Abstract
The nervous control of expiratory muscles is less well understood than that of the inspiratory muscles, particularly in the rat. The patterns of respiratory discharges in adult rats were therefore investigated for the muscles of the caudal intercostal spaces, with hypercapnia and under either anaesthesia or decerebration. With neuromuscular blockade and artificial ventilation, efferent discharges were present for both inspiration and expiration in both external and internal intercostal nerves. This was also the case for proximal internal intercostal nerve branches that innervate only internal intercostal and subcostalis muscles. If active, this region of muscle in other species is always expiratory. Here, inspiratory bursts were almost always present. The expiratory activity appeared only gradually and intermittently, when the anaesthesia was allowed to lighten or as the pre-decerebration anaesthesia wore off. The intermittent appearance is interpreted as the coupling of a slow medullary expiratory oscillator with a faster inspiratory one. The patterns of nerve discharges, in particular the inspiratory or biphasic activation of the internal and subcostalis layers, were confirmed by observations of equivalent patterns of EMG discharges in spontaneously breathing preparations, using denervation procedures to identify which muscles generated the signals. Some motor units were recruited in both inspiratory and expiratory bursts. These patterns of activity have not previously been described and have implications both for the functional role of multiple respiratory oscillators in the adult and for the mechanical actions of the muscles of the caudal intercostal spaces, including subcostalis, which is a partly bisegmental muscle.
Introduction
In the cat, the dog and in humans, the patterns of activation of expiratory muscles have been described and their nervous control is partly understood, although to a lesser extent than for the inspiratory muscles (Iscoe, 1998; De Troyer et al. 2005; Saywell et al. 2007). In the rat, very few studies have been published on the control of the expiratory muscles, although such knowledge should now be considered particularly important. In recent years, the rat is the species which has given fundamental insights into the mechanisms generating the respiratory rhythm (reviewed by Feldman & Del Negro, 2006). Among these mechanisms, an important feature has been the clear existence of at least two independent oscillators, one of which may be specialized for the control of expiration (Janczewski et al. 2002; Mellen et al. 2003). Nearly all of the experiments on these mechanisms have been done on neonatal or young animals, and many of these on in vitro preparations. Thus, information on the activation of the respiratory muscles in more physiological conditions, particularly in the adult, is vital to understand the generality of the conclusions drawn from the experiments on the more reduced and immature preparations.
The experiments described here were initiated for a different reason, which is the potential use of expiratory axons for the assessment of repair and plasticity following experimental spinal cord injury. The rat is the species most used in such studies, the caudal thoracic segments frequently being involved as the site of injury. In these studies axonal regeneration or plasticity may be reported, but nearly always assessed by anatomical means, because the neurones which are the direct targets for most descending axonal systems, and for which physiological measurements could be made, are not precisely known. Our hope was that expiratory bulbospinal neurones (EBSNs) might provide an exception, because in the cat the targets of these axons are known: they have strong direct connections to motoneurones of thoracic segments, readily demonstrated by cross-correlation methods (Cohen et al. 1985; Kirkwood, 1995; Saywell et al. 2007). Segments close to the injury site are likely to be most important in terms of connections, especially for those of regenerated axons (Fawcett, 2002). Thus, the original aim of the experiments described here was the establishment in the rat of a preparation where expiratory discharges would reliably be available for making similar correlation measurements for the caudal thoracic segments. More generally, a description of the normal motor output from these segments should also be of general interest for comparison with data obtained subsequent to injury and/or repair.
The presence or absence of an expiratory motor output in an anaesthetized preparation is very dependent on the particular experimental conditions, such as the level of anaesthesia and its history (Kirkwood, 1995; Saywell et al. 2007). Recordings were therefore made from intercostal nerves of the caudal thoracic segments in rats under a variety of anaesthetic and decerebration protocols, to establish which one was the most suitable for the connectivity estimates. The connectivity data obtained (de Almeida & Kirkwood, 2006) will be submitted for publication in due course. However, in the course of these experiments, it became apparent that the patterns of the intercostal nerve discharges, in their own right, provided new evidence with regard to the fundamental issue of the operation of the respiratory rhythm generator in the adult, and in particular with respect to the activity of a separate ‘expiratory’ oscillator.
It was also very soon apparent that the spatial distribution of inspiratory and expiratory activity across the surface of the thorax was different from that in the other species investigated so far (cat, dog, baboon, human). The pattern in other species can be simply described by two general principles: (1) the regional distribution of activity within each muscle layer follows the distribution of mechanical advantage in that layer; and (2) inspiratory activation is restricted to the external layer and the parasternal muscle, whereas interosseus expiratory activation is restricted to the internal layer (De Troyer et al. 2005). The rat appears to offer a clear challenge to the generality of those principles, because we found that some areas of a single intercostal layer were activated in both inspiration and expiration. In order to verify this, a short series of experiments with EMG recordings in spontaneous respiration was added to those measuring nerve discharges under neuromuscular blockade.
Some of the recordings, either of nerve discharges or of EMG, suggested the surprising result that individual motoneurones might be excited in both phases of respiration. This observation was confirmed and further analysed in additional experiments with intracellular recording of the central respiratory drive potentials in individual thoracic motoneurones. These additional experiments are described in the accompanying paper (de Almeida & Kirkwood, 2010). The independent operation of inspiratory and expiratory oscillators that was established in the present experiments then gave particularly useful insights into how different synaptic components may be combined to make up the overall central respiratory drive potential.
Finally, the paper includes a short section with some details of the anatomy of the caudal intercostal muscles that have not been described before in the rat. These details are needed in the interpretation of some of the electrophysiological results.
A preliminary report has been published (de Almeida & Kirkwood, 2008).
Methods
Experiments were performed on adult female Sprague–Dawley rats (Harlan, UK) weighing between 180 and 299 g, as approved by the Ethical Review Process of the Institute of Neurology, in accordance with UK legislation (Animals (Scientific Procedures) Act, 1986) and commensurate with the recommendations of Drummond (2009). A range of different anaesthetic regimens were used, which are listed in Table 1, together with the numbers of animals used under each regimen.
Table 1.
Anaesthetic regimens employed
| Anaesthetic and dose | No. of animals |
|---|---|
| Ketamine/xylazine | |
| Induction, i.p., 100 mg kg−1, 10 mg kg−1, respectively, then supplements of ketamine/xylazine (same proportions), i.v., as required. | 6 |
| Urethane | |
| 1.4 g kg−1, i.p. | 3 |
| Ketamine only | |
| 100 mg kg−1, i.p., then supplements, i.v., as required. | 1 |
| Halothane only | |
| Induction 5%, maintenance 1–2% for surgery and 0.4–1% for recording. | 2 |
| Fentanyl and ketamine, then α-chloralose | |
| Induction, fentanyl, 2 mg kg−1, plus ketamine 50 mg kg−1, i.p., maintained with ketamine and fentanyl i.p., then i.v. as required. For recordings, α-chloralose (20 mg kg−1, i.v.). | 1 |
| Halothane then α-chloralose | |
| Halothane: induction 5%, maintenance 1–2% during surgery, titrated to α-chloralose (30–80 mg kg−1, i.v.) for recording. | 12 |
| Ketamine/xylazine then decerebration | |
| As above, until decerebration. | 5 |
| Halothane then decerebration | |
| Induction 5%, maintenance 1–2% during surgery. Lowered to 0.5% at start of decerebration; turned off once decerebration completed. | 21 |
Suppliers: ketamine, Vetalar, Pfizer, UK; xylazine, Rompun, Bayer plc, UK; urethane, Sigma; halothane, Merial Animal Health Ltd, UK; fentanyl, Sublimaze, Janssen-Cilag, UK; α-chloralose; α-Chloralose-HBC complex, Sigma (doses cited above are the equivalent doses of α-chloralose; actual doses of the complex were 10 times higher).
Surgical procedures
Atropine sulphate (60 μg, i.m., Hameln Pharmaceuticals Ltd, UK) was administered to minimize airway fluid secretion. Rectal temperature was maintained at 37–38°C with a thermostat-controlled heating blanket (Harvard). The jugular vein was cannulated for the administration of anaesthetic supplements and fluids, and the trachea for mechanical ventilation. The right carotid artery was cannulated for the measurement of blood pressure and bilateral vagotomies performed. In the decerebrate experiments, the left carotid artery was also prepared, with a loose tie around it. Sodium lactate solution (Hartmann's solution, Baxter Healthcare Ltd) was administered periodically (up to 1 ml h−1) and a plasma substitute (Gelofusin, Braun Medical Ltd) was infused as required (both i.v.), to stabilize the animal's fluid balance and to maintain blood pressure within physiological limits. A mean arterial pressure above 80 mmHg was maintained for nearly all of the recordings, though occasionally, towards the end of an experiment, a decline to 60 mmHg was accepted, but only if the pattern of respiratory discharges remained stable (see Results).
