Summary
Background
Control of tuberculosis in settings with high HIV prevalence is a pressing public health priority. We tested two active case-finding strategies to target long periods of infectiousness before diagnosis, which is typical of HIV-negative tuberculosis and is a key driver of transmission.
Methods
Clusters of neighbourhoods in the high-density residential suburbs of Harare, Zimbabwe, were randomised to receive six rounds of active case finding at 6-monthly intervals by either mobile van or door-to-door visits. Randomisation was done by selection of discs of two colours from an opaque bag, with one disc to represent every cluster, and one colour allocated to each intervention group before selection began. In both groups, adult (≥16 years) residents volunteering chronic cough (≥2 weeks) had two sputum specimens collected for fluorescence microscopy. Community health workers and cluster residents were not masked to intervention allocation, but investigators and laboratory staff were masked to allocation until final analysis. The primary outcome was the cumulative yield of smear-positive tuberculosis per 1000 adult residents, compared between intervention groups; analysis was by intention to treat. The secondary outcome was change in prevalence of culture-positive tuberculosis from before intervention to before round six of intervention in 12% of randomly selected households from the two intervention groups combined; analysis was based on participants who provided sputum in the two prevalence surveys. This trial is registered, number ISRCTN84352452.
Findings
46 study clusters were identified and randomly allocated equally between intervention groups, with 55 741 adults in the mobile van group and 54 691 in the door-to-door group at baseline. HIV prevalence was 21% (1916/9060) and in the 6 months before intervention the smear-positive case notification rate was 2·8 per 1000 adults per year. The trial was completed as planned with no adverse events. The mobile van detected 255 smear-positive patients from 5466 participants submitting sputum compared with 137 of 4711 participants identified through door-to-door visits (adjusted risk ratio 1·48, 95% CI 1·11–1·96, p=0·0087). The overall prevalence of culture-positive tuberculosis declined from 6·5 per 1000 adults (95% CI 5·1–8·3) to 3·7 per 1000 adults (2·6–5·0; adjusted risk ratio 0·59, 95% CI 0·40–0·89, p=0·0112).
Interpretation
Wide implementation of active case finding, particularly with a mobile van approach, could have rapid effects on tuberculosis transmission and disease.
Funding
Wellcome Trust.
Introduction
Africa has been in the grip of a severe epidemic of tuberculosis since the onset of the HIV epidemic: the region includes all but one of 15 countries with the highest tuberculosis incidences and accounts for 79% of the global burden of 1·4 million cases of HIV-related tuberculosis.1 Unlike industrialised countries, most tuberculosis disease in endemic areas is due to recently transmitted rather than remotely acquired infection2 and most new infections are acquired from casual rather than close household contacts.3 As such, interventions targeting tuberculosis transmission in the community have high potential to rapidly improve control, even in settings with high prevalence of HIV infection.4
Tuberculosis is characterised by a long period of infectiousness before diagnosis, leading to a high burden of infectious tuberculosis in the general community.4 Despite substantial investment in facility-based diagnosis and treatment, community control of undiagnosed tuberculosis remains poor in Africa.5–9 However, delays in reporting of tuberculosis symptoms and subsequent diagnosis can be overcome with community-level access to diagnostic services.10
Periodic active case finding for tuberculosis was widely implemented, mainly using chest radiography, during a period of rapid decline in tuberculosis incidence in the northern hemisphere and some Asian countries,11,12 and remains an integral part of tuberculosis control in high-risk groups.11,13–15 Community-wide interventions can add substantially to facility-based services16 and have the potential to fundamentally alter the epidemiology of transmissible diseases.17 For tuberculosis, however, despite millions of participants in active case finding during the last century, the broad effect on disease control has remained uncertain11 because of the technical difficulty in assessment of meaningful outcomes.18 Since no diagnostic test is available for recent tuberculosis infection, the main goal to reduce tuberculosis trans-mission rates cannot be measured. The closest proxy outcome is the prevalence of infectious tuberculosis in the community, assessment of which requires thousands of individuals to be screened.18
We report the results of DETECTB, a cluster-randomised study investigating community-level active case finding for tuberculosis in Harare, Zimbabwe, which is an urban setting with high prevalence of HIV infection. Two strategies for active case finding were compared: door-to-door enquiry for chronic cough,19 and neighbourhood visits by a mobile van.20 The cluster-randomised design was chosen to allow cumulative yield of smear-positive tuberculosis to be directly compared between groups over six intervention rounds, spaced at 6-monthly intervals. The broad effect of the strategies was assessed through prevalence surveys before and after the intervention to investigate whether culture-positive tuberculosis had become less prevalent in the population.
Methods
Study population
46 study clusters were demarcated in residential suburbs of Harare, Zimbabwe, aiming for a total population of 100 000–120 000 adults aged 16 years or older. Cluster boundaries were based on census enumeration areas modified with street maps to provide 0·5 km or more between cluster boundaries, with exclusion of informal settlements, apartment blocks, or hostels. Each cluster was estimated to include 2000–3000 adults on the basis of the last census (2002), and only formal serviced residential neighbourhoods were eligible for inclusion.
Approval was granted by the ethics committees of Biomedical Research and Training Institute (Harare, Zimbabwe), Medical Research Council of Zimbabwe (Harare, Zimbabwe), and London School of Hygiene and Tropical Medicine (London, UK). Written informed consent was provided by all participants in the prevalence surveys and before HIV testing. The protocol request to waive the requirement for signed consent forms for the active case-finding intervention was approved for three reasons: individuals with suspected tuberculosis were defined and investigated in line with national policy, except for the community location; informed consent could not be taken from participants who submitted specimens through other household members; and the potential for harm was minimal. Leaflets provided with all specimen containers specified that this study was for research purposes and provided other essential information for participants (webappendix p 1).
Randomisation and masking
Study clusters were randomly allocated to receive six rounds of active case finding at 6-monthly intervals by either mobile van (5 days per cluster per round) or door-to-door enquiry for chronic cough (one enquiry per household), with no standard of care intervention. Randomisation was done by selection of red and black coloured discs (23 of each colour), which were otherwise identical, from an opaque bag held above eye-level. Discs were withdrawn at a public meeting by community advisory board members representing each cluster. Before selection began, black was allocated to represent the door-to-door group, and red to represent the mobile van group. Community health workers and cluster residents were not masked to the intervention. However, laboratory work and clinical management was done without reference to the intervention group, and interim data were not analysed by intervention group until the final analysis, allowing investigators and laboratory staff to be masked to intervention allocation. A monitoring system ensured that the two interventions were delivered as intended (webappendix p 2).
