Abstract
Objective
We sought to explore the effects of non-selective COX and selective COX-2 inhibition on collateral development in a model of chronic myocardial ischemia. We hypothesized that COX-2 inhibitors will negatively effect angiogenic and inflammatory pathways.
Methods
Yorkshire swine were made chronically ischemic by placing an ameroid constrictor on the left circumflex coronary artery. Swine were divided into three groups and given: no drug (control, n=7), a non-selective COX inhibitor (naproxen 400mg daily, NAP, n=7), or a selective COX-2 inhibitor (celecoxib 200mg daily, CBX, n=7). After 7 weeks, coronary angiography was performed. Myocardial function and microvascular reactivity were assessed. Serum and myocardial tissue were analyzed for prostaglandin levels and markers of inflammation and angiogenesis.
Results
The CBX group demonstrated significantly increased mean arterial pressure and decreased left ventricular function. Myocardial perfusion in the CBX group was similar to the control, but less than in the NAP group. Coronary microvascular contraction in the collateral dependent territory was increased in the NAP group, but minimally affected in the CBX group. Oxidative stress and apoptosis were increased in the CBX group. Expression of angiogenic markers, VEGF and phospho-eNOS (ser1177), and tissue levels of prostacyclin were decreased in both the CBX and NAP groups. The NAP group had diminished expression of endostatin.
Conclusion
The effects of selective and non-selective COX inhibition are more complex than previously published, but they do not decrease collateral dependent blood flow to the myocardium in our model of chronic myocardial ischemia.
Keywords: angiogenesis, cardiovascular diseases, prostaglandins
INTRODUCTION
Selective inhibitors of the inducible isoform of cyclooxygenase (COX-2) were developed to provide relief of pain associated with arthritis, surgery, and other conditions, while lowering the incidence of gastrointestinal complications compared to non-selective, non-steroidal anti-inflammatory drugs (nsNSAIDs).1 However, selective COX-2 inhibitors have been purported to increase the risk of adverse cardiovascular events, such as myocardial infarction, and mortality. One widely prescribed selective COX-2 inhibitor, rofecoxib, was removed from the market in 2004 due to an increased incidence of adverse cardiovascular events observed during the VIGOR trial which examined the effects of rofecoxib on arthritic pain relief.2 On the other hand, nsNSAIDs have also been implicated in increased cardiovascular events.3, 4 As a consequence, both selective and non-selective NSAIDs inhibitors carry a “black box warning” from the United States Food and Drug Administration due to the perceived risk of increased cardiovascular events.
COX is responsible for the production of thromboxane (TXA2) in platelets, which facilitates thrombosis and is a vasoconstrictor in most vascular beds. TXA2 is antagonized by low dose aspirin. On the other hand, in endothelium, COX catalyzes the formation of prostacyclin (PGI2), a substance known to be anti-thrombotic and a vasodilator. The key to potential cardiovascular side effects with selective COX-2 inhibitors was purported to be related to the balance between the pro- and anti-thrombotic prostaglandins, the so called FitzGerald hypothesis. In 2006, Cheng et al 5 published a study which demonstrated in mice that selective inhibition, knockout of COX-2 or deletion of the PGI2 receptor, yielded an increase in thrombotic events and elevated blood pressure. Inhibition of COX-1, which simulates the effects of aspirin, reversed these effects.
Another theory consistent with increased cardiovascular risk from long-term selective COX-2 inhibition has been proposed by Wu et al. 6 In this study, inhibition of COX-2 in cell culture led to decreased angiogenesis. Also, the formation of vascular structures in Matrigel was accelerated in COX-2 over-expressing cells but attenuated in COX-2 siRNA-transfected cells. This suggests that selective COX-2 inhibitors may lead to decreased angiogenesis and coronary collateral formation. With fewer (or less developed) protective collateral vessels, patients may suffer more devastating outcomes during ischemic cardiac events. Coronary collateral development has been associated with reduced risk of death and myocardial injury after coronary artery occlusion.7
At this time, no studies have examined the role of non-selective COX or selective COX-2 inhibitors on angiogenesis or myocardial perfusion in a large animal model of chronic myocardial ischemia. We hypothesized that inhibition of COX-2 will alter the production of TXA2 and PGI2, negatively effect coronary collateral formation and pro-angiogenic pathways, and lead to diminished collateral dependent myocardial perfusion.
METHODS
Study Design
Yorkshire miniswine (Parsons Research, Amherst, MA) were divided into three groups. One received celecoxib 200 mg orally per day (CBX, n=8), another received naproxen 400 mg orally per day (NAP, n=10), and the third received no drug (control, n=8). All animals received drug treatment for seven weeks beginning at the time of ameroid placement. All animals underwent ameroid constrictor placement on the proximal left circumflex coronary artery (LCx) (Research Instruments SW, Escondido, CA). For all surgical procedures, anesthesia was induced with ketamine (10 mg/kg IM) and thiopental 2.5%, and maintained with a gas mixture of oxygen at 1.5 – 2 L/min and 3.0% isoflurane. The animals were intubated and mechanically ventilated at 16 breaths/min. During the first procedure, the pericardium was opened through a left mini-thoracotomy, and a titanium ameroid constrictor (1.75 mm internal diameter) was placed around the proximal LCx. Seven weeks after ameroid placement, swine were again anesthetized, the heart was exposed and physiologic measurements were taken, followed by euthanasia. The swine were not given any medication to prevent or treat potential arrhythmias.
