Abstract
Previously, we demonstrated that enhancing cholinergic activity during a working memory (WM) task improves performance and reduces blood flow in the right anterior middle/superior frontal cortex, an area known to be important for WM. The purpose of this study was to evaluate the interaction between WM task demands and cholinergic enhancement on neural responses in the prefrontal cortex. Regional cerebral blood flow (rCBF) was measured using H2 15O and positron emission tomography, as 10 young healthy volunteers performed a parametrically varied match-to-sample WM for faces task. For each item, a picture of a face was presented, followed by a delay (1, 6, 11, or 16 sec), then by the presentation of two faces. Subjects were instructed to identify which face they previously had seen. For control items, nonsense pictures were presented in the same spatial and temporal manner. All conditions were performed during an intravenous infusion of saline and physostigmine (1 mg/hr). Subjects were blind to the substance being infused. Reaction time increased significantly with WM delay, and physostigmine decreased reaction time across delay conditions. Significant task-related rCBF increases during saline infusion were seen in superior frontal, middle frontal, and inferior frontal regions, and the response magnitudes in the regions increased systematically with task difficulty. In all of these prefrontal regions, physostigmine administration significantly reduced rCBF during task, particularly at longer task delays, so that no correlation between task delay and rCBF was observed. In the ventral visual cortex, physostigmine increased rCBF at longer task delays in medial regions, and decreased rCBF over delay conditions in lateral cortical areas. These results indicate that, during cholinergic potentiation, brain activity in prefrontal regions is not modulated by increases in WM task demands, and lends further support to the hypothesis that cholinergic modulation enhances visual processing, making the task easier to perform, and thus, compensate for the need to recruit prefrontal cortical regions as task demands increase.
INTRODUCTION
Working memory (WM) denotes a cognitive process that temporarily maintains an active representation of information for further processing or recall (Baddeley, 2003; Baddeley, Logie, Bressi, Della Sala, & Spinnler, 1986). The role of the cholinergic system in WM as well as in other cognitive functions is well established, in that enhancing cholinergic activity improves performance on WM and attention tasks (Furey, Pietrini, Alexander, Mentis, et al., 2000; Furey, Pietrini, Alexander, Schapiro, & Horwitz, 2000; Furey, Pietrini, & Haxby, 2000; Furey et al., 1997; Robbins, 1997; Glasky, Melchior, Pirzadeh, Heydari, & Ritzmann, 1994; Terry, Jackson, & Buccafusco, 1993), whereas blocking normal cholinergic function impairs performance (Robbins, 1997; Dawson & Iversen, 1993; Rusted & Warburton, 1988). In the last several years, functional brain imaging studies have begun to reveal neural mechanisms associated with cholinergic effects on cognitive function in humans (Sarter, Nelson, & Bruno, 2005; Bentley, Vuilleumier, Thiel, Driver, & Dolan, 2003a; Furey, Pietrini, Alexander, Schapiro, et al., 2000; Furey, Pietrini, & Haxby, 2000; Sarter & Bruno, 2000; Furey et al., 1997; Mesulam, 1995).
In a series of studies with positron emission tomography (PET) and functional magnetic resonance imaging (fMRI), we investigated how enhancement of cholinergic activity by the anticholinesterase agent physostigmine modulates neural response to a WM task across brain regions (Freo et al., 2005; Furey, Pietrini, Alexander, Schapiro, et al., 2000; Furey, Pietrini, & Haxby, 2000; Furey et al., 1997), and showed a selective reduction in task-specific neural activity in prefrontal cortical areas known to be critical for WM function. We also demonstrated that cholinergic enhancement improved task performance, and that the magnitude of improvement correlated with the magnitude of change in neural activity in multiple WM brain regions. Specifically, improvement in task performance correlated with increased neural activity in medial visual areas associated with early perceptual processing and with decreased activity in prefrontal WM regions (Furey, Pietrini, Alexander, Schapiro, et al., 2000). An fMRI study that evaluated the influence of cholinergic modulation on each WM component (i.e., encoding, maintenance, recognition) also showed that prefrontal activity was diminished across the WM task components, and further demonstrated that increased cholinergic function was associated with selective increases in neural response to task-relevant stimuli in ventral temporal and occipital visual cortical regions, particularly during stimulus encoding (Furey, Pietrini, & Haxby, 2000). In addition to the increase in selectivity of visual processing, these earlier studies also demonstrated reduced activity in lateral visual cortical regions during WM and cholinergic enhancement (Freo et al., 2005; Furey, Pietrini, & Haxby, 2000; Furey et al., 1997) while the medial visual cortex showed relative increases in blood flow that correlated with decreases in reaction time (Furey, Pietrini, Alexander, Schapiro, et al., 2000), results that may suggest a sharpened representation of the visual stimulus through signal-to-noise processes that both increase response to signal and decrease response to noise. Together, these findings have led us to the hypothesis that cholinergic augmentation improves the efficiency of perceptual processing of task-relevant information, and thus, provides an enhanced visual percept during stimulus encoding. This, in turn, may reduce the effort required to perform the WM task and the need to recruit prefrontal cortical areas (Furey, Pietrini, & Haxby, 2000).
This hypothesis is supported also by reports which indicate that increasing WM task difficulty, such as by modulating visuoperceptual integrity (Grady et al., 1996), length of the WM retention interval (Grady et al., 1998; Barch et al., 1997; Haxby, Ungerleider, Horwitz, Rapoport, & Grady, 1995), or memory load (Braver et al., 1997), is associated with systematic increases in the magnitude of neural response in WM regions of the prefrontal cortex. A parametric task could be utilized to evaluate the influence of cholinergic modulation on neural activity in prefrontal cortical regions that respond specifically to changes in WM task demands.
In the present study, we measured neural responses while subjects performed a visual WM task whose maintenance delay duration was varied parametrically before and during pharmacological modulation of cholinergic activity using physostigmine. We modulated WM delay to more directly assess the interaction between cholinergic enhancement and neural responses to WM task demands (Barch et al., 1997). Previously, we hypothesized that cholinergic modulation reduced task difficulty by enhancing the perceptual processing of relevant visual information. In this experiment, we expected that prefrontal brain regions that respond to task difficulty by selectively increasing response as task demands increase also would show selective responses to cholinergic enhancement, whereas other prefrontal areas that do respond during task performance, but are not modulated differentially by increasing task demands, would not be modulated by cholinergic enhancement. In addition, visual processing areas in occipital and/or temporal extrastriate regions would show a reduction in the overall extent of activity and an increase in neural activity in those regions responding during the visual WM task, under cholinergic modulation.
