Abstract
The mouse trigeminal (V) system undergoes significant postnatal structural and functional developmental changes. Histological modules (barrelettes, barreloids and barrels) in the brainstem, thalamus and cortex related to actively moved (whisking) tactile hairs (vibrissae) on the face allow detailed studies of development. High-resolution (3H)-2-deoxyglucose (2DG) emulsion autoradiography with cytochrome oxidase histochemistry was used to analyze neuronal activity changes related to specific whisker modules in the developing and mature mouse V system provoked by passive (experimenter-induced) and active (animal-induced) displacements of a single whisker (D4). We tested the hypothesis that neuronal activity patterns change in relation to the onset of active touch (whisking) on postnatal day (P) 14. Quantitative image analyses revealed: 1) on P7, when whisker like patterns of modules are clear, heightened 2DG activity in all appropriate modules in the brainstem, thalamus and cortex, 2) on P14, a transitory activity pattern coincident with the emergence of whisking behavior that presages, 3) strong labeling of the spinal V subnucleus interpolaris and barrel cortex produced by single-whisker-mediated active touch in adults, and 4) at all above-listed ages and structures, significant suppression of baseline activity in some modules surrounding those representing the stimulated whisker. Differences in activity patterns before and after the onset of whisking behavior may be due to neuronal activity induced by whisking, and by strengthening of modulatory projections that alter activity of subcortical inputs produced by whisking behavior during active touch.
Keywords: 2-deoxyglucose, barrels, vibrissae, brainstem, thalamus, cortex
Introduction
The rodent whisker-barrel system is a model for studies of mechanisms in neural development, plasticity and information processing (Woolsey and Van der Loos, 1970; Woolsey, 1990; Jones and Diamond, 1995). However, relatively little is known of the development of neuronal activity in this system. Neuronal activity is the basis for many mechanisms underlying pattern formation, plasticity and information processing (Simons and Land, 1987; reviews by Erzurumlu and Kind, 2001; Kleinfeld et al., 2006). Armstrong-James (1975) first recorded whisker activation in the rat barrel cortex on P6, finding that responses to whisker deflections were transient and receptive fields were larger than in adults. Kossut and Hand (1984) first used 2DG in the developing whisker-barrel system to show that whisker deflection-induced 2DG uptake first occurs in barrel cortex on P4–6. Melzer et al. (1994) demonstrated increased 2DG uptake first on P2 in the spinal V subnuclei interpolaris (SpVi) and caudalis (SpVc), on P4 in V nucleus principalis (PrV) and the ventroposteromedial thalamus (VPM), and on P7 in the barrel cortex. Wu and Gonzalez (1997) showed increased 2DG uptake in newborn rats in all V brainstem subnuclei and in VPM. Cortical barrels were not activated until P6.
In mature animals, findings from 2DG uptake during whisker deflection are inconsistent. In adult rat and mouse, some noted stimulus-evoked 2DG uptake in VPM (Durham et al., 1981; Gonzalez and Sharp, 1985; Sharp et al., 1988), whereas others have not (Melzer et al., 1985, 1994; Welker et al., 1992). Jacquin et al. (1993) studied unrestrained and actively whisking hamsters that often failed to produce 2DG labeling in the thalamus; PrV was always labeled but to varying degrees; while SpVi and SpVc were robustly activated. This is puzzling, as the direct pathway to the cortex is via “lemniscal” structures - PrV and VPM. Sharp et al. (1988) studied awake, restrained rats while they stroked select whiskers, which produced reliable 2DG uptake in appropriate regions of the thalamus and all V brainstem subnuclei. The basis for these inconsistencies likely reflects variations in the active vs. passive nature of the stimuli used in these reports.
The present study had 3 goals: 1) compare 2DG labeling in the whisker pathway in two stimulation paradigms: “active touch” where exploring animals direct whisker contact with objects in their environment (see Durham and Woolsey, 1977; McCasland and Woolsey, 1988; Jacquin et al., 1993), and “passive touch” where the experimenter stimulates the whiskers without anesthesia (see, e.g., Kossut and Hand, 1984; Sharp et al., 1988; Wu and Gonzalez, 1995); 2) assess activation with active vs. passive touch in development; and 3) relate functional activity to the emergence of whisking behavior. The hypothesis was that, in perinatal animals that have yet to display whisking behavior, active and passive whisker deflection produce similar patterns of 2DG uptake in appropriate somatotopic whisker modules in all trigeminal pathway loci. Further, with the onset of whisking behavior on P14, modulatory systems emerge that come to limit activity in specific portions of the barrel neuraxis related to specific behavioral conditions.
Materials and Methods
Subjects and Tissue Preparation
A total of 38 Swiss Webster mice (Charles River Laboratories International, Inc., 251 Ballardvale Street, Wilmington, MA 01887), 16 at P7, 12 at P14 (P0 = birth), and 10 adults (>60 days), were studied. Housing, maintenance, and preparation adhered to federal (NIH) and institutional (ASC-WU) animal care guidelines.
Under gentle restraint (for P7 and P14 mouselings) or light pentobarbital anesthesia (for adults), all of the major vibrissae on both sides of the face, except the left D4 whisker (see Fig. 1 for whisker and barrel neuraxis nomenclature), were trimmed at skin level. Anesthetized animals were given 8–18 hrs to recover prior to 2DG experiments. Animals had access to water, but not to food, 4 to 18 hrs prior to 2DG studies. For “passive activation”, following 1–2 hrs of accommodation to gentle restraint, the left D4 vibrissa was stimulated by hand (~1–2 Hz) for 5 min in all directions prior to injection of 2DG. After intraperitoneal injection (1.0 mCi/10g body weight – a higher dose than that described by McCasland and Woolsey (1988)) of (3H)2DG (American Radiolabeled Chemicals, 2.5 mCi/mL), manual D4 whisker stimulation at 2–4 Hz resumed for the next 30 minutes. For “active whisking”, the animals received intraperitoneal 2DG injections and were placed in cages containing novel objects that they freely explored for 35 minutes in a dimly lit room.
Figure 1.

Schematic diagram of the mouse whisker-barrel pathway. To the left, whiskers are labeled on the face. Whisker rows are labeled A –E along with selected whisker arc numbers; whisker straddlers are labeled α-δ. Related modules evaluated in this study are in the brainstem, thalamus and cerebral cortex (barrelettes, barreloids and barrels) and they are labeled similarly. Regions of interest, namely structures related to the stimulated D4 whisker (black) and 8 surrounding whiskers (C3–5, D3, D5 and E3–5) were identified and outlined on CO-stained sections through all parts of the whisker to barrel pathway in each animal and silver grains related to 3H 2DG label activity of each element quantified. Abbreviations: PrV = principal trigeminal nucleus; SpVo = spinal trigeminal subnucleus oralis; SpVi = spinal trigeminal subnucleus interpolaris; SpVc = spinal trigeminal subnucleus caudalis; VPM = ventroposteromedial nucleus of the thalamus; Po = posterior nucleus of the thalamus; a = anterior; d = dorsal; m = medial.