Procedures
Both active case-finding strategies used community workers to identify chronic cough (≥2 weeks) in the community, followed by collection of two sputum samples per adult for fluorescence microscopy but not culture. Mobile van and door-to-door teams rotated through the clusters, taking 24 weeks to complete the 23 clusters allocated to the intervention group. The intervention period (from first cluster of round one to last cluster of round six) was from January, 2006, to November, 2008. The mobile van was located in each cluster for 5 days per intervention round from 9 am to 4 pm, including Saturday, and used a loudspeaker to publicise leafleting and services provided by one team of three lay field workers. Individuals reporting symptoms to staff waiting by the van provided sputum samples, and could report symptoms and obtain containers on behalf of other individuals within their household. Door-to-door enquiry for chronic cough and leafleting was done by two teams of three lay field workers. Households were visited up to three times per round between 9 am and 4 pm, including one weekend visit, until at least one member was present. Specimen containers and instructions were left if symptoms were volunteered for any household members. Leaflets explained the study rationale and stressed the benefits to family and friends of early diagnosis of tuberculosis, and the important role of HIV-negative tuberculosis in persistence of transmission. No other attempt at community mobilisation was made.
Adults who volunteered symptoms provided two sputum specimens: one immediate and one early morning specimen obtained before eating. Smears were made from centrifuged sputum (Sorvall Legend, Kendro Scientific, Langenselbold, Germany), stained with Auramine-O (Sigma-Aldrich Chemie, Buch, Spain), and read by fluorescence microscopy (Leica DM 1000, Leitz, Wetzlar, Germany); specimens were not cultured. In both intervention groups, specimens were transported and processed centrally, with positive smears reported to the participant's home within 4 days, confirmation of the participant's usual residential address, and referral for treatment. Negative results were not reported back, but participants could access facility-level follow-up at their own discretion.21 Subsequent facility management included confirmatory chest radiography, repeat sputum microscopy and culture, and diagnostic HIV testing and counselling, with referral to adjacent municipal services for treatment of tuberculosis and HIV management (webappendix p 3).21
We also used the municipal electronic tuberculosis register to identify patients with tuberculosis who were registered to addresses within study clusters. These diagnoses were then classified as routine or active case finding through cross-reference and reconciliation with study records.
Every dwelling in the study clusters was visited to ascertain the number of households within the dwelling (defined as sharing meals), and the number of adult residents per household. This enumeration survey was done in 2005–06 before the first round of intervention and in 2008 before the sixth round of intervention to provide the sampling frame for two cross-sectional prevalence surveys. Survey methods have been previously described.22 Allocated household identifiers were then used as the sample frame to produce a random sample of 12% of households from each of the 46 study clusters by use of the sample command in Stata. All consenting adult members (≥16 years) of selected households were then screened for tuberculosis from culture of two sputum specimens, with subsequent case ascertainment if positive (webappendix p 4). All specimens were cultured by use of Lowenstein-Jensen slopes made within the laboratory to detect subclinical in addition to symptomatic infectious tuberculosis. Participants reporting cough, haemoptysis, fever, night sweats, or weight loss also had sputum microscopy and chest radiography. Participants were asked to provide venous blood for HIV testing, with voluntary counselling and testing for those who wanted to know their results. HIV testing was done with Determine (Abbott Diagnostics, Johannesburg, South Africa) and either Unigold (Trinity Biotech, Dublin, Ireland) or SD Bioline (Standard Diagnostics, Suwon, South Korea).22 Recruitment and specimen collection for the second prevalence survey was completed in December, 2008, with final clinical follow-up of individuals with suspected tuberculosis in April, 2009.
The primary outcome measure was the cumulative yield of smear-positive tuberculosis per 1000 adult residents diagnosed by six rounds of active case finding, and was compared at the cluster level between intervention groups. The secondary outcome measure was the change in the point prevalence of infectious (culture-positive) tuberculosis from before intervention to before round six of intervention in the two intervention groups combined. This outcome was analysed at an individual level (not cluster level).
Statistical analysis
The primary outcome sample size23 assumed a cumulative yield of smear-positive tuberculosis of 7·5 per 1000 residents, with 100 000 adults providing 80% power at a 5% level of significance to detect a 30% increase in cumulative yield per 1000 adults by the more effective intervention with the coefficient of variation k=0·20, and a 35% difference with k=0·25. For the secondary outcome, we assumed 20% of selected individuals would not participate and prevalence of culture-positive tuberculosis of about 10 per 1000 participants before intervention, with a 12% random sample of residents providing 80% power to detect a 40–50% reduction in prevalence from before intervention, dependent on the extent of household clustering and design effect.
For the primary endpoint, analysis was by intention to treat. The mean cumulative yield of smear-positive tuberculosis per 1000 adults in each intervention group was compared with the t test, and the unadjusted risk ratio (RR) was calculated as the ratio of these means.24 For multivariate analysis, a linear regression model of cumulative yield per 1000 adults included community-level covariates, but not intervention group. The adjusted cumulative yield for each cluster was then analysed in place of recorded values, with the number of degrees of freedom of the t distribution reduced by the number of covariates included. As preplanned, a similar approach was used to compare participation in each intervention group from numbers of adult residents submitting sputum. For the secondary endpoint, analysis was based on participants who provided sputum in the prevalence surveys before intervention and before round six of intervention. The unadjusted and adjusted RR measuring the change in prevalence of culture-positive tuberculosis was estimated by use of generalised estimating equation regression models with the log link function, accounting for neighbourhood clustering.
The need for adjusted analyses was prespecified as limited to covariates meeting definitions of imbalance; in the event that both intervention groups and cross-sectional surveys were well balanced, the use of unadjusted analyses was justified. However, to increase robustness, we present post-hoc analyses adjusted for four cluster-level variables for comparison of the primary endpoint across intervention groups: household crowding (proportion of individuals living in households with ≥2 people per room), male sex (proportion of men in adult population), and HIV infection, which are the main risk factors for prevalent tuberculosis,22 and rates of diagnosis of smear-positive tuberculosis before intervention. All analyses were done with Stata (version 11.0).