METHODS
Ten (5 men, 5 women; mean age ± SD = 26 ± 1 years) right-handed healthy volunteers were studied. All participants underwent clinical, neurological, and psychiatric examinations and laboratory tests (including routine blood and urine tests; liver, renal, and endocrinological panels; EEG; EKG; chest X-rays; structural brain MRI) to rule out the history or presence of any relevant medical, neurological, or psychiatric disorders and use of substance that could affect brain function or metabolism. All subjects were nonsmokers and had been medication-free for at least 4 weeks prior to the study, including over-the-counter medications. Written informed consent was obtained from all participants prior to their enrolment into the study and after explanation of the study procedure and risks involved (according to protocol 93-AG-193 approved by the National Institute on Aging Intramural Review Board).
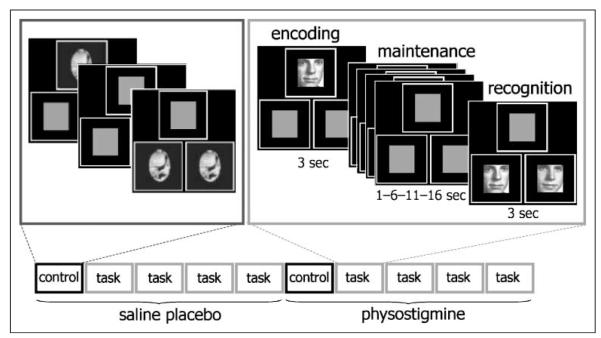
Prior to PET scanning, catheters were placed in a radial artery for drawing blood samples and in antecubital veins of both arms for injection of the isotope and for infusion of drug and/or saline solution. Absolute regional cerebral blood flow (rCBF) was measured using H2 15O and PET (Scanditronix PC2048-15B PET Scanner, Uppsala, Sweden; full width at half maximum = 6.5 mm) over ten 4-min scans during a parametrically varied visual WM task or a sensorimotor control task. For each WM item, a picture of a face was presented for 3 sec, followed by a delay of 1, 6, 11, or 16 sec, and finally, two faces were shown side-by-side for 3 sec (Figure 1). Pictures were black-and-white images of male and female faces. Target and distracter stimuli were always of the same sex. Subjects were instructed to indicate which of the two faces shown during recognition matched the face presented during encoding by pressing hand-held response buttons. For the sensorimotor control task, nonsense pictures were presented in the same spatial and temporal manner, but there was no memory component in the task. Subjects were instructed to press both response buttons following the presentation of side-by-side nonsense pictures (Figure 1). The five task conditions were presented in randomized order to each subject, first during an intravenous infusion of saline solution and, subsequently, during intravenous infusion of physostigmine (1.93 mg/hr for 10 min, followed by 0.82 mg/hr to completion of the study; Furey et al., 1997), for a total of 10 scans. Prior to the infusion of physostigmine, 0.2 mg of the peripheral cholinergic antagonist, glycopyrrolate, was administered intravenously to reduce potential cholinergic side effects (Oduro, 1975). A 30-min delay occurred following the physostigmine infusion, prior to cognitive testing. Heart rate, respiratory rate, blood pressure, and blood oxygen saturation levels were monitored continuously throughout each study. All subjects were unaware of when they would receive physostigmine infusion during the PET scan examination.
Figure 1.
Experimental paradigm. Ten 4-min PET scans were obtained using a parametrically varied visual WM task (face recognition) and a sensorimotor control task. During the match-to-sample WM task, a stimulus array composed of three equal-sized squares, one centered above two positioned side-by-side, was presented. Each trial began with the presentation of a picture of a face in the top square, followed by a delay interval that varied between 1, 6, 11, or 16 sec, followed by two faces shown in the bottom two squares. Participants were asked to indicate which of the two faces matched the face shown in the top square. The control task included the presentation of nonsense stimuli, and participants were asked to press both buttons. The five task conditions (control and 4 WM delays) were presented in randomized order for each subject, first during an intravenous placebo infusion of saline and, subsequently, during intravenous infusion of physostigmine.
PET using H2 15O produces a temporal window during which data are acquired for each scan. In this study, data were acquired for the same period of time during each task delay condition, and as a result, the number of trials per delay condition varied so that more trials were obtained in the short task delay conditions and fewer trials were obtained in the long delay conditions. As a result, as the task delay increased, the percentage of the PET acquisition period attributed to visual processing (i.e., face stimuli) decreased and the percentage of the acquisition period attributed to information maintenance (i.e., delay period) increased.
Reaction time and accuracy data were obtained for five of the subjects (the remaining performance data were not retrievable due to hardware dysfunction) and were analyzed using repeated measures analysis of variance (repeated Reaction time by Delay length by Infusion condition). Drug effects were assessed using one-tailed tests based on previously reported findings (Furey, Pietrini, & Haxby, 2000; Furey et al., 1997).
Using SPM99 (Wellcome Department of Cognitive Neurology, Institute of Neurology, University College, London; Friston et al., 1995: www.fil.ion.ucl.ac.uk/spm/), PET images were registered to correct for between-scan movements, spatially normalized to the Talairach and Tournoux (1988) brain atlas, and smoothed using a 12 × 12 × 12 mm Gaussian filter.
Brain regions showing statistically significant rCBF increases in response to the WM task during saline solution and during physostigmine infusion were determined separately by contrasting all task scans combined (1, 6, 11, and 16 sec delays) to the sensorimotor control condition (individual voxel level of p < .05, with a minimum of 50 contiguous significant voxels). These results were used to create masks that restricted the search volume in subsequent analyses, and therefore, we utilized a less conservative statistical criteria so that search volumes for drug effects were inclusive. The correction for multiple comparisons in analyses implementing the masks was determined based on the restricted search volumes.
Brain regions showing significant Task × Drug interactions were identified by contrasting rCBF during WM conditions combined over task delays (relative to the sensorimotor control task) between the drug and placebo conditions. To identify rCBF decreases during task associated with physostigmine infusion, the interaction contrast was masked by the activation map from the placebo condition. To identify rCBF increases during task associated with physostigmine, the interaction contrast was masked by the activation map from the physostigmine condition. For all interactions, statistical significance was assumed at a voxel level of p < .005 and a corrected cluster level of p ≤ .05.
To identify brain areas showing a positive or negative linear relation between rCBF response and task delay, voxel by voxel linear trends analyses were performed within the masked regions that responded to task under saline or physostigmine infusion; significance was assumed at a voxel level of p < .005 and required 25 contiguous significant voxels.
Twenty-millimeter spheres were placed on local maxima from significant interaction effects and linear trends analyses, and volume mean rCBF values were obtained. These volume means were used to display experimental effects.
RESULTS
Reaction time increased with increasing task delay (n = 5; F = 19.23, p < .001). Physostigmine significantly decreased reaction time relative to saline placebo (F = 5.4, p < .05), and this effect did not differ across task delays (Figure 2). Performance accuracy exceeded 95% correct on all delay conditions, under both placebo and physostigmine conditions. No change in performance accuracy was observed following physostigmine administration (p > .20).