In most experiments, neuromuscular blockade was used (pancuronium bromide; 0.3 mg h−1i.v., Hospira UK Ltd). Before neuromuscular blockade, anaesthesia was maintained at a level where the animal showed no more than a minimal withdrawal reflex to a noxious paw pinch. After neuromuscular blockade, the depth of anaesthesia was assessed from the recordings of blood pressure and the respiratory discharges. Only minimal transient changes of blood pressure, heart rate or respiratory pattern following a similar pinch were allowed before anaesthetic supplements were administered. Particular care was taken to monitor anaesthetic levels with the use of ketamine/xylazine, where frequent supplements were needed (see Results) and with halothane alone, which was eventually reduced to an inspired concentration of 0.5% or 0.4% in the two animals involved, though only at times of 12 h and 11 h, respectively, after induction. Artificial ventilation was carried out using O2-enriched air at a rate of 100 cycles min−1. Expired CO2 was monitored at the trachea, the stroke volume adjusted to bring the end-tidal CO2 fraction to about 4% and then CO2 was added to the gas mixture to increase the end-tidal CO2 level to a value adequate to give brisk respiratory discharges in the thoracic intercostal nerves, usually around 7–8%.
Animals were supported, prone, by vertebral clamps usually on the T6 and T12 vertebrae and by a strong ligature tied into the lumbar fascia. The head was supported in different ways according to the experiment. If there was to be no craniotomy, the nose was tied onto a bite-bar. For medullary recordings in anaesthetized animals, a plate was screwed to the skull. For decerebrate preparations a custom-made clamp was used with bilateral spikes screwed into the frontal and premaxillary bones. In experiments involving either medullary recordings or those involving spinal cord intracellular recordings, a laminectomy of 2–4 vertebrae (T7–T10) was made and the dura opened. In experiments with medullary recordings, a partial occipital craniotomy and a partial laminectomy at C1 were made and the dura opened. In these experiments a pair of stimulating electrodes (cut back tungsten microelectrodes) was placed in the left ventral spinal cord, usually at T11, for antidromic identification of bulbospinal neurones. A small patch of pia was removed from the surface of the medulla on the right side, caudal to Obex and a pressure plate was lightly applied to the medullary dorsum to minimize movement.
For a variable number of intercostal spaces on the left side (T5–T11, depending on the experiment), the external and/or the internal intercostal nerves (Smith & Hollyday, 1983) were cut and their central ends were mounted on pairs of platinum wire electrodes for recording or for stimulation. Some experiments also included the preparation of external and/or internal intercostal nerves from the right side of one or two intercostal spaces. More details are included in the Results section.
In experiments involving intracellular recordings, bilateral pneumothoraces were made to minimize intrathoracic pressure fluctuations and thus to reduce the movement of the cord. An end-expiratory pressure of 2–3 cmH2O was applied to prevent atelectasis. For all experiments except those involving EMG recordings, a paraffin oil pool was constructed from skin flaps, which submerged both the dissected nerves and the exposed spinal cord.
For decerebration, the left carotid artery was tied. A wide craniotomy was made, with ligation of the sagittal sinus. Animals were artificially ventilated and all brain tissue rostral to the superior colliculus was removed by use of a blunt spatula and aspiration. To minimize further bleeding, the exposed brainstem was covered with Spongostan (Johnson & Johnson Medical, UK) soaked in saline.
At the end of the experiment, the animal was killed with an anaesthetic overdose.
Recordings
Nerve discharges
Many of these recordings come from experiments where cross-correlation analyses were intended between EBSNs and the nerve discharges, so the procedure was simply to make successive recordings, typically 30 min duration, of the discharges of each EBSN, together with efferent discharges in up to five of the nerves. The EBSN properties and the cross-correlation analyses will be reported elsewhere, but some of the EBSN discharge patterns are relevant to interpreting the expiratory motoneurone discharges, so will briefly be reported here. The EBSNs were recorded in the right medulla in a similar region to that reported by Shen & Duffin (2002), centred at about 1.5 mm caudal and lateral from Obex and around 1.5 mm deep. Glass microelectrodes, tip diameter 2.6–2.8 μm, filled with 3 m NaCl were used, with conventional amplification. Units were identified as bulbospinal by antidromic activation from T11. A few shorter recordings were also made to document the progression of the activity patterns throughout the experiment. Neuromuscular blockade, with an elevated level of CO2 (see above) was employed throughout (see above). Nerve and EBSN recordings were band-pass filtered (300 Hz–3 kHz) and usually stored both on magnetic tape and in a computer via a 1401 interface and Spike 2 software (Cambridge Electronic Design, Cambridge, UK).
Nerve discharge data also came from the intracellular recording experiments (de Almeida & Kirkwood, 2010), which consisted of a series of recordings, each from one motoneurone, together with the efferent discharges from an external intercostal nerve (T6 or, for one experiment, T9). The latter were required as an indicator of respiratory phase (see Results for definition of this). Periods of recordings from two or three intercostal nerves were also made in some of these experiments specifically for analyses of the patterns of nerve discharges.
EMG recordings
Because the patterns observed in the nerve discharges were different from those in other species and since different species are known to differ in the details of their intercostal innervation (see Discussion), it was considered important to verify the activity patterns of the muscle layers independently of the nerve discharges. Recordings were made from three rats anaesthetized with halothane then α-chloralose and from three decerebrates (after halothane). All these six animals breathed spontaneously via a Y-shaped tracheal cannula, with CO2 being sampled just proximal to the Y-junction. They were supplied, via one arm of the Y, with a gas mixture: air supplemented with O2 and CO2, the CO2 fraction being adjusted to provide an end-tidal level similar to that in the nerve or intracellular recording experiments (see above). A small resistance (a short length of smaller diameter tube) was placed on the other branch of the Y, exiting to the atmosphere. A flow signal, the pressure difference across this resistance, was used to indicate the timing of inspiration and expiration, as was a recording of efferent discharges from an external intercostal nerve on the right side, for comparison with the other experiments. This nerve, on platinum wire electrodes, was submerged under petroleum jelly.
On the left side, the skin and superficial muscles were reflected, as was the fascia over transversospinalis, longissimus dorsi and iliocostalis muscles. Serratus posterior muscles were reflected with this fascia. Transversospinalis, longissimus dorsi and iliocostalis were removed and surface EMG recordings were made using platinum wires placed approximately 2 mm apart at various locations on the exposed intercostal muscles of the left side (T6–T11). The order at which recordings were made varied between experiments, according to the results obtained. For individual intercostal spaces, levator costae and/or external intercostal muscle were removed and nerve branches cut as required, so as to determine the source of a given phase of muscle activity at a given site, largely through a process of elimination. In order to preserve good vision of the fine nerve branches etc., an oil pool was not used, but unrecorded muscle was kept moist by saline-soaked cotton and exposed surfaces of recorded muscles were moistened periodically with saline. EMG recordings were filtered, usually with the same band-pass filter settings as the nerve recordings (see above), and the data similarly stored. These filter settings were used because they helped eliminate the movement artefact that sometimes occurred with wire electrodes on the muscle surface.
Anatomy
Observations of the general arrangement and the innervation of the dorsal musculature of the caudal thoracic segments were obtained during the dissections for the nerve and EMG recordings. Additional information about the arrangement of the intercostal muscle layers was obtained post mortem from three other rats which had been perfused for histology in a different project. These had been anaesthetized with ketamine plus xylazine or α-chloralose (as above) and transcardially perfused with a saline rinse followed by 0.5 l of 10% formol saline or of 4% paraformaldehyde in phosphate buffer. The dorsal part of the thoracic wall and the spinal column were removed and photographed from the pleural surface, following which, for one animal, a piece containing the proximal parts of a few intercostal spaces was excised, decalcified in HCl and formic acid, rinsed then incubated in 0.1 m phosphate buffer with 25% sucrose for 48 h. Frozen sections were cut in the parasagittal plane at 100 μm, mounted on subbed slides, stained with toluidine blue, dehydrated, coverslipped with DePeX (VWR International Ltd, UK) and examined with a microscope (Axioskop, Carl Zeiss, Germany). Outlines of the muscle layers were traced by the use of a drawing tube attached to the microscope.