Post-stimulation tissue processing followed the method of McCasland and Woolsey (1988). Following D4 whisker stimulation, animals were deeply anesthetized with pentobarbital (60 mg/kg, intraperitoneally) for 2–4 hrs to increase brain glycogen levels prior to their sacrifice by intracardiac perfusion (Nelson et al., 1968; McCasland and Woolsey, 1988). Cold fixative (modified PLP fixative; McLean and Nakane, 1974; Durham et al., 1981) was delivered transcardially using a peristaltic pump. After perfusion, brains were removed, postfixed and cryoprotected overnight in the same fixative containing 30% sucrose at 4° C, and sectioned serially at 40 µm on a freezing microtome. Cortices were sectioned tangent to the pia over the barrel field; the thalamus and brainstem in the transverse plane. Sections were processed for cytochrome oxidase (CO) histochemistry (Wong-Riley et al., 1978), mounted on subbed slides and air-dried. Afterwards, slides were defatted with xylenes, washed, and dipped in Kodak NTB2 photographic emulsion diluted 1:1 with distilled water. After 90 days, the emulsion was developed and fixed, and the sections cleared and coverslipped.
Data Analysis
Figure 2 illustrates at two different magnifications the approach used for generating quantitative data and images from combined high-resolution 2DG autoradiography and CO histochemistry. A Zeiss LSM 310 confocal microscope was used to scan and store CO staining with transmitted illumination (Fig. 2a), and silver grains under epi-illumination (Fig. 2c). Optimal sections through the 9-whisker module array (C3–5, D3–5, E3–5) were captured as separate digital “layers”. On CO images, each of the whisker-related patches in array was manually outlined (e.g., D3–5 in Fig. 2b) using Voxel View software on an SGI workstation. Patch outlines were then overlaid onto the superimposed image of silver grains (Fig. 2c). Figure 2d illustrates inversion of the bright field images. Fig. 2e shows 2DG label in relation to CO staining that is the basis for images in Figs. 3–5.
Figure 2.
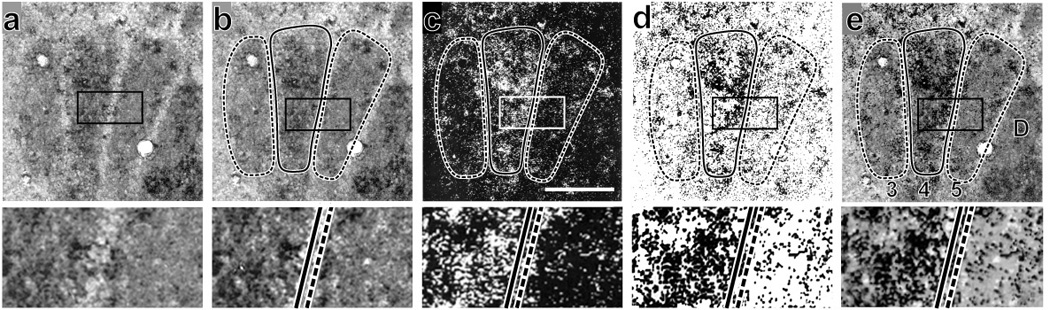
Combining high-resolution images from cytochrome oxidase (CO) histochemistry and 2DG autoradiography. Each top panel has below it a 2.5 × enlarged image of rectangled area showing details of the D4 and D5 barrels and septum dividing them. a. Grayscale image of a CO-stained cortical section illuminated with transmitted light clearly shows that the D4 barrel and surrounding barrels are sharply segregated. b. The same image with the barrels outlined: D4 solid line and D3, D5 with dashed lines. c. Silver grains under epi-illumination with the barrel outlines from b overlaid. d. Fig. 1c image inverted so that silver grains are black. e. The image in d overlaid on the CO image in panel b. Many silver grains are clustered, presumably over neurons, especially within the D4 barrel. Anterior is up; medial left; bar in panel c = 100 µm for upper tier of panels; 40 µm for bottom tier.
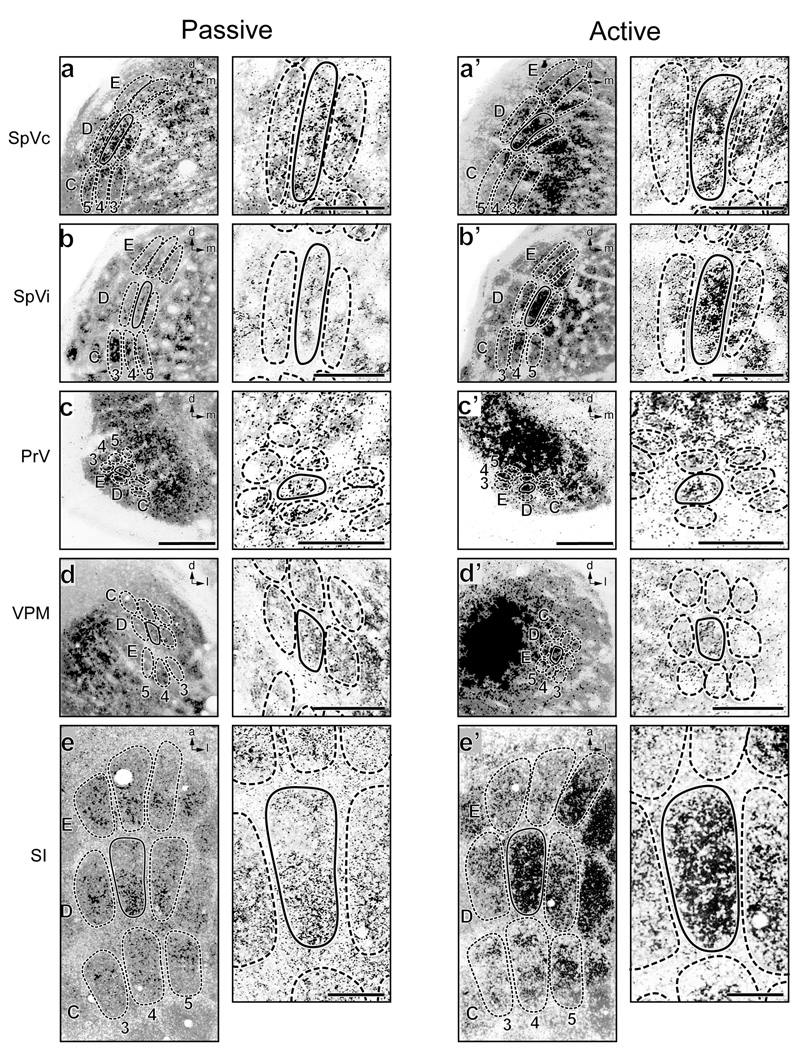
Figure 3.