Figure 2.
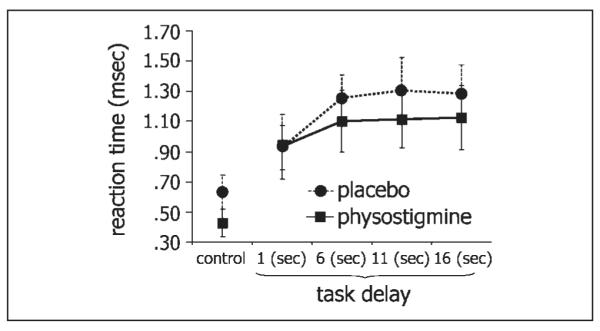
Effect of physostigmine on reaction time across task delays. Mean reaction time (±SE) is shown for the control task and for each of the WM delay conditions under placebo (dotted lines) and physostigmine (black lines). Physostigmine significantly reduced reaction time (p = .04).
Brain regions showing increases in rCBF during the WM task under placebo and drug conditions, and regions showing significant interactions in rCBF between task and drug conditions, are summarized in Table 1 and have been superimposed onto the right hemisphere of a brain template in Figure 3.
Table 1.
Cortical Regions with Significant rCBF Increases or Decreases during a Parametrically Varied Working Memory Task before and during Physostigmine Infusion
| Placebo |
Physostigmine |
Task × Drug Interactiona |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Brain Areas | BA | Hem | Z | x | y | zb | Z | x | y | z | Z | x | y | z |
| Superior frontal | 8/9 | R | 2.63 | 19 | 37 | 33 | 5.23 | 22 | 32 | 33 | ||||
| 9 | R | 2.3 | 24 | 41 | 27 | |||||||||
| 9 | R | 2.78 | 22 | 39 | 30 | |||||||||
| Anterior middle frontal | 9/46 | R | 3.26 | 22 | 43 | 13 | ||||||||
| 10/46 | R | 3.46 | 24 | 47 | 16 | 3.99 | 20 | 39 | 19 | |||||
| 45 | R | 3.01 | 20 | 37 | 9 | 4.88 | 22 | 45 | 2 | |||||
| 10/46 | L | 2.36 | −24 | 41 | 16 | |||||||||
| Inferior frontal | 8/6 | R | 2.59 | 26 | 3 | 25 | 4.07 | 33 | 3 | 25 | ||||
| 8/6 | R | 2.33 | 26 | −3 | 24 | 3.87 | 29 | −5 | 38 | |||||
| 8/6 | R | 1.97 | 36 | 3 | 32 | |||||||||
| Inferior frontal/insula | 46 | R | 2.26 | 38 | 30 | 15 | ||||||||
| 45 | R | 2.58 | 33 | 28 | 5 | 3.41 | 31 | 22 | 4 | |||||
| 45 | L | 2.28 | −40 | 12 | 14 | |||||||||
| 47/13 | L | 2.64 | −36 | 16 | 1 | |||||||||
| 45/13 | L | 2.35 | −43 | 16 | 1 | |||||||||
| Anterior cingulate | 32 | R | 2.16 | 15 | 32 | −6 | ||||||||
| 24/32 | R | 2.03 | 12 | 30 | −2 | |||||||||
| 32 | R | 2.96 | 10 | 18 | 36 | |||||||||
| 32 | R | 2.86 | 19 | 35 | 2 | |||||||||
| 32 | R | 2.35 | 6 | 28 | 1 | 2.78 | 3 | 39 | 5 | |||||
| 32 | R | 3.8 | 1 | 41 | 12 c | |||||||||
| 32 | R | 3.1 | 3 | 49 | 6 c | |||||||||
| 32 | L | 1.98 | −1 | 34 | 1 | 3.1 | −1 | 41 | 2 c | |||||
| Insula | 13 | R | 3.36 | 31 | 14 | 18 | 3.60 | 31 | 18 | 11 | ||||
| 13 | R | 3.58 | 38 | 24 | 19 | |||||||||
| Hippocampus | R | 2.15 | 27 | −36 | 5 | |||||||||
| Temporal | 27 | R | 2.6 | 17 | −36 | 1 | 2.06 | 15 | −30 | −5 | ||||
| 27 | R | 2.04 | 12 | −29 | −12 | |||||||||
| 23/31 | R | 4.48 | 22 | −50 | 25 | |||||||||
| 31 | R | 3.71 | 24 | −60 | 21 | |||||||||
| 31/19 | R | 3.59 | 29 | −65 | 21 | |||||||||
| 39/19 | R | 1.84 | 26 | −52 | −14 | |||||||||
| 39/19 | R | 2.59 | 20 | −42 | −20 | |||||||||
| 28 | L | 2.01 | −22 | −13 | −15 | |||||||||
| Occipital | 18 | R | 5.41 | 36 | −71 | −18 | 3.21 | 38 | −71 | −18 | 4.21 | 34 | −83 | −8 |
| 18 | R | 1.84 | 26 | −65 | −18 | |||||||||
| 18 | R | 2.48 | 1 | −71 | −4 | |||||||||
| 18 | R | 2.70 | 15 | − 71 | − 7 c | |||||||||
| 19 | R | 3.91 | 22 | −71 | 17 | 4.26 | 20 | −79 | −1 | 4.14 | 34 | −56 | 0 | |
| 19 | R | 3 | 17 | −85 | −1 | 4.13 | 13 | −67 | 0 | 3.90 | 45 | −58 | −21 | |
| 19 | R | 1.92 | 3 | −60 | −3 | |||||||||
| 19 | R | 2.09 | 17 | −46 | −10 | |||||||||
| 18 | L | 4.01 | − 15 | − 67 | − 7 | |||||||||
| 18 | L | 3.29 | −32 | −71 | −18 | 3.65 | −34 | −87 | −5 | |||||
| 18 | L | 3.23 | −34 | −83 | −15 | 3.37 | −38 | −75 | −18 | |||||
| 19 | L | 3.64 | −29 | −89 | 2 | 4.45 | −20 | −79 | 10 | 4.25 | −31 | −91 | −12 | |
| Basal ganglia | R | 3.36 | 10 | −21 | 2 | 1.83 | 3 | −30 | −2 | |||||
| R | 3.28 | 12 | 1 | −7 | ||||||||||
| R | 3.17 | 12 | 8 | −3 | ||||||||||
| L | 2.45 | −31 | −15 | −8 | ||||||||||
Search volumes for analyses of interaction effects were restricted by the masks that identified brain regions responding to task during placebo and during drug. Statistical significance was assumed at an individual voxel level of p < .005 and a corrected cluster level of p < .05. Loci shown in BOLD indicate regions with increases in rCBF during physostigmine. All other loci represent reductions in rCBF during physostigmine.
