Abstract
Methamphetamine is a drug of abuse that can induce oxidative stress and neurotoxicity to dopaminergic neurons. We have previously reported that oxidative stress promotes the liberation of intracellular Zn2+ from metal-binding proteins, which, in turn, can initiate neuronal injurious signaling processes. Here, we report that methamphetamine mobilizes Zn2+ in catecholaminergic rat pheochromocytoma (PC12) cells, as measured by an increase in Zn2+-regulated gene expression driven by the metal response element transcription factor-1. Moreover, methamphetamine-liberated Zn2+ was responsible for a pronounced enhancement in voltage-dependent K+ currents in these cells, a process that normally accompanies Zn2+-dependent cell injury. Overnight exposure to methamphetamine induced PC12 cell death. This toxicity could be prevented by the cell-permeant zinc chelator N,N,N′,N′-tetrakis(2-pyridylmethyl)-ethylenediamine (TPEN), and by over-expression of the Zn2+-binding protein metallothionein 3 (MT3), but not by tricine, an extracellular Zn2+ chelator. The toxicity of methamphetamine to PC12 cells was enhanced by the presence of co-cultured microglia. Remarkably, under these conditions, TPEN no longer protected but, in fact, dramatically exacerbated methamphetamine toxicity, tricine again being without effect. Over-expression of MT3 in PC12 cells did not mimic these toxicity-enhancing actions of TPEN, suggesting that the chelator affected microglial function. Interestingly, P2X receptor antagonists reversed the toxicity-enhancing effect of TPEN. As such, endogenous levels of intracellular Zn2+ may normally interfere with the activation of P2X channels in microglia. We conclude that Zn2+ plays a significant but complex role in modulating the cellular response of PC12 cells to methamphetamine exposure in both the absence and presence of microglia.
Keywords: Zinc, methamphetamine, toxicity, potassium currents, microglia, purinergic receptor
Methamphetamine is a widely abused psychostimulant with a very high risk of dependence (Winslow et al., 2007). Chronic methamphetamine use can produce a myriad of neurological and psychiatric deficits, and, importantly, can also lead to permanent and pronounced abnormal brain structural changes in humans (Thompson et al., 2004; Chang et al., 2007). Several molecular mechanisms have been proposed to account for the neurotoxic properties of methamphetamine, most seemingly converging on pro-oxidant and oxidative stress-induced processes (Yamamoto and Bankson, 2005; Cadet et al., 1997; Krasnova and Cadet, 2009; Yamamoto et al., 2010). Additionally, microglial activation following methamphetamine treatment has been tightly linked to the degenerative changes observed with this drug of abuse (Guilarte et al., 2003; LaVoie et al., 2004; Thomas et al., 2004b; Thomas et al., 2007; Sekine et al., 2008; Yoshimoto et al., 2008). Activated microglia can create a neurotoxic milieu (Colton and Gilbert, 1987; van der Veen et al., 1997), and it has been shown that inhibition of microglial activation can attenuate neuronal injury in models of methamphetamine toxicity (Thomas and Kuhn, 2005a; Zhang et al., 2006).
Oxidative processes, including those associated with microglial activation, can lead to neuronal injury via the liberation of Zn2+ from intraneuronal, metal-binding proteins such as metallothionein (MT; Aizenman et al., 2000; McLaughlin et al., 2001; Knoch et al., 2008; Aras et al., 2009a). Cytoplasmic free Zn2+ has been shown to trigger several cellular signaling cascades leading to the activation of necrotic, apoptotic and autophagic cell injurious processes in neurons (Sensi et al., 2009). Interestingly, Zn2+ itself, when present in the extracellular milieu, can also activate microglia (Kauppinen et al., 2008). In this study, we investigated whether methamphetamine exposure could, in fact, mobilize Zn2+ in a catecholaminergic rat pheochromocytoma (PC12) cell line. We further evaluated whether this Zn2+ component was important for methamphetamine-induced toxicity, as well as the relative contribution of microglia to this process. To our surprise, we found that Zn2+ plays a critical but complex role in methamphetamine-mediated injury to PC12 cells as chelation of this metal could have either protective or destructive consequences depending, respectively, on the absence or presence of microglia.
EXPERIMENTAL PROCEDURES
Reagents and cell culture procedures
A green fluorescent protein-expressing plasmid (pGFP) was purchased from Clontech (Palo Alto, CA, USA). Luciferase DNA (pUHC13-3) was a kind gift from Dr. H. Bujard (Heidelberg, Germany). MRE-firefly luciferase reporter (pLuc-MCS/4MREa) was a gift from Dr. D.P. Giedroc (Bloomington, IN, USA), and Renilla reniformis luciferase reporter gene (pRL-TK) was purchased from Promega (Madison, WI, USA). Construction of the plasmid encoding a GFP/metallothionein 3 (MT3) fusion protein was described earlier (Aras et al., 2009a). The anti-P2X2 receptor antibody was purchased from Abcam (Cambridge, MA, USA). SteadyLite Plus Luminescence Reporter Gene Assay System was purchased from PerkinElmer Inc (Waltham, MA, USA) and Dual-Glo Luciferase Assay System was purchased from Promega (Madison, WI, USA). LipofectAMINE 2000 was purchased from Invitrogen (Carlsbad, CA, USA). Rat adrenal pheochromocytoma (PC12) cells were purchased from American Type Culture Collection (Rockville, MD, USA) and a rat brain microglial cell line (Cheepsunthorn et al., 2001) was a kind gift from Dr J.R. Connor (University Park, PA, USA). PC12 cells were maintained in F-12K Nutrient Mixture (Kaighn’s modification) supplemented with 2.5% fetal bovine serum, 15% horse serum, 2 mM L-glutamine, 24 U/ml penicillin and 24 mg/ml streptomycin. Microglia were maintained in DMEM supplemented with 10% fetal bovine serum, 2 mM L-glutamine, 24 U/ml penicillin and 24 mg/ml streptomycin.
Methamphetamine exposure
We utilized concentrations of methamphetamine that approximate the in vivo conditions known to induce neurotoxicity. A perusal of the literature of methamphetamine-induced toxicity in mice indicates that the majority of investigators use paradigms that range from single injections of doses of 30–40 mg/kg, or multiple (e.g. 4) injections, 2 to 4 hr apart, of doses of 3–10 mg/kg (rev. by Krasnova and Cadet, 2009). A 20 g adult mouse has approximately 1.5 ml of blood (6–8% of weight). A single 10 mg/kg dose would result in a maximal blood concentration of methamphetamine of approximately 800–900 µM, assuming a homogeneous volume of distribution and 100% absorption; but even if only 50% of this dose makes it into the brain, the final concentrations reached for a single injection are within the ranges used in most of the experiments of the present study.