This trial is registered, number ISRCTN84352452.
Role of the funding source
The funder had no role in any aspect of study design or analysis, data collection, data analysis, data interpretation, writing of the report, or the decision to submit for publication. ELC, TB, and TD had full access to all data. ELC had final responsibility for the decision to submit for publication.
Results
The 46 study clusters eligible for inclusion in the study had a combined population size of 110 432 adults at baseline, increasing to 124 244 after five rounds of intervention (figure 1). Eligible suburbs included eight of the ten suburbs with the highest rates of diagnosis of smear-positive tuberculosis in Harare. In the 12% of households randomly selected for survey of tuberculosis and HIV prevalence, 10 092 adults (81% of 12 426) provided sputum before intervention and 11 211 (77% of 14 569) provided sputum after five rounds of intervention, with lower participation in men (65% [3970/6151] before intervention, 57% [4061/7185] after intervention) than in women (98% [6121/6275] before intervention, 97% [7150/7384] after intervention; webappendix p 5).
Figure 1.
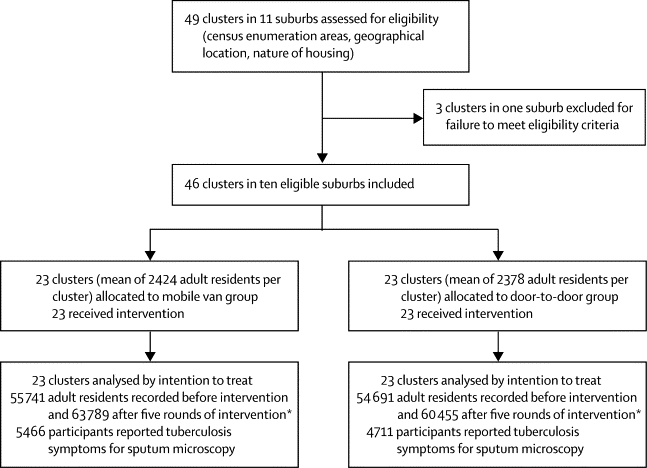
Trial profile
*Analysis based on mean population from the two household enumeration surveys.
Characteristics assessed at baseline in the household enumeration and tuberculosis and HIV prevalence surveys were similar between intervention groups (table 1). Overall, HIV prevalence was 21% (1916/9060) and the prevalence of culture-positive tuberculosis was 6·5 per 1000 adults. Previous tuberculosis treatment was reported by 3% (334/10 089) of participants. During the 6 months before intervention, diagnosis of smear-positive tuberculosis was diagnosed in 2·8 per 1000 adults (table 1). The trial was completed as planned with no adverse events.
Table 1.
Participant characteristics at baseline by source of study data
| Mobile van group | Door-to-door group | ||
|---|---|---|---|
| Household enumeration survey before intervention | |||
| Total number of households | 20 700 | 20 719 | |
| Total number of adults* | 55 741 | 54 691 | |
| Mean number of adults per cluster (range) | 2424 (1390–3940) | 2378 (1180–3182) | |
| Men | 24 222/49 221 (49%) | 23 650/47 481 (50%) | |
| HIV and tuberculosis prevalence survey before intervention† | |||
| Number of participants | 5371 | 4721 | |
| Culture-positive tuberculosis (per 1000 adults) | 35/5371 (6·5) | 31/4721 (6·6) | |
| Smear-positive tuberculosis (per 1000 adults) | 19/5371 (3·5) | 21/4721 (4·4) | |
| HIV infection | 1048/4842 (22%) | 868/4218 (21%) | |
| Age (years) | 31·8 (13·7) | 30·5 (12·3) | |
| <25 | 2129/5371 (40%) | 2003/4721 (42%) | |
| 25–44 | 2401/5371 (45%) | 2071/4721 (44%) | |
| ≥45 | 841/5371 (16%) | 647/4721 (14%) | |
| Previous tuberculosis treatment | 195/5368 (4%) | 139/4721 (3%) | |
| Current smoker | 463/5370 (9%) | 404/4720 (9%) | |
| Household crowding (≥2 people per room) | 2655/5361 (50%) | 2189/4713 (46%) | |
| Education (secondary or higher) | 4412/5367 (82%) | 4030/4719 (85%) | |
| Routine tuberculosis register‡ | |||
| Smear-positive tuberculosis case notification in preceding 6 months (per 1000 adults per year) | 86/55 741 (3·1) | 68/54 691 (2·5) | |
Data are n/N (%) or mean (SD), unless otherwise stated. Summary data are based on available data combined across all clusters within each intervention group, unless otherwise stated.
Information on number of adult residents was missing for 1% of households.
12 426 adults were randomly selected to participate in the survey but data are based on 10 092 adults who provided sputum for tuberculosis screening.
Routinely diagnosed tuberculosis cases registered to an address within study clusters for the 6 months before the start of intervention.
In analysis of the primary outcome, detection of smear-positive tuberculosis and the cumulative yield was higher in the mobile van group than in the door-to-door group during the six rounds of intervention (table 2), and yield per 1000 adults was higher for the mobile van group than the door-to-door group at every round (figure 2). The difference between the intervention groups remained significant after adjustment for cluster-level variables (table 2). The cumulative tuberculosis yield per 1000 adults increased with increasing cluster HIV prevalence in the mobile van group but not in the door-to-door group (interaction p=0·0070). The difference between the two groups was greatest in clusters with HIV prevalence of 20% or higher (adjusted RR 2·05, 95% CI 1·33–3·15), with little difference below 20% (1·08, 0·74–1·57). This effect was not simple and direct, however, because in patients diagnosed with tuberculosis who consented to diagnostic HIV testing, HIV prevalence was similar between intervention groups: 72% (156/217) in the mobile van group, and 67% (74/111) in the door-to-door group.
Table 2.