2DG and CO double-labeled sections through 5 portions of the V neuraxis in 2 P7 mouselings, one subjected to the passive D4 whisker deflection paradigm (left micrographs), the other to active D4 whisker deflection (right micrographs). Each panel has a corresponding higher magnification image to the immediate right of it to more readily discern 2DG label in/around the D4 module. a,a’. Left SpVc. Clusters of silver grains including heavily labeled cells are visible in the passive D4 and other whisker modules (barrelettes). b,b’. Left SpVi. Heavily labeled D4 patches can be seen in both animals. c,c’. Left PrV. The D4 patch is labeled only in the passive case. d,d’. Right VPM nucleus of the thalamus showing barreloids. D4 modules are labeled in both passive and active deflection. e,e’. The 9-barrel array in the right SI cortex. Passive and active deflection both activated the D4 and many surrounding barrels, a region of the SI cortex wider than that activated in adult animals. Also note the small cluster of heavily labeled cells near the center of the D4 barrel in e’. Calibration bars of 100 µm and 50 µm apply to all panel pairs at lower and higher magnifications, respectively. d=dorsal, m= medial, l= lateral, a=anterior.
Figure 5.
2DG and CO double-labeled sections in 2 adult cases under the passive (left column) or active (right column) stimulation conditions. All conventions are the same as in Figure 3. Active whisker deflections produced especially heavy 2DG labeling of D4 patches in SpVc, SpVi and S1 cortex; in the latter, D4 labeling is very dense. This contrast with surrounding barrels is significant and ~67% greater than what occurred in P14 cases and ~88% greater than what occurred in P7 cases.
To quantify the activity (2DG labeling), the total area of each module, that is directly proportional to the total number of pixels in that module, was determined. For each dark-field scan, the number of pixels with values greater than 239 (an arbitrary threshold for reflecting silver grains approximating 95% of the 255 maximal grayscale value) was divided by the total number of pixels in the module, and multiplied by 100 to yield a percentage of bright pixels for each module (~ 2DG label density). Data were normalized by calculating the average percentage of bright pixels for all 9-modules related to the stimulated D4 whisker and the surrounding C3–5, D3, D5, and E3–5 whiskers, then dividing the percentage of bright pixels for each module by this 9-module (barrel, barreloid, barrelette) average. This was then expressed as the percent of average bright voxels for each module. The average percentage of all 9 whisker modules was compared to the average percentage of the D4 module (D41 in Table 1), and to the average percentage of the 8 surrounding modules (SURROUND2 in Table 1). Multi-factor analysis of variance (ANOVA) followed by posthoc group comparisons (Tukey t-tests corrected for multiple comparisons) identified significant silver grain densities in each V structure (brainstem to cortex) at each age with one or the other stimulus paradigm (Table 1 and cartooned in Fig. 6). For all data presented, average D4 patch 2DG values are compared to the averaged average values of all surrounding whisker modules at all levels of the whisker pathway.
Table 1.
Normalized (%) raw 2DG labeling density (bright pixels/module (CO) pixels) data that were used to perform the statistical tests illustrated in Figure 6. Values are presented ± standard deviations. P values are derived from post hoc Tukey paired comparison tests. Note that each ’surround’ data point is a mean (+/− SD) of all of the 8 modules surrounding the stimulated D4 whisker module indicated by structure, age and stimulation condition. Because they are shown as a % of the average signal intensity within the referenced 9-modules, inclusive of the D4 module, the surround data shown are usually lower than 100%.
| PASSIVE DEFLECTION |
ACTIVATED NUCLEUS |
D41 | SURROUND2 | NON- ACTIVATED (CONTROL) NUCLEUS |
D4 | SURROUND | ACTIVE DEFLECTION | ACTIVATED NUCLEUS | D4 | SURROUND | NON-ACTIVATED (CONTROL) NUCLEUS | D4 | SURROUND |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
P7 (n = 10) |
L SpVc | 109 ± 7*** | 99 ± 9 | R SpVc | 107 ± 7 | 99 ± 15 | P7 | L SpVc | 109 ± 5*** | 99 ± 16 | R SpVc | 101 ± 7 | 100 ± 14 |
| L SpVi | 124 ± 14**** | 97 ± 27 | R SpVi | 106 ± 6 | 99 ± 18 | (n = 6) | L SpVi | 118 ± 12*** | 98 ± 21 | R SpVi | 110 ± 18 | 100 ± 14 | |
| L PrV | 110 ± 11* | 99 ± 22 | R PrV | 103 ± 9 | 100 ± 24 | L PrV | 101 ± 41 | 100 ± 28 | R PrV | 105 ± 9 | 99 ± 23 | ||
| R VPM | 118 ± 22* | 98 ± 16 | L VPM | 105 ± 9 | 99 ± 15 | R VPM | 116 ± 12* | 98 ± 30 | L VPM | 95 ± 3 | 101 ± 23 | ||
| R SI | 107 ± 12**** | 98 ± 17 | L SI | 111 ± 7 | 99 ± 14 | R SI | 118 ± 15* | 98 ± 22 | L SI | 108 ± 11 | 99.21 | ||
|
P14 (n = 8) |
L SpVc | 119 ± 14** | 98 ± 24 | R SpVc | 103 ± 7 | 100 ± 20 | P14 | L SpVc | 114 ± 16 | 98 ± 16 | R SpVc | 103 ± 11 | 100 ± 19 |
| L SpVi | 118 ± 12*** | 98 ± 28 | R SpVi | 102 ± 11 | 100 ± 25 | (n = 4) | L SpVi | 127 ± 8*** | 100 ± 41 | R SpVi | 106 ± 11 | 99 ± 40 | |
| L PrV | 115 ± 9*** | 98 ± 23 | R PrV | 101 ± 10 | 100 ± 19 | L PrV | 113 ± 7** | 98 ± 17 | R PrV | 117 ± 32 | 103 ± 27 | ||
| R VPM | 111 ± 8** | 100 ± 15 | L VPM | 103 ± 6 | 100 ± 15 | R VPM | 113 ± 32 | 101 ± 27 | L VPM | 103 ± 9 | 100 ± 15 | ||
| R SI | 129 ± 21*** | 96 ± 27 | L SI | 109 ± 24 | 99 ± 35 | R SI | 142 ± 27* | 95 ± 26 | L SI | 107 ± 21 | 99 ± 19 | ||
|
Adult (n = 5) |
L SpVc | 115 ± 7*** | 98 ± 18 | R SpVc | 106 ± 15 | 99 ± 17 | Adult | L SpVc | 103 ± 10 | 100 ± 20 | R SpVc | 129 ± 27** | 99 ± 22 |
| L SpVi | 122 ± 6**** | 97 ± 38 | R SpVi | 108 ± 13 | 99 ± 21 | (n = 5) | L SpVi | 137 ± 10**** | 95 ± 43 | R SpVi | 104 ± 13 | 100 ± 31 | |
| L PrV | 114 ± 9** | 98 ± 24 | R PrV | 105 ± 17 | 99 ± 28 | L PrV | 109 ± 8 | 99 ± 20 | R PrV | 93 ± 12 | 101 ± 27 | ||
| R VPM | 109 ± 6** | 99 ± 10 | L VPM | 103 ± 8 | 100 ± 14 | R VPM | 112 ± 4**** | 99 ± 13 | L VPM | 111 ± 13 | 99 ± 29 | ||
| R SI | 115 ± 19 | 98 ± 31 | L SI | 115 ± 19 | 98 ± 31 | R SI | 198 ± 6* | 88 ± 44 | L SI | 107 ± 3 | 99 ± 26 |
average of all normalized (%) D4 values ± standard deviation (%)
average of all 8 Surround values in the 9-module array ± standard deviation
boldface if D4 is significantly different from Surround:* = p<0.05; **= p<0.01; *** = p<0.005; **** = p<0.001;
(Tukey's paired comparisons)
Figure 6.