Zn2+-selective luciferase expression assay
PC12 cells were transfected with the metal response element (MRE)-firefly luciferase reporter (pLuc-MCS/4MREa) together with the Renilla luciferase reporter (pRL-TK) using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) as described earlier (Hara and Aizenman, 2004; Aras et al., 2009a). The MRE-luciferase construct contains four tandem repeats of the MREa sequence from the human metallothionein-IIA (hMT-IIA) gene upstream of a promoter driving firefly luciferase expression (Chen et al. 2004). In transfected cells, activation of MRE by the Zn2+-selective MRE transcription factor 1 (MTF-1) drives the transcription of the firefly luciferase gene, increasing enzymatic luciferase activity. Renilla luciferase is a non-inducible reporter used to standardize transfection efficiency. Following transfection cells were supplemented with 10 µM ZnCl2, to saturate all possible metal binding sites (Palmiter, 1994), and treated with methamphetamine 48 hours later. Following a 3 hour drug treatment, cells were rinsed and returned to a humidified 37°C incubator for 24 hours. Using a Dual-Glo luciferase assay system (Promega, Madison, WI), firefly and Renilla luciferase luminescence was measured using a 96-well microplate reader (Wallac 1420, PerkinElmer Life Sciences, Waltham, MA).
Electrophysiological measurements
PC12 cells were plated onto 12-mm coverslips in six-well plates at a density of 175,000 cells/well. Electrophysiological recordings were performed 30–320 minutes after initiating methamphetamine treatment using the whole-cell configuration of the patch-clamp technique as described previously (Aras et al., 2009b). The extracellular solution contained (in mM): 115 NaCl, 2.5 KCl, 2.0 MgCl2, 10 HEPES, 10 D-glucose; pH was adjusted to 7.2 with concentrated KOH; 0.250 µM tetrodotoxin was added to inhibit voltage gated sodium channels. The intracellular (electrode) solution contained (in mM): 100 K-gluconate, 11 EGTA, 10 KCl, 1 MgCl2, 1 CaCl2 × 2H2O, 10 HEPES; pH was adjusted to 7.2 with concentrated KOH; 0.22 mM ATP was added and osmolarity was adjusted to 280 mOsm with sucrose. All measurements were obtained under voltage clamp with an Axopatch 200B amplifier (Molecular Devices, Sunnyvale, CA) and pClamp software (Molecular Devices) using 2–3 MOhm electrodes. Partial compensation (80%) for series resistance was provided in all instances. Currents were filtered at 2 kHz and digitized at 10 kHz (Digidata; Molecular Devices). K+ currents were evoked with a series of 200 ms voltage steps from a holding potential of −80mV to +50mV in 10mV increments. Before the start of the depolarization, a pre-pulse to −10mV was given for 30 ms to inactivate A-type K+ currents. Current density calculations were made by dividing the steady-state current amplitudes at +50 mV by the cell capacitance.
Toxicity assays
PC12 cells were seeded into 24-well culture plates at a density of 100,000 cells/well and transfected with a 1:5 mixture of a constitutive luciferase-expressing plasmid (pUHC13-3) and a GFP expression vector (pGFP) using LipofectAMINE 2000. In some experiments, a GFP/MT3-expressing plasmid substituted for GFP. Methamphetamine exposure was performed 24 hours following transfection, with or without the addition of untransfected microglia (25,000 cells/well). 24 hours post-treatment, the cultures were rinsed (which removed the majority of the microglia), and PC12 cell viability was assessed by measuring luciferase reporter signal, as described previously (Boeckman and Aizenman, 1996; Aras et al., 2008). Results using these assays were confirmed by visualizing GFP expression (see Fig. 5), but all quantification was performed with the luciferase expression assay.
Fig. 5.
Characterization of the methamphetamine toxicity-enhancing actions of TPEN. (A) Luciferase-expressing PC12 cells were exposed to 300 µM methamphetamine in the absence (n=31) or presence (n=25) of microglia for 24 hours and assayed for viability. The effects of intracellular and extracellular Zn2+ were also examined for each of these groups by the addition of 3 µM TPEN (n=4), 3 µM TPEN pre-bound to 3 µM Zn2+ (n=3), or 1 mM tricine (n=4). Note that the presence of microglia enhances methamphetamine toxicity. Note also TPEN protects PC12 cells from methamphetamine toxicity (see Fig. 3), but enhances the stimulant’s toxicity when microglia are present. TPEN pre-bound to Zn2+ and tricine lack any effect. Values are expressed as percent of vehicle control and represent mean ± s.e.m; ***p<0.001 (ANOVA/Bonferroni, PC12 vs PC12/microglia). (B) PC12 cells expressing either luciferase and an empty control vector, or luciferase and MT3 were exposed to 300 µM methamphetamine with our without microglia for 24 hours and assayed for viability. While MT3 mimics the protective actions of TPEN in the absence of microglia, it does not have the toxicity-enhancing actions of the chelator when microglia are present. Values are expressed as percent of vehicle control and represent mean ± s.e.m (n=8); **p<0.01, ***p<0.001 (ANOVA/Bonferroni, vector vs. MT3). (C) Luciferase-expressing PC12 cells cultured in the presence of microglia were exposed to 300 µM methamphetamine and 3 µM TPEN alone (n=4), or in the presence of either suramin (100 µM; n=4) or TNP-ATP (0.03 µM; n=3). Note that the P2X receptor blockers inhibit the methamphetamine toxicity-enhancing effects of the Zn2+ chelator. Values are expressed as percent of vehicle control and represent mean ± s.e.m; *p<0.05 (unpaired t test). Inset: immunoblot demonstrating expression of P2X2 receptors in microglia (M); brain (B) and lung (L) expression are shown for comparison (molecular weight marker = 50 KDa).
Immunoblotting
Microglia were briefly rinsed twice with ice-cold PBS and exposed to a cell lysis buffer supplemented with a protease inhibitor cocktail (Biosource, Camarillo, CA). Debris was pelleted by centrifugation for 10 min, and the remaining lysates were used immediately or stored at −20 °C. Rat brain and lung tissue were processed similarly after gentle homogenization. The protein concentrations of the samples were measured with a bicinchoninic acid assay. Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) was carried out using the Mini Protean 3 System (Bio-Rad, Hercules, CA). Prior to electrophoresis, protein samples were treated with a reducing sample buffer and boiled at 100 °C for 5 min. Samples with equal amounts of protein were run on 7.5% SDS-PAGE gel. For immunoblotting, separated protein bands were transferred onto a 0.2 µm nitrocellulose membrane (Bio-Rad). The membranes were then blocked with 1% BSA in phosphate buffered saline with 0.05% Tween 20 at room temperature for 1 h, and probed with anti P2X2 receptor antibody, washed and incubated with goat secondary antibody conjugated to horseradish peroxidase at room temperature for 1 h. Blots were then visualized using SuperSignal West Pico Chemiluminescent Substrate Kit (Pierce, Rockford, IL).