Talairach and Tournoux (1988) brain atlas coordinates: x = distance in millimeters to the right (+) or to the left (−) of the midline; y = distance anterior (+) or posterior (−) to the anterior commissure; z = distance superior (+) or inferior (−) to a horizontal plane through the anterior and posterior commissures.
Region significant at the voxel level of p < .05, uncorrected for the cluster.
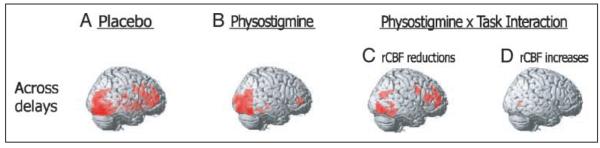
Figure 3.
Effect of physostigmine on brain activity during WM. Brain regions showing significant increases in rCBF during a parametrically varied visual WM task as compared to a sensorimotor control condition during placebo (A) and physostigmine (B) infusions are projected onto the right hemisphere of a brain template and displayed in the top row. The extent to which these effects are expressed in each of the delay conditions is displayed in each of the columns below. The Task by Drug interactions are displayed with significant rCBF decreases (C) and increases (D) shown separately. For the placebo and physostigmine conditions, the overlays are thresholded as described for the comparable overall analysis. The overlays for the interaction conditions are the result of a subtraction.
Under placebo conditions, increases in task-related rCBF were observed bilaterally throughout the prefrontal cortex with loci in inferior frontal and anterior middle frontal cortical areas, as well as in the insular cortex, and in occipital and temporal visual extrastriate regions. Increases were observed unilaterally in right superior frontal and anterior cingulate cortical regions (Table 1 and Figure 3A).
In the presence of physostigmine, task-specific rCBF increases were observed bilaterally in occipital and right temporal visual extrastriate regions, and in the right anterior cingulate, with no additional activations in prefrontal regions (Table 1 and Figure 3B). Note the paucity of prefrontal activation during physostigmine in Figure 3B. The Drug × Task interaction (Table 1 and Figure 3C) identified significantly less rCBF during physostigmine and task in the right superior frontal, anterior middle frontal, insular, and inferior frontal cortex, as well as in bilateral occipital and right temporal visual extrastriate areas. Significantly greater rCBF during drug relative to placebo was observed in the left medial visual cortical regions. Increases observed in the right medial visual cortex were seen only with less strict statistical criteria (voxel level, p < .05; see Table 1) (Figure 3D). A trend (p = .07) toward significantly greater rCBF during drug relative to placebo was observed in the right anterior cingulate cortex (Table 1).
The results of the linear trends analysis are displayed in Figure 4 and are reported in Table 2. During placebo, rCBF showed a positive linear trend with increased task delay (i.e., rCBF increased as task delay increased) in right anterior middle and inferior frontal cortical regions (Figure 4), and showed a negative linear trend with increased task delay (i.e., rCBF decreased as task delay increased) in occipito-temporal visual extrastriate regions.
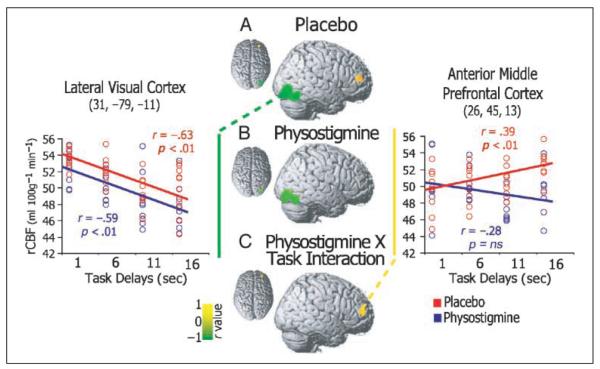
Figure 4.
Brain areas showing correlations between rCBF response and task difficulty. Regions showing significant positive (yellow) and negative (green) correlations between rCBF and task delay conditions have been superimposed on the right hemisphere of a brain template for placebo (top), physostigmine (middle), and the Task × Drug interaction (bottom). Example scatterplots with fitted regression lines as observed during placebo (red) and physostigmine (blue) are shown for a representative lateral visual cortical region (left plot) and an anterior middle frontal cortical locus (right plot).
Table 2.
Cortical Regions Showing Positive or Negative Correlations between rCBF Response and Task Difficulty before and during Physostigmine Infusion
| Placeboa |
Physostigmine |
Task × Drug Interaction |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Brain Areas | BA | Hem | Z Score | x | y | zb | Z Score | x | y | z | Z Score | x | y | z |
| Positive Correlation | ||||||||||||||
| Anterior middle frontal | 9/46 | R | 2.97 | 26 | 45 | 13 | 3.45 | 26 | 45 | 13 | ||||
| Inferior frontal | 45 | R | 2.89 | 33 | 28 | 5 | ||||||||
| Cingulate | 32 | R | 2.83 | 19 | 37 | 2 | 2.17 | 20 | 32 | −2 | ||||
| 34 | R | 2.1 | 19 | 4 | −11 | |||||||||
| Basal ganglia | R | 2.59 | 8 | 1 | 3 | 2.9 | 13 | −3 | 3 | |||||
| R | 2.13 | 6 | 10 | 4 | 1.91 | 17 | 4 | −4 | ||||||
| R | 2.05 | 8 | 16 | −3 | ||||||||||
| Negative Correlation | ||||||||||||||
| Occipital | 18 | R | 4.65 | 31 | −79 | −11 | 4.59 | 31 | −69 | −21 | ||||
| R | 4.01 | 41 | −63 | −18 | 3.77 | 31 | −83 | −15 | ||||||
| R | 2.93 | 13 | −87 | −22 | 3.74 | 26 | −85 | −22 | ||||||
| 18 | L | 2.87 | −34 | −85 | −19 | 2.93 | −27 | −71 | −18 | |||||
| 18 | L | 2.58 | −25 | −67 | −14 | 2.8 | −40 | −71 | −18 | |||||
| 19 | L | 2.18 | −29 | −91 | −16 | 2.63 | −25 | −85 | 2 | |||||
Search volumes for correlation analyses were restricted by the masks that identified brain regions responding to task during placebo and during drug. Statistical significance was assumed at an individual voxel level of p < .005 and required 50 contiguous significant voxels.
Talairach and Tournoux (1988) brain atlas coordinates: x = distance in millimeters to the right (+) or to the left (−) of the midline; y = distance anterior (+) or posterior (−) to the anterior commissure; z = distance superior (+) or inferior (−) to a horizontal plane through the anterior and posterior commissures.