Results
Anatomy of the dorsal region of caudal intercostal spaces
Successive stages of the dissection of a typical intercostal space, following the removal of the long back extensors, are shown in Fig. 1A, indicated as three adjacent spaces. Initially, as shown on the left-most (rostral) space, the external surface of the external intercostal and levator costae muscles were exposed. Removal of levator costae (Fig. 1A, middle space) revealed the proximal border of the internal intercostal muscle, plus the external face of the innermost layer, the subcostalis muscle (Smith & Hollyday, 1983), whose fibres run parallel to the internal intercostal layer. In the cat, the equivalent layer, ‘intracostalis’, is often absent or very thin, perhaps only 1 or 2 fibres thick (Sears, 1964a), as it is in the dog (A. De Troyer, personal communication). However, in the rat we found it to be considerably thicker, as illustrated in the histological section (Fig. 1C). The section also shows that this muscle layer passes under each rib, so that many or all of the muscle fibres of this layer must span at least two segments, as reported for human subcostalis muscle in the caudal segments (Walmsley, 1916) and as mentioned as ‘bisegmented’ by Sakamoto et al. (1996). In fact, this layer is readily visible by viewing the pleural surface of the rib cage (Fig. 1B). Visual inspection suggests the layer is present, with fibres crossing the ribs, from rib 12 to at least as rostral as rib 3 and is thicker proximally than distally, but we have not systematically investigated this.
Figure 1. Anatomical details of caudal intercostal segments in the rat.
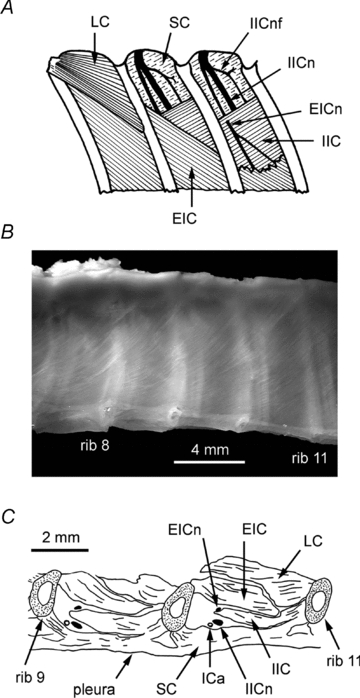
A, schematic diagram of the dissection for recording intercostal nerve discharges or EMG. Dorsal view of the left side: the intercostal space at the left shows the appearance after the removal of transversospinalis, longissimus dorsi and iliocostalis muscles; the middle one shows the appearance after levator costae has been removed; the final intercostal space shows the appearance after removal of the proximal part of the external intercostal layer (see text for more details). B, view of the pleural surface of the rib cage in a fixed specimen, showing the proximal part of intercostal spaces 7–10. The fibres of subcostalis muscle can be seen between the ribs and the pleura, running diagonally (top right to bottom left). The internal intercostal nerve (and in some cases the intercostal artery) can also be seen through this layer of muscle, caudal to each rib. C, drawing of a parasagittal histological section through the proximal part of two adjacent intercostal spaces. The plane of section is through the proximal border of both the external and the internal intercostal layers. Because these borders run diagonally (see A), neither muscle fully spans the intercostal space in this section. More laterally the internal layer would appear thicker. Note particularly that parts of subcostalis run below each rib. Abbreviations: EIC, external intercostal muscle; IIC, internal intercostal muscle; LC, levator costae muscle; SC, subcostalis muscle; EICn, external intercostal nerve; IICn, internal intercostal nerve; IICnf, internal intercostal nerve filament; ICa, intercostal artery.
Because the internal layer is not present in the most proximal region of the space, the intercostal nerves, which run in the space between subcostalis and the internal intercostal muscle were visible (beneath the posterior intercostal membrane) as soon as levator costae was removed. The external intercostal nerve was seen to divide from the internal intercostal nerve proximally, but to obtain a suitable length for convenient mounting on electrodes, some of the external intercostal muscle was removed, so as to expose the nerve after it has penetrated the internal layer near its proximal border, as illustrated in the most caudal intercostal space in Fig. 1A. Similarly, some of the internal intercostal muscle was removed to prepare the internal intercostal nerve (not illustrated).
As in the cat (Sears, 1964a,b;), each of the two intercostal nerves gives off a number of fine branches, ‘filaments’, which innervate their respective muscle layers. For the external layer, the most proximal of these (Fig. 1A, most caudal intercostal space) was usually tied in with the remaining external intercostal nerve for recording or stimulation. In some experiments the most proximal filament of the internal intercostal nerve was separately prepared for recording. This filament was always present, in the position illustrated in Fig. 1A, and is the direct analogue of a similar filament in the cat (cf. Bainton & Kirkwood, 1979). Here, this filament always diverged to innervate both subcostalis and internal intercostal muscles. We were particularly concerned that this most proximal filament might also innervate the external layer more distally. However, we never saw any branches of this filament entering the external layer, nor did this layer twitch when this filament was cut, which was seen for the other two layers.
Finally, in the cat, serratus muscle (serratus dorsalis cranialis) is innervated by a branch of the external intercostal nerve (Meehan et al. 2004, cf. Tani et al. 1994). We investigated whether this was also true for the rat (spaces T8–T10). Two innervation arrangements were observed, either from a distal branch of one of the external intercostal nerve filaments or, as illustrated by Smith & Hollyday (1983), from a branch directly from the internal intercostal nerve, penetrating through both intercostal muscle layers to reach serratus. At least one example of each arrangement was confirmed by muscle twitches, but such confirmation was not always achieved, which means that not all the branches were positively confirmed as being motor nerve branches. However, note that these branches were always very thin and at risk of being damaged in the rather destructive dissection required. Sometimes both branches were seen for the same intercostal space, on one occasion apparently joining to make a common trunk entering serratus muscle.
Efferent discharges in the intercostal nerves
General occurrence of expiratory discharges
Recordings were made from three categories of nerves, the external intercostal nerve, the most proximal filament of the internal intercostal nerve (referred to as ‘internal intercostal nerve filament’) and the remainder of the internal intercostal nerve (referred to as ‘internal intercostal nerve’). All of these could show both inspiratory and expiratory bursts of activity (e.g. internal intercostal nerve, Fig. 2A; external intercostal nerve, Fig. 2B), but in all types of preparation the occurrence of expiratory bursts was dependent on the time in the experiment, as in Fig. 2A.
Figure 2. Cycle-to-cycle variability in expiratory discharges.
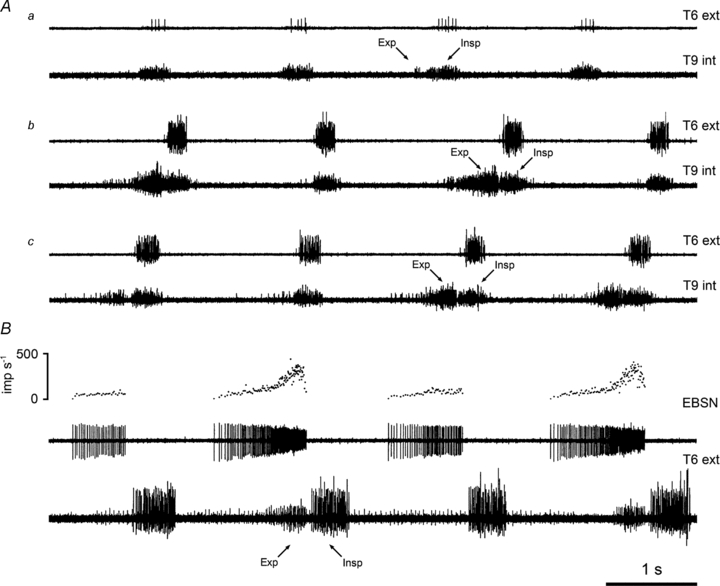
A, efferent discharges in the T6 external and the T9 internal intercostal nerves at three different times during one experiment with urethane anaesthesia: a, 9 h; b, 10.5 h; c, 13 h after induction. B, demonstration that intermittent expiratory efferent discharges represent a pattern of events in the medulla (ketamine/xylazine-anaesthetized rat): recordings from an EBSN (middle trace), its instantaneous frequency (top trace) and the T6 external intercostal nerve (bottom trace). Expiratory (Exp) and inspiratory (Insp) bursts indicated. Time calibration applies to both panels.