RESULTS
Methamphetamine triggers Zn2+-regulated gene expression
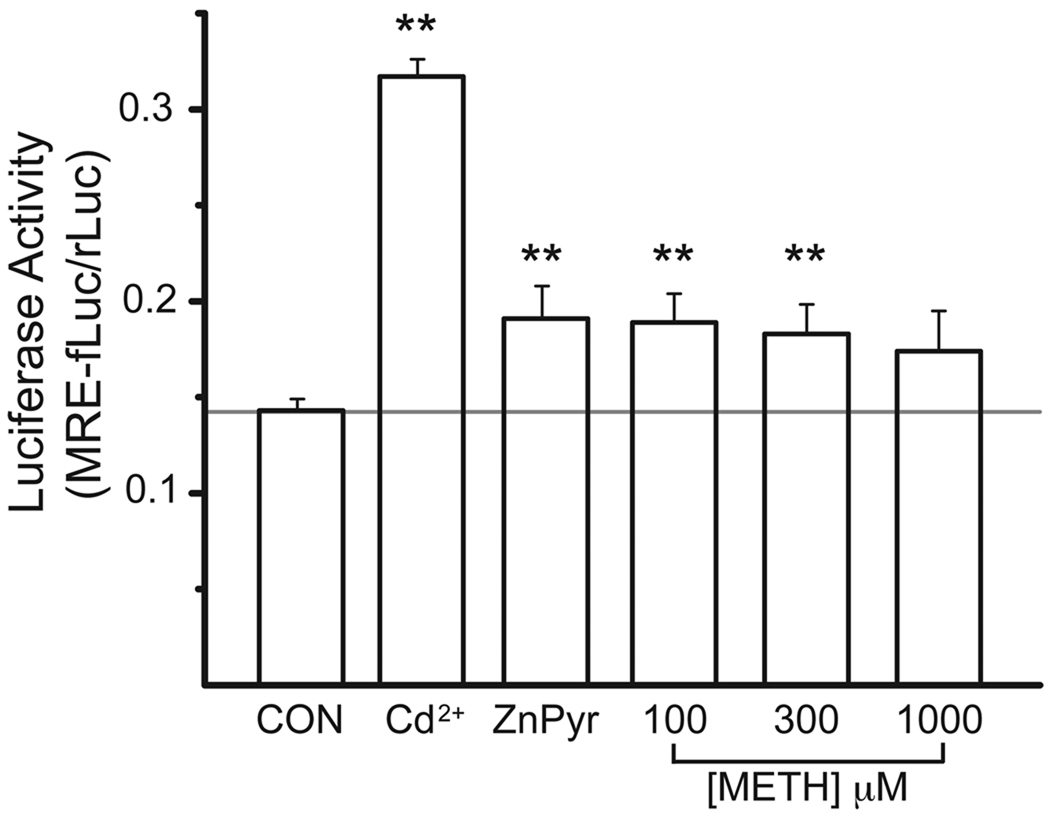
We first evaluated whether methamphetamine exposure could induce increases in intracellular free Zn2+ in PC12 cells. Initially, we loaded methamphetamine-treated cells with the Zn2+-specific indicator Fluozin-3 in order to quantify relative increases in intracellular concentrations of the metal using single cell microspectrofluorimetry (imaging). This technique requires continuous perfusion of the cells plated on glass in order to quench baseline fluorescent signals with the Zn2+ chelator TPEN. This allows for the measurement of the levels of pre-existing free metal (Knoch et al., 2008; Aras et al., 2009a). Although methamphetamine-treated cells appeared to show an increase in background fluorescence, it became very difficult to consistently quantify this effect. Methamphetamine caused the cells to become loosely attached to the glass surface, resulting in a partial loss of cells during the TPEN perfusion. More importantly however, the cells remaining partly attached to the dish had a significant amount of movement during the perfusion, leading to our inability to sustain a sufficient number cells under focus. Attempts to perform similar measurement on a plate fluorimeter were also not highly successful due to a continuous drift in the baseline in vehicle-treated cells. We therefore turned our attention to a different method that allows for the quantification of relative Zn2+ changes in the cytosol using a gene reporter assay (Hara and Aizenman, 2004). This assay takes advantage of the highly Zn2+-selective metal regulatory element (MRE) transcription factor 1 (MTF-1) driving the expression of MRE-firefly luciferase (Chen et al. 2004). Cells transfected with MRE-luciferase, together with a constitutive Renilla luciferase marker (to control for variations in transfection efficacy; see Experimental Procedures) were exposed to increasing concentrations of methamphetamine (100–1000 µM) for 3 hours. A day later, luciferase expression was quantified. As a positive control we utilized Cd2+ exposure (20 µM, 3 hours). This metal does not by itself promote MTF-1 DNA binding and MRE-luciferase expression, but is able to potently displace Zn2+ from its high affinity protein binding sites thereby indirectly affecting gene expression (Zhang et al., 2003). We observed a significant increase in MRE-luciferase expression in cells exposed to either 100 or 300 µM methamphetamine, with no statistically significant changes observed at 1000 µM (Fig. 1), likely because at this concentration we began to see substantial toxicity at the time of the assay, even for this relatively short drug exposure time (see below). While no methamphetamine treatment drove MRE-luciferase expression to the levels observed following Cd2+ treatment, the effects of 100–300 µM methamphetamine were comparable to those induced by 1 µM zinc added in the presence of 0.5 µM of the zinc ionophore pyrithione (ZnPyr; Fig. 1). These results demonstrate that methamphetamine exposure produces a mobilization of intracellular Zn2+ to levels sufficient to drive the MTF-1/MRE-luciferase expression system.
Fig. 1.
Methamphetamine triggers Zn2+-regulated gene expression in PC12 cells. MRE-firefly luciferase (MRE-fLuc) and Renilla luciferase (rLuc)-expressing PC12 were stimulated with vehicle (CON), 20 µM Cd2+ (as CdCl2), 1 µM Zn2+ (as ZnCl2) plus 0.5 µM pyrithione (ZnPyr), or increasing concentrations of methamphetamine (METH) for a total of three hours. MRE-fLuc/rLuc values (mean ± s.e.m., n=7) were measured 24 hours later. Note that the increase in MRE-fLuc expression induced by 100 and 300 µM METH is similar to that produced by ZnPyr exposure; **p<0.01 (ANOVA/Dunnet vs. CON).