Participation and cumulative yield of smear-positive tuberculosis cases in the community during six rounds of intervention
| Mobile van group | Door-to-door group |
Unadjusted |
Adjusted |
|||
|---|---|---|---|---|---|---|
| Risk ratio (95% CI) | p value | Risk ratio (95% CI)* | p value | |||
| Analysis of participation | ||||||
| Number of participants | 5466 | 4711 | .. | .. | .. | .. |
| Cumulative participation rate per 1000 adults† | 91·5 | 81·8 | .. | .. | .. | .. |
| Mean (SD) cumulative participation rate per 1000 adults per cluster‡ | 95·9 (35·6) | 84·2 (31·5) | 1·14 (0·91–1·42) | 0·24 | 1·03 (0·85–1·24) | 0·77 |
| Primary outcome analysis | ||||||
| Smear-positive cases | 255 | 137 | .. | .. | .. | .. |
| Cumulative yield per 1000 adults† | 4·27 | 2·38 | .. | .. | .. | .. |
| Mean (SD) cumulative yield per 1000 adults per cluster‡ | 4·22 (1·95) | 2·46 (1·33) | 1·71 (1·27–2·31) | 0·0010 | 1·48 (1·11–1·96) | 0·0087 |
| Analysis of smear-negative participants | ||||||
| Number meeting tuberculosis case definition§ | 241 | 214 | .. | .. | .. | .. |
| Cumulative yield per 1000 adults† | 4·03 | 3·72 | .. | .. | .. | .. |
| Mean (SD) cumulative yield per 1000 adults per cluster‡ | 4·27 (2·40) | 3·80 (2·06) | 1·12 (0·81–1·56) | 0·48 | 0·88 (0·71–1·09) | 0·22 |
Adjusted for cluster-level variation in household crowding, male sex, HIV infection, and rates of diagnosis of smear-positive tuberculosis before intervention; analysis of participation was also adjusted for mean age.
Based on mean of adult population from two enumeration surveys (59 770 in mobile van group and 57 581 in door-to-door group).
One participant contributed two disease episodes (smear-positive tuberculosis in two separate rounds).
Participants who were smear negative on community specimens and met the case definition for tuberculosis on follow-up in the mobile van and door-to-door groups: 44 and 36, respectively, were smear positive on follow-up; 59 and 48, respectively, were smear negative and culture confirmed; and 138 and 130, respectively, met definitions for culture-negative tuberculosis including response to tuberculosis treatment (described by Dimairo and colleagues21).
Figure 2.
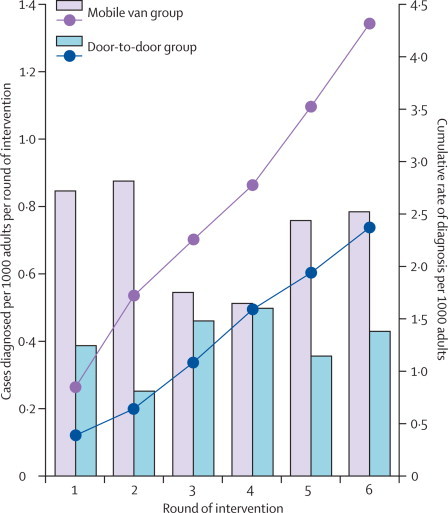
Detection of smear-positive tuberculosis through active case finding
Solid bars show number of cases per 1000 adults diagnosed in each round of intervention. Lines show the cumulative rate of diagnosis per 1000 adults. The increase in population during the course of the study is assumed to have occurred at a constant rate.
49% (194/392) of smear-positive patients diagnosed through active case finding were men. Although tuberculosis symptoms included chronic cough in most cases (82% [207/253] in the mobile van group [data were missing for two individuals], and 84% [115/137] in the door-to-door group), most patients had not previously sought health care. Patients with chronic cough were interviewed about previous consultations with health-care providers. Of those who agreed to interview, active case finding was the first consultation for 146 of 199 (73%) in the mobile van group, and 91 of 109 (83%) in the door-to-door group (p=0·0438).
Rates of reporting of tuberculosis symptoms were similar in the two groups, with the exception of round one, in which participation in the mobile van group was substantially higher than in the door-to-door group (data not shown). During the six rounds of intervention, 10 177 participants submitted sputum, with a similar cumulative participation rate in both intervention groups, but a higher proportion of smear-positive participants recorded in the mobile van group (4·7% [255/5466]) than in the door-to-door group (2·9% [137/4711]; table 2). At cluster level, cumulative rates for submission of sputum and diagnostic yield were significantly correlated (r=0·55). Notably, 3013 participants (55% of 5466) in the mobile van group and 3101 (66% of 4711) in door-to-door group were women.
As described in the Methods, smear-negative participants could attend follow-up, although few did so: 27% (1387/5202) from the mobile van group and 29% (1336/4568) from the door-to-door group. Across both intervention groups, an additional 455 smear-negative participants with suspected tuberculosis met the case definition for tuberculosis (table 2), although 268 (59%) were smear and culture negative.
In the period from the start of round one to the end of round six in each cluster, a total of 472 smear-positive patients were diagnosed through active case finding, including 80 patients (44 in the mobile van group and 36 in the door-to-door group) whose first smears were negative, but who were smear positive on follow-up investigations. Routine health services diagnosed an additional 670 cases of smear-positive tuberculosis in adult residents of study clusters (367 in the mobile-van group and 303 in the door-to-door group). Therefore, active case finding contributed 472 (41%) of 1142 smear-positive diagnoses made during the course of intervention.
In analysis of the secondary outcome, the overall prevalence of culture-positive tuberculosis declined substantially by 44% (95% CI 17–62) from before intervention to before round six of intervention, and the reduction remained significant even after adjustment (table 3). Similar results were recorded for smear-positive disease, for two alternative case definitions of tuberculosis that included all positive tuberculosis cultures combined irrespective of whether tuberculosis was confirmed on follow-up, and for all tuberculosis cases meeting case definitions for disease irrespective of culture result (table 3).
Table 3.