After the heart was harvested, myocardial samples were rapidly frozen in liquid nitrogen (molecular studies), placed in 4°C Krebs solution (microvessel reactivity studies) or 10% formalin (immunohistochemistry studies).
All experiments were approved by the Beth Israel Deaconess Medical Center Institutional Animal Care and Use Committee. Animals were cared for in compliance with the Harvard Medical Area Institutional Animal Care and Use Committee and in accordance with the ‘Principles of Laboratory Animal Care’ formulated by the National Society for Medical Research and the ‘Guide for the Care and Use of Laboratory Animals’ (NIH publication no. 5377-3 1996).
Measurement of Global and Regional Myocardial Function
Indices of global and regional left ventricular (LV) function were obtained prior to animal sacrifice including: systolic blood pressure (SBP), developed LV pressure (DLVP), positive (+dP/dt) and negative (−dP/dt) first derivatives of LV pressure, and longitudinal and horizontal segmental shortening in the area at risk (AAR) for 10 sequential beats using a Sonometrics system (Sonometrics Corp. London, ON, Canada).
X-ray Coronary Angiography
X-ray coronary angiography with iohexol (Omnipaque, GE Healthcare, Princeton, NJ) was carried out in order to ensure occlusion of the LCx and to assess collateral formation. Recorded images were interpreted by an interventional cardiologist blinded to the treatment groups. Angiographic collateral formation was assessed according to the blush score and Rentrop grading system, depending on the presence and extension of the collateral filling of coronary epicardial vessels.
Microvessel Studies
After cardiac harvest, coronary arterioles (80–180µm in diameter) from the ischemic territory were dissected from the surrounding tissue and placed in an isolated microvessel chamber as described previously.8 After precontraction of microvessels by 30–50% of the baseline diameter with the thromboxane A2 analog U46619 (0.1–1.0 µM), the microvascular responses to vasorelaxing agents sodium nitroprusside (SNP, 10−9 to 10−4 mol/L, endothelium independent cGMP-mediated vasodilator) and adenosine diphosphate (ADP, 10−9 to 10−4 mol/L, an endothelium dependent vasodilator) were evaluated. Responses to vasoconstricting agents endothelin-1 (ET-1, 10−12 to 10−7) and serotonin (5-HT, 10−9 to 10−5) were assessed in non-precontracted vessels. All drugs were applied extraluminally. Responses were defined as percent relaxation of the preconstricted diameter for ADP and SNP, and % constriction of baseline diameter for ET-1 and serotonin. All reagents were obtained from Sigma-Aldrich (St Louis, MO).
Myocardial Perfusion Analysis
Myocardial perfusion was determined with isotope-labeled microspheres (ILMs), 15 µm diameter (BioPAL Worcester, MA) using previously reported methods.9 Briefly, 1.5×107 gold-labeled microspheres were injected during temporary LCx occlusion at the time of ameroid placement to identify the area at risk (AAR). Lutetium (rest conditions) and Europium (ventricular pacing at 150 beats/minute) labeled ILMs were injected prior to sacrifice. Following euthanasia, left ventricular sections were collected for ILM assay. The samples were exposed to neutron beams and microsphere densities were measured using a gamma counter.
Immunoblotting Studies
Tissue lysate was made from the ischemic myocardium. Sixty micrograms of total protein was fractionated by 4–20% gradient, SDS polyacrylamide gel electrophoresis (Invitrogen, San Diego, CA) and transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Bedford, MA). Each membrane was incubated with specific primary antibodies. Antibodies were obtained from Cell Signaling (Danvers, MA), BD Biosciences (San Jose, CA), and Abcam (Cambridge, MA). Immune complexes were visualized with the enhanced chemiluminescence detection system (Amersham, Piscataway, NJ). Bands were quantified by densitometry of autoradiograph films. Ponceau staining was used to ensure equal protein loading.
Immunofluorescence Double Staining for Dividing and Total Endothelial Cells
Frozen sections (12µm in thickness) of myocardium from the ischemic territories were formalin fixed. Antibodies against proliferation marker Ki-67 (Abcam Inc., Cambridge, MA) and PECAM-1 (CD-31, R&D Systems, Minneapolis, MN) were simultaneously applied to the sections and incubated. Detection was obtained using appropriate secondary antibodies (Jackson ImmunoReaserch, West Grove, PA). Sections were mounted in Vectashield with 4',6-diamidino-2-phenylindole (DAPI, Vector Laboratories, Burlingame, CA). Photomicrographs were taken with a Zeiss Axiolab microscope (Carl Zeiss Inc, Thornwood, NY) equipped with a digital camera at 200x magnification (Photodoc, Upland, CA). Total and dividing endothelial cells were counted in a blinded fashion. Data is presented as number of Ki-67 positive endothelial cells/mm2.