Zn2+-dependent enhancement of K+ currents by methamphetamine
One of the hallmark features of neuronal injury following the mobilization of intracellular Zn2+ by oxidants is a delayed enhancement of voltage-dependent K+ currents (McLaughlin et al., 2001). This process facilitates the efflux of cytoplasmic K+, which is a necessary component for the completion of several cell injury programs (Hughes and Cidlowski, 1999; Yu, 2003). We thus evaluated whether methamphetamine exposure led to a surge in K+ currents, and whether chelating Zn2+ could prevent this process. Electrophysiological recordings were performed from methamphetamine-treated PC12 at various times following the drug exposure. Cells exposed to 1 mM methamphetamine exhibited significantly larger K+ current densities, when compared to vehicle-treated control cells, beginning approximately 180 minutes after initiation of treatment (Fig. 2). This is a very similar time course as that observed in cortical neurons following exposure to oxidants (McLaughlin et al., 2001). We also used a higher concentration of methamphetamine (3 mM) to test whether the current surge could be induced sooner. Indeed, cells exposed to this higher concentration of methamphetamine had significantly elevated currents as soon as recordings were started, approximately 30–90 minutes following the initiation of the treatment (Figs. 2B, 2C). Nevertheless, we had difficulty recording from cells treated with 3 mM methamphetamine beginning approximately after 2 hours of exposure. Regardless of the difference in the time course for K+ current enhancement by both concentrations of methamphetamine, the pooled current density data revealed that the overall level of current surge induced by the drug was the same, and nearly 2-fold above baseline, currents (Fig. 2C). We next evaluated whether the presence of 3 µM TPEN could prevent the current surge. Indeed, this Zn2+ chelator could totally abrogate the K+ current surge induced by methamphetamine without affecting the basal, control currents (Fig. 2C). These data indicate that methamphetamine exposure does in fact lead to a pronounced K+ current surge in PC12 cells and that this surge can be prevented by the presence of a zinc chelator.
Fig. 2.
Methamphetamine induces a TPEN-sensitive voltage-dependent K+ current surge in PC12 cells. (A) Representative whole-cell potassium currents recorded under control conditions (CON), and after exposure to 3 mM methamphetamine (METH). Currents were evoked by a series of voltage steps from a holding potential of −80 mV to +50 mV in 10 mV increments. Calibration, 1.25 nA, 25 ms. (B) Time course of potassium current enhancement following exposure to either 1 mM (closed circles) or 3 mM METH (open circles). Each point represents a single cell. Currents were evoked by a single voltage step from −80 mV to +50 mV, measured at steady state, and normalized to cell capacitance. The solid horizontal line represents the mean current density for 16 untreated cells recorded at various times. Note the K+ current enhancement occurs within 30 minutes following 3 mM METH exposure and 180 minutes after 1 mM METH exposure. (C) Mean ± s.e.m. current densities of PC12 cells under control conditions (n=16), or following exposure to 1 mM METH (n=16), 3 mM METH (n=23,), 3 µM TPEN (n=14), or 3 mM METH plus 3 µM TPEN (n=17). **p<0.01; ANOVA/Dunnet. Note TPEN completely suppresses the current surge induced by the stimulant.
Methamphetamine toxicity has an intracellular Zn2+ component
We evaluated the toxicity induced by increasing concentrations of methamphetamine to PC12 cells. Initially, we monitored the cells following a 3-hour exposure to concentrations as high as 3 mM of the drug. Immediately following the treatment we noted that PC12 cells exposed to 1 and 3 mM methamphetamine showed a pronounced presence of intracellular vacuoles, as reported by others (Cubells et al., 1994). However, very little cell death ensued during the next 24 hours and, in fact, the majority of the cells seemed to recover from the transient insult. In contrast, the continuous presence of 100–1000 µM methamphetamine for 24 hours did lead to a small but consistent concentration-dependent loss in cell viability. The cultures remained, however, overall fairly resistant to the lethal actions of the drug (Fig. 3). The presence of 3 µM TPEN together with methamphetamine was sufficient to significantly enhance viability against 100 and 300 µM methamphetamine (Fig. 3). In contrast to the actions of TPEN, which is membrane permeable, the cell impermeant Zn2+ chelator tricine (1 mM; Paoletti et al., 1997) did not antagonize at all the lethal actions of the stimulant (Fig. 3). As such, any Zn2+ mediating the toxic effects of the drug triggers the injurious process without accessing the extracellular compartment.
Fig. 3.
Effects of Zn2+ chelators on methamphetamine toxicity. Luciferase-expressing PC12 cells were treated with vehicle or with increasing concentrations of methamphetamine alone (n=31), or in the presence of either 3 µM TPEN (n=4) or 1 mM tricine (n=4) for 24 hr. Viability was examined visually (see Fig. 5) and quantified by measuring luciferase expression in surviving cells. Values are expressed as percent of vehicle control and represent mean ± s.e.m.; **p<0.01 (unpaired, two-tailed t-test). Note that toxicity to 300 µM methamphetamine was reversed by TPEN, but not by tricine.
Microglia enhance methamphetamine toxicity: a complex role for Zn2+
Microglial activation is known to play an important role in methamphetamine neurotoxicity in vivo. As such, we sought to examine the potential role Zn2+ may play in any interactions occurring between PC12 cells and microglia following methamphetamine exposure. First, we exposed the cultures to methamphetamine in the absence or presence of microglia (microglia were added at the same time as the methamphetamine). We observed that in the presence of microglia, methamphetamine neurotoxicity to PC12 cells increased significantly, albeit not too dramatically (Figs. 4 and 5A). In the presence of 3 µM TPEN, however, the viability of the PC12 cells exposed to methamphetamine together with microglia decreased dramatically (Figs. 4 and 5A). This result was very surprising since, as mentioned above, TPEN at this concentration was cytoprotective against methamphetamine when microglia were not present. TPEN previously bound with equimolar Zn2+ lost both its cytoprotective and cytodestructive properties, respectively, in the absence and presence of microglia (Fig. 5A). In contrast to the effects of TPEN, the extracellular Zn2+ chelator tricine did not enhance methamphetamine toxicity in the presence of microglia, again indicating that all the putative actions of Zn2+ described thus far are mediated inside the cells.
Fig. 4.
