nerve cord bathed in saline (top) and in 10 μM ImI (bottom). Traces above rasters show corresponding raw voltage data from one electrode. Arrows mark the time and location of the stimulus. Solid lines below records indicate portion of response that is fictive swimming; dotted lines indicate portions that are not. b. Average duration of responses to electrical stimulation in saline and in ImI. c. Average number of recognizable fictive swim cycles in response to a single stimulus, in saline (gray) and in ImI (black). Paired t-tests for N = 30 anterior (M3–8), 27 middle (M9–12), and 15 posterior (M13–16) ganglia from 19 leeches (significance as in Figure 2). d. Cycle periods during fictive swimming in saline (gray) and ImI (black). Data shown are mean of the median period for each preparation with SSD across preparations. e. Burst propagation delay between neighboring ganglia in saline (gray), and ImI (black). f. Spike trains recorded from motor neuron DE-3 in ganglion M10 during swimming in an intact leech, first in control conditions and then after injection with ImI. g. Dorsal and ventral motor neuron bursts from ganglion M11 of an isolated nerve cord in saline and in ImI. h. Quality of dorsal–ventral coordination (QDV, see text). The total number of recorded ganglia is indicated above each bar. (Due to unfavorable anatomy, no recordings were obtained from ventral excitor motorneurons from posterior ganglia in ImI.) i. Histograms of ISIs of DE-3 in M6 in the first 30 s after stimulation (6 stimuli in saline, 12 in ImI; one typical preparation). Note logarithmic axes.
We also recorded electrophysiologically from DE-3 in ganglion M10 of several intact animals using cuff electrodes (see Supplemental Methods). Simultaneous video recording allowed us to directly identify episodes of swimming. In control conditions, swim bursts were very regular (Figure 3f) and had periods of 408 ± 61 ms (N = 3), matching behavioral observations. After injection with 20 nmol ImI (in 250 μL saline) periods lengthened to 566 ± 114 ms (N = 3), again matching behavioral observations. Burst patterns became less regular, but—unlike in recordings from isolated cords—excessively long burst periods (> 1.5 s) were never observed in swimming intact animals.
Interganglionic coordination
For a swim stroke to effectively propel a leech forward, coordination between segments is as important as the quality of the motor neuron output within individual segments. Even in isolated cords—which lack sensory feedback—interganglionic coordination was tightly controlled when cords were bathed in saline, as evidenced by the precise anteroposterior propagation of bursts (Figure 3a). Interganglionic propagation delays were slightly longer in the middle of the cord than near the ends (Figure 3e), perhaps because ganglia are physically closer together at the ends of the cord, but the variability of delays was low, and bursts always propagated anteroposteriorly. In the presence of ImI, propagation was severely disrupted. Except at the posterior end of the cord, median propagation delays were no longer significantly different from zero, indicating that bursts either propagated equally often in both directions, or that they did not propagate at all, but rather occurred simultaneously in several ganglia.
Dorsoventral coordination
In addition to interganglionic coordination, dorsoventral coordination is critical for effective swimming. To produce undulation, rather than tensing or shortening, dorsal and ventral contractions must occur in antiphase, and under control conditions they did (Figure 3g): The fraction of ventral spikes that occurred outside of dorsal bursts (QDV) was close to one (Figure 3h), indicating near perfect alternation of dorsal and ventral bursts without overlap. Remarkably, in ImI, despite the general degradation of the swim pattern, dorsal and ventral bursts still alternated without much overlap, and QDV did not decrease significantly relative to controls. Thus, dorsoventral coordination was preserved in ImI, despite the disruption of many other parameters of the swim motor pattern.
Discussion
Treating intact leeches with α-conotoxin ImI severely compromised their usually elegant and effective swimming, yet they still swam. Similarly, exposing isolated ventral nerve cords to the toxin disrupted the rhythmic production of motor neuron bursts that drive swimming behavior, but did not abolish it.
Following treatment with ImI, leeches swam in a petal-shaped circular pattern (Figure 1b) rather than in the typical straight path of control animals (Figure 1a), apparently as a result of exaggerated bending toward the dorsal side as the swim wave passed through the body and a maintained backward craning of the head. Because the neuronal circuitry underlying swimming is well-studied [6], we have formed hypotheses regarding the basis of the disruption.