During physostigmine infusion, rCBF in occipitotemporal areas still showed a significant linear trend with task delay, and the magnitude of the correlation did not differ from placebo (Figure 4A). In contrast, no significant linear trend was observed in the prefrontal cortex (Figure 4B) during physostigmine infusion. The Drug by Task interaction of the linear model indicates that the differences in linear trends observed during placebo and drug were significant in inferior and middle frontal cortical areas (Figure 4C).
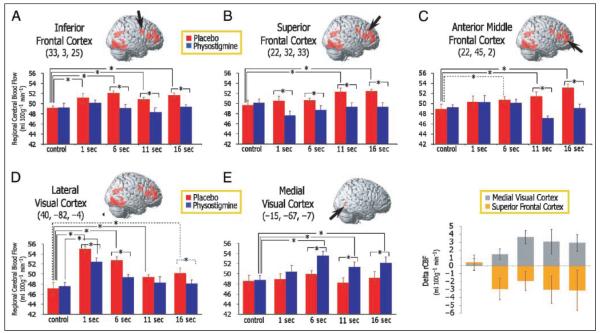
Mean volumes (20 mm) centered around local maxima that identified peak differences between drug and placebo conditions in the overall Drug by Task interaction analysis were obtained for selected loci and used for illustrative purposes to reveal patterns of drug effects on rCBF. Bar graphs in Figure 5 show mean (±SE) rCBF during the control task and across task delay conditions as measured during placebo and physostigmine. Individual t tests were used to explain the overall effect in each region, and asterisks are placed in the figures to identify differences observed between placebo and drug conditions, or between task conditions within an infusion condition. In areas of the inferior frontal cortex (Figure 5A), rCBF increased similarly during each task delay relative to the sensorimotor control task under placebo, (i.e., no linear trend between rCBF and task delay; also see Figure 4) and showed reductions during physostigmine infusion that were selective to the WM delay conditions (nonsignificant to the 1-sec delay) with no change in the rCBF response to the control task. In the superior frontal cortex (Figure 5B), rCBF increases showed a significant linear trend with task delay during placebo (the rCBF response in the voxel in Figure 5B showed a linear trend, but the extent of the correlation includes only 17 voxels, and therefore, does not appear in Figure 4). In direct t-test comparisons between the rCBF response during the control task and each of the WM delay conditions, only rCBF increases observed during the long delay conditions (11 and 16 sec) were significant during placebo (Figure 5B). Significantly lower rCBF was seen during drug relative to the placebo condition for each WM delay conditions, with no change during the control task. A third pattern was observed in the anterior middle frontal cortex (Figure 5C), where rCBF increases during placebo correlated with task delay (Figure 4), and reductions in rCBF during physostigmine were restricted to the longer task delays (11 and 16 sec). As in the superior frontal cortex, rCBF increases during individual delays were significantly greater than rCBF response to the control task only during the longer task delays under placebo conditions (Figure 5C).
Figure 5.
Effect of physostigmine on volume mean rCBF across task delays. Volumes of 20 mm were averaged around loci identified in the Task × Drug interaction, and mean rCBF (±SE) values are plotted for the control task and each WM delay as obtained during placebo (red) and physostigmine (blue). Representative volumes are shown from the inferior frontal cortex (A), the superior frontal cortex (B), the anterior middle frontal cortex (C), the lateral visual extrastriate cortex (D), and the medial visual cortex (F). Results of t tests performed to characterize significant Task × Drug interactions are indicated with asterisks overlying lines that indicate the comparisons; asterisks with solid lines are significant at p < .05 and asterisks with dashed lines represent trends at p < .10.
Mean rCBF from lateral visual extrastriate regions for each of the task delay conditions is shown in Figure 5D. Significant rCBF increases were seen relative to the control task under placebo conditions, and a negative linear trend was observed between these increases and the task delay conditions. Reductions in rCBF were observed under physostigmine infusion selectively during the task conditions (i.e., not during the control task), but the linear trend between rCBF and task delay remained unchanged (Figures 4 and 5D). In contrast, areas of medial visual regions (Table 1 and Figure 3D) that were not activated during task under placebo showed significant increases in rCBF under physostigmine infusion, and these increases were similar across task delays (Figure 5E). Figure 5F shows physostigmine-induced rCBF increases in the medial visual cortex and rCBF reductions in the superior frontal imposed on the same axis to highlight the extent to which changes in the activity in these regions mirror each other. Again, note the absence of an effect during the control task for both brain regions.
DISCUSSION
The results of this study support the hypothesis that augmentation of cholinergic activity reduces task demands, as evidenced by both the reduction in prefrontal cortical activity in regions that respond differentially to task demands and to the improvement in task performance. The modulation of activity in visual processing regions, which included both a reduction in activity in lateral areas (although these regions are still responding during the task) and an increase in activity in medial visual areas, may enhance visual processing to result in an improved visual percept that renders the task easier and diminishes the need to recruit the prefrontal cortex to perform the visual WM task.
Under placebo conditions, anterior middle and superior frontal cortical regions showed linear increases in activity as WM delay increased, suggesting that these regions respond to changes in WM task demands. Both superior frontal and anterior middle frontal areas showed diminished neural activity during cholinergic enhancement and no longer showed modulation in activity in association with changing task demands, consistent with the hypothesis that cholinergic enhancement reduces task difficulty.
The improvement in task performance also is consistent with the interpretation that the WM task became easier to perform during cholinergic enhancement, and provides strong support to our interpretation that changes in neural activity reflect enhanced neural processing. Despite the fact that we have performance data on only half of the subjects, this effect of physostigmine on reaction time is consistent with effects that we reported previously using physostigmine and this face WM task without variation in the delay conditions (Freo et al., 2005; Furey, Pietrini, Alexander, Schapiro, et al., 2000; Furey, Pietrini, & Haxby, 2000; Furey et al., 1997); others have reported using other tasks (Bentley, Husain, & Dolan, 2004; Bentley, Vuilleumier, Thiel, Driver, & Dolan, 2003b). Thus, the behavioral finding primarily represents a replication of previously reported effects of cholinergic enhancement. In the current dataset, we also see evidence of a reduction in reaction time during the sensorimotor control condition. Previously, we found that cholinergic enhancement selectively modulated performance during task conditions with no effect on the control condition with sample sizes larger than we have in the current reaction time analysis (Furey, Pietrini, Haxby, & Drevets, 2007; Furey, Pietrini, & Haxby, 2000). The effect on the control condition observed in the current study should be considered within the larger literature.
Different prefrontal brain regions showed different patterns of response to cholinergic potentiation. The anterior middle frontal cortex showed systematic increases in neural response as task difficulty increased under placebo conditions, and in direct comparisons, only longer delays were associated with significant increases in activity over the control task. This region, which responded systematically to task demand in the absence of drug, consistently has been associated with WM maintenance processes (D’Esposito, Postle, & Rypma, 2000; D’Esposito, Postle, Ballard, & Lease, 1999). Physostigmine infusion had no effect on response in this region to the control task or to shorter task delays, but did reduce neural response selectively to the longer delay conditions when the maintenance conditions were more demanding.