Tissue Thromboxane and Tissue and Serum Prostacyclin Levels
Tissue levels of stable thromboxane and prostacyclin breakdown products, 11-dehydrothromboxane B2 (11-d-TXB2) and 6-keto prostaglandin F1α (6-k-PGF1α), respectively were measured by ELISA (Neogen Corp, Lexington, KY). Tissue lysates from the ischemic myocardium underwent liquid-liquid exchange, extraction, and concentration according to the manufacturer’s recommendations. The samples were put into an antibody coated plate along with HRP conjugated 6-k-PGF1α. The plate was washed and HRP substrate added. The plate was incubated and read on at 650 nm, and sample results were plotted against the standard curve.
Serum levels of 6-k-PGF1α were measured by ELISA (Neogen Corp). Serum samples underwent liquid-liquid exchange, extraction, concentration, and assay as described above.
Myocardial Protein Oxidative Stress
Dinitrophenylhydrazine-derivatized myocardial tissue homogenates were separated by 10% polyacrylamide gel electrophoresis and transferred to PVFD membranes (Chemicon International, Inc. Temecula, CA). The membranes were incubated with primary antibody specific to dinitrophenylhydrazine, followed by incubation with a horseradish peroxidase-linked secondary antibody. Immune complexes were visualized with the enhanced chemiluminescence detection system (Amersham).
Myocardial Lipid Oxidative Stress
Measurement of lipid peroxidation in the ischemic myocardium was carried out using a commercial kit to assess levels of free malondialdehyde (MDA), a reactive carbonyl compound produced during decomposition of lipid peroxides (Oxis International, Foster City, CA). Homogenates were incubated with N-methyl-2-phenylindole in acetonitrile, probucol, and concentrated hydrochloric acid. Samples were centrifuged and the MDA containing supernatant removed. Absorbance was measured at 586nm and values were analyzed against a standard curve. Levels of MDA were corrected for protein concentration between samples.
Quantification of Apoptotic cells
Apoptotic cells in the myocardium were identified using the ApopTag detection kit according to manufacturer’s specifications (Chemicon Inc., Temecula, CA). At least one cm2 of tissue was analyzed from each animal (4 per group). The number of TUNEL-positive cardiomyocytes is expressed as positive cells/cm2.
Data Analysis
All results are expressed as mean ± standard error of the mean (SEM). Microvessel responses are expressed as percent relaxation of the preconstricted diameter, or contraction of the baseline diameter, and were analyzed using two-way, repeated measures analysis of variance (ANOVA) with a post-hoc Bonferroni test, which was applied to interactions of treatment and dose. Western blots were analyzed after digitalization (ScanJet 4c; Hewlett-Packard, Palo Alto, CA) with NIH ImageJ 1.33 software (National Institute of Health, Bethesda, MD). For data analysis, levels of phosphorylated proteins were normalized to total expression levels. Comparisons between the three groups were analyzed by one-way, repeated measures ANOVA with Newman-Keuls Multiple Comparison post-hoc test, using GraphPad Prism 4 (GraphPad Software Inc., San Diego, CA).
RESULTS
Animal Model
Over the course of this study, there were 5 mortalities; one in the control group, one in the CBX group, and three in the NAP group. The animals that died in the NAP and CBX groups developed symptoms consistent with heart failure, tachypnea and abdominal ascites. These animals died an average of 32±1.2 days after ameroid placement. There were seven animals included in each group for analysis, as two additional animals were added to the NAP group.
Functional Data
Heart rate was significantly lower in the CBX group than in the control group (control 138±17, NAP 108±17, and CBX 90±4, p=0.04). Both mean arterial pressure and left ventricular pressure (LVP) were significantly higher in the CBX group. In addition, segmental shortening was significantly higher in the CBX group vs. control and NAP groups. The positive first derivative of LV pressure over time (+dP/dt), a pre- and after-load independent measure of global left ventricular function, was lower in the CBX group vs. the NAP and control groups (Figure 1A–D).
Figure 1.
Myocardial functional data. (A) Mean arterial blood pressure was increased in the COX group, *p<0.001. (B) Left ventricular pressure (LVP) was increased in the COX group, †p=0.004. (C) Vertical segmental shortening was increased in the COX group, ‡p=0.02. (D) Positive first derivative of developed pressure over time (+dP/dt) was decreased in the COX group, §p= 0.04.