It could be several hours after the initial recordings for substantial expiratory discharges to appear. A steady state, with a constant pattern, might then ensue, followed eventually by a decline in both expiratory and inspiratory bursts, often the expiratory one first. The decline in the phasic bursts, often accompanied by an increase in tonic activity, usually coincided with a general deterioration of the preparation, as indicated by a falling blood pressure, and signalled the termination of the experiment. Animals in which a constant pattern did not develop were those in which either the appearance of the expiratory discharges was very slow, or which deteriorated relatively early. Although the time course varied between the different types of preparation, the sequence by which expiratory discharges appeared was consistent, save for the exceptions noted in the next section. It usually started by the occurrence of a short expiratory discharge in occasional respiratory cycles (perhaps 1 in 10), immediately preceding the inspiratory bursts which were used to define the cycles (Fig. 2Aa). The proportion of cycles including an expiratory burst usually then increased, often via stages with relatively regular patterns, such as an expiratory burst occurring in 1 cycle in 4, 1 in 3 or 1 in 2, eventually (but not always) arriving at a stage where every cycle showed such a burst (Fig. 2Ac). As the expiratory excitation increased, the duration of the expiratory burst often increased to occupy a greater proportion of expiration.
Note that despite the use of preparations under neuromuscular blockade and the absence of a phrenic recording there was never any difficulty in identifying the expiratory and inspiratory phases, most simply by reference to the illustrations from Duffin and his colleagues, who did include a phrenic recording in their experiments (e.g. Tian & Duffin, 1996). Identification of the phases was also helped by the fact that, although in some preparations, at least initially, only inspiratory discharges were seen, expiratory ones were never seen on their own. Further confirmation that our identifications were correct came from the spontaneously breathing animals in the EMG recording experiments (see below).
A large part of the variability of the expiratory discharges occurred at a supraspinal level, as was clear from the EBSN discharges, which were represented in most of the preparations reported here (43/51). Whenever variability in expiratory nerve discharges was seen, an equivalent variability was observed for the EBSNs. Most often, when an expiratory nerve discharge occurred in only some cycles, then the equivalent discharge seen in an EBSN was a weak expiratory ramp discharge in all cycles, with an additional strong burst in the last part of the expiration, corresponding to the nerve discharge (Fig. 2B). A few of the EBSNs only fired in those cycles that included expiratory motoneurone discharges, or only fired one or two spikes in those cycles that did not (see examples in de Almeida & Kirkwood, 2010). Note that the variable occurrence of the expiratory burst is not likely to be equivalent to the variable occurrence of phase II expiration (Ballantyne & Richter, 1986), since even the relatively slow ramp in the firing of EBSNs usually showed a delay after the end of inspiration, as in Fig. 2B. We presume this delay represents the duration of phase I expiration. Also note that the respiratory cycle was never locked to the ventilator in a 1:1 fashion (the rate was set high, 100 cycles min−1), but could sometimes be locked to a sub-harmonic of the ventilator frequency, such as 2:1 or 3:1, as judged by the respiratory frequency taking values very close to 50 or 33 cycles min−1.
Occurrence of expiratory discharges in intercostal nerves of different preparations under neuromuscular blockade
(1) Ketamine/xylazine. For 3 out of the 6 animals, expiratory activity was completely absent in both external and internal intercostal nerves. In the other 3, it was present intermittently for the external intercostal nerve, as it was in the internal intercostal nerve for 1 out of 2 animals tested. The variation was sometimes as described above (e.g. Fig. 2B), but this anaesthetic was an exception to the general rule in that sometimes the expiratory discharges first appeared as an occurrence in every cycle, during brief periods of apparently elevated excitability where the inspiratory discharges also increased. Further, the temporal variation depended on the timing of anaesthetic supplements, as illustrated in Fig. 3. Supplements were frequent for this anaesthetic (typically every 15–30 min).
Figure 3. Effect of an anaesthetic supplement with ketamine/xylazine anaesthesia.
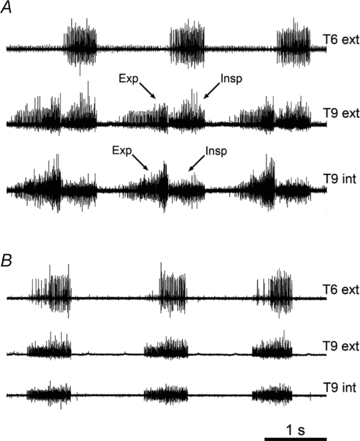
Recordings from two external and one internal intercostal nerve, as indicated. A, just before a supplement of anaesthetic. B, a few minutes after the supplement (2.6 mg kg−1 ketamine, 0.26 mg kg−1 xylazine).
(2) Urethane. In all three urethane experiments, no expiratory activity was detected at any time in the external intercostal nerves. Internal intercostal nerve discharges were recorded in two of these experiments, with expiratory activity present in one. This took time to develop (Fig. 2A) and was only present for 1–2 h before disappearing again.
(3) Ketamine only. One animal tested, with recording only from one external intercostal nerve. No expiratory discharges were seen.
(4) Halothane only. Two animals tested. Expiratory discharges eventually appeared in both external and internal intercostal nerves, including periods with expiratory activity in each cycle. In one animal, this only occurred 3 h after the halothane concentration was reduced to 0.5% (12 h after induction), in the other, after it was reduced to 0.4% (11 h after induction).
(5) Fentanyl plus ketamine, then α-chloralose. Fentanyl was tested in one animal, because we considered it possible that the expiratory activity might be promoted by μ-opioids, like the pre-inspiratory oscillator in the neonate described by Onimaru et al. (2006). Under fentanyl plus ketamine no expiratory activity was detected, but it did appear in both external and internal intercostal nerves about 2 h after switching to α-chloralose and persisted for a further 5.5 h.
(6) Halothane then α-chloralose. All recordings were made after switching to α-chloralose. Expiratory discharges were seen in the external intercostal nerves of 7 out of 9 animals and in the internal intercostal nerves of 7 out of 8 animals (examples shown in Fig. 4A and B). These discharges appeared only after some delay from the time of switching to α-chloralose (range 45 min to 8 h, median 3 h).
Figure 4. Typical combinations of expiratory and inspiratory efferent discharges, in various rat preparations.
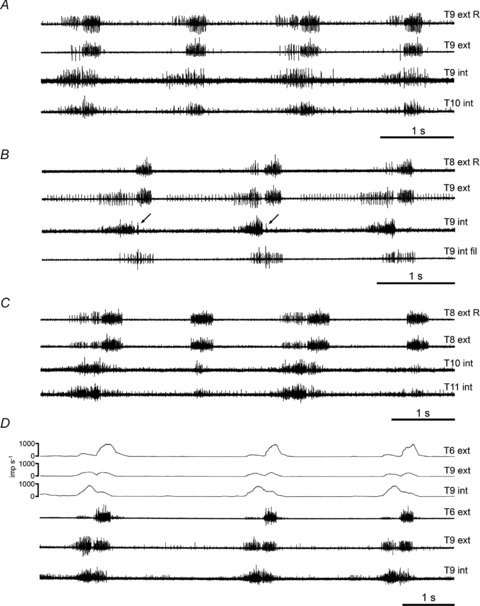
A and B, two different experiments, α-chloralose following halothane; C, decerebrate following ketamine/xylazine; D, decerebrate following halothane. Combinations of external and internal intercostal nerves in each animal, as indicated (R, nerve on right side (others on left side); int fil, filament of internal intercostal nerve innervating proximal internal intercostal muscle plus subcostalis). Arrows in B indicate very short inspiratory bursts in two of the three respiratory cycles (see text). Upper 3 traces in D show the (multi-unit) firing frequencies for each of the nerves in the lower 3 traces (spikes converted to events off-line by threshold crossing in Spike 2 software). Note that expiratory bursts are present in every cycle for A, B and D, but only in alternate cycles for C.
(7) Ketamine/xylazine then decerebration. Expiratory activity appeared in both external and internal intercostal nerves in all of four animals that had a sufficient survival time (example in Fig. 4C), but it took from 3 to 8 h to develop after decerebration. In a 5th animal, it appeared in an internal intercostal nerve in the last record taken, 3 h after decerebration.