The superior frontal cortex also showed increased neural activity as task demands increased during placebo, and again only longer delays were associated with significant increases in activity in direct comparisons. However, in this region, we observed significant reductions in neural activity at each of the task delays during physostigmine administration, including the shorter delay conditions. Previous WM functional studies demonstrated that more dorsally located prefrontal regions subserve the manipulation and retrieval components of WM more than the maintenance of information (D’Esposito et al., 1999, 2000). As our WM paradigm has no manipulation requirement, this region likely is contributing to the retrieval aspects of our task. The reduction in neural activity observed during each of the delay conditions suggests that the retrieval of information may also be less demanding following cholinergic enhancement, reducing the need to recruit this prefrontal area. Moreover, requirements for the retrieval of information are similar across delay conditions, and therefore, we would expect that the influence of cholinergic enhancement also would be similar over delays, consistent with our observations in this area.
The inferior frontal region showed a third pattern of response to drug where increased neural activity was observed to each of the task delay conditions during placebo, but the magnitude of response did not vary with delay, suggesting that this region responds to WM requirements during the variable delays, but it is not affected directly by task demands. During cholinergic enhancement, activity in this region decreased similarly across delay conditions. Thus, prefrontal brain regions recruited during WM that are delay independent but, nonetheless, important to WM function, also are modulated by cholinergic enhancement.
Areas of the medial visual cortex selectively increased neural activity during physostigmine infusion in response to the WM task, with no change in response to the control task, whereas the more lateral regions of visual processing areas had reduced activity. As cholinergic function is associated with signal-to-noise processing (Everitt & Robbins, 1997; Murphy & Sillito, 1991; Sato, Hata, Masui, & Tsumoto, 1987; Sillito & Kemp, 1983), both the increased activity observed in medial visual areas, together with the decreased activity in lateral visual processing areas, would be consistent with a cholinergically mediated enhancement of perceptual processing. Lateral ventral extrastriate regions showed negative correlations between WM task delay and neural activity under placebo, consistent with the inherent reduction in stimulus input over time as WM delay increases. Although the overall magnitude of response decreased in lateral visual cortical areas during physostigmine, this region continued to respond during the task and the negative correlation was uninfluenced by drug. This reduction in activity is consistent with our previous findings (Freo et al., 2005; Furey, Pietrini, & Haxby, 2000; Furey et al., 1997), and likely reflects signal-to-noise modulation (Furey, Pietrini, & Haxby, 2000) (see below). In contrast, medial visual cortical regions that were not recruited differentially relative to the control task during placebo showed significant increases in neural activity during enhancement of cholinergic activity selectively during WM task conditions. This is one of two cortical regions that showed increased activity in response to cholinergic enhancement. This finding is consistent with our previous results demonstrating a negative correlation between physostigmine-induced decreases in task reaction time and increases in rCBF in early visual processing regions (Furey, Pietrini, Alexander, Schapiro, et al., 2000). Others also have observed task-specific increases in activity in visual extrastriate areas following cholinergic enhancement (Kumari, Aasen, Ffytche, Williams, & Sharma, 2006; Rombouts, Barkhof, Van Meel, & Scheltens, 2002). Bentley et al. (2004) showed that during a spatial WM task, physostigmine reduces activity in the inferior prefrontal cortex, as well as reduced activity in the primary visual cortex and increased activity in extrastriate visual regions. Other studies similarly have observed both increases and decreases in neural activity in different visual processing areas during cholinergic enhancement and cognitive tasks (Freo et al., 2005; Bentley et al., 2003a; Furey, Pietrini, Alexander, Schapiro, et al., 2000). Such findings similarly have been discussed in terms of signal-to-noise processing effects of acetylcholine (Bentley et al., 2004; Furey, Pietrini, & Haxby, 2000).
The selectivity of the effects reported by our study should be highlighted. Previously, we demonstrated that physostigmine-induced reductions in right prefrontal cortical activity were highly selective to the task condition, when compared to a resting state rather than a taskspecific control condition (Furey et al., 1997). The earlier result was critical to demonstrate that physostigmine did not alter blood flow at rest while modulating neural activity during the task. Here we have demonstrated that the cholinergically mediated rCBF changes in prefrontal cortical regions and in visual extrastriate processing areas are selective to task conditions, with no effect on neural activity during a sensorimotor control task that had no WM component.
The mechanism by which acetylcholine modulates neural activity is as yet unclear. The increased activity in the visual cortex may occur via top–down attentional influences of prefrontal regions that continue to respond under conditions of increased cholinergic function during the WM task, such as the anterior cingulate cortex. Alternatively, increased activity in the visual cortex may occur through direct effects of cholinergic activity on cortical stimulus processing. Evidence suggests that acetylcholine may modulate neural responses through signal-to-noise mechanisms (Hasselmo, 1995; Murphy & Sillito, 1991; Sato et al., 1987). Investigators have demonstrated that the direct application of acetylcholine to the visual cortex enhances and sharpens the selectivity of neural responses to specific stimulus features, including stimulus direction and orientation (i.e., increased responses to the preferred features and reduced responses to the less preferred features). These findings indicate that, in the absence of cholinergic modulation of the prefrontal cortex, increasing cholinergic activity enhances neural responses during the processing of visual stimuli. In the current context, increased cholinergic activity may improve the perceptual representation of the encoded stimulus by boosting signal-to-noise, either by increasing responses to signal or by reducing responses to noise. The enhanced perceptual representation then renders the task easier, across delay conditions, and reduces the need for prefrontal contributions when performing WM.
An alternative explanation of our findings might be that physostigmine has direct effects on the prefrontal cortex to inhibit activity, and the medial visual cortex is recruited further to compensate for this effect. One argument against this alternative comes from our previous work (Furey et al., 1997) demonstrating that, during a rest condition, no change in neural activity is observed in the prefrontal cortex during increased cholinergic function with physostigmine, but rather task-specific decreases exclusively were observed.
An attractive alternative explanation for the reductions in neural activity seen in prefrontal WM regions, as well as in lateral visual processing areas, is the possibility that increased cholinergic function may enhance neural efficiency. A growing literature has demonstrated that increased neural efficiency is associated with reduced overall neural activity, resulting in increased focality of neural response (Rypma et al., 2006; Sayala, Sala, & Courtney, 2006; Rypma, Berger, Genova, Rebbechi, & D’Esposito, 2005). This alternative would argue that the entire WM network requires less activity due to overall improved efficiency. Although this interpretation cannot be ruled out, the increase in neural activity observed in medial visual cortical areas during cholinergic enhancement, that were not recruited prior to drug, would be difficult to explain. Alternatively, we are arguing that the WM system is working more efficiently, requiring less input from prefrontal brain regions and lateral visual regions as a result of an enhanced representation of the visual information in the medial visual cortex.