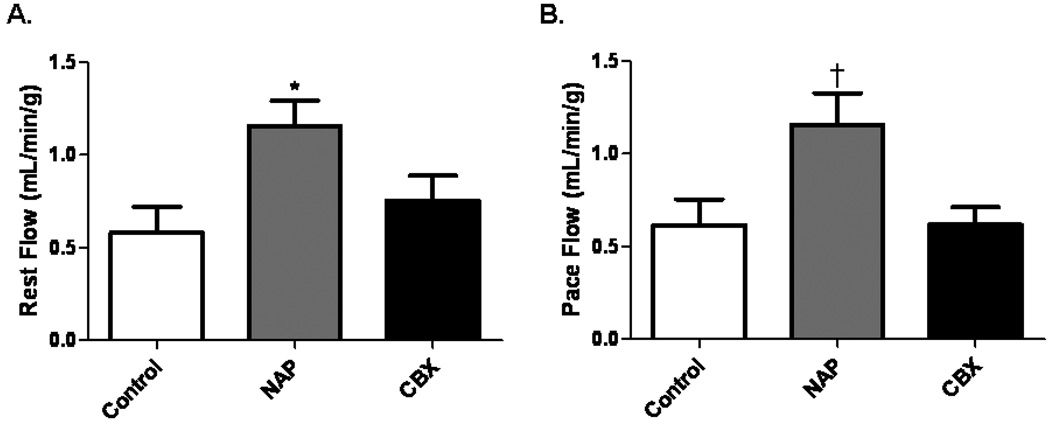
Collateral Dependent Blood Flow in the Ischemic Territory
There was a significant increase in absolute blood flow at rest in the NAP group. In all groups, absolute blood flow was unaffected by ventricular pacing (150 beats/minute) (Figure 2A and B). Collateral dependent myocardial blood flow was similar in the CXB and control groups at rest and with pacing. There was no difference in LAD flow between the groups at rest (control 0.86±0.17 ml/min/g, NAP 1.22±0.18 ml/min/g, and CBX 1.00±0.18 ml/min/g, p=0.36), or during paced conditions (control 0.96±0.13 ml/min/g, NAP 0.86±0.06 ml/min/g, and CBX 0.8600±0.18 ml/min/g, p=0.83).
Figure 2.
Blood flow in the collateral dependent territory. (A) Blood flow to the ischemic myocardium at rest was increased in the NAP group, *p=0.03. (B) There was significantly increased blood flow to the ischemic territory during ventricular pacing in the NAP group, †p=0.02.
Coronary Angiography
In all cases total LCx occlusion was observed during the final coronary angiogram, and there was no difference in observed Rentrop scores (control 0.89±0.3, NAP 1.4±0.38, and CBX 1.6±0.37, p=0.35). There was also no difference in observed blush scores between the groups (control 0.17±0.17, NAP 0.8±0.37, and CBX 0.5±0.22, p=0.25).
Microvascular Function
There was no difference in baseline microvessel diameter between groups (control 156±13µm, NAP 140±10µm, and CBX 142±10µm, p=0.60). Likewise, there was no difference in the preconstricted diameter (control 96±5 µm, NAP 86±13µm, CBX 94±7µm, p=0.78) or % precontraction (control −36.6±2%, NAP −36.0± 3.1%, CBX−34.1±1.4%, p=0.73) of microvessels between groups. There was a greater contraction response in the NAP group to endothelin-1 vs. control or CXB groups. Serotonin induced contraction was greater in the NAP group vs. both control and CXB groups. When examining the microvascular response to ADP, an endothelial dependent vasorelaxing agent, the CBX group demonstrated decreased relaxation response at the highest dose of ADP as compared to the control and NAP groups. There was no difference between groups when the vessels were subjected to SNP, an endothelial independent vasorelaxing agent (Figure 3A–D).
Figure 3.
Microvascular responses. (A) Microvessel response to endothelin-1 demonstrates significantly increased contraction in the NAP group at 10−8 (*p=0.001 vs. control and CBX) and 10−9 (†p=0.001 vs. control). The percentage contraction was not different between the control and CBX groups at any concentration. (B) Contraction responses to serotonin demonstrate increases in the NAP group at 10−6 (‡p=0.001 vs. control and CBX) and 10−5 (§p=0.04 vs. control). (C) Relaxation responses to ADP, an endothelial dependent vasorelaxation agent, showed decreased microvascular responses in the CBX group (∥p=0.01 vs. control and NAP). (D) Responses to sodium nitroprusside (SNP) were not different between the groups (p=0.22).
Levels of Prostacyclin and Thromboxane in the Ischemic Myocardium and Serum
The NAP group exhibited a marked decrease in tissue levels of PGF1α, while in the CBX group it was slightly reduced vs. control. The NAP and CBX groups both had markedly lower serum levels of PGF1α vs. control. Both the NAP and CBX groups demonstrated a trend toward increased tissue levels of TXB2, though these differences did not reach statistical significance (Figure 4A–C).
Figure 4.
Serum and tissue levels of prostacyclin and thomboxane. (A) Tissue levels of PGF1α shows a marked decrease in the NAP group (*p<0.001 vs. control and CBX) and a significant decrease in the CBX group as well (†p<0.05 vs. control). (B) Serum PGF1α levels were significantly higher in the control group (‡p= 0.004 vs. NAP and CBX). (C) Tissue levels of TXB2 were slightly increased in the NAP and CBX groups (p=0.25 vs. control).
Levels of Tissue Oxidant Stress
The CBX group demonstrated significantly higher levels of protein oxidative stress in the ischemic territory of the left ventricle (Figure 5A). Levels of tissue malondialdehyde (lipid oxidative stress) were similar among the groups (Figure 5B).
Figure 5.
Levels of protein and lipid oxidative stress in the normal and ischemic myocardium. (A) Protein peroxidation levels in the ischemic territory (AAR) were increased in the ischemic territory (*p=0.001 vs. control and NAP). (B) There was a trend toward decreased lipid oxidative stress in the ischemic territory of the NAP group (p=0.07).