(8) Halothane then decerebration. Expiratory discharges were seen in the internal intercostal nerves of all 16 animals tested and in the external intercostal nerves of 16 out of 18. These discharges (example in Fig. 4D) appeared within 1–3.5 h after decerebration (median 2 h). One of the two animals without discharges in the external intercostal nerve (not tested for the internal intercostal nerve) was one with a short survival time after decerebration.
It is evident from the above that the sampling of animals across different anaesthetic regimes was uneven. The sampling of the numbers and categories of nerves was also uneven, being dependent on the type of experiment (e.g. nerve recordings or intracellular recordings). Further, the sampling times, quoted above for the delay to the appearance of expiratory discharges, are approximate and dependent on factors such as the times of making recordings from successive medullary neurones. Nevertheless, because of our requirement to promote expiratory activation, it became evident that the most profitable regimens to use (with the possible exception of halothane alone) were halothane followed by either α-chloralose or decerebration, and these two provided most of the data presented here. As far as we can tell, the relative strengths of activation (e.g. in comparing different intercostal spaces, see below) were independent of the overall levels of activation, either within one regimen, or across all of the regimens. No obvious differences were seen according to whether or not the animals were prepared for intracellular recording.
Patterns of inspiratory and expiratory discharges in the different nerves
External intercostal nerve. In all animals, inspiratory bursts were observed in at least one external intercostal nerve (T5–T10, mostly T6 and/or T9). Comparisons were made between the more rostral and the more caudal segments in 25 animals (T6 vs. T9 for 21 of these). Assessments were made in terms of (multiunit) firing rate during the burst, often simply by eye (e.g. presence or absence of a burst), but also guided by frequency counts as illustrated in Fig. 4D. However, because of variability between individual runs, and uneven sampling, the judgements were made in broad terms. The inspiratory bursts were judged stronger rostrally in 11 animals, about the same in 9, and stronger caudally in only 5. Expiratory discharges were additionally seen, as described in the previous section. These were never seen in the more rostral segment without being present caudally and, when present, they were always stronger for T8–T10 than for T6–T7 (comparisons made in 24 animals) (e.g. Figs 3 and 4D).
Internal intercostal nerve. All of the recordings from the internal intercostal nerve were made from relatively caudal segments, with numbers of animals represented for each segment as follows (T8, 1; T9, 36; T10, 23; T11, 1). The occurrence of expiratory discharges was as listed above. Most often an inspiratory burst was present in addition to the expiratory one, but it could be that inspiratory bursts were seen alone or expiratory alone. There did not seem to be an obvious pattern to the occurrence or strength of the inspiratory bursts according to the anaesthetic regime, or according to the segment (within the limited sampling of segments made). Considering combined groups, inspiratory bursts were seen in the internal intercostal nerves of 14 out of 19 anaesthetized animals and in 19 out of 21 of the decerebrates. Figures 3 and 4 show the range of patterns observed. Note that for the external intercostal nerves, the time course of individual inspiratory bursts was almost always an incrementing ramp, as is typical for the phrenic nerve and is shown by both external and internal intercostal nerves in the cat (De Troyer et al. 2005). For some internal intercostal nerves here, this was also the case (Fig. 3), but for many, the inspiratory bursts were decrementing (Fig. 4B and C). Sometimes this was extreme, to the extent that the bursts appeared as only a few spikes at the very start of inspiration (e.g. Fig. 4B). When present, the decrementing time course occurred whether or not the inspiratory burst was preceded by an expiratory one (Fig. 4C).
Internal intercostal filament. In general, the most obvious hypothesis to explain the presence of inspiratory discharges in an internal intercostal nerve is that they are destined for parasternal, interchondral muscle (see Introduction), although this hypothesis is hard to sustain for the relatively strong discharges in T10 or T11 (Fig. 4A and C), since the ribs 11–13 do not attach to costal cartilages (Greene, 1968). However, the proximal internal intercostal filament allowed a direct approach to the question, because it appears to innervate specifically internal intercostal muscle and/or subcostalis muscle.
The internal intercostal filament was prepared in five animals, with recordings under ketamine/xylazine in one, halothane in one, α-chloralose following halothane in two and in one decerebrate following halothane. The T9 filament was used in three animals, the T10 in one, and both T9 and T10 in the fifth animal. Five of the six filaments showed the same pattern, namely bursts (approximately equal intensity) in both inspiration and expiration, as illustrated in Fig. 4B. However, in the ketamine/xylazine animal and initially in the two α-chloralose animals, the recordings showed bursts only in inspiration, the expiratory bursts appearing later in the α-chloralose animals, about 3.5 and 4 h after the switch to α-chloralose. The inspiratory bursts in the filament could also be decrementing. Close inspection of the recording of Fig. 4B on an expanded time-scale (not illustrated) revealed that each burst started with a single spike from each of several (perhaps 6 or 7) units, but then apparently only 2 or 3 of the units continued to fire throughout inspiration.
Serratus nerves. Recordings were attempted on a few occasions. Efferent discharges were seen in one of these: a single unit with a very low amplitude spike, firing a few spikes consistently in inspiration. The nerve concerned was a branch directly from the internal intercostal nerve in T11, in a decerebrate following ketamine/xylazine. At the time of recording, brisk inspiratory discharges, with weak expiratory discharges in alternate cycles, were present in the T8 external intercostal nerve. No weight should be put on the absence of discharges in the other serratus nerves tested because, as mentioned previously, they could easily have been damaged during the dissection.
EMG recordings
Two of the observations from the nerve recordings are contrary to expectations derived from published data from other species: the expiratory activation in the external intercostal nerve and the inspiratory activation in the internal intercostal nerve, particularly in its proximal filament. Although our observations during the dissections indicated innervation of the external and the internal intercostal muscles, respectively, by these two nerves, it is possible that very fine branches innervating other layers had been missed. We therefore made EMG recordings from the dorsal regions of these two muscle layers in spontaneously breathing animals, to check which patterns of activation were actually present in the muscles.
EMG recordings were made on the left with a simultaneous recording from a contralateral external intercostal nerve on the right, so as to directly link the EMG recordings with the nerve recordings described above. Airflow was also recorded, to confirm the identification of the respiratory phases (Figs 5–8). Initial recordings from the surface of levator costae muscles in several segments showed prominent inspiratory discharges, as expected from other species (Hilaire et al. 1983; Goldman et al. 1985; De Troyer & Farkas, 1989). To avoid cross-talk from these discharges (see below), recordings from intercostal muscles were generally made with levator costae muscle removed from that segment and the two adjacent segments.
Figure 5. EMG recordings in 3 different spontaneously breathing rats.
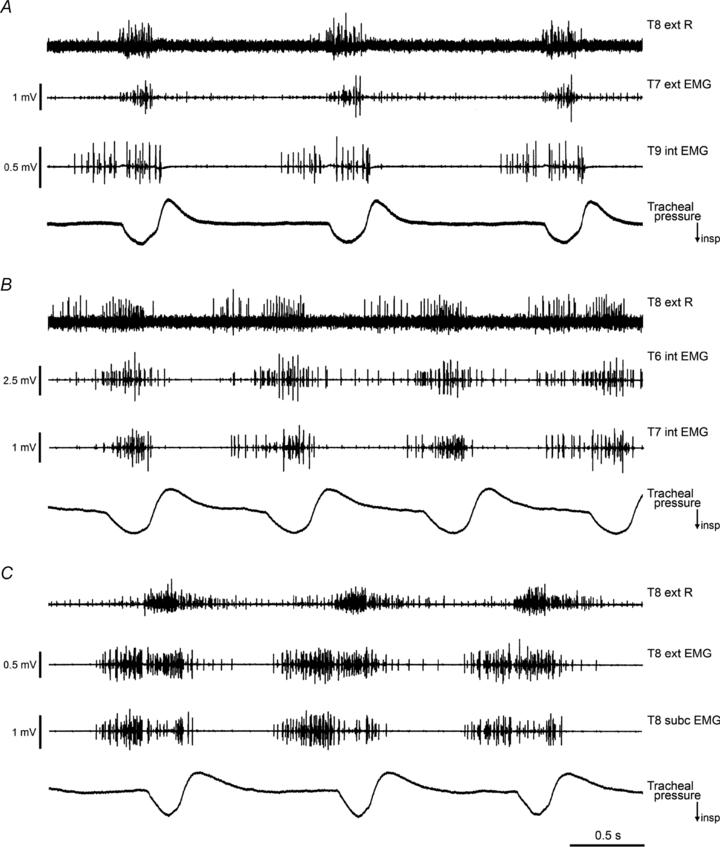
In each animal, the efferent discharges from the T8 external intercostal nerve on the right side are shown (T8 ext R), together with two EMG recordings on the left, from the surface of the external (ext) or internal (int) intercostal muscle layers or subcostalis (subc), as indicated. The tracheal pressure record indicates the phase of respiration, inspiration downwards. The intercostal nerves and muscles for all segments on the left were intact when these records were taken. Levator costae muscles had been removed from T7–T10 for A and from T6–T10 for B and C. Time calibration applies to all 3 panels. A, decerebrate following halothane, 2 h 25 min after decerebration. B, α-chloralose following halothane, 4 h 45 min after switching to α-chloralose. C, decerebrate following halothane, 2 h 50 min after decerebration.