Schematized summary of the results of statistical tests performed on 2DG label intensity from every animal’s experimental 9-whisker module arrays for all ages, structures and stimulation conditions studied. Significant multi-factor ANOVA dictated posthoc Tukey paired comparisons the results of which are illustrated here by dark shading (“activation” or increased 2DG label contrast relative to that of the remaining 8 modules, significant at the p < .05 level), light shading (NS or nonsignificant effects on 2DG signal contrast), and no shading (“suppression” or decreased 2DG label contrast relative to that of the remaining 8 modules, p < .05). As summarized in Table I, a significant change in 2DG label contrast was only seen once in the control “non-activated” contralateral structures. The grid Key on the far right indicates the whisker modules represented in each of the 30 illustrated experimental conditions. The center box in each 9-module grid is the D4 whisker representation. See METHODS for details of quantified image analyses and statistics.
Results
The results of the present study can be briefly summarized as follows. On P7, when whisker- like patterns of CO staining are clear, heightened 2DG activity was observed in all appropriate barrel-like modules in the brainstem, thalamus and cortex. On P14, a transitory 2DG labeling pattern was observed coincident with the emergence of whisking behavior that presages, in adulthood, strong labeling of the spinal V subnucleus interpolaris and barrel cortex produced by single-whisker-mediated active touch. Conversely, in all of the above-listed ages and structures, significant suppression of baseline activity was observed in some modules surrounding those representing the stimulated whisker. Detailed presentation of these and associated results follow.
Qualitative Observations
Rhythmic, coordinated whisking behavior was absent on P7, but, small irregular whisker movements were observed. Rapid (8–15 Hz) whisking, rhythmic protraction and retraction of vibrissae, begins abruptly just before P14 (e.g. Sullivan et al., 2003). Thus, the P7 “active touch” paradigm reflects unrestrained mouselings rubbing their muzzles against each other, their dams, the cage and/or bedding. This behavior, while clearly not “active whisking,” represents the mouselings’ purposeful movements guided in part by sensory input from vibrissae that modifies behavior. For the active touch paradigm, mice were placed in a novel clean cage containing unfamiliar objects in a dimly lit room that induces exploratory behaviors.
2DG label in modules representing caudal vibrissae was reliably low; however, adjoining regions representing intact small rostral and ventral whiskers often were heavily labeled (e.g., Figs. 3a, 3b’, 3c, 3c’, 3d, 4a’, 4c, 4c’, 4d, 4d’, 5c, 5c’, 5d, 5d’). Individual cells were labeled and clearly evident, especially in layer IV barrels (e.g., Figs. 4e, 4e’, 5e, 5e’). In cortex, row E barrels usually contained higher levels of 2DG than row C barrels (Fig. 6, and see Durham and Woolsey, 1985; McCasland and Woolsey, 1988). 2DG label in the D5 barrel was often nearly as high as in the D4 barrel (Fig. 6; see Shin et al., 2005).
Figure 4.
2DG and CO double-labeled sections in 2 P14 cases under the passive (left column) or active (right column) stimulation conditions. All conventions are the same as in Figure 3. Note the pronounced 2DG labeling of the D4 patches at all levels produced by active touch, in contrast to the pattern seen with passive touch.
In P7 cases, passive D4 whisker deflection activated the D4 module at every level along the whisker-to-barrel pathway (Fig. 3). Label in the D4 patch was most pronounced in the SpVi (Fig. 3b), whereas 2DG labeling was less intense in the D4 modules of the other locations (Figs. 3a, c, d, e). Indeed, in the thalamus and barrel cortex, all modules surrounding the heavily labeled D4 were labeled. Active D4 whisker deflection in P7 mice activated all D4 foci, but was not as effective as passive stimulation in evoking contrast between the D4 and surrounding neuropil (Fig. 3a’–e’). Differential D4 labeling was most distinct in the SpVc, SpVi, and SI cortex (Figs. 3a’, b’, e’). By contrast, in PrV and VPM, a difference in label density between the D4 and surrounding patches, if any, was subtle (Figs. 3c’, d’). PrV instead showed increased 2DG label in portions of the pattern representing the smaller whiskers on the upper lip and nose.
In P14 cases, passive whisker deflection also activated the D4 modules at all levels of the whisker pathway (Fig. 4). This was greatest in the SpVc and cortex (Figs. 4a, e). Active whisking was more effective in selectively activating the D4 modules than on P7 (Fig. 4 vs. Fig. 3). As in P7 mice, 2DG uptake increased most dramatically in portions of structures representing the smaller upper lip and nose hairs (Figs. 4c’, d’). Some cases showed substantial labeling in the cortical D4 barrel (Fig. 4e’).
In adult passive touch cases, in contrast with the pronounced D4 labeling seen in all stations on P7 and P14, D4 whisker stimulation did not lead to heavy 2DG uptake in D4 modules, although labeling of the D4 module was above background throughout the pathway (Fig. 5). However, active touch in adults produced the clearest delineation of the D4 modules of all of the stimulation/age conditions studied here (Fig. 5). Increased 2DG labeling and contrast with surrounding modules were particularly prominent in the SpVi (Fig. 5b’) and barrel cortex (Fig. 5e’).
It is also noteworthy that from P7 to adulthood, the higher-order “lemniscal” structures, VPM and S1, displayed a clear reduction in the number of modules around D4 activated by D4 whisker stimuli; i.e. the labeling became more focused upon the D4 barreloid and barrel. Moreover, there are ages and stimulus conditions where 2DG uptake was not reliably affected in the PrV, VPM and S1 cortex. Indeed, based upon the literature (but with different stimulus paradigms), it was surprising to find that, in adulthood, passive deflection of the D4 whisker did not activate the D4 barrel cortex, whereas it was robustly activated by active whisking.
Quantitative Analyses of Increased 2DG Uptake
Figure 6 shows the major findings derived from statistics performed on the raw data contained in Table 1. To first summarize, on P7, active and passive touch via the D4 whisker produced statistically reliable increases in 2DG labeling in each of the V nuclei and barrel cortex, with the exception of the PrV in the active touch paradigm. On P14, a different pattern was observed coincident with the emergence of whisking behavior. In adults, lemniscal and paralemniscal structures were differentially labeled. Such differences between the passive vs. active single whisker stimulation paradigms are evident in the autoradiograms in Figure 5.