TPEN can both decrease and enhance methamphetamine toxicity. GFP-expressing PC12 cells were exposed to vehicle (CON), 300 µM methamphetamine (METH), and 300 µM methamphetamine in the presence of 3 µM TPEN (METH+TPEN) for 24 hr alone or in the presence of microglia. Note GFP-expressing cells in the METH+TPEN group is near control levels in the absence of microglia, but plummets when microglia are included in the treatment. Note also that not all PC12 cells are GFP positive (top-left panel). The quantification of the toxicity represented here is shown in Fig. 5.
In subsequent experiments we transfected PC12 cells with a plasmid encoding the Zn2+ binding protein MT3 (Aras et al., 2009a) prior to methamphetamine exposure in either the presence or absence of microglia. We reasoned that over-expression of MT3 should mimic at least some of the effects of TPEN. Indeed, we found that cells expressing MT3 were protected from 300 µM methamphetamine toxicity when microglia were absent from the cultures (Fig. 5B). Moreover, in the presence of microglia, MT3-expressing PC12 cells were also protected (Fig. 5B). In other words, the toxicity-enhancing actions of TPEN could not be mimicked at all by MT3 expression in PC12 cells. These results suggest that i) chelating Zn2+ in PC12 cells can be protective vs. methamphetamine toxicity in the absence or presence of microglia, and ii) the methamphetamine toxicity-enhancing actions of TPEN when microglia are present are likely the result of the actions of the chelator in the microglial cells themselves.
We began to search for possible ways by which TPEN may enhance microglial toxicity following methamphetamine exposure in the co-cultures. Pro-apoptotic processes as well as activation of cycloxygenase-2 and nitric oxide synthase have been closely linked to methamphetamine toxicity and associated microglial activation (Cadet et al., 1997; Jayanthi et al., 2001; Jayanthi, 2004; Thomas et al., 2004a; Thomas and Kuhn, 2005b; Jayanthi et al., 2005). However, neither the broad-spectrum cysteine protease inhibitor Boc-aspartyl-FMK (10 µM), the cycloxygenase-2 blocker NS-398 (10 µM), nor the nitric oxide synthase inhibitor N (G)-nitro-L-arginine methyl ester (30 µM) could prevent the TPEN enhanced toxicity (n=4; not shown). We turned our attention on purinergic receptors, as P2X and P2Y receptors are widely expressed in microglia, and both are also known to mediate the activation of these cells (Franke et al., 2007). Moreover, ATP can be released from PC12 cells (Fabbro et al., 2004). First, we observed that the broad-spectrum P2X and P2Y receptor antagonist suramin (100 µM) inhibited methamphetamine/microglia toxicity (no TPEN; 68.2 ± 2.7% viability control vs. 87.5 ± 5.4% viability suramin; n=4, *p<0.01, t-test). Importantly, suramin was effective in reversing the toxic-enhancing actions of TPEN under these circumstances (Fig. 5C), suggesting that the zinc chelator may, in fact, facilitate the activation of purinergic receptors during methamphetamine exposure. We next turned our attention to P2X receptors. A certain number of P2X receptor subtypes have intracellular cysteine and histidine binding sites that could potentially be targets for Zn2+, including P2X2 receptors (Coddou et al., 2009), which are expressed in our microglial cell line (Fig. 5C, inset). Although the P2X2/3 receptor antagonist 2′,3′-O-(2,4,6-trinitrophenyl) adenosine 5′-triphosphate (TNP-ATP; 0.03 µM) could not mimic the protective effects of suramin vs. methamphetamine/microglia toxicity (no TPEN; 76.7 ± 7.5% viability, n=3; not significantly different from control), this drug did effectively reverse the toxicity-enhancing actions of TPEN (Fig. 5C). As such, we propose that ATP released from PC12 cells injured by methamphetamine increases microglia activation, a process that is enhanced by chelating intracellular Zn2+ in the microglia leading to increased activation of P2X receptors.
DISCUSSION
Our present results suggest that Zn2+ may be intimately involved in the neurotoxic actions of methamphetamine. Although PC12 cells, in the absence of microglia, are relatively resistant to the lethal effects of the stimulant, the cell permeant Zn2+ chelator TPEN can abate this toxicity, at least within a certain methamphetamine concentration range. As in previously described Zn2+-dependent neurotoxic paradigms (e.g. Du et al., 2002), TPEN ceases to be protective when it is pre-bound with equimolar Zn2+. Importantly, since the membrane impermeant Zn2+ chelator tricine (Paoletti et al., 1997) is not protective, any Zn2+ liberated by the oxidative conditions produced by methamphetamine (Yamamoto and Bankson, 2005; Krasnova and Cadet, 2009; Yamamoto et al., 2010) must originate from intracellular binding sites, such as MT (Aizenman et al., 2000; Maret, 2008; Aras et al., 2009a; Sensi et al., 2009). However, over-expression of this metal binding protein can provide an excess of potential binding sites for Zn2+ and is thereby also protective (Chung et al., 2008; West et al., 2008; Aras et al., 2009a). Of interest, a previous study demonstrated that pre-treatment of human dopaminergic neuroblastoma (SK-N-SH) cells with 50 µM ZnCl2 for 12 hours led to an up-regulation of MT isoforms 1 and 2. This, in turn, correlated with increased resistance to methamphetamine toxicity in these cells (Ajjimaporn et al., 2005). Increased expression of MT can also protect SK-N-SH cells from methamphetamine-induced changes in α-synuclein expression (Ajjimaporn et al., 2007).
The situation is altered dramatically by the presence of microglia. Not only does the inclusion of these cells exacerbate the neurotoxic properties of methamphetamine, as suggested by prior in vivo observations (Guilarte et al., 2003; LaVoie et al., 2004; Thomas et al., 2004b; Thomas et al., 2007; Sekine et al., 2008), but also under these conditions, the participation of Zn2+ in the overall process becomes much more complex. TPEN chelation of Zn2+ in this case renders methamphetamine much more toxic to PC12 cells. A possible scenario to account for our various results is as follows: Methamphetamine can directly injure PC12 cells, at least partly via an intracellular Zn2+-dependent process that is mildly lethal. The injured PC12 cells release a number of chemical signals, likely including ATP, which, in turn, help activate the co-cultured microglial cells. The activated microglia then participate in the injury process towards PC12 cells, thereby enhancing the lethal effects of methamphetamine. Under these conditions, TPEN now potentiates the toxic actions of microglia, presumably by removing a putative block by intracellular Zn2+ of P2X channels present in the microglial cells themselves. Although clearly other scenarios are possible, we believe that this is one of the simplest explanations for our observations.