Figure 8. Isolation of single motor units in subcostalis, firing in inspiration or in both inspiration and expiration.
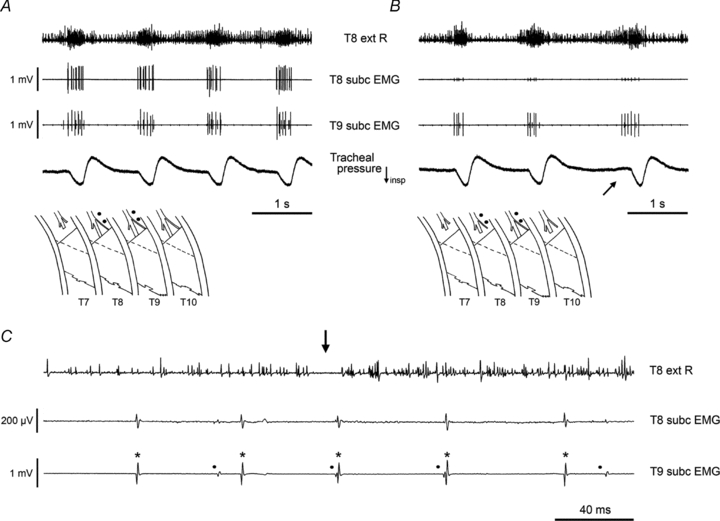
Diagrams in A and B indicate the recording sites on left subcostalis muscle in T8 and T9. EMG recordings are shown, together with the external intercostal nerve of T8 on the right side (top trace). Before (A) and after (B) section of the internal intercostal nerve and its filament in T8 and in T10. Intercostal space T7 had been extensively denervated and external intercostal muscle removed as indicated (see text for more details). Note increased expiratory discharge in the external nerve recording in the 3rd cycle of B, accompanied by an expiratory deviation in the tracheal pressure trace (arrow). C, extract from the nerve and EMG recordings in B, shown on an expanded time scale. The expiratory–inspiratory transition for the 3rd cycle in B is shown: note the gap in the external intercostal nerve discharge between the two phases (arrow). Two motor units remain active in the T9 record in B and C, their spikes indicated by the asterisks and dots in C. Both units are also detectable, though much attenuated, in the T8 record. Both units are active in both phases of respiration. Voltage calibration in A also applies to B. α-Chloralose following halothane.
Figure 5 shows recordings made at a relatively early stage in three of these experiments, before any intercostal nerves had been sectioned or intercostal muscle removed. At these times, both expiratory and inspiratory bursts had developed in each animal. Note that the inspiratory and expiratory bursts in the EMG are now defined as such by their relationship to the flow trace. Note also that the relationship of the bursts in the external intercostal nerve recording with the flow trace (particularly the separate inspiratory and expiratory components in panel B) confirms the correctness of the previous identifications of inspiratory and expiratory bursts, made in the nerve recordings under neuromuscular blockade. The EMG discharges at this time in the experiment were usually at their strongest. In Fig. 5C the expiratory activity was strong enough to create a visible expiratory deviation in the flow trace towards the end of expiration. The external intercostal nerve recording did not always show an expiratory discharge in these experiments. In the examples shown it is only convincingly present in panel B, though a weak burst had just started to develop at the time of the recording in C. It was notable that in only 2/6 of these animals did expiratory discharges in the nerve appear in an all-or-nothing fashion. In both of these, intense expiratory bursts appeared towards the end of the experiment (6 h after decerebration for one, 7.5 h after switching to α-chloralose in the other) but in both, the occurrences of the bursts were irregular (at their most frequent, separated by 1–6 cycles and 2–8 cycles, respectively). The corresponding EMG bursts were also intense, but in both animals the EMG recordings had previously shown plentiful expiratory activity for at least 5 h prior to this. In the remaining four animals, when the expiratory bursts appeared, their intensity increased gradually and in all cycles.
In two of the decerebrate animals in the EMG series, in contrast to any of the nerve recording experiments, a prominent post-inspiratory component was present in some of the recordings (usually in recordings with an inspiratory component) and in the external intercostal nerve, as shown in Fig. 5C. This component was clearest in the very earliest recordings, and faded as the expiratory component grew. Details of the patterns of activity in the different muscle layers and their verification follow.
Internal intercostal muscle (including subcostalis)
In all six animals the predominant pattern of discharge was inspiratory, with a variable expiratory component, i.e. a pattern very similar to the internal intercostal filament recordings described above. Figure 6A–D illustrates one set of observations which confirmed this, where we recorded from the proximal part of the internal intercostal muscle in T10. At the time of the recording, levator costae muscle and the dorsal part of the external intercostal muscle (up to about 7 mm distally) had been removed from both T9 and T10, the whole ventral ramus had been cut at the medial border of subcostalis in T9, and the external intercostal nerve had been cut in T10. The recording shows one relatively large motor unit active in expiration. As was common in such recordings, it appeared that this unit also fired through inspiration (Fig. 6E). However, this is not absolutely certain because, although there was only one unit with large spikes in expiration, two or three units with spikes of similar amplitude and shape were recruited during inspiration. Section of the internal intercostal nerve filament in T10 eliminated all of these large potentials, but left medium-sized potentials in inspiration (Fig. 6B).
Figure 6. Effects of nerve section on EMG recordings in one intercostal space.
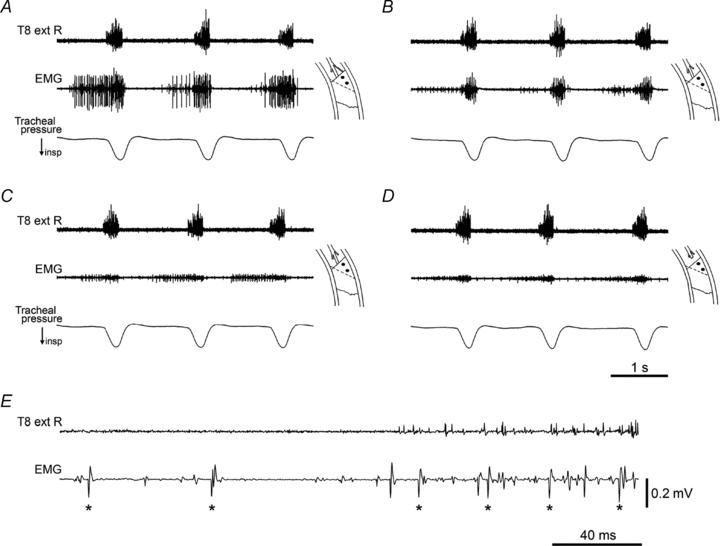
A–D, EMG recordings at one site shown in time order, with successive nerve or muscle sections. Diagrams indicate the recording site, at the proximal border of the left internal intercostal muscle in T10. In this and subsequent figures, the black dots indicate the positions of the two recording wires. Top trace is the recording from the external intercostal nerve of T8 on the right side. A, the proximal part of the external intercostal muscle in T10 and its nerve had been removed (dotted line in diagrams indicate its original proximal border), T9 had been denervated and the external intercostal nerve cut in T11. B, following section of the internal intercostal nerve filament. C, following additional removal of levator costae muscle in T11. D, following additional section of the internal intercostal nerve in T10. See text for more details. E, extract from the nerve and EMG recordings in A (start of 2nd inspiration), shown on an expanded time scale. Large EMG spikes (asterisks) show a probable single motor unit that was active in both inspiration and expiration. Time calibration in D applies to A–D. Voltage calibration in E applies to A–E. α-Chloralose following halothane.
This effect of the nerve filament section is good evidence that the large spikes represented activity in the internal intercostal or subcostalis muscles. The same effect was seen for eight individual segments (all of T6–T10 being represented, in 5 animals), either for recordings from this proximal part of the internal intercostal muscle or from subcostalis. In general, however, there are potential confounding factors with such measurements. One is that of cross-talk. This is well recognized as being likely to occur both within one intercostal space, where recording from one intercostal layer can very easily show activity from the other layer(s), and between adjacent spaces (Legrand & De Troyer, 1999). In our experiments, the former was probably universal, and the latter could also be a strong effect, as may be seen in Fig. 6. Here, the medium-amplitude inspiratory spikes in Fig. 6B (remaining after section of the internal intercostal nerve filament) were eliminated by removal of T11 levator costae muscle (Fig. 6C), and therefore almost certainly arose via cross-talk. The remaining small expiratory spikes in Fig. 6C were eliminated by section of the remaining internal intercostal nerve of T9, leaving an even smaller, mostly inspiratory, signal of about 0.1 mV in amplitude (Fig. 6D). Such a low amplitude discharge almost always remained after section of all the intercostal nerves of a segment, even with section of all the neighbouring intercostal nerves. We presume it originated from a remote source, such as the diaphragm.
Direct observations of cross-talk also came from dual recordings. Figure 7 shows a pair of recordings from the proximal region of the internal intercostal muscle of T6 and T7. At the time of the recording, spaces T8 and T9 had been extensively denervated, the external intercostal muscle removed from the proximal regions of both T6 and T7, and the internal intercostal nerve sectioned in T7. Despite the T7 nerve section, a brisk discharge was present in the T7 record (inspiratory, but sometimes with a small expiratory component, as in the first respiratory cycle in Fig. 7A). Cross-talk from T6 is the best explanation for this discharge, since, as illustrated in an expanded version of the same recordings (Fig. 7C), it may be seen that virtually all of the large unit potentials in the T7 record are synchronous with similar potentials in T6. Consistent with this, when T6 ventral ramus was cut proximally, the T7 discharge disappeared and the T6 discharge was severely reduced (Fig. 7B), the remaining signal presumably originating in T5.
Figure 7. Effects of nerve section on EMG recordings in two adjacent intercostal spaces.
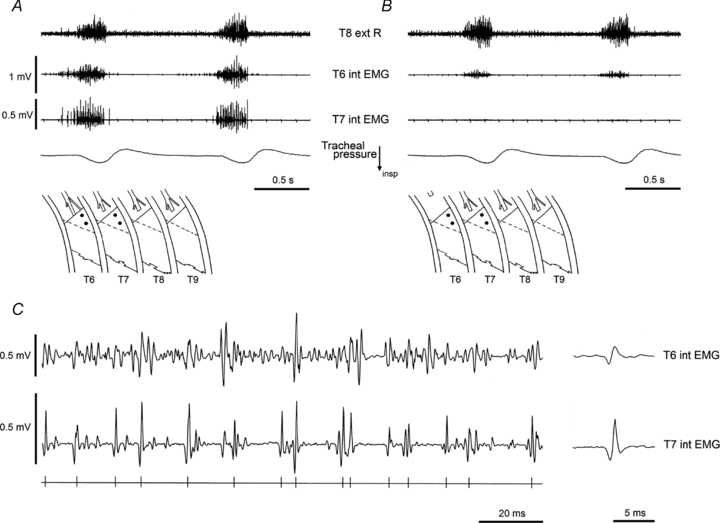
Diagrams in A and B indicate the recording sites at the proximal border of the left internal intercostal muscle in T6 and T7. EMG recordings shown together with the external intercostal nerve of T8 on the right side (top trace). Before (A) and after (B) section of the internal intercostal nerve and its filament in T6. Intercostal spaces T7–T9 had been extensively denervated (see text for more details). C, extract from the nerve and EMG recordings in A (middle of 2nd inspiration), shown on an expanded time scale, to illustrate cross-talk. Bottom trace indicates times of occurrence of the larger spikes in T7 record. Individual EMG spikes can be found in the T6 raw record corresponding to each of the selected T7 spikes. Average voltages from each channel, triggered from these times, are shown at the right. α-Chloralose following halothane.
The maximum size of spikes positively identified as arising by cross-talk between spaces (seen in paired recordings from subcostalis) was 1 mV, but potentials as large as 0.7 mV were also seen in a recording from the surface of an external intercostal muscle, synchronous with spikes recorded from subcostalis of the adjacent space. It is not surprising that the largest examples of cross-talk involved subcostalis, given that the muscle fibres may span two segments (Fig. 1B and C). For such an instance, the term ‘cross-talk’ may not be strictly accurate, but we have retained it for simplicity.
A second confounding factor is the possibility that nerve section could lead to a general reduction of motoneurone excitability, with the expiratory discharges most likely to be affected. This may be particularly the case for the internal intercostal nerve, which carries large numbers of afferents, including muscle spindles from internal intercostal and abdominal muscles. In theory, this factor could compromise any deduction made about the origin of a particular discharge that was based on the elimination of that discharge by nerve section. However, we believe that the deductions mentioned above about the origin of the inspiratory discharges in the proximal internal intercostal and subcostalis layers, on account of their elimination by section of the internal intercostal nerve filament, are relatively safe from such criticism, because the numbers of afferents carried in this filament are relatively small.
With regard to both of the possible confounding factors, the observations that are safest overall are those of surviving activity recorded from electrodes on the layer of muscle in question, where all the surrounding areas had been denervated. One such example is illustrated in Fig. 8A, where both recordings are inspiratory. Here the recordings came from the subcostalis layer of two segments, T8 and T9, and the surrounding denervation is shown in the inset diagram. The discharges in both segments are relatively ‘thin’, with only 2 or 3 large units in each, probably on account of the decreased excitability resulting from the extensive nerve section. Inspection of individual spikes for these large units showed their timings to be quite different in each record, so there was no cross-talk between them. It is possible that the recording in T9 might have arisen by cross-talk from T10, but the recording in T8 could not have arisen in this way.
Consistent with this, subsequent section of T8 internal intercostal nerve and filament eliminated the large spikes in the T8 record, but left the two spikes (one large, one intermediate in size) in the T9 record. Moreover, these two units survived section of the T10 internal intercostal nerve and filament, as shown in Fig. 8B and C. The recording of Fig. 8B started about 7 s after the T10 nerve section, during the period of raised excitability which often followed the nerve section. Excitability was raised to the extent that in the third cycle in Fig. 8B, expiration in general was promoted, as is shown both by the enhanced expiratory burst in the contralateral external intercostal nerve recording and by the small expiratory deflection in the airflow (arrow). At this time, both of the two units in the recording from subcostalis in T9 fired a few spikes at the end of this expiration as well as in inspiration. This is verified in the expanded recording of this cycle in Fig. 8C. Some cross-talk was present so that each of the units in T9 also appeared as small spikes in the T8 record. In this example, the cross-talk is of positive value, in that it gives a particularly clear signature to both of the motor units, so that their identification as firing in both phases of respiration is certain.
The answer to the first question addressed by the EMG recordings is thus positive: the proximal part of internal intercostal and subcostalis muscles are activated during inspiration, as well as during expiration. At least some motoneurones are activated during both phases.
External intercostal muscle
The second question, whether the external intercostal muscle is activated during expiration, turned out to be much harder to answer. Recordings from the dorsal part of this muscle did often show both inspiratory and expiratory bursts (Fig. 5C). However, successive stages in the necessary control procedures, i.e. sectioning of the various nerves, nearly always led to a reduction, and then the abolition of the expiratory component. This could be ascribed either to elimination of cross-talk, particularly when the sectioned nerve was the internal intercostal nerve of the same segment, or to peripheral deafferentation leading to decreased excitability, as described above. The EMG experiments have therefore not yet answered this question.
Discussion
There are two important new observations in this study. The first is the variability of the expiratory drive to thoracic motoneurones, in particular its all-or-nothing occurrence in individual respiratory cycles. This variability, observed in the adult rat in vivo, has general significance with regard to respiratory rhythm generation. The second observation is that of inspiratory or biphasic activation of the proximal internal intercostal muscles. This represents a spatial pattern which is different from other species, and which could have implications for our understanding of the functional role of the intercostal muscles. Some new details of the anatomy and the innervation of these segments have also been presented. These are important for methodological reasons, allowing assignment of the observed activity to the correct muscle layers.
Innervation of the intercostal muscles of the caudal segments
The situation in the cat is very straightforward (Sears, 1964a,b; Meehan et al. 2004). Each intercostal space is innervated by the motoneurones of one segment of the spinal cord. The external muscle layer is innervated entirely from the branches of a single division of the ventral ramus, the external intercostal nerve, while the internal layer, plus the (vestigial) intracostalis (here, subcostalis) is innervated from the remainder, the internal intercostal nerve. Exactly the same arrangement is seen in the dog (Legrand & De Troyer, 1999; A. De Troyer, personal communication). The question is, how similar is the arrangement in the rat? This question arose simply because, despite the general appearance of a scheme of innervation that is almost identical to that in the cat and the dog, the patterns of discharges seen in the nerves were different. Moreover in other species, the scheme of innervation certainly is different. In the macaque and in the human, the main intercostal nerve trunk divides into multiple branches. Although a branch similar to the external intercostal nerve may be present, innervation of the external layer is not exclusively via this branch and various branches can supply both the external and the internal muscle layers (Sakamoto et al. 1993, 1996). In the rabbit, something rather similar pertains (T. A. Sears, personal communication). However, the EMG recording experiments here, including a variety of nerve section procedures, gave absolutely no evidence (within the caveats already expressed) to support any possibility other than selective innervation of each muscle layer by its respective, separate nerve. Of course, many of the nerve section procedures employed here did not provide completely definitive observations related to innervation, because of the interfering effects of cross-talk or changes in motoneurone excitability. However, the main point is that, in individual recordings where all possibilities of non-selective innervation had been eliminated by nerve section or muscle removal, the unusual patterns of motoneurone discharge remained. This was certainly the case for the inspiratory or biphasic excitation of the internal intercostal or subcostalis layer(s). Expiratory excitation of the external intercostal muscle remained unproven from the EMG experiments, but supporting evidence is provided in the accompanying paper (de Almeida & Kirkwood, 2010).
The question of the exclusive innervation of each intercostal space from one spinal segment must next be considered, in particular because of the recent claims of Giraudin et al. (2008), following retrograde tracing experiments and EMG recordings from an in vitro neonatal rat preparation. They concluded that each intercostal space is innervated by motoneurones of several spinal cord segments. However, these authors took little account of the possibility of spread of tracer at the site of application and no account of possible cross-talk at the recording site. Thus, with regard to the segmental origin of the motoneurones innervating a given intercostal space, we have discounted their results and instead taken as a starting point the clear result from Smith & Hollyday (1983) that each intercostal nerve contains motor axons from only one spinal segment (see Meehan et al. 2004 for other references). We then need to consider the question of whether those axons innervate only the muscles of one space. In humans there are intercommunicating nerve branches between the segmental nerves (Sakamoto et al. 1996). These have not been reported in the cat, dog or macaque, nor did we see any evidence for them in the rat, although no detailed search for them was made from the pleural side (which should be the most critical). However, their possible existence in the rat must be considered because of our observations of prominent ‘cross-talk’ between adjacent segments. This possibility could be additional to our alternative explanation of bisegmental muscle fibres in subcostalis.
In fact, there is evidence in the rat for some motor axons running below the rib from one segment to the next, not as intercommunicating branches between segmental nerves, but rather, during development, as terminal branches of internal intercostal motor units that have some muscle fibres in the segment rostral to the nerve concerned (Dennis et al. 1981). Most of these extra-segmental branches disappear with elimination of multineuronal innervation around the time of birth. Such motor units with muscle fibres in both segments could provide an explanation for some of our cross-talk observations. Note, however, that the extrasegmental innervation observed by Dennis et al. (1981) did not involve bisegmental fibres since, in their study, the muscle fibre activation in the rostral segment survived section of the muscle fibres in the caudal segment. Also, note that at least some of the large cross-talk potentials in our study could not have arisen from the innervation of motor units described by Dennis et al. (1981), which was derived from axons in the more caudal segment. In contrast, in the example of Fig. 7, the potentials in T7 were eliminated by section of the nerve in T6. Bisegmental muscle fibres innervated (in this case) from the rostral segment seem to provide the best explanation here. It is not clear why Dennis et al. (1981) did not observe bisegmental fibres; perhaps such fibres are rare in the more rostral intercostal spaces or the more ventral regions they may have investigated, or are rare at early ages.
In summary, all the available evidence supports the view that the external and internal/subcostalis layers in the rat are innervated exclusively by the external and internal intercostal nerves, respectively. However, for a given intercostal space, some parts of the internal intercostal or subcostalis muscles may be innervated from an adjacent segment, in addition to their own segment.
Expiratory activity in the thoracic nerves under different conditions
In the present study, the appearance of expiratory activity was dependent both on the anaesthetic regimen employed and the time in the experiment. Detailed comparisons between the different anaesthetics should not be attempted from our data. The experiments were not set up for systematic comparisons; rather they represent a progression to establish a suitable preparation for using correlation and spike-triggered averaging methods. To that end, two regimens, halothane followed either by α-chloralose or by decerebration gave the most consistent expiratory discharges. The choice between these two may not be critical: with α-chloralose, the preparations were more stable and could last longer, but the expiratory discharges took more time to develop.
We can certainly conclude that all the anaesthetics depress expiratory activity, but the most interesting aspect is the manner by which the activity appeared, the gradually increasing frequency of the all-or-nothing occurrence of expiratory bursts as the level of anaesthesia became lighter. Recordings such as Fig. 2B show a behaviour almost identical to that displayed by the in situ preparation of Abdala et al. (2009), which was interpreted as indicating the operation of an independent expiratory ‘oscillatory mechanism’ activated under conditions of a high respiratory drive. The late expiratory activity in that study was associated with the integrity of the retrotrapezoid/parafacial region of the rostral medulla, thus implicating a mechanism similar to that proposed by Feldman and colleagues (Feldman & Del Negro, 2006) for the neonate. It seems very likely that such a mechanism was operating in our preparations. On this assumption, it is of interest that when the activity first appeared, the ‘expiratory’ oscillator frequency was apparently very low (10 or more times slower than the ‘inspiratory’ oscillator). The rate increased as anaesthesia lightened, until entrainment became 1:1. At some intermediate stage, the situation can therefore be regarded as the converse of that reported by Janczewski et al. (2002) and by Mellen et al. (2003), where the pre-Bötzinger oscillator was slowed by μ-opioids and the rostrally located expiratory oscillator determined the respiratory frequency. Inspiration then occurred at ‘quantal’ intervals. Here we suggest that the expiratory oscillator was much more severely slowed by anaesthetics than the inspiratory one, and then ‘quantal expiration’ occurred.
One difference between our preparation and that of Abdala et al. (2009), made from juvenile animals, is the low occurrence of post-inspiratory excitation. This was also the case in intracellular recordings from expiratory motoneurones (de Almeida & Kirkwood, 2010), where the smallest, sub-threshold effects might be detected. Here, the only post-inspiratory activity that was seen (e.g. Fig. 4A, T10 int; Fig. 4D, T6 ext; Fig. 5C) was associated with inspiratory activity, as is common for phrenic or external intercostal discharges. EBSN discharges rarely showed it. The double burst ‘pre-I’ pattern (Suzue, 1984) which includes the post-inspiratory component has been regarded as one of the signatures of the rostral expiratory oscillator in the medulla (‘hallmark’ in the discussion of Abdala et al. 2009). We suggest that the variable, all-or-nothing occurrence of expiration in our recordings is sufficient evidence for a separate oscillator being active, without this signature being present.
The recordings of Iizuka & Fregosi (2007), from adults in vivo (urethane anaesthetized or decerebrate), also showed partial synchronization between inspiratory and strong expiratory bursts in hypercapnia, with little signs of a post-inspiratory component. We suggest (cf. Fortuna et al. 2008) that the occurrence of such a component depends mostly on age. Fortuna et al. (2008) took a slightly different view from their experiments in adults because they saw a post-inspiratory component in hypoxia, but not in hypercapnia. We suspect, however, that the expiratory oscillator had simply not been recruited in their experiments with hypercapnia, consistent with the concentration of halothane they quoted (‘∼1%’). This was rather higher than was the case in our two animals at the times when intermittent strong expiratory discharges eventually appeared, under the