Prefrontal brain regions that showed increased neural response as a function of WM load had reduced activity following the administration of scopolamine, a cholinergic antagonist (Bullmore et al., 2003), although one might expect, based on our findings, that the prefrontal cortex would increase responses to task during scopolamine. This also may be explained via signal-to-noise mechanisms, whereby regions responsive to load showed diminished selectivity in response, resulting in increased noise, producing an overall reduction in measured signal. In this case, however, we would expect to see some impairment in behavioral performance consistent with impaired processing, but no effect on behavior was observed (Bullmore et al., 2003). This discrepancy also may be due simply to the different tasks used in each of these studies.
Researchers have shown that prefrontal regions that are implicated in cognitive task performance also are involved in motor response preparation, at least for some tasks (Schumacher, Cole, & D’Esposito, 2007). As a result, we should note that the cholinergically mediated reduction in reaction time may affect activity in prefrontal regions as a result of associated changes in motor response preparation.
One might question the experimental design where the drug infusion always followed the placebo infusion. Previously, we showed that task-associated rCBF response magnitude did not vary over repetitions of a WM task in prefrontal brain regions, either during physostigmine or during placebo (Furey, Pietrini, Alexander, Mentis, et al., 2000). Although the benefits of randomization will not be argued, we designed the study with the knowledge that task repetition per se would not alter the rCBF measurement. The alternative in a within-subjects design would be to randomize the infusion conditions over two separate occasions, but the invasiveness of the arterial line renders this option unfavorable.
In conclusion, the results reported here extend our previous findings by demonstrating that augmentation of cholinergic system activity during a visual WM for faces task reduces WM demands, and thus the task becomes easier, diminishing the need for prefrontal involvement. As in previous studies, we observed modulation in visual processing areas during physostigmine infusion that is consistent with enhanced processing of visual input. The absence of modulation in neural response in prefrontal WM regions as task demands change is consistent with the hypothesis that improved perceptual representations of stimuli reduces task difficulty and the need to recruit prefrontal areas.
Acknowledgments
This work was supported by the National Institute on Aging intramural program, and in part by grants from the I.R.I.S. Foundation (Livorno, Italy).
We thank Peter Herscovitch and the technologists of the NIH positron emission tomography program, and Joanna Szczepanik and Richard Desmond for technical support.
REFERENCES
- Baddeley A. Working memory and language: An overview. Journal of Communication Disorders. 2003;36:189–208. doi: 10.1016/s0021-9924(03)00019-4. [DOI] [PubMed] [Google Scholar]
- Baddeley A, Logie R, Bressi S, Della Sala S, Spinnler H. Dementia and working memory. Quarterly Journal of Experimental Psychology A. 1986;38:603–618. doi: 10.1080/14640748608401616. [DOI] [PubMed] [Google Scholar]
- Barch DM, Braver TS, Nystrom LE, Forman SD, Noll DC, Cohen JD. Dissociating working memory from task difficulty in human prefrontal cortex. Neuropsychologia. 1997;35:1373–1380. doi: 10.1016/s0028-3932(97)00072-9. [DOI] [PubMed] [Google Scholar]
- Bentley P, Husain M, Dolan RJ. Effects of cholinergic enhancement on visual stimulation, spatial attention, and spatial working memory. Neuron. 2004;41:969–982. doi: 10.1016/s0896-6273(04)00145-x. [DOI] [PubMed] [Google Scholar]
- Bentley P, Vuilleumier P, Thiel CM, Driver J, Dolan RJ. Cholinergic enhancement modulates neural correlates of selective attention and emotional processing. Neuroimage. 2003a;20:58–70. doi: 10.1016/s1053-8119(03)00302-1. [DOI] [PubMed] [Google Scholar]
- Bentley P, Vuilleumier P, Thiel CM, Driver J, Dolan RJ. Effects of attention and emotion on repetition priming and their modulation by cholinergic enhancement. Journal of Neurophysiology. 2003b;90:1171–1181. doi: 10.1152/jn.00776.2002. [DOI] [PubMed] [Google Scholar]
- Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, Noll DC. A parametric study of prefrontal cortex involvement in human working memory. Neuroimage. 1997;5:49–62. doi: 10.1006/nimg.1996.0247. [DOI] [PubMed] [Google Scholar]
- Bullmore E, Suckling J, Zelaya F, Long C, Honey G, Reed L, et al. Practice and difficulty evoke anatomically and pharmacologically dissociable brain activation dynamics. Cerebral Cortex. 2003;13:144–154. doi: 10.1093/cercor/13.2.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Esposito M, Postle BR, Ballard D, Lease J. Maintenance versus manipulation of information held in working memory: An event-related fMRI study. Brain and Cognition. 1999;41:66–86. doi: 10.1006/brcg.1999.1096. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Postle BR, Rypma B. Prefrontal cortical contributions to working memory: Evidence from event-related fMRI studies. Experimental Brain Research. 2000;133:3–11. doi: 10.1007/s002210000395. [DOI] [PubMed] [Google Scholar]
- Dawson GR, Iversen SD. The effects of novel cholinesterase inhibitors and selective muscarinic receptor agonists in tests of reference and working memory. Behavioural Brain Research. 1993;57:143–153. doi: 10.1016/0166-4328(93)90130-i. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Central cholinergic systems and cognition. Annual Review of Psychology. 1997;48:649–684. doi: 10.1146/annurev.psych.48.1.649. [DOI] [PubMed] [Google Scholar]
- Freo U, Ricciardi E, Pietrini P, Schapiro MB, Rapoport SI, Furey ML. Pharmacological modulation of prefrontal cortical activity during a working memory task in young and older humans: A PET study with physostigmine. American Journal of Psychiatry. 2005;162:2061–2070. doi: 10.1176/appi.ajp.162.11.2061. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline J-B, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: A general linear approach. Human Brain Mapping. 1995;2:189–210. [Google Scholar]
- Furey ML, Pietrini P, Alexander GE, Mentis MJ, Szczepanik J, Shetty U, et al. Time course of pharmacodynamic and pharmacokinetic effects of physostigmine assessed by functional brain imaging in humans. Pharmacology, Biochemistry and Behavior. 2000;66:475–481. doi: 10.1016/s0091-3057(00)00186-6. [DOI] [PubMed] [Google Scholar]