While we had difficulty imaging the Zn2+ liberated by methamphetamine using standard fluorescence techniques that we have successfully used in prior studies (Knoch et al., 2008; Aras et al., 2009a), we were able to measure a consistent increase in MTF-1/MRE-luciferase expression following a three-hour drug exposure. This method utilizes a highly, if not exclusively specific molecular signature for cellular events that are accompanied by increases in cytoplasmic free Zn2+ (Chen et al. 2004; Hara and Aizenman, 2004; Aras et al., 2009a). In addition, we observed a TPEN-sensitive increase in K+ current density following methamphetamine exposure, again reinforcing the notion that toxic exposure to this drug leads to Zn2+-specific signaling processes. Indeed, we have ample evidence that increases in K+ currents in both neurons and cell lines following oxidative or nitrosative injury are due to cell signaling pathways that are activated by the liberation of Zn2+ from intracellular binding sites (McLaughlin et al., 2001; Redman et al., 2007; Redman et al., 2009). We thus feel confident that methamphetamine can and does liberate Zn2+ from intracellular binding sites and that this metal can participate in the cytotoxic actions of the stimulant.
The methamphetamine toxicity-enhancing actions of TPEN in the presence of microglia were indeed surprising. The fact that MT3 over-expression in the PC12 cells could not mimic these toxic actions of TPEN suggested to us that the likely site of action for the chelator was at the microglia. We hypothesized that methamphetamine-injured PC12 cells could, among other factors, release ATP, which in turn would help trigger microglia activation (Franke et al., 2007). Since suramin, but also the P2X2/3 blocker ATP-TNP could reverse the toxicity-enhancing actions of TPEN, we deduced that P2X receptors were involved in the process. Indeed, a potential modulatory effect of intracellular Zn2+ on P2X receptor activation could be mediated via redox sensitive intracellular cysteine residues, such as the one recently reported for P2X2 receptors at cysteine430 (Coddou et al., 2009). Through this site, P2X2 receptor activation is enhanced following its oxidation. Coordination of that site by Zn2+ ions, together with other nearby cysteine or histidine residues could, in theory, prevent, or at least limit its oxidation. TPEN-mediated chelation of the metal would, in turn, then allow for the potentiation of receptor function as the microglia become activated and an oxidative environment is generated. We confirmed with immunoblots that our microglial cell line expresses P2X2 receptors, although clearly, other P2X receptor subtypes could be involved as well.
Extracellular Zn2+ has been shown to directly activate microglia via a yet undefined signaling process (Kauppinen et al., 2008). Thus, the overall picture may indeed be even more complex in injurious situations in the brain known to be accompanied by increases in the release of vesicular Zn2+ into the extracellular space from excitatory synapses (Dietz et al., 2009; Sensi et al., 2009). However, to our knowledge, activation of excitatory, zinc-containing pathways have not been implicated in any models of methamphetamine toxicity, but clearly, this possible scenario must be kept in mind when extrapolating our present in vitro findings to any in vivo models. Nevertheless, our work does strongly suggest that neurotoxic methamphetamine exposure, just as other pathological conditions known to be accompanied by oxidative damage, has a Zn2+ component that conceivably could be targeted to protect neurons, as in other systems (Pal et al., 2004; Maret, 2008; Sensi et al., 2009). However, given the complex role this metal plays, especially once microglial cells become part of the injurious process, Zn2+-directed therapies may generate pronounced undesirable effects.
Acknowledgments
This study was partially supported by the USA National Institutes of Health grants DA002950 and NS043277 to EA. An American Recovery and Reinvestment Act summer stipend supplement to grant DA002950 provided partial support for RS.
Abbreviations
- GFP
green fluorescent protein
- MRE
metal regulatory element
- MT
metallothionein
- MTF
metal regulatory element transcription factor
- PC12
rat pheochromocytoma cells
- SDS-PAGE
sodium dodecyl sulfate–polyacrylamide gel electrophoresis
- TPEN
N,N,N′,N′-tetrakis(2-pyridylmethyl)-ethylenediamine
- TNP-ATP
2′,3′-O-(2,4,6-trinitrophenyl) adenosine 5′-triphosphate
REFERENCES
- Aizenman E, Stout AK, Hartnett KA, Dineley KE, McLaughlin B, Reynolds IJ. Induction of neuronal apoptosis by thiol oxidation: putative role of intracellular zinc release. J Neurochem. 2000;75:1878–1888. doi: 10.1046/j.1471-4159.2000.0751878.x. [DOI] [PubMed] [Google Scholar]
- Ajjimaporn A, Swinscoe J, Shavali S, Govitrapong P, Ebadi M. Metallothionein provides zinc-mediated protective effects against methamphetamine toxicity in SK-n_SH cells. Brain Res Bull. 2005;67:466–475. doi: 10.1016/j.brainresbull.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Ajjimaporn A, Phansuwan-Pujito P, Ebadi M, Govitrapong P. Zinc protects SK-N-SH cells from methamphetamine-induced alpha-synuclein expression. Neurosci Lett. 2007;419:59–63. doi: 10.1016/j.neulet.2007.03.073. [DOI] [PubMed] [Google Scholar]
- Aras MA, Hartnett KA, Aizenman E. Assessment of cell viability in primary neuronal cultures. Curr Protoc Neurosci Suppl. 2008;44:7.18.1–7.1815. doi: 10.1002/0471142301.ns0718s44. [DOI] [PubMed] [Google Scholar]
- Aras MA, Hara H, Hartnett KA, Kandler K, Aizenman E. PKC regulation of neuronal zinc signaling mediates survival during preconditioning. J Neurochem. 2009a;110:106–117. doi: 10.1111/j.1471-4159.2009.06106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aras MA, Saadi RA, Aizenman E. Zn2+ regulates Kv2.1 voltage-dependent gating and localization following ischemia. European Journal of Neuroscience. 2009b;30:2250–2257. doi: 10.1111/j.1460-9568.2009.07026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeckman FA, Aizenman E. Pharmacological properties of acquired excitotoxicity in Chinese hamster ovary cells transfected with N-methyl-D-aspartate receptor subunits. J Pharmacol Exp Ther. 1996;279:515–523. [PubMed] [Google Scholar]