Image analyses and statistical evaluations are necessarily complex because they considered several different factors in a multi-factor ANOVA: three ages, 10 CNS structures (five activated and five contralateral non-activated), nine whisker-related patches encompassing the D4 and surrounding modules in each activated structure, and two stimulation paradigms, as follows:
Activated vs. Contralateral Non-activated Sites
This first level of analysis revealed differences in 2DG uptake between the left V brainstem nuclei, right VPM, right cortex (all activated) and these same structures on the contralateral (non-activated) side. Significant differences occurred between activated and non-activated sites after both passive (ANOVA, F = 4.49; df = 9/2747; p < 0.0001) and active (F = 3.44; df = 9/1418; p = 0.001) touch when data were pooled from the varied structures, barrel-like modules, and ages. No significant variance was observed in non-activated sites when all of the data were pooled from the varied structures, ages or stimulation conditions. Posthoc Tukey paired comparisons indicated that differences in 2DG uptake within the activated sides reflected variance attributable to specific barrel-like modules, CNS structures within which they occurred, ages, and stimulation paradigms.
D4 vs. Surrounding Barrel-like Modules
Contrast between the D4 and eight surrounding modules in five separate activated structures were analyzed. ANOVA showed significant inter-module differences in the combined data from all five structures by age in both stimulation paradigms (P7: F = 30.0; df = 4/1435;p < 0.00001; P14: F = 15.3; df = 4/1075; p < 0.00001; Adult: F = 10.6; df = 4/895; p < 0.00001). Posthoc comparisons showed differences in 2DG labeling between the D4 and surrounding modules, as follows: On P7, D4 statistically differed from C3, C4 and C5; on P14, D4 differed from all other modules; in adults, D4 differed from all other modules, except D5.
Age
Pooled data from the active and passive touch groups, showed significant differences between the D4 and surrounding modules in all whisker representations in the trigeminal pathway on P7. At P14 and adulthood, D4 contrast was significant in every CNS structure, save for the SpVc (p = 0.086 on P14, p = 0.2 in adults). The contrast in SI cortex in adults exceeded that seen on P14, which exceeded that seen on P7.
Active vs. Passive Touch
The active vs. passive nature of the stimulus was a significant main effect (F = 2.32; df = 1/4183; p = 0.018), which co-varied with age (F = 2.94; df = 2/4180; p = 0.0001). Passive whisker deflection was effective in driving 2DG uptake in P7 and P14 mice, but was substantially less pronounced in adults. Conversely, active whisking more strongly activated appropriate foci in P14 and adult animals than in P7 mice. This significant effect was largely attributed to extraordinary levels of active touch-induced 2DG uptake in the adult SpVi and barrel cortex.
Quantitative Analyses of Decreased 2DG Uptake
Figure 6 also indicates that 2DG labeling was significantly reduced relative to baseline in a number of structures, ages and stimulus conditions. A reliable reduction in activity (“suppression”) occurred, even as early as P7, in modules adjoining the D4 module at all levels of the V neuraxis, in both stimulation paradigms. Such suppression was not more widely distributed later in development. Regardless of level, age or stimulus, the D4 module never displayed significant stimulus-induced suppression. The findings in Figure 6 indicate that stimulus-evoked suppression occurred most reliably in the C-row (dorsal to the D-row on the face) whisker modules. Further, when an adjoining D-row module was reliably suppressed, it was always D3 and not D5. And, the only module to display E-row suppression was in P14 S1 cortex under passive stimulation.
Discussion
A differential pattern of 2DG labeling is produced in a one-whisker stimulation paradigm. In developing mice, changes in stimulus-evoked activation of specific whisker-related modules first appear at the onset of whisking behavior. It specifies either the “active” state, in which the mouse self-directs scanning of its environment, or an “alert” state, in which afferent input is evoked without behavioral whisking. These findings support the hypothesis that stimulus-evoked activity changes at the onset of active touch (whisking) on P14. Further, they indicate the important role of the SpVi-based paralemniscal pathway in active touch, including descending modulatory projections to the V brainstem nuclei (intersubnuclear, corticotrigeminal) to dampen activity in the PrV-VPM lemniscal pathway during active touch that develop later.
Technical Issues and Limitations
Durham et al. (1981) modified the Sokoloff et al. (1977) (14C)2DG method with (3H)2DG in perfused and fixed tissue. Systemic fixation improved tissue preservation, and the lower energy emission of (3H) compared to (14C), combined with emulsion rather than film autoradiography, improved localization. McCasland and Woolsey (1988) refined the technique to increase the tissue levels of 2DG. The method is compatible with a number of histological procedures, including CO histochemistry. Double labeling provides cellular resolution of 2DG uptake and superior localization of 2DG label in register with whisker module boundaries throughout the V whisker pathway.
The passive touch paradigm employed here did not use mechanically controlled stimulation. However, with restraint, mice adapted quickly and evidently stopped whisking altogether once single whisker manual stimulation began (but see below). Given the significant differences in 2DG labeling between active whisking vs. passive touch of single whiskers, that we report, further detailed studies that unequivocally eliminate whisking during passive touch and that provide greater stimulus control in awake behaving animals are of interest. For instance, stimulus control approaches using ‘head-fixed’ and single whisker operant conditioning paradigms (e.g. Bermejo et al., 1996) should be particularly well suited to test detailed hypotheses based on the present findings (Table 1). Specifically, future studies could parse out specific features of “active” vs. “passive” touch as defined in the present work that produce activity in specified components of the whisker-barrel neuraxis.
Some undetected active whisking could have occurred during our delivery of 35 minutes of continuous manual whisker deflections while gently restraining the mice that we term “passive touch”. Stimulus parameters were not monitored quantitatively. But investigator controlled “passive touch” parameters are clearly different than those controlled by the animal in the “active touch” exploratory whisking paradigm. A significant finding here is the demonstration for the first time that these two paradigms produced robustly different 2DG activity patterns along the whisker-barrel neuraxis. This provides a context to characterize actual stimulus and behaviorally relevant feature(s) that account for such robust differences in activity across the mouse V neuraxis with age, CNS locus and mode of whisker utilization.
The possibility that animal-initiated whisking might have occurred during our 35 minute “passive touch” epoch cannot be ruled out. This is important because others (Fanselow and Nicholelis, 1999; Hentschke et al., 2006; Crochet and Petersen, 2006; Ferezou et al., 2006, 2007; Lee et al., 2008) have documented with a wide array of strategies a suppression of the passive stimulus-evoked activity in VPM and barrel cortex when animals are actively whisking at the time of experimenter-controlled whisker deflection. Conversely, increased VPM and cortical neuronal activity are reported in the absence of whisking. The latter is seemingly inconsistent with the diminished 2DG uptake we observe with “passive touch”, Our findings of dampened VPM and cortical 2DG activity during “passive touch” could, therefore, either be a consequence of some whisking that occurred whilst we stimulated whiskers or more likely suppression of behaviorally irrelevant inputs over time as has been long known in many different systems and paradigms. In the present context it is important to point out that the time frames of the signals recorded (2DG vs single and multi-unit activities) are very different as metabolically driven 2DG uptake is integrated over periods of minutes not over seconds.