Quantification of Capillary Growth and Density
Both the NAP and CBX groups demonstrated fewer dividing endothelial cells than the control group. In assessing the total number of endothelial cells in the ischemic myocardium, there were also significantly fewer cells in the NAP and CBX groups when compared to the control (Figure 6A–E).
Figure 6.
Quantification of dividing and total endothelial cells. Double staining for endothelium (CD-31, green) and dividing cells (Ki-67, red) with DAPI (blue) allow for the visualization of dividing endothelial cells in the ischemic territory which appear pink in the combined image. (A) In this representative section for the control group there are many dividing endothelial cells. (B) A representative myocardial section from the ischemic area of the NAP group demonstrates fewer dividing endothelial cells. (C) Representative image from the CBX group. All images are taken at 200x magnification. (D) Quantification of Ki-67 positive endothelial cells demonstrated significant increases in the control group as compared to the NAP and CBX groups (*p=0.003). There was no difference between NAP and CBX groups. (E) Similarly, the total number of endothelial cells in the ischemic territory was significantly lower in the NAP and CBX groups as compared to the control (†p=0.003).
Levels of Apoptosis
The number of apoptotic cells in the ischemic myocardium of the CBX group were significantly increased compared to the control and NAP groups (control 0.017±0.002 cells/cm2, NAP 0.050±0.023 cells/cm2, CBX 0.092±0.047 cells/cm2, p=0.002).
Immnoblotting
In examining pro-angiogenic proteins, VEGF and VEGFR2 expression were decreased in the ischemic myocardium of both NAP and CBX groups vs. control. Similarly, expression of phospho-eNOS (ser1177) was diminished in both NAP and CBX groups. Expression of c-Jun N-terminal kinase (JNK) was increased in the CBX group as compared to the control group. Expression of the anti-angiogenic protein endostatin was significantly lower in the NAP group than in the control and CBX groups (Figure 7A–E). Expression of phospho-Akt (ser473) was increased 1.4 fold in the NAP and CBX groups, but this did not reach significance (p=0.10). There was also a trend toward increased TNFα expression in the CBX group vs. control and NAP (p=0.08). There was no difference in the myocardial expression levels of NFkB (p=0.11), ERK (p=0.76), or apoptosis inducing factor (AIF) (p=0.55) between the groups.
Figure 7.
Immunoblotting in the ischemic myocardium. (A) Levels of VEGF were highest in the control group and decreased in the NAP and CBX groups (*p=0.001 vs. NAP and CBX). (B) Expression of phospho-eNOS was highest in the control group (†p=0.002 vs. NAP and CBX). (C) VEGFR2 was likewise downregulated in the NAP and CBX groups (‡p=0.04 vs. NAP and CBX). (D) JNK expression levels were increased in the CBX group (§p=0.04 vs. control). There was not a significant difference between the control and NAP groups. (E) Endostatin, an anti-angiogenic protein, was significantly downregulated in the NAP group as compared to the control and CBX groups (∥p=0.02). Post-hoc analysis reveals that there was no difference between the control and CBX groups.
DISCUSSION
The principle findings of this study are that the non-selective COX inhibitor naproxen increased collateral dependent perfusion in a chronic model of myocardial ischemia, while the selective COX-2 inhibitor celecoxib did not alter collateral dependent perfusion when compared to non-treated animals. Serum levels of PGI2 were reduced to a similar extent by both drugs, and tissue levels of TXA2 were slightly and similarly increased vs. non-treated animals. Capillary density in the collateral dependent myocardium was diminished in both NAP and CBX groups.
Previous studies have demonstrated that non-selective COX10 and selective COX-2 inhibitors11 diminish coronary collateral perfusion. Most previous studies have examined the effects of indomethacin and other non-specific COX inhibitors on acute collateral dependent perfusion or regulation of vasomotor tone, rather than chronic effects on collateral development.12 In the current study, we treated pigs with either naproxen or celecoxib for 7 weeks. Blood flow to the collateral dependent region was increased with non-selective COX inhibition, though there was no increase in vessel density or in the level of vasodilatory proteins. There was no difference between the groups in regards to the microvessel responses to SNP, an endothelium independent vasorelaxation agent. However, the CBX group demonstrated slightly decreased relaxation responses to ADP, an endothelium dependent vasorelaxation agent. The etiology of improved perfusion to the ischemic territory in the NAP group is not clear, but may be related to preserved microvascular relaxation and withdrawal of a vasoconstrictor effect of COX inhibition. Endostatin, a powerful endogenous inhibitor of angiogenesis, has been shown to reduce blood flow locally.13 Thus it is possible that the lower expression of endostatin in the ischemic territory of the NAP group contributed to improved blood flow.
The CBX group demonstrated significantly increased mean arterial and left ventricular developed pressures, as well as worsened global left ventricular function compared to the other two groups. Nearly all NSAIDs are associated with the development of systemic hypertension.14 Causes of increased blood pressure may include sodium retention15, decreased prostaglandin concentrations16 and renal vasoconstriction secondary to local enothelin-1 production.17 In our study, naproxen was not associated with hypertension. It is unclear why the CBX animals had lower heart rates than the control group. We did not assess stroke volume or cardiac output, as the change in heart rate may have been compensatory for other hemodynamic changes.