- Furey ML, Pietrini P, Alexander GE, Schapiro MB, Horwitz B. Cholinergic enhancement improves performance on working memory by modulating the functional activity in distinct brain regions: A positron emission tomography regional cerebral blood flow study in healthy humans. Brain Research Bulletin. 2000;51:213–218. doi: 10.1016/s0361-9230(99)00219-1. [DOI] [PubMed] [Google Scholar]
- Furey ML, Pietrini P, Haxby JV. Cholinergic enhancement and increased selectivity of perceptual processing during working memory. Science. 2000;290:2315–2319. doi: 10.1126/science.290.5500.2315. [DOI] [PubMed] [Google Scholar]
- Furey ML, Pietrini P, Haxby JV, Alexander GE, Lee HC, VanMeter J, et al. Cholinergic stimulation alters performance and task-specific regional cerebral blood flow during working memory. Proceedings of the National Academy of Sciences, U.S.A. 1997;94:6512–6516. doi: 10.1073/pnas.94.12.6512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furey ML, Pietrini P, Haxby JV, Drevets WC. Selective effects of cholinergic modulation on task performance during selective attention. Neuropsychopharmacology. 2007;33:913–923. doi: 10.1038/sj.npp.1301461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasky AJ, Melchior CL, Pirzadeh B, Heydari N, Ritzmann RF. Effect of AIT-082, a purine analog, on working memory in normal and aged mice. Pharmacology, Biochemistry and Behavior. 1994;47:325–329. doi: 10.1016/0091-3057(94)90017-5. [DOI] [PubMed] [Google Scholar]
- Grady CL, Horwitz B, Pietrini P, Mentis MJ, Ungerleider LG, Rapoport SI, et al. Effect of task difficulty on cerebral blood flow during perceptual matching of faces. Human Brain Mapping. 1996;4:227–239. doi: 10.1002/(SICI)1097-0193(1996)4:4<227::AID-HBM1>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Bookstein F, Horwitz B, Rapoport SI, Haxby JV. Age-related changes in regional cerebral blood flow during working memory for faces. Neuroimage. 1998;8:409–425. doi: 10.1006/nimg.1998.0376. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME. Neuromodulation and cortical function: Modeling the physiological basis of behavior. Behavioural Brain Research. 1995;67:1–27. doi: 10.1016/0166-4328(94)00113-t. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Ungerleider LG, Horwitz B, Rapoport SI, Grady CL. Hemispheric differences in neural systems for face working memory: A PET–rCBF study. Human Brain Mapping. 1995;3:68–82. [Google Scholar]
- Kumari V, Aasen I, Ffytche D, Williams SC, Sharma T. Neural correlates of adjunctive rivastigmine treatment to antipsychotics in schizophrenia: A randomized, placebo-controlled, double-blind fMRI study. Neuroimage. 2006;29:545–556. doi: 10.1016/j.neuroimage.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Cholinergic pathways and the ascending reticular activating system of the human brain. Annals of the New York Academy of Sciences. 1995;757:169–179. doi: 10.1111/j.1749-6632.1995.tb17472.x. [DOI] [PubMed] [Google Scholar]
- Murphy PC, Sillito AM. Cholinergic enhancement of direction selectivity in the visual cortex of the cat. Neuroscience. 1991;40:13–20. doi: 10.1016/0306-4522(91)90170-s. [DOI] [PubMed] [Google Scholar]
- Oduro KA. Glycopyrrolate methobromide: 2. Comparison with atropine sulphate in anaesthesia. Canadian Anaesthetists’ Society Journal. 1975;22:466–473. doi: 10.1007/BF03004861. [DOI] [PubMed] [Google Scholar]
- Robbins TW. Arousal systems and attentional processes. Biological Psychology. 1997;45:57–71. doi: 10.1016/s0301-0511(96)05222-2. [DOI] [PubMed] [Google Scholar]
- Rombouts SA, Barkhof F, Van Meel CS, Scheltens P. Alterations in brain activation during cholinergic enhancement with rivastigmine in Alzheimer’s disease. Journal of Neurology, Neurosurgery and Psychiatry. 2002;73:665–671. doi: 10.1136/jnnp.73.6.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusted JM, Warburton DM. The effects of scopolamine on working memory in healthy young volunteers. Psychopharmacology (Berlin) 1988;96:145–152. doi: 10.1007/BF00177553. [DOI] [PubMed] [Google Scholar]
- Rypma B, Berger JS, Genova HM, Rebbechi D, D’Esposito M. Dissociating age-related changes in cognitive strategy and neural efficiency using event-related fMRI. Cortex. 2005;41:582–594. doi: 10.1016/s0010-9452(08)70198-9. [DOI] [PubMed] [Google Scholar]
- Rypma B, Berger JS, Prabhakaran V, Bly BM, Kimberg DY, Biswal BB, et al. Neural correlates of cognitive efficiency. Neuroimage. 2006;33:969–979. doi: 10.1016/j.neuroimage.2006.05.065. [DOI] [PubMed] [Google Scholar]
- Sarter M, Bruno JP. Cortical cholinergic inputs mediating arousal, attentional processing and dreaming: Differential afferent regulation of the basal forebrain by telencephalic and brainstem afferents. Neuroscience. 2000;95:933–952. doi: 10.1016/s0306-4522(99)00487-x. [DOI] [PubMed] [Google Scholar]
- Sarter M, Nelson CL, Bruno JP. Cortical cholinergic transmission and cortical information processing in schizophrenia. Schizophrenia Bulletin. 2005;31:117–138. doi: 10.1093/schbul/sbi006. [DOI] [PubMed] [Google Scholar]
- Sato H, Hata Y, Masui H, Tsumoto T. A functional role of cholinergic innervation to neurons in the cat visual cortex. Journal of Neurophysiology. 1987;58:765–780. doi: 10.1152/jn.1987.58.4.765. [DOI] [PubMed] [Google Scholar]
- Sayala S, Sala JB, Courtney SM. Increased neural efficiency with repeated performance of a working memory task is information-type dependent. Cerebral Cortex. 2006;16:609–617. doi: 10.1093/cercor/bhj007. [DOI] [PubMed] [Google Scholar]
- Schumacher EH, Cole MW, D’Esposito M. Selection and maintenance of stimulus–response rules during preparation and performance of a spatial choice-reaction task. Brain Research. 2007;1136:77–87. doi: 10.1016/j.brainres.2006.11.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillito AM, Kemp JA. Cholinergic modulation of the functional organization of the cat visual cortex. Brain Research. 1983;289:143–155. doi: 10.1016/0006-8993(83)90015-x. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Thieme; New York: 1988. [Google Scholar]
- Terry AV, Jr., Jackson WJ, Buccafusco JJ. Effects of concomitant cholinergic and adrenergic stimulation on learning and memory performance by young and aged monkeys. Cerebral Cortex. 1993;3:304–312. doi: 10.1093/cercor/3.4.304. [DOI] [PubMed] [Google Scholar]