Prevalence of tuberculosis disease before intervention and before round six of intervention
|
Before intervention (n=10 092) |
Before round six of intervention (n=11 211) |
Unadjusted |
Adjusted |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Number of cases | Prevalence per 1000 adults (95% CI) | Number of cases | Prevalence per 1000 adults (95% CI) | Risk ratio (95% CI)* | p value | Risk ratio (95% CI)* | p value | ||
| Prespecified analysis of culture-positive confirmed tuberculosis (secondary outcome)† | |||||||||
| Overall | 66 | 6·5 (5·1–8·3) | 41 | 3·7 (2·6–5·0) | 0·56 (0·38–0·83) | 0·0036 | 0·59 (0·40–0·89) | 0·0112 | |
| HIV status‡ | .. | .. | .. | .. | .. | .. | .. | 0·1724§ | |
| Positive | 34 | 17·7 (12·3–24·7) | 24 | 13·0 (8·4–19·3) | 0·74 (0·44–1·24) | .. | 0·75 (0·45–1·26) | .. | |
| Negative | 29 | 4·1 (2·7–5·8) | 13 | 1·6 (0·9–2·8) | 0·40 (0·21–0·78) | .. | 0·42 (0·22–0·81) | .. | |
| Post-hoc subgroup analyses | |||||||||
| Sex | .. | .. | .. | .. | .. | .. | .. | 0·2206§ | |
| Women | 35 | 5·7 (4·0–7·9) | 18 | 2·5 (1·5–4·0) | 0·44 (0·25–0·78) | .. | 0·46 (0·25–0·83) | .. | |
| Men | 31 | 7·8 (5·3–11·1) | 23 | 5·7 (3·6–8·5) | 0·73 (0·43–1·25) | .. | 0·75 (0·43–1·31) | .. | |
| Intervention group | .. | .. | .. | .. | .. | .. | .. | 0·8244§ | |
| Mobile van | 35 | 6·5 (4·5–9·1) | 21 | 3·6 (2·2–5·5) | 0·54 (0·32–0·93) | .. | 0·62 (0·36–1·07) | .. | |
| Door-to-door | 31 | 6·6 (4·5–9·3) | 20 | 3·7 (2·3–5·7) | 0·56 (0·32–0·99) | .. | 0·56 (0·31–1·02) | .. | |
| Prespecified analysis of smear-positive confirmed tuberculosis¶ | |||||||||
| Overall | 40 | 4·0 (2·8–5·4) | 25 | 2·3 (1·5–3·3) | 0·56 (0·34–0·93) | 0·0239 | 0·60 (0·36–1·00) | 0·0504 | |
| Post-hoc analysis of all culture-positive tuberculosis (including unconfirmed cases)¶ | |||||||||
| Overall | 88 | 8·7 (7·0–10·7) | 55 | 4·9 (3·7–6·4) | 0·56 (0·40–0·79) | 0·0009 | 0·60 (0·42–0·85) | 0·0043 | |
| Prespecified analysis of all tuberculosis (including culture-negative cases)¶ | |||||||||
| Overall | 91 | 9·0 (7·3–11·1) | 62 | 5·5 (4·2–7·1) | 0·62 (0·45–0·85) | 0·0034 | 0·66 (0·48–0·92) | 0·0150 | |
Neighbourhood clustering was accounted for in unadjusted analyses; in adjusted analyses, further adjustment was made for household crowding, sex, HIV infection, and past tuberculosis treatment at an individual level.
As per protocol, includes one repeatedly smear-positive case in the survey before intervention for which cultures failed to grow mycobacteria because of contamination, and six culture-positive cases from households selected in the survey after intervention that were detected during round six of the intervention (three from each intervention group).
HIV status was not ascertained for three participants with culture-positive tuberculosis and 1029 participants who did not have tuberculosis (culture negative) at the survey before intervention, and for four participants with culture-positive tuberculosis and 1356 participants who did not have tuberculosis (culture negative) at the survey after intervention.
p value for interaction.
As specified in the trial protocol and previously described,22 independent evidence of tuberculosis disease was needed before participants with positive tuberculosis cultures were accepted as confirmed tuberculosis, which is standard practice whenever diagnostic tests are used for screening purposes; case ascertainment used repeat smears, cultures, radiography, and response to tuberculosis treatment.
As anticipated in planned analyses defined in our protocol, the reduction in prevalence of culture-positive tuberculosis associated with intervention was greater in participants without HIV infection (58% reduction, 95% CI 19 to 78) than in those with HIV infection (25%, −26 to 55), although not significantly so (table 3). Since women had more complete participation in both prevalence surveys and both intervention groups than did men, post-hoc analysis examined the effect by sex. Although the estimated reduction was greater in women (54%, 17 to 75) than in men (25%, −31 to 57), this difference was not significant. A further post-hoc analysis showed little difference in the effect of intervention group on prevalence, with a reduction of 38% (−7 to 64) in the mobile van group versus 44% (−2 to 69) in the door-to-door group (table 3).
During the intervention period, the population in the study area increased by 13%. Overall HIV prevalence fell slightly from 21% to 19%, with no other major changes (webappendix p 6).
Discussion
We have shown that untargeted periodic active case finding for symptomatic smear-positive tuberculosis repeated once every 6 months made a substantial contribution to diagnosis of smear-positive tuberculosis in an urban population with high HIV prevalence, and to control of infectious tuberculosis (panel). The mobile van delivery strategy significantly outperformed door-to-door enquiry for chronic cough, especially in neighbourhoods with high HIV prevalence. By the start of intervention round six, infectious tuberculosis in the community had fallen by more than 40% from rates before intervention, to rates well below those reported elsewhere in the region.5–9 This major improvement in tuberculosis control in a population with high HIV prevalence suggests that such an intervention could provide rapid reductions in tuberculosis transmission in the community, and could lead to declining rates of new tuberculosis cases in individuals with and without HIV infection within a few years.
Panel. Research in context.
Systematic review
Before the study started, we searched PubMed for relevant articles with search terms including “tuberculosis AND case finding” as MeSH headings, “tuberculosis” and “case finding” as search terms, “tuberculosis AND (community OR population OR household)”, “tuberculosis AND case-finding AND clinical trial”, and “(active OR enhanced OR intensified) AND tuberculosis AND (case-finding OR case finding)”. References in relevant articles were also screened. In view of the long history of active case finding, we did not set any criteria for assessing quality to avoid exclusion of articles published before standard reporting guidelines were developed.