- Cadet JL, Ordonez SV, Ordonez JV. Methamphetamine induces apotosis in immortalized neural cells: protection by the proto-oncogene, bcl-2. Synapse. 1997;25:176–184. doi: 10.1002/(SICI)1098-2396(199702)25:2<176::AID-SYN8>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Chang L, Alicata D, Ernst T, Volkow N. Structural and metabolic brain changes in the striatum associated with methamphetamine abuse. Addiction. 2007;102 Suppl 1:16–32. doi: 10.1111/j.1360-0443.2006.01782.x. [DOI] [PubMed] [Google Scholar]
- Cheepsunthorn P, Radov L, Menzies S, Reid J, Connor JR. Characterization of a novel brain-derived microglial cell line isolated from neonatal rat brain. Glia. 2001;35:53–62. doi: 10.1002/glia.1070. [DOI] [PubMed] [Google Scholar]
- Chen X, Zhang B, Harmon PM, Schaffner W, Peterson DO, Giedroc DP. A novel cysteine cluster in human metal-responsive transcription factor 1 is required for heavy metal-induced transcriptional activation in vivo. J Biol Chem. 2004;279:4515–4522. doi: 10.1074/jbc.M308924200. [DOI] [PubMed] [Google Scholar]
- Chung RS, Hidalgo J, West AK. New insight into the molecular pathways of metallothionein-mediated neuroprotection and regeneration. J Neurochem. 2008;104:14–20. doi: 10.1111/j.1471-4159.2007.05026.x. [DOI] [PubMed] [Google Scholar]
- Coddou C, Codocedo JF, Li S, Lillo JG, Acuña-Castillo C, Bull P, Stojilkovic SS, Huidobro-Toro JP. Reactive oxygen species potentiate the P2X2 receptor activity through Intracellular Cys430. J Neurosci. 2009;29:12284–12291. doi: 10.1523/JNEUROSCI.2096-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colton CA, Gilbert DL. Production of superoxide anions by a CNS macrophage, the microglia. FEBS Lett. 1987;223:284–288. doi: 10.1016/0014-5793(87)80305-8. [DOI] [PubMed] [Google Scholar]
- Cubells JF, Rayport S, Rajendran G, Sulzer D. Methamphetamine neurotoxicity involves vacuolation of endocytic organelles and dopamine-dependent intracellular oxidative stress. J Neurosci. 1994;14:2260–2271. doi: 10.1523/JNEUROSCI.14-04-02260.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz RM, Weiss JH, Shuttleworth CW. Contributions of Ca2+ and Zn2+ to spreading depression-like events and neuronal injury. J Neurochem. 2009;109 Suppl 1:145–152. doi: 10.1111/j.1471-4159.2009.05853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du S, McLaughlin B, Pal S, Aizenman E. In vitro neurotoxicity of methylisothiazolinone, a commonly used industrial and household biocide, proceeds via a zinc and extracellular signal-regulated kinase mitogen-activated protein kinase-dependent pathway. J Neurosci. 2002;22:7408–7416. doi: 10.1523/JNEUROSCI.22-17-07408.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbro A, Skorinkin A, Gandolfo M, Nistri A, Giniatullin R. Quantal release of ATP from clusters of PC12 cells. J Physiol. 2004;15:505–517. doi: 10.1113/jphysiol.2004.068924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke H, Schepper C, Illes P, Krügel U. Involvement of P2X and P2Y receptors in microglial activation in vivo. Purinergic Signalling. 2007;3:435–445. doi: 10.1007/s11302-007-9082-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilarte TR, Nihei MK, McGlothan JL, Howard AS. Methamphetamine-induced deficits of brain monoaminergic neuronal markers: distal axotomy or neuronal plasticity. Neuroscience. 2003;122:499–513. doi: 10.1016/s0306-4522(03)00476-7. [DOI] [PubMed] [Google Scholar]
- Hara H, Aizenman E. A molecular technique for detecting the liberation of intracellular zinc in cultured neurons. J Neurosci Meth. 2004;137:175–180. doi: 10.1016/j.jneumeth.2004.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes FM, Jr, Cidlowski JA. Potassium is a critical regulator of apoptotic enzymes in vitro and in vivo. Adv Enzyme Regul. 1999;39:157–171. doi: 10.1016/s0065-2571(98)00010-7. [DOI] [PubMed] [Google Scholar]
- Jayanthi S, Deng X, Bordelon M, McCoy MT, Cadet JL. Methamphetamine causes differential regulation of pro-death and anti-death Bcl-2 genes in the mouse neocortex. FASEB J. 2001;15:1745–1752. doi: 10.1096/fj.01-0025com. [DOI] [PubMed] [Google Scholar]
- Jayanthi S, Deng X, Noailles PA, Ladenheim B, Cadet JL. Methamphetamine induces neuronal apoptosis via cross-talk between endoplasmic reticulum and mitochondria-dependent death cascades. FASEB J. 2004;18:238–251. doi: 10.1096/fj.03-0295com. [DOI] [PubMed] [Google Scholar]
- Jayanthi S, Deng X, Ladenheim B, McCoy MT, Cluster A, Cai NS, Cadet JL. Calcineurin/NFAT-induced up-regulation of the Fas ligand/Fas death pathway is involved in methamphetamine-induced neuronal apoptosis. Proc Natl Acad Sci USA. 2005;102:868–873. doi: 10.1073/pnas.0404990102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauppinen TM, Higashi Y, Suh SW, Escartin C, Nagasawa K, Swanson RA. Zinc triggers microglial activation. J Neurosci. 2008;28:5827–5835. doi: 10.1523/JNEUROSCI.1236-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoch ME, Hartnett KA, Hara H, Kandler K, Aizenman E. Microglia induce neurotoxicity via intraneuronal Zn(2+) release and a K(+) current surge. Glia. 2008;56:89–96. doi: 10.1002/glia.20592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnova IN, Cadet JL. Methamphetamine toxicity and messengers of death. Brain Research Review. 2009;60:379–407. doi: 10.1016/j.brainresrev.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaVoie MJ, Card JP, Hastings TG. Microglial activation precedes dopamine terminal pathology in methamphetamine-induced neurotoxicity. Exp Neurol. 2004;187:47–57. doi: 10.1016/j.expneurol.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Maret W. Metallothionein redox biology in the cytoprotective and cytotoxic functions of zinc. Exp Geront. 2008;43:378–381. doi: 10.1016/j.exger.2007.11.005. [DOI] [PubMed] [Google Scholar]