Circuits Subserving Active vs. Passive Touch
In adult mice, activity is significantly higher in the barrel cortex and SpVi during single-whisker mediated exploratory behavior than with manual deflections of the same whisker. Active touch-induced labeling in the cortex and SpVi were the highest signals obtained for any age or condition. By comparison, the same animals had significant 2DG label in the VPM, but this translated into only modest levels of contrast; labeling in the PrV and SpVc was not significant. Cortical 2DG label was conspicuously absent with experimenter-induced whisker deflection, although all subcortical centers were significantly activated. Significant contrast, however, between stimulated D4 and surrounding whisker modules was obtained only in the SpVi, with lesser but still significant contrast in the VPM and SpVc. These findings suggest that sensory input from passive whisker deflection, that typically elicits bouts of active whisking (Ferezou et al., 2006), does not engage descending cortical circuitry until actual whisking develops and “solicited” input arrives.
Numerous studies have shown that different stimulus conditions (whisker movement, animal mobility, reward paradigm, signal recording, etc.) of alert, mice and rats activate cortex in particular patterns (Krupa et al., 2004; Ferezou et al., 2006, 2007; Fanselow and Nicolelis, 1999; Hentschke et al., 2006; Furuta et al., 2008; Lee et al., 2008). Further distinctions are known from electrophysiological studies comparing sedentary vs. exploring rodents’ central patterns of activation. For example, Fanselow and Nicolelis, (1999) demonstrated that cuff stimulation of the infraorbital nerve produced robust increases in neuronal activity in the VPM and SI when the animals were not whisking. In contrast, when the animals engaged in self-directed whisking, total activity levels were reduced. It is worth noting that single whisker stimulation was not described and correlation of recording sites with particular whisker representations in VPM and SmI were not detailed by Fanselow and Nicolelis. Similar suppression of activity was described by Trageser and Keller (2004) in recordings of activity from cells in the posterior thalamus; they suggested that a descending cortico-thalamic projection exerts top-down modulation of activity. Lee et al. (2008) reported that passive whisker stimulation activated cells in the whisker-to-barrel pathway, that was significantly reduced in whisking mice. These distinctions were eliminated by surgical ablation of brainstem connectivity, thus suggesting a brainstem level gating of ascending input.
These many seeming discrepancies between reports in the literature are most likely attributable to differences in whisker stimulation, monitoring responses, - i.e., unit recording, optical imaging, metabolic marker averaging, etc., - head fixed, freely moving, restrained animals, etc., thus making direct comparisons of whisker stimulation in actively whisking vs. non-whisking animals difficult. Clearly progress is being made in precisely delineating the nature of the whisker deflection stimulus during recordings of cortical activity evoked by active touch. This ought to permit a more direct and informative synthesis of underlying functions/connections/behaviors (Krupa et al., 2004; Hentschke et al., 2006; Crochet and Petersen, 2006; Ferezou et al., 2006, 2007).
However, given the findings from different approaches discussed above, our 2DG labeling results are notable: first, because whisker module activity is monitored at 5 stations along the pathway, and second, because of the emphasis on the lemniscal PrV-VPM-cortex pathway in current formulations of whisker information processing. PrV has heavy projections to VPM and is the brainstem nucleus required for development of whisker-related patterns in the VPM and cortex (Killackey and Fleming, 1985). Yet, SpVi’s heightened activity during active touch is consistent with a key function of the paralemniscal pathway in whisking behavior. These findings may have several different, but not mutually exclusive, bases. First, the dense GABAergic input to the PrV from SpVi cells that discharge to single whisker deflection (Furuta et al., 2008) would be expected to suppress PrV cell activity with activation of SpVi. VPM thalamus, a principal target of the PrV, would then have decreased responses to the same stimuli. This was observed here in the whisking animals, particularly adults. Second, increased and multiwhisker module activity in SpVi during active touch could relate to it being particularly responsive to whisker inputs during whisking. Studies of SpVi unit activity in whisking animals are required to test this hypothesis. Third, the pairing of the SpVi and barrel cortex during active touch may reflect connections between the two, via the “ascending limb” of the posterior thalamic nucleus (Williams et al., 1994) and via the direct cortico-SpVi projection (Jacquin et al., 1990b). If so, heightened SpVi and cortical activity should span multiple whisker modules, given the fact that SpVi projection neurons always respond to multiple whiskers (Jacquin et al., 1986). This was found in the present study where the cortical D5 barrel and the SpVi D5 module were as active as the D4 modules during D4 whisker active touch. Fourth, the paucity of SpVc activity in adults differs from our prior study (Jacquin et al., 1993) in hamsters documenting strong SpVc contrast in single whisker-mediated active touch. Possibly, in the present experiments, the mouse SpVc is responding only under conditions of passive touch to engage connections with brainstem oromotor reflex circuits, to alert animals to potentially deleterious, or at least unintended, orofacial stimuli. SpVc is a target of a dense GABAergic projection from the SpVi (Jacquin et al., 1990a; Furuta et al., 2008). SpVi-based dampening of SpVc activity could explain the weak responses there. Similarly, levels of activity in the C row, and frequently in the D3 module, appear to be considerably lower than average locus levels at all levels of the V pathway. This is pronounced in SpVi under all experimental conditions. Earlier data suggested that center-surround inhibitory activity in subcortical loci rely on intersubnuclear circuitry (Jacquin et al., 1990; Timofeeva et al., 2004). In actively exploring P14 and adult mice, this relationship is particularly robust. Here, suppression of surround responses in cortex could reflect ascending activation of all cells within a barrel column, including inhibitory projections to adjacent barrels (Wróbel et al., 1998).
Development of Active versus Passive Touch
At P7, nearly all D4 modules in lemniscal and paralemniscal structures in the whisker pathway were activated by D4 whisker deflection, with the exception of the PrV. Passive whisker deflection was more effective than active touch in increasing focal 2DG uptake, labeling appropriate modules in the SpVc, SpVi, PrV, VPM, and cortex, consistent with previous developmental 2DG studies (Melzer et al., 1994; Wu and Gonzales, 1997). This “permissive” activity pattern likely reflects immaturity among descending and local modulatory circuits (e.g., White et al., 1997) that develop concomitantly with whisking motor circuitry. At P14, when whisking behavior is initiated, the labeling pattern in the passive touch paradigm is very similar to that found at P7. However, active touch at this age yields an activity pattern that approaches that of adults (see above), suggesting that the sensorimotor pathway is transitioning rapidly into a mature “whisking” system. Unfortunately, little is known regarding the status of V intersubnuclear and cortico-V pathways and connections in and around P14. Given the activity changes we show from a lemniscal- to a paralemniscal-dominant system that occur concurrently with the emergence of whisking behavior, a testable hypothesis is that intersubnuclear and cortico-V connections undergo an important transformation at this point and afterward. It is also very likely that late-developing intracortical connections (e.g. Miller et al., 2001), singularly or in concert with V intersubnuclear and corticofugal projections, account for whisking-induced changes in activation patterns in the entire whisker-barrel neuraxis.