Interpretation
Findings of our study show that the mobile van method was substantially more effective than was door-to-door enquiry for identification of patients with previously undiagnosed smear-positive tuberculosis. Repeated implementation of periodic active case finding can substantially reduce undiagnosed infectious tuberculosis in the community, which is likely to be associated with reduced transmission rates.
Active case finding has been an integral part of tuberculosis control in industrialised countries since the 1920s.11,12,15 Early programmes used radiological screening of otherwise untargeted adults, reporting yields as high as 30 cases of previously undiagnosed tuberculosis per 1000 screened in New York City during the early 1930s.11 Intensive interventions in native Alaskans in the 1950s, in whom prevalence was extremely high, led to rapid and major reductions in tuberculosis incidence, mortality, and transmission in the population within a few years.25 Elsewhere, however, the effect is difficult to discern from pre-existing downward trends.11,25 Policy from the 1970s recommended targeted screening of close contacts of patients with tuberculosis, recent immigrants, prisoners, homeless people, and people with HIV infection, but not general populations.15 In these high-prevalence groups, active case finding can reduce tuberculosis incidence through prevention of secondary cases.13,26
During the past 15 years, global scale-up of facility-based tuberculosis diagnostic and treatment services has greatly improved treatment success rates, but has had disappointingly little effect on tuberculosis incidence.15 Failure to adequately control undiagnosed tuberculosis in poor communities, together with an increasing prevalence of factors favouring tuberculosis transmission and disease, seem to be the key issues and enhanced case finding is being reconsidered as a possible next step in global control.15 However, little evidence is available to guide contemporary choices about who should be screened, how screening should be done and with what frequency, and how to deliver services effectively.11,15
We chose to use fluorescence microscopy to screen sputum samples from adults volunteering symptoms, rather than the more sensitive alternative of radiological screening of all adults, because smear-positive patients are by far the most infectious and decentralised microscopy is already well supported globally and has low unit costs. Between three and eight per 1000 adults surveyed in four African countries from 2002 to 2009 were smear positive, about half of whom reported chronic cough, providing a simple target linked to infectiousness that has a high positive predictive value for smear positivity at community level, and consistency with facility-based approaches.5,8,9,11,27 Moreover failure to provide diagnosis at subclinical stages is not necessarily a long-term barrier to tuberculosis control.10,28
In our study, active case finding provided the first investigation for 77% of smear-positive participants, despite the fact that all participants were symptomatic and lived within 2 km of a primary clinic. This finding adds to accumulating evidence that the slow rate at which patients with tuberculosis report to health facilities is a major rate-limiting step in global efforts to control tuberculosis.5,15,29–31 Competing priorities for time and money, fear of diagnosis with an HIV-related disease, and the hope of spontaneous resolution all contribute to this delay.31,32
The finding that the mobile van attracted significantly more smear-positive participants than did door-to-door enquiry for chronic cough was counterintuitive, but especially striking in clusters with high HIV prevalence that were also the poorest and most crowded. This finding was associated with a higher rate of smear positivity in the mobile van group than in the door-to-door group, not an increased rate of participation. Reporting of symptoms is more proactive than is response to direct enquiry, and more participants in the mobile van group reported previous consultations with health-care providers than did those in the door-to-door group. The mobile van group was potentially associated with stigma because consultation for an HIV-related disease was done in front of neighbours, but this intervention provided increased opportunity for encouragement from others, and time to decide to seek the intervention and find a convenient moment to do so.32 Follow-up house-to-house enquiry added little to case finding through a mobile clinic in Thailand,20 but the study did not investigate the possibility that mobile clinics were more effective than was house-to-house enquiry. Mobile services are often used to provide outreach services, including HIV testing, and report high participation.33 Unannounced door-to-door enquiries for chronic cough are likely to be less sensitive than are more intensive approaches to home-based case finding (for example, face-to-face interview and screening of adult members of randomly selected households detected additional tuberculosis cases in the door-to-door group, despite this survey immediately following the last round of intervention in our study), but contributed 40% of all cases of smear-positive tuberculosis diagnosed in South Korea during the 1970s.15
Assessment of the combined effect of our two intervention strategies through prevalence surveys provides a clearer measure of the effect on tuberculosis control than could be obtained from our primary outcome alone: counting cases diagnosed provides little insight into how much smear-positive person-time has been averted, and does not capture potentially important indirect effects from reduced tuberculosis transmission and more timely reporting of tuberculosis symptoms to routine health-care providers between intervention rounds. By contrast with the case finding outcome, the effect of intervention on prevalence was very similar between the mobile van and door-to-door groups. Subanalyses for prevalence had very low power and the 95% CIs are widely overlapping. But, if these overlaps truly indicate absence of difference, then patients in the door-to-door group were on average at an earlier stage in their health-seeking process than were those diagnosed through the mobile van and so were further in time from routine diagnosis, in which case the two intervention groups will have had equivalent effect on tuberculosis transmission.
The prevalence data also suggest suboptimal intervention effect in men and in individuals with HIV infection. Although neither interaction was significant, men tend to have a higher prevalence of undiagnosed tuberculosis than do women,5,22,34 and health-seeking behaviour varies substantially by sex.31 In the active case-finding component of this study, participation, but not diagnosis, was higher in women than in men. For HIV infection, we anticipated that the 6-monthly intervals of intervention might be intrinsically more likely to affect HIV-negative tuberculosis (low incidence but typical delay to diagnosis or death of ≥1 year) than HIV-positive tuberculosis (high incidence but a brief delay to diagnosis or death).4,10,22
Our intervention clusters were fairly homogeneous, with no slums or rural clusters, and we assessed only two of many case-finding approaches.11 High-density urban populations are the obvious target for active case finding in view of their accessibility and high tuberculosis burden,5,9 but effective rural strategies have also been described.35 Other limitations of our study include the separation of clusters by areas receiving no intervention to avoid cross-contamination, which will have diluted any effect on transmission rates. Our secondary outcome was an uncontrolled before-and-after comparison, vulnerable to coincidental time trends. However, we believe that such trends are unlikely to explain the striking reduction in infectious tuberculosis because population characteristics remained similar during the intervention period, with little change in coverage of antiretroviral therapy36 and deterioration rather than strengthening of routine health and tuberculosis services during the study period. Last, suboptimal participation by men in the prevalence surveys could have biased our subanalysis by sex, although the substantial reduction in infectious tuberculosis in women strongly supports our conclusions about the effectiveness of active case finding.