First, consider what ImI does not disrupt. Normal swimming is a bilaterally symmetric behavior: homologous pairs of motor neurons on the two sides of each ganglion activate longitudinal muscles on both sides of the segment in synchrony. This bilateral symmetry in muscle contraction is not hard-wired: during local bending [18], longitudinal muscles on one side of the leech contract while homologous contralateral muscles relax. Synchronous contractions probably depend on two features of the circuitry: bilateral pairs of homologous motor neurons are electrically coupled to one another [19] and pairs of homologous neurons receive input from the swim CPG simultaneously [6]. The persistence of this symmetry following ImI treatment strongly suggests that the toxin does not affect these connections among and onto the motor neurons.
In addition, the strictly alternating contraction of dorsal and ventral longitudinal muscles and the strictly alternating activity in the motor neurons that drive these muscles were largely unchanged following treatment with ImI (Figure 3g, h). This alternation, too, is not a given: during crawling and shortening, dorsal and ventral longitudinal muscles in a segment co-contract [6]. Contraction in antiphase depends at least in part on connections between excitatory and inhibitory motor neurons within each ganglion: During swimming, when motor neurons that excite dorsal longitudinal muscles are active, motor neurons that inhibit ventral longitudinal muscles are also active. These inhibitory motor neurons not only project to muscles in the periphery, they also make inhibitory synapses onto motor neurons that drive those same muscles [6]. The swim CPG feeds onto this set of chemical-synaptically connected motor neurons, providing another layer of control [6], and the strict preservation of antiphasic activation of dorsal and ventral longitudinal muscles after ImI treatment suggests that the toxin spares this level of circuitry as well.
What ImI did change was the timing of the motor output and especially its intersegmental coordination. When isolated nerve cords were exposed to ImI, the coordinated front-to-back propagation of activity broke down. Instead, bursts of activity occurred simultaneously in several ganglia (Figure 3a, e). However, in ImI-injected whole animals, propagation was moderately well preserved (Figure 2b, d), suggesting that peripheral biomechanical feedback through stretch receptors located in the muscles of the body wall [8, 20] partially rescued intersegmental coordination in spite of disorganization among the CPGs. (Differences between intact animals and isolated cords cannot be explained by possible concentration differences, since—in both preparations—a wide range of concentrations produced qualitatively the same results.)
Why were interganglionic connections more affected than the connections within the CPG and from the CPG neurons onto the motor neurons? It is possible that the former are less numerous or synaptically weaker than the latter. More interestingly, interganglionic synapses could differ pharmacologically from their intraganglionic counterparts, rendering them intrinsically more sensitive to ImI. (Across phyla, ImI acts as an antagonist to nicotinic acetylcholine receptors [10, 11, 12].) If so, ImI could be used to differentially block intersegmental particular components of the CPG, which would allow us to deepen our understanding of how the swimming CPG, a relatively small set of interconnected neurons, generates well-coordinated and plastic locomotory behavior.
An exploration of the balance between intersegmental control and intraganglionic local control during swimming in the leech, aided by a drug like ImI which spares motorneuronal connections but disrupts interneuronal networks, will help reveal fundamental principles of motor control against which metachronal locomotion in these and other species can be compared.