Active touch on P7 includes purposeful movements of the head and neck, but not the whiskers. Such a search strategy was sufficient to produce a single whisker-mediated 2DG labeling pattern that differed from that found after passive touch, suggesting that the whiskers provide meaningful spatial information even without concomitant control of whisking. There is a precedent for this result in prior studies of the development of whisker-mediated behaviors (Sullivan et al., 2003). Moreover, the failure of active touch on P7 to produce significant contrast in the PrV suggests that the paralemniscal system may suffice to drive these early whisker-mediated behaviors and that modulatory systems may be in place at this age that engage to dampen lemniscal responses to single whisker stimulation. An alternative hypothesis is that functional V brainstem projections to the cerebellum, superior colliculus and other subcortical sites (reviewed by Kleinfeld et al., 2006) may have a more rapid developmental time course and suffice to explain early whisker-mediated behaviors.
Acknowledgements
This work was supported by the National Institutes of Health grants R01-NS29885, R01-NS046036, P01-NS049048 and the Spastic Paralysis Foundation of the Illinois-Eastern Iowa District of the Kiwanis International. We thank Jon Christenson, Joop Arends and Joe DeMaro for expert technical assistance.
References
- Armstrong-James M. The functional status and columnar organization of single cells responding to cutaneous stimulation in neonatal rat somatosensory cortex. J. Physiol. (Lond.) 1975;246:501–538. doi: 10.1113/jphysiol.1975.sp010902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermejo R, Gao P, Harvey M, Zeigler HP. Conditioned “whisking” in the rat. Somatosens. Mot. Res. 1996;13:225–234. doi: 10.3109/08990229609052578. [DOI] [PubMed] [Google Scholar]
- Crochet S, Petersen CC. Correlating whisker behavior with membrane potential in barrel cortex of awake mice. Nat Neurosci. 2006;9:608–610. doi: 10.1038/nn1690. [DOI] [PubMed] [Google Scholar]
- Curtis JC, Kleinfeld D. Seeing what the mouse sees with its vibrissae: a matter of behavioral state. Neuron. 2006;50:524–526. doi: 10.1016/j.neuron.2006.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham D, Woolsey TA. Barrels and columnar cortical organization: evidence from 2-deoxyglucose (2-DG) experiments. Brain Res. 1977;137:168–174. doi: 10.1016/0006-8993(77)91022-8. [DOI] [PubMed] [Google Scholar]
- Durham D, Woolsey TA. Functional organization in cortical barrels of normal and vibrissae-damaged mice: A (3H)2-deoxyglucose study. J. Comp. Neurol. 1985;235:97–110. doi: 10.1002/cne.902350108. [DOI] [PubMed] [Google Scholar]
- Durham D, Woolsey TA, Kruger L. Cellular localization of 2-[3H]deoxy-D-glucose from paraffin-embedded brains. J. Neurosci. 1981;1:519–526. doi: 10.1523/JNEUROSCI.01-05-00519.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erzurumlu RS, Kind PC. Neural activity: sculptor of ‘barrels’ in the neocortex. Trends Neurosci. 2001;24:589–595. doi: 10.1016/s0166-2236(00)01958-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow E, Nicolelis MAL. Behavioral modulation of tactile responses in the rat somatosensory system. J. Neurosci. 1999;19:7603–7616. doi: 10.1523/JNEUROSCI.19-17-07603.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferezou I, Bolea S, Petersen CH. Visualizing the cortical representation of whisker touch: voltage-sensitive dye imaging in freely moving mice. Neuron. 2006;50:617–629. doi: 10.1016/j.neuron.2006.03.043. [DOI] [PubMed] [Google Scholar]
- Ferezou I, Haiss F, Gentet LJ, Aronoff R, Weber B, Petersen CH. Spatiotemporal dynamics of cortical sensorimotor integration in behaving mice. Neuron. 2007;56:907–923. doi: 10.1016/j.neuron.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Furuta T, Timofeeva E, Nakamura K, Togo M, Kaneko T, Deschênes M. Inhibitory gating of vibrissal inputs in the brainstem. J. Neurosci. 2008;28:1789–1798. doi: 10.1523/JNEUROSCI.4627-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez MF, Sharp FR. Vibrissal tactile stimulation: (14C) 2-deoxyglucose uptake in rat brainstem, thalamus, and cortex. J. Comp. Neurol. 1985;231:457–472. doi: 10.1002/cne.902310405. [DOI] [PubMed] [Google Scholar]
- Hentschke H, Haiss F, Schwarz C. Central signals rapidly switch tactile processing in rat barrel cortex during whisker movements. Cerebral Cortex. 2006;16:1142–1156. doi: 10.1093/cercor/bhj056. [DOI] [PubMed] [Google Scholar]
- Jacquin MF, Mooney RD, Rhoades RW. Morphology, response properties, and collateral projections of trigeminothalamic neurons in brainstem subnucleus interpolaris of rat. Exp. Brain Res. 1986;61:457–468. doi: 10.1007/BF00237571. [DOI] [PubMed] [Google Scholar]
- Jacquin MF, Chiaia N, Haring JH, Rhoades RW. Intersubnuclear connections within the trigeminal brainstem complex. Somatosens. Mot. Res. 1990a;7:399–420. doi: 10.3109/08990229009144716. [DOI] [PubMed] [Google Scholar]
- Jacquin MF, Weigand M, Renehan WE. Structure-function relationships in rat brainstem subnucleus interpolaris. VIII. Cortical inputs. J. Neurophysiol. 1990b;64:3–27. doi: 10.1152/jn.1990.64.1.3. [DOI] [PubMed] [Google Scholar]
- Jacquin MF, McCasland JS, Henderson TA, Rhoades RW, Woolsey TA. 2-DG uptake patterns related to single vibrissae during exploratory behaviors in the hamster trigeminal system. J. Comp. Neurol. 1993;332:38–58. doi: 10.1002/cne.903320104. [DOI] [PubMed] [Google Scholar]
- Jones EG, Diamond IT. Cerebral Cortex. Volume 11. NY: Plenum Press; 1995. The Barrel Cortex of Rodents. [Google Scholar]
- Killackey HP, Fleming K. The role of the principal sensory nucleus in central trigeminal pattern formation. Brain Res. 1985;354:141–145. doi: 10.1016/0165-3806(85)90077-x. [DOI] [PubMed] [Google Scholar]
- Kleinfeld D, Ahissar E, Diamond ME. Active sensation: insights from the rodent vibrissa sensorimotor system. Curr. Opin. Neurobiol. 2006;16:435–444. doi: 10.1016/j.conb.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Kossut M, Hand PJ. The development of the vibrissal cortical column: A 2-deoxyglucose study in the rat. Neurosci. Lett. 1984;46:1–6. doi: 10.1016/0304-3940(84)90189-7. [DOI] [PubMed] [Google Scholar]