The role of COX-2 as a pro-angiogenic factor has been well described.18 Both nonselective COX and selective COX-2 inhibition led to significantly decreased endothelial density and proliferation. Both drugs caused downregulation of VEGF, VEGFR2, and phospho-eNOS, essential components of the angiogenic pathway. The decrease in endostatin in the NAP group was unexpected. A decrease in this protein could have resulted in increased collateral formation, but in this case it did not, at least at the capillary level. There may have also been other unmeasured anti-angiogenic proteins such as angiostatin and tumastatin in the drug treated groups. Surprisingly, this reduction in expression of pro-angogenic mediators was not associated with a reduction in collateral dependent perfusion. Blood flow to the myocardium is controlled by numerous vasoactive substances and autoregulation, which may have influenced the results.
Interestingly, we did observe an increase in expression of the pro-apoptotic protein JNK in the ischemic myocardium of the CBX animals. Levels of apoptosis at the time of animal sacrifice were increased in the CBX group and this may have been mediated in part by JNK signaling. It has been reported that JNK leads to apoptosis via LKB1.19 JNK has been shown to be activated by increased levels of oxidative stress.20 We did not see upregulation of AIF, another protein involved with apoptosis.
Additionally, elevated levels of protein oxidative stress were found in the ischemic myocardium of the CBX group, but not the NAP group. Previous studies have demonstrated increases in reactive oxygen species (ROS) and oxidative stress with some, but not all, COX-2 inhibitors, and this is thought to result in higher rates of apoptosis.21 Increased levels of oxidant stress have been linked to left ventricular remodeling and the development of heart failure.22 Although most NSAIDs have been implicated in increasing oxidant stress, we did not observe this in the myocardium at the current dose of naproxen in this model.
The first selective COX-2 inhibitors were introduced around 2000. There were early concerns for a theoretical increased risk of cardiovascular side effects, because it was thought that prostacyclin levels could be diminished while platelet and tissue levels of thromboxane would not be altered. This was supported by a study that demonstrated a decrease in urinary metabolites of prostacyclin, but no reduction in urinary metabolites of thromboxane in healthy volunteers taking celecoxib.23 However, this study did not consider that COX-2 produces prostaglandins such as prostacyclin in many tissues including the lungs and colon, and the concentration of urinary metabolites is not necessarily a reflection of vascular or perivascular tissue concentrations. In the current study, both COX-2 inhibition and non-selective COX inhibition resulted in markedly reduced serum PGI2, as well as decreased PGI2 and slightly increased TXA2 levels in the ischemic myocardium. The decrease in PGI2 tissue levels in the CBX group was more modest than in the NAP group.
The risk of adverse events may be dose-dependent. For example, the Adenomatous Polyp Celecoxib (APC) trial found celecoxib to be associated with increased adverse events when administered at doses greater than that generally prescribed for arthritic conditions.24 Additionally, in a pooled analysis examining celecoxib use in about 8,000 patients (approximately 16,000 patient-years of follow up) from recent randomized controlled clinical trials, the risk of cardiovascular death, myocardial infarction (MI), stroke, heart failure and thromboembolism were shown to be dose dependent, with a hazard ratio of 1.1 for a dose of 400 mg daily and 3.1 for a dose of 400 mg twice a day.25 At dosages recommended for treatment of osteoarthritis (200 mg once per day), celecoxib has not been associated with an increased incidence of adverse events in any clinical trial to date. Assuming swine demonstrate similar dose dependent effects, we decided to utilize relatively high-doses of celecoxib and naproxen to create conditions similar to studies in which cardiac complications were observed.
There are several limitations of this study. First, it was performed in a porcine model of chronic myocardial ischemia. While in most situations, the porcine coronary circulation closely mimics the physiology and pathophysiology of the human coronary circulation, this may not necessarily be the case for COX inhibition. While the experimental methods in this paper have been extensively utilized in the past, it is possible that they do not accurately represent all changes in the swine related to the drug treatment in this study. For example, the microsphere flow data can be influenced by cardiac output. Second, a non-diseased porcine model was utilized, as opposed to an elderly, diabetic or hypercholesterolemic model. Most patients with arthritic conditions requiring NSAIDs have some endothelial dysfunction due to preexisting conditions. Third, there were more animal deaths in the NAP group as compared to the other two groups. These animals were not included in any part of the analysis, and this may introduce a potential survival bias. While we discontinued treatment drugs 24 hours prior to the final experiment, this period of time will not allow for full washout of the drug and it is likely that residual drug was present. We attempted to minimize the acute effects of COX inhibition in an attempt to examine the long term effects on collateral dependent perfusion. It is likely that continuing the drugs until the time of the final experiment may have produced different findings reflecting continued inhibition of COX or COX-2. Additionally, only one high dose of each drug was examined and different doses may result in different effects. Finally, not all COX-2 inhibitors display similar specificity for COX-2 and many have different toxicology profiles. It is incorrect to assume that because one drug in a group is associated with an adverse effect that there is a “class effect” and that all members share the effect or share it to the same degree. Drugs in this group likely function along a spectrum of potential actions on multiple pathways.