Active case finding for tuberculosis in the general community was discouraged for several decades because of high costs of implementation and insufficient strength of treatment programmes.11,12,15 With the successful global scale-up of effective tuberculosis treatment, however, our results suggest that active case finding needs re-evaluation in general populations wherever tuberculosis incidence or prevalence is high. Effectiveness should ideally be assessed as cases averted or reduction in prevalence, and these outcomes, not cases found, should be used for cost-effectiveness analysis together with capture of transmission dynamics.37 In countries with severe generalised epidemics of HIV infection and tuberculosis, including the middle-income countries of South Africa and Botswana, the affordability of active case finding in the general population needs to be weighed against the extremely high financial and societal costs of allowing the dual epidemic to rage on.1,38 The effect on HIV-negative tuberculosis that we report is in the range needed for countries to meet the Millennium Development Goal relating to tuberculosis prevalence,15 and was achieved in under 3 years. Interventions should aim to effectively engage men, and, in settings of high HIV prevalence, should ideally be accompanied by interventions promoting HIV diagnosis linked to intensified tuberculosis prevention with antiretroviral therapy and isoniazid preventive therapy.4
Acknowledgments
Acknowledgments
This study was funded by the Wellcome Trust. We thank all participants and our community advisers and trial steering committee members.
Contributors
ELC, AEB, GJC, BGW, and RJH designed the study. TD, RJH, TB, and ELC contributed to data management, and TD did the analysis with input from RJH, TB, and ELC. ED, TB, and BM coordinated the trial and managed data. SM, SSM, and PRM contributed to the study design and logistics. All authors contributed to writing of the report and have seen and approved the final draft.
Conflicts of interest
ELC has received expenses to attend technical advisory and working groups as a consultant from WHO, and expenses to present data at a meeting and a conference from Keystone Symposia; and ELC's institution has received a grant from the Wellcome Trust. AEB is a board member for and has received payment for lectures from the Biomedical Research and Training Institute; and has received travel and accommodation expenses from Pump Aid, the Wellcome Trust, and the Royal Society. All other authors declare that they have no conflicts of interest.
Web Extra Material
References
- 1.WHO . Global tuberculosis control: epidemiology, strategy, financing. WHO/HTM/TB/2009.411. World Health Organization; Geneva: 2009. [Google Scholar]
- 2.Glynn JR, Crampin AC, Yates MD. The importance of recent infection with Mycobacterium tuberculosis in an area with high HIV prevalence: a long-term molecular epidemiological study in northern Malawi. J Infect Dis. 2005;192:480–487. doi: 10.1086/431517. [DOI] [PubMed] [Google Scholar]
- 3.Verver S, Warren RM, Munch Z. Proportion of tuberculosis transmission that takes place in households in a high-incidence area. Lancet. 2004;363:212–214. doi: 10.1016/S0140-6736(03)15332-9. [DOI] [PubMed] [Google Scholar]
- 4.Corbett EL, Marston B, Churchyard GJ, De Cock KM. Tuberculosis in sub-Saharan Africa: opportunities, challenges and change in the era of antiretroviral treatment. Lancet. 2006;367:926–937. doi: 10.1016/S0140-6736(06)68383-9. [DOI] [PubMed] [Google Scholar]
- 5.Ayles H, Schaap A, Nota A. Prevalence of tuberculosis, HIV and respiratory symptoms in two Zambian communities: implications for tuberculosis control in the era of HIV. PLoS One. 2009;4:e5602. doi: 10.1371/journal.pone.0005602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wood R, Middelkoop K, Myer L. Undiagnosed tuberculosis in a community with high HIV prevalence: implications for tuberculosis control. Am J Respir Crit Care Med. 2007;175:87–93. doi: 10.1164/rccm.200606-759OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.den Boon S, White NW, van Lill SW. An evaluation of symptom and chest radiographic screening in tuberculosis prevalence surveys. Int J Tuberc Lung Dis. 2006;10:876–882. [PubMed] [Google Scholar]
- 8.Guwatudde D, Zalwango S, Kamya MR. Burden of tuberculosis in Kampala, Uganda. Bull World Health Organ. 2003;81:799–805. [PMC free article] [PubMed] [Google Scholar]
- 9.Sekandi JN, Neuhauser D, Smyth K, Whalen CC. Active case finding of undetected tuberculosis among chronic coughers in a slum setting in Kampala, Uganda. Int J Tuberc Lung Dis. 2009;13:508–513. [PMC free article] [PubMed] [Google Scholar]
- 10.Corbett EL, Bandason T, Cheung YB. Epidemiology of tuberculosis in a high HIV prevalence population provided with enhanced diagnosis of symptomatic disease. PLoS Med. 2007;4:e22. doi: 10.1371/journal.pmed.0040022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Golub JE, Mohan C, Comstock GW, Chaisson RE. Active case-finding of tuberculosis: historical perspective and future prospects. Int J Tuberc Lung Dis. 2005;9:1183–1203. [PMC free article] [PubMed] [Google Scholar]
- 12.Uplekar M, Raviglione MC. The “vertical-horizontal” debates: time for the pendulum to rest (in peace)? Bull World Health Organ. 2007;85:413–414. doi: 10.2471/07.041756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frieden TR, Fujiwara PI, Washko RM, Hamburg MA. Tuberculosis in New York City—turning the tide. N Engl J Med. 1995;333:229–233. doi: 10.1056/NEJM199507273330406. [DOI] [PubMed] [Google Scholar]
- 14.de Vries G, van Hest RA, Richardus JH. Impact of mobile radiographic screening on tuberculosis among drug users and homeless persons. Am J Respir Crit Care Med. 2007;176:201–207. doi: 10.1164/rccm.200612-1877OC. [DOI] [PubMed] [Google Scholar]