- Krupa DJ, Weist MC, Shuler MG, Laubach M, Nicolelis MAL. Layer-specific somatosensory cortical activation during active tactile discrimination. Science. 2004;304:1989–1922. doi: 10.1126/science.1093318. [DOI] [PubMed] [Google Scholar]
- Lee S, Carvel GE, Simons DJ. Motor modulation of afferent somatosensory circuits. Nature Neurosci. 2008;11:1430–1438. doi: 10.1038/nn.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lottem E, Azouz R. Mechanisms of tactile information transmission through whisker vibration. J. Neurosci. 2009;29:11686–11697. doi: 10.1523/JNEUROSCI.0705-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCasland JS, Woolsey TA. High resolution 2-deoxyglucose mapping of functional cortical columns in mouse barrel cortex. J. Comp. Neurol. 1988;278:555–569. doi: 10.1002/cne.902780407. [DOI] [PubMed] [Google Scholar]
- McLean IW, Nakane PK. Periodate-lysine paraformaldehyde fixative: A new fixative for immunoelectron microscopy. J. Histochem. Cytochem. 1974;22:1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Melzer P, Van der Loos H, Dörfl J, Welker E, Robert P, Emery D, Berrini JC. A magnetic device to stimulate selected whiskers of freely moving or restrained small rodents: its application in a deoxyglucose study. Brain Res. 1985;348(2):229–240. doi: 10.1016/0006-8993(85)90441-x. [DOI] [PubMed] [Google Scholar]
- Melzer P, Welker E, Dörfl J, Van der Loos H. Maturation of normal metabolic response to vibrissa stimulation in the developing whisker-to-barrel pathway of the mouse. Brain Res. 1994;77:227–250. doi: 10.1016/0165-3806(94)90199-6. [DOI] [PubMed] [Google Scholar]
- Miller B, Blake NMJ, Erinjeri JP, Reistad C, Sexton-Steele TR, Woolsey TA. Postnatal growth of intrinsic connections in mouse barrel cortex. J. Comp. Neurol. 2001;436:17–31. [PubMed] [Google Scholar]
- Nelson SR, Schulz DW, Passonneau JB, Lowrey OH. Control of glycogen levels in brain. J. Neurochem. 1968;15:1271–1279. doi: 10.1111/j.1471-4159.1968.tb05904.x. [DOI] [PubMed] [Google Scholar]
- Sharp FR, Gonzalez MF, Morgan CW, Norton MT, Sharp JW. Common fur and mystacial vibrissae parallel sensory pathways: 14 C 2-deoxyglucose and WGA-HRP studies in the rat. J. Comp. Neurol. 1988;270:446–469. doi: 10.1002/cne.902700312. [DOI] [PubMed] [Google Scholar]
- Shin J-W, Lee D-J, Jung H-S, Sohn N-W. Metabolic barrel representations with various patterns of neonatal whisker deafferentation in rats. Int. J. Dev. Neurosc. 2005;23:537–544. doi: 10.1016/j.ijdevneu.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Simons DJ, Land PW. Early experience of tactile stimulation influences organization of somatic sensory cortex. Nature. 1987;326:694–697. doi: 10.1038/326694a0. [DOI] [PubMed] [Google Scholar]
- Sokoloff L, Reivich M, Kennedy C, Des Rosiers MH, Patlak CS, Pettigrew KD, Sakurada O, Shinohara M. The (14C) deoxyglucose method for the measurement of local cerebral glucose utilization: Theory, procedure, and normal values in the conscious and anesthetized albino rat. J. Neurochem. 1977;28:897–916. doi: 10.1111/j.1471-4159.1977.tb10649.x. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Landers M, Fleming J, Young T, Polan J. Characterizing the functional significance of neonatal rat vibrissae. Somatosens. Mot. Res. 2003;20:157–162. doi: 10.1080/0899022031000105190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timofeeva E, Lavallée P, Arsenault D, Deschênes M. Synthesis of multiwhisker-receptive fields in subcortical stations of the vibrissal system. J. Neurophysiol. 2004;91:1510–1515. doi: 10.1152/jn.01109.2003. [DOI] [PubMed] [Google Scholar]
- Trageser JC, Keller A. Reducing the uncertainty: Gating of peripheral inputs by zona incerta. J. Neurosci. 2004;24:8911–8915. doi: 10.1523/JNEUROSCI.3218-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welker E, Rao SB, Dörfl J, Melzer P, van der Loos H. Plasticity in the barrel cortex of the adult mouse: effects of chronic stimulation upon deoxyglucose uptake in the behaving animal. J. Neurosci. 1992;12:153–170. doi: 10.1523/JNEUROSCI.12-01-00153.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White EL, Weinfeld L, Lev DL. A survey of morphogenesis during the early postnatal period in PMBSF barrels of mouse SmI cortex with emphasis on barrel D4. Somatosens. Mot. Res. 1997;14:34–55. doi: 10.1080/08990229771204. [DOI] [PubMed] [Google Scholar]
- Williams MN, Zahm DS, Jacquin MF. Differential foci and synaptic organization of the principal and spinal trigeminal projections to the thalamus in the rat. Eur. J. Neurosci. 1994;6:29–53. doi: 10.1111/j.1460-9568.1994.tb00286.x. [DOI] [PubMed] [Google Scholar]
- Wong-Riley MTT, Merzenich MM, Leake PA. Changes in endogenous enzymatic reactivity to DAB induced by neuronal activity. Brain Res. 1978;141:185–192. doi: 10.1016/0006-8993(78)90629-7. [DOI] [PubMed] [Google Scholar]
- Woolsey TA. Peripheral alteration and somatosensory development. In: Coleman JR, editor. Development of Sensory Systems in Mammals. NY: Wiley; 1990. pp. 461–516. [Google Scholar]
- Woolsey TA, Rovainen CM, Cox SB, Henegar MH, Liang GE, Liu D, Moskalenko Y, Sui J, Wei L. Neuronal units linked to microvascular modules in cerebral cortex: response elements for imaging the brain. Cerebral Cortex. 1996;6:647–660. doi: 10.1093/cercor/6.5.647. [DOI] [PubMed] [Google Scholar]
- Woolsey TA, Van der Loos H. The structural organization of layer IV in the somatosensory region (SI) of mouse cerebral cortex. The description of a cortical field composed of discrete cytoarchitectonic units. Brain Res. 1970;17:205–242. doi: 10.1016/0006-8993(70)90079-x. [DOI] [PubMed] [Google Scholar]
- Wróbel A, Kublik E, Musiaø P. Gating of the sensory activity within barrel cortex of the awake rat. Exp. Brain. Res. 1998;123:117–123. doi: 10.1007/s002210050552. [DOI] [PubMed] [Google Scholar]
- Wu CC, Gonzalez MF. Functional development of the vibrissae somatosensory system of the rat: A (14C)2-Deoxyglucose metabolic mapping study. J. Comp. Neurol. 1997;384:323–336. doi: 10.1002/(sici)1096-9861(19970804)384:3<323::aid-cne1>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]