In conclusion, both a selective COX-2 and a non-selective COX inhibitor altered local and systemic physiology, but did not alter myocardial perfusion as compared to the control group. While COX inhibition did not lead to reduced blood flow to the ischemic territory, both non-selective and selective inhibition led to lower levels of local vasodilators, decreased prostaglandin levels, and diminished capillary density and endothelial cell division. There were, however, some important differences between the effects of naproxen and celecoxib such as the effects on hemodynamics, oxidative stress, apoptosis, and alteration of microvascular responses. The impact of selective and non-selective COX inhibition appears to be very complex, but based on this work it appears that collateral dependent myocardial blood flow is not reduced with COX inhibition in this model.
Acknowledgments
Grants
Funding for this project was provided to F.W.S. by NHLBI (RO1HL46716, RO1HL69024, and RO1HL85647), NIH 5T32-HL0074 (M.P.R.) and the Irving Bard Memorial Fellowship (M.P.R., L.M.C.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
Dr. Frank W. Sellke has research support from Ikaria (Clinton, NJ) and Orthologic (Tempe, AZ), and is a consultant for Novo Nordisk (Princeton, NJ), and Cubist Pharmaceuticals (Lexington, MA). Dr. Sellke is a consultant for the law firms representing Pfizer (Princeton, NJ) in the Bextra/Celebrex litigation, but no funding was received for this study, and there was no consultation or notification regarding this study.
REFERENCES
- 1.Coon KD, Inge LJ, Swetel K, Felton V, Stafford P, Bremner RM. Genomic characterization of the inflammatory response initiated by surgical intervention and the effect of perioperative cyclooxygenase 2 blockade. J Thorac Cardiovasc Surg. 139(5):1253–1260. doi: 10.1016/j.jtcvs.2010.01.022. 1260 e1251–1252. [DOI] [PubMed] [Google Scholar]
- 2.Bombardier C, Laine L, Reicin A, Shapiro D, Burgos-Vargas R, Davis B, Day R, Ferraz MB, Hawkey CJ, Hochberg MC, Kvien TK, Schnitzer TJ. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. VIGOR Study Group. N Engl J Med. 2000;343(21):1520–1528. doi: 10.1056/NEJM200011233432103. 1522 p following 1528. [DOI] [PubMed] [Google Scholar]
- 3.Farkouh ME, Greenberg BP. An evidence-based review of the cardiovascular risks of nonsteroidal anti-inflammatory drugs. Am J Cardiol. 2009;103(9):1227–1237. doi: 10.1016/j.amjcard.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 4.Ott E, Nussmeier NA, Duke PC, Feneck RO, Alston RP, Snabes MC, Hubbard RC, Hsu PH, Saidman LJ, Mangano DT. Efficacy and safety of the cyclooxygenase 2 inhibitors parecoxib and valdecoxib in patients undergoing coronary artery bypass surgery. J Thorac Cardiovasc Surg. 2003;125(6):1481–1492. doi: 10.1016/s0022-5223(03)00125-9. [DOI] [PubMed] [Google Scholar]
- 5.Cheng Y, Wang M, Yu Y, Lawson J, Funk CD, Fitzgerald GA. Cyclooxygenases, microsomal prostaglandin E synthase-1, and cardiovascular function. J Clin Invest. 2006;116(5):1391–1399. doi: 10.1172/JCI27540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu G, Luo J, Rana JS, Laham R, Sellke FW, Li J. Involvement of COX-2 in VEGF-induced angiogenesis via P38 and JNK pathways in vascular endothelial cells. Cardiovasc Res. 2006;69(2):512–519. doi: 10.1016/j.cardiores.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 7.Meier P, Gloekler S, Zbinden R, Beckh S, de Marchi SF, Zbinden S, Wustmann K, Billinger M, Vogel R, Cook S, Wenaweser P, Togni M, Windecker S, Meier B, Seiler C. Beneficial effect of recruitable collaterals: a 10-year follow-up study in patients with stable coronary artery disease undergoing quantitative collateral measurements. Circulation. 2007;116(9):975–983. doi: 10.1161/CIRCULATIONAHA.107.703959. [DOI] [PubMed] [Google Scholar]
- 8.Tofukuji M, Metais C, Li J, Franklin A, Simons M, Sellke FW. Myocardial VEGF expression after cardiopulmonary bypass and cardioplegia. Circulation. 1998;98(19 Suppl):II242–II246. discussion II247-248. [PubMed] [Google Scholar]
- 9.Boodhwani M, Mieno S, Feng J, Sodha NR, Clements RT, Xu SH, Sellke FW. Atorvastatin impairs the myocardial angiogenic response to chronic ischemia in normocholesterolemic swine. J Thorac Cardiovasc Surg. 2008;135(1):117–122. doi: 10.1016/j.jtcvs.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 10.Altman JD, Klassen CL, Bache RJ. Cyclooxygenase blockade limits blood flow to collateral-dependent myocardium during exercise. Cardiovasc Res. 1995;30(5):697–704. [PubMed] [Google Scholar]