- McLaughlin B, Pal S, Tran MP, Parsons AA, Barone FC, Erhardt JA, Aizenman E. p38 activation is required upstream of potassium current enhancement and caspase cleavage in thiol oxidant-induced neuronal apoptosis. J Neurosci. 2001;21:3303–3311. doi: 10.1523/JNEUROSCI.21-10-03303.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal S, He K, Aizenman E. Nitrosative stress and potassium channel-mediated neuronal apoptosis: is zinc the link? Pflugers Arch. 2004;448:296–303. doi: 10.1007/s00424-004-1256-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti P, Ascher P, Neyton J. High-affinity zinc inhibition of NMDA NR1-NR2A receptors. J Neurosci. 1997;17:5711–5725. doi: 10.1523/JNEUROSCI.17-15-05711.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter RD. Regulation of metallothionein genes by heavy metals appears to be mediated by a zinc-sensitive inhibitor that interacts with a constitutively active transcription factor, MTF-1. Proc Natl Acad Sci USA. 1994;91:1219–1223. doi: 10.1073/pnas.91.4.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman PT, Hartnett KA, Aras MA, Levitan ES, Aizenman E. Regulation of apoptotic potassium currents by coordinated zinc-dependent signalling. J Physiol. 2009;587:4393–4404. doi: 10.1113/jphysiol.2009.176321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman PT, He K, Hartnett KA, Jefferson BS, Hu L, Rosenberg PA, Levitan ES, Aizenman E. Apoptotic surge of potassium currents is mediated by p38 phosphorylation of Kv2.1. Proc Natl Acad Sci U S A. 2007;104:3568–3573. doi: 10.1073/pnas.0610159104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekine Y, Ouchi Y, Sugihara G, Takei N, Yoshikawa E, Nakamura K, Iwata Y, Tsuchiya KJ, Suda S, Suzuki K, Kawai M, Takebayashi K, Yamamoto S, Matsuzaki H, Ueki T, Mori N, Gold MS, Cadet JL. Methamphetamine causes microglial activation in the brains of human abusers. J Neurosci. 2008;28:5756–5761. doi: 10.1523/JNEUROSCI.1179-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sensi SL, Paoletti P, Bush AI, Sekler I. Zinc in the physiology and pathology of the CNS. Nat Rev Neurosci. 2009;10:780–791. doi: 10.1038/nrn2734. [DOI] [PubMed] [Google Scholar]
- Thomas DM, Dowgiert J, Geddes TJ, Francescutti-Verbeem D, Liu X, Kuhn DM. Microglial activation is a pharmacological specific marker for the neurotoxic amphetamines. Neurosci Lett. 2004;367:349–354. doi: 10.1016/j.neulet.2004.06.065. [DOI] [PubMed] [Google Scholar]
- Thomas DM, Francesutti-Verbeem DM, Liu X, Kuhn DM. Methamphetamine neurotoxicity in dopamine nerve endings of the striatum is associated with microglial activation. J Pharmacol Exp Ther. 2004b;311:1–7. doi: 10.1124/jpet.104.070961. [DOI] [PubMed] [Google Scholar]
- Thomas DM, Francesutti-Verbeem DM, Kuhn DM. The newly synthesized pool of dopamine determines the severity of methamphetamine-induced neurotoxicity. J Neurochem. 2007;105:605–616. doi: 10.1111/j.1471-4159.2007.05155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DM, Kuhn DM. MK-801 and dextromethorphan block microglial activation and protection against methamphetamine-induced toxicity. Brain Res. 2005a;1050:190–198. doi: 10.1016/j.brainres.2005.05.049. [DOI] [PubMed] [Google Scholar]
- Thomas DM, Kuhn DM. Cyclooxygenase-2 is an obligatory factor in methamphetamine-induced neurotoxicity. J Pharmacol Exp Ther. 2005b;313:870–876. doi: 10.1124/jpet.104.080242. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, Simon SL, Geaga JA, Hong MS, Sui Y, Lee JY, Toga AW, Ling W, London ED. Structural abnormalities in the brains of human subjects who methamphetamine. J Neurosci. 2004;24:6028–6036. doi: 10.1523/JNEUROSCI.0713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Veen RC, Hinton DR, Incardonna F, Hofman FM. Extensive peroxynitrite activity during progressive stages of central nervous system inflammation. J Neuroimmunol. 1997;77:1–7. doi: 10.1016/s0165-5728(97)00013-1. [DOI] [PubMed] [Google Scholar]
- West AK, Hidalgo J, Eddins D, Levin ED, Aschner M. Metallothionein in the central nervous system: Roles in protection, regeneration and cognition. Neurotoxicology. 2008;29:489–503. doi: 10.1016/j.neuro.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow BT, Voorhees KI, Pehl KA. Methamphetamine abuse. American Family Physician. 2007;76:1169–1674. [PubMed] [Google Scholar]
- Yamada J, Sawada M, Nakanishi H. Cell cycle-dependent regulation of kainite-induced inward currents in microglia. Biochem Biophys Res Commun. 2006;349:913–919. doi: 10.1016/j.bbrc.2006.08.126. [DOI] [PubMed] [Google Scholar]
- Yamamoto BK, Bankson MG. Amphetamine neurotoxicity: cause and consequence of oxidative stress. Crit Rev Neurobiol. 2005;17:87–117. doi: 10.1615/critrevneurobiol.v17.i2.30. [DOI] [PubMed] [Google Scholar]
- Yamamoto BK, Moszczynska A, Gudelsky GA. Amphetamine toxicities. Ann NY Acad Sci. 2010;1187:101–121. doi: 10.1111/j.1749-6632.2009.05141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimoto S, Ouchi Y, Sugihara G, Takei N, Yoshikawa E, Nakamura K, Iwata Y, Tsuchiya KJ, Suda S, Suzuki K, Kawai M, Takebayashi K, Yamamoto S, Matsuzaki H, Ueki T, Mori N, Gold MS, Cadet JL. Methamphetamine causes microglial activation in the brains of human abusers. J Neurosci. 2008;28:5756–5761. doi: 10.1523/JNEUROSCI.1179-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu SP. Regulation and critical role of potassium homeostasis in apoptosis. Prog Neurobiol. 2003;70:363–386. doi: 10.1016/s0301-0082(03)00090-x. [DOI] [PubMed] [Google Scholar]
- Zhang B, Georgiev O, Hagmann M, Guenes C, Cramer M, Faller P, Vasak M, Schaffner W. Activity of metal-responsive transcription factor 1 by toxic heavy metals and H2O2 in vitro is modulated by metallothionein. Mol Cell Biol. 2003;23:8471–8485. doi: 10.1128/MCB.23.23.8471-8485.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Kitaichi K, Fujimoto Y, Nakayama H, Shimizu E, Iyo M, Hashimoto K. Protective effects of minocycline on behavioral changes and neurotoxicity in mice alter administration of methamphetamine. PNPBP. 2006;30:1381–1393. doi: 10.1016/j.pnpbp.2006.05.015. [DOI] [PubMed] [Google Scholar]