- 15.Lönnroth K, Castro KG, Chakaya JM. Tuberculosis control and elimination 2010–50: cure, care, and social development. Lancet. 2010;375:1814–1829. doi: 10.1016/S0140-6736(10)60483-7. [DOI] [PubMed] [Google Scholar]
- 16.Lewin SA, Dick J, Pond P. Lay health workers in primary and community health care. Cochrane Database Syst Rev. 2005;1 doi: 10.1002/14651858.CD004015.pub2. CD004015. [DOI] [PubMed] [Google Scholar]
- 17.Mermin J, Ekwaru JP, Liechty CA. Effect of co-trimoxazole prophylaxis, antiretroviral therapy, and insecticide-treated bednets on the frequency of malaria in HIV-1-infected adults in Uganda: a prospective cohort study. Lancet. 2006;367:1256–1261. doi: 10.1016/S0140-6736(06)68541-3. [DOI] [PubMed] [Google Scholar]
- 18.Dye C, Bassili A, Bierrenbach AL. Measuring tuberculosis burden, trends, and the impact of control programmes. Lancet Infect Dis. 2008;8:233–243. doi: 10.1016/S1473-3099(07)70291-8. [DOI] [PubMed] [Google Scholar]
- 19.Sung CK. Case-finding in the Korean national tuberculosis programme. Bull Int Union Tuberc. 1976;51:381–382. [PubMed] [Google Scholar]
- 20.Elink Schuurman MW, Srisaenpang S, Pinitsoontorn S, Bijleveld I, Vaeteewoothacharn K, Methapat C. The rapid village survey in tuberculosis control. Tuber Lung Dis. 1996;77:549–554. doi: 10.1016/s0962-8479(96)90054-4. [DOI] [PubMed] [Google Scholar]
- 21.Dimairo M, MacPherson P, Bandason T. The risk and timing of tuberculosis diagnosed in smear-negative TB suspects: a 12 month cohort study in Harare, Zimbabwe. PLoS One. 2010;5:e11849. doi: 10.1371/journal.pone.0011849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Corbett EL, Bandason T, Cheung YB. Prevalent infectious tuberculosis in Harare, Zimbabwe: burden, risk factors and implications for control. Int J Tuberc Lung Dis. 2009;13:1231–1237. [PMC free article] [PubMed] [Google Scholar]
- 23.Hayes RJ, Bennett S. Simple sample size calculation for cluster-randomized trials. Int J Epidemiol. 1999;28:319–326. doi: 10.1093/ije/28.2.319. [DOI] [PubMed] [Google Scholar]
- 24.Hayes RJ, Moulton LH. Cluster randomised trials. Chapman & Hall/CRC Press; Boca Raton, FL: 2009. [Google Scholar]
- 25.Grzybowski S, Styblo K, Dorken E. Tuberculosis in Eskimos. Tubercle. 1976;57:S1–S58. doi: 10.1016/0041-3879(76)90059-3. [DOI] [PubMed] [Google Scholar]
- 26.Shenoi SV, Escombe AR, Friedland G. Transmission of drug-susceptible and drug-resistant tuberculosis and the critical importance of airborne infection control in the era of HIV infection and highly active antiretroviral therapy rollouts. Clin Infect Dis. 2010;50(suppl 3):S231–S237. doi: 10.1086/651496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Corbett EL, Zezai A, Cheung YB. Provider-initiated symptom screening for tuberculosis in Zimbabwe: diagnostic value and the effect of HIV status. Bull World Health Organ. 2010;88:13–21. doi: 10.2471/BLT.08.055467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krivinka R, Drapela J, Kubik A. Epidemiological and clinical study of tuberculosis in the district of Kolin, Czechoslovakia (1965–1972) Bull World Health Organ. 1974;51:59–69. [PMC free article] [PubMed] [Google Scholar]
- 29.Needham DM, Bowman D, Foster SD, Godfrey-Faussett P. Patient care seeking barriers and tuberculosis programme reform: a qualitative study. Health Policy. 2004;67:93–106. doi: 10.1016/s0168-8510(03)00065-4. [DOI] [PubMed] [Google Scholar]
- 30.Kemp JR, Mann G, Simwaka BN, Salaniponi FM, Squire SB. Can Malawi's poor afford free tuberculosis services? Patient and household costs associated with a tuberculosis diagnosis in Lilongwe. Bull World Health Organ. 2007;85:580–585. doi: 10.2471/BLT.06.033167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Storla DG, Yimer S, Bjune GA. A systematic review of delay in the diagnosis and treatment of tuberculosis. BMC Public Health. 2008;8:15. doi: 10.1186/1471-2458-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mavhu W, Dauya E, Bandason T. Chronic cough and its association with TB-HIV co-infection: factors affecting help-seeking behaviour in Harare, Zimbabwe. Trop Med Int Health. 2010;15:574–579. doi: 10.1111/j.1365-3156.2010.02493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khumalo-Sakutukwa G, Morin SF, Fritz K, NIMH Project Accept Study Team Project Accept (HPTN 043): a community-based intervention to reduce HIV incidence in populations at risk for HIV in sub-Saharan Africa and Thailand. J Acquir Immune Defic Syndr. 2008;49:422–431. doi: 10.1097/QAI.0b013e31818a6cb5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Borgdorff MW, Nagelkerke NJ, Dye C, Nunn P. Gender and tuberculosis: a comparison of prevalence surveys with notification data to explore sex differences in case detection. Int J Tuberc Lung Dis. 2000;4:123–132. [PubMed] [Google Scholar]
- 35.Datiko DG, Lindtjorn B. Health extension workers improve tuberculosis case detection and treatment success in southern Ethiopia: a community randomized trial. PLoS One. 2009;4:e5443. doi: 10.1371/journal.pone.0005443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.WHO. UNAIDS. UNICEF . Towards universal access: scaling up priority HIV/AIDS interventions in the health sector. Progress report 2008. World Health Organization; Geneva: 2008. [Google Scholar]
- 37.Currie CS, Floyd K, Williams BG, Dye C. Cost, affordability and cost-effectiveness of strategies to control tuberculosis in countries with high HIV prevalence. BMC Public Health. 2005;5:130. doi: 10.1186/1471-2458-5-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karim SS Abdool, Churchyard GJ, Karim Q Abdool, Lawn SD. HIV infection and tuberculosis in South Africa: an urgent need to escalate the public health response. Lancet. 2009;374:921–933. doi: 10.1016/S0140-6736(09)60916-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