- 11.Rossoni G, Muscara MN, Cirino G, Wallace JL. Inhibition of cyclo-oxygenase-2 exacerbates ischaemia-induced acute myocardial dysfunction in the rabbit. Br J Pharmacol. 2002;135(6):1540–1546. doi: 10.1038/sj.bjp.0704585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dai XZ, Bache RJ. Effect of indomethacin on coronary blood flow during graded treadmill exercise in the dog. Am J Physiol. 1984;247(3 Pt 2):H452–H458. doi: 10.1152/ajpheart.1984.247.3.H452. [DOI] [PubMed] [Google Scholar]
- 13.Sorensen DR, Read TA, Porwol T, Olsen BR, Timpl R, Sasaki T, Iversen PO, Benestad HB, Sim BK, Bjerkvig R. Endostatin reduces vascularization, blood flow, and growth in a rat gliosarcoma. Neuro Oncol. 2002;4(1):1–8. doi: 10.1215/15228517-4-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson AG, Nguyen TV, Day RO. Do nonsteroidal anti-inflammatory drugs affect blood pressure? A meta-analysis. Ann Intern Med. 1994;121(4):289–300. doi: 10.7326/0003-4819-121-4-199408150-00011. [DOI] [PubMed] [Google Scholar]
- 15.Zhang J, Ding EL, Song Y. Adverse effects of cyclooxygenase 2 inhibitors on renal and arrhythmia events: meta-analysis of randomized trials. JAMA. 2006;296(13):1619–1632. doi: 10.1001/jama.296.13.jrv60015. [DOI] [PubMed] [Google Scholar]
- 16.Funk CD, FitzGerald GA. COX-2 inhibitors and cardiovascular risk. J Cardiovasc Pharmacol. 2007;50(5):470–479. doi: 10.1097/FJC.0b013e318157f72d. [DOI] [PubMed] [Google Scholar]
- 17.Johnson AG, Nguyen TV, Owe-Young R, Williamson DJ, Day RO. Potential mechanisms by which nonsteroidal anti-inflammatory drugs elevate blood pressure: the role of endothelin-1. J Hum Hypertens. 1996;10(4):257–261. [PubMed] [Google Scholar]
- 18.Wu G, Mannam AP, Wu J, Kirbis S, Shie JL, Chen C, Laham RJ, Sellke FW, Li J. Hypoxia induces myocyte-dependent COX-2 regulation in endothelial cells: role of VEGF. Am J Physiol Heart Circ Physiol. 2003;285(6):H2420–H2429. doi: 10.1152/ajpheart.00187.2003. [DOI] [PubMed] [Google Scholar]
- 19.Lee JH, Koh H, Kim M, Park J, Lee SY, Lee S, Chung J. JNK pathway mediates apoptotic cell death induced by tumor suppressor LKB1 in Drosophila. Cell Death Differ. 2006;13(7):1110–1122. doi: 10.1038/sj.cdd.4401790. [DOI] [PubMed] [Google Scholar]
- 20.Mendelson KG, Contois LR, Tevosian SG, Davis RJ, Paulson KE. Independent regulation of JNK/p38 mitogen-activated protein kinases by metabolic oxidative stress in the liver. Proc Natl Acad Sci U S A. 1996;93(23):12908–12913. doi: 10.1073/pnas.93.23.12908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ryan EP, Bushnell TP, Friedman AE, Rahman I, Phipps RP. Cyclooxygenase-2 independent effects of cyclooxygenase-2 inhibitors on oxidative stress and intracellular glutathione content in normal and malignant human B-cells. Cancer Immunol Immunother. 2008;57(3):347–358. doi: 10.1007/s00262-007-0374-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suematsu N, Tsutsui H, Wen J, Kang D, Ikeuchi M, Ide T, Hayashidani S, Shiomi T, Kubota T, Hamasaki N, Takeshita A. Oxidative stress mediates tumor necrosis factor-alpha-induced mitochondrial DNA damage and dysfunction in cardiac myocytes. Circulation. 2003;107(10):1418–1423. doi: 10.1161/01.cir.0000055318.09997.1f. [DOI] [PubMed] [Google Scholar]
- 23.McAdam BF, Catella-Lawson F, Mardini IA, Kapoor S, Lawson JA, FitzGerald GA. Systemic biosynthesis of prostacyclin by cyclooxygenase (COX)-2: the human pharmacology of a selective inhibitor of COX-2. Proc Natl Acad Sci U S A. 1999;96(1):272–277. doi: 10.1073/pnas.96.1.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Solomon SD, McMurray JJ, Pfeffer MA, Wittes J, Fowler R, Finn P, Anderson WF, Zauber A, Hawk E, Bertagnolli M. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med. 2005;352(11):1071–1080. doi: 10.1056/NEJMoa050405. [DOI] [PubMed] [Google Scholar]
- 25.Solomon SD, Wittes J, Finn PV, Fowler R, Viner J, Bertagnolli MM, Arber N, Levin B, Meinert CL, Martin B, Pater JL, Goss PE, Lance P, Obara S, Chew EY, Kim J, Arndt G, Hawk E. Cardiovascular risk of celecoxib in 6 randomized placebo-controlled trials: the cross trial safety analysis. Circulation. 2008;117(16):2104–2113. doi: 10.1161/CIRCULATIONAHA.108.764530. [DOI] [PMC free article] [PubMed] [Google Scholar]