Supplementary Material
Acknowledgments
We thank Dr. Baldomero Olivera and his laboratory for supplying Conus venoms and ImI in the early phases of this work. This work was supported by NIH Research Grants MH43396 and NS35336 (to WBK), by a Senior Research Fellowship from the Broad Foundations (to DAW) and by Microsoft Research Labs. DAW holds a Career Award at the Scientic Interface from the Burroughs Wellcome Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Marder E, Calabrese RL. Principles of rhythmic motor pattern generation. Physiol Rev. 1996;76:687–717. doi: 10.1152/physrev.1996.76.3.687. [DOI] [PubMed] [Google Scholar]
- 2.Mulloney B, Skinner FK, Namba H, Hall WM. Intersegmental coordination of swimmeret movements: mathematical models and neural circuits. Ann N Y Acad Sci. 1998;860:266–280. doi: 10.1111/j.1749-6632.1998.tb09055.x. [DOI] [PubMed] [Google Scholar]
- 3.Mulloney B, Hall WM. Local and intersegmental interactions of coordinating neurons and local circuits in the swimmeret system. J Neurophysiol. 2007;98:405–413. doi: 10.1152/jn.00345.2007. [DOI] [PubMed] [Google Scholar]
- 4.Grillner S, Wallen P. Cellular bases of a vertebrate locomotor system-steering, intersegmental and segmental co-ordination and sensory control. Brain Res Brain Res Rev. 2002;40:92–9106. doi: 10.1016/s0165-0173(02)00193-5. [DOI] [PubMed] [Google Scholar]
- 5.Ayali A, Fuchs E, Ben-Jacob E, Cohen A. The function of intersegmental connections in determining temporal characteristics of the spinal cord rhythmic output. Neuroscience. 2007;147:236–246. doi: 10.1016/j.neuroscience.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kristan WB, Jr, Calabrese RL, Friesen WO. Neuronal control of leech behavior. Prog Neurobiol. 2005;76:279–327. doi: 10.1016/j.pneurobio.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Brodfuehrer PD, Debski EA, O’Gara BA, Friesen WO. Neuronal control of leech swimming. J Neurobiol. 1995;27:403–418. doi: 10.1002/neu.480270312. [DOI] [PubMed] [Google Scholar]
- 8.Yu X, Nguyen B, Friesen WO. Sensory feedback can coordinate the swimming activity of the leech. J Neurosci. 1999;19:4634–4643. doi: 10.1523/JNEUROSCI.19-11-04634.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kristan WB, Calabrese RL. Rhythmic swimming activity in neurons of isolated nerve cord of leech. J Exp Biol. 1976;65:643–668. doi: 10.1242/jeb.65.3.643. [DOI] [PubMed] [Google Scholar]
- 10.McIntosh JM, Yoshikami D, Mahe E, Nielsen DB, Rivier JE, Gray WR, Olivera BM. A nicotinic acetylcholine-receptor ligand of unique specificity, alpha-conotoxinimi. J Biol Chem. 1994;269:16733–16739. [PubMed] [Google Scholar]
- 11.Pereira EF, Alkondon M, McIntosh JM, Albuquerque EX. Alpha-conotoxinimi: a competitive antagonist at alpha-bungarotoxin-sensitive neuronal nicotinic receptors in hippocampal neurons. J Pharmacol Exp Ther. 1996;278:1472–1483. [PubMed] [Google Scholar]
- 12.Kehoe J, McIntosh JM. Two distinct nicotinic receptors, one pharmacologically similar to the vertebrate alpha7-containing receptor, mediate cl currents in aplysia neurons. J Neurosci. 1998;18:8198–8213. doi: 10.1523/JNEUROSCI.18-20-08198.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wagenaar DA, Hamilton MS, Huang T, Kristan WB, French KA. A hormone- activated central pattern generator for courtship. Curr Biol. 2010;20:487–495. doi: 10.1016/j.cub.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wagenaar DA, Kristan WB., Jr Automated video analysis of animal movements using gabor orientation filters. Neuroinformatics. 2010;8:33–42. doi: 10.1007/s12021-010-9062-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brodfuehrer PD, Friesen WO. Control of leech swimming activity by the cephalic ganglia. J Neurobiol. 1986;17:697–705. doi: 10.1002/neu.480170612. [DOI] [PubMed] [Google Scholar]
- 16.Brodfuehrer PD, Kogelnik AM, Friesen WO, Cohen AH. Effect of the tail ganglion on swimming activity in the leech. Behav Neural Biol. 1993;59:162–166. doi: 10.1016/0163-1047(93)90912-2. [DOI] [PubMed] [Google Scholar]
- 17.Kristan WB, Stent GS, Ort CA. Neuronal control of swimming in medicinal leech. 3. Impulse patterns of motor neurons. J Comp Physiol. 1974;94:155–176. [Google Scholar]
- 18.Kristan WB. Sensory and motor neurons responsible for the local bending response in leeches. J Exp Biol. 1982;96:161–180. [Google Scholar]
- 19.Fan RJ, Marin-Burgin A, French KA, Friesen WO. A dye mixture (neurobiotin and alexa 488) reveals extensive dye-coupling among neurons in leeches; physiology confirms the connections. J Comp Physiol A. 2005;191:1157–1171. doi: 10.1007/s00359-005-0047-8. [DOI] [PubMed] [Google Scholar]
- 20.Cang J, Yu X, Friesen WO. Sensory modification of leech swimming: interactions between ventral stretch receptors and swim-related neurons. J Comp Physiol A. 2001;187:569–79. doi: 10.1007/s003590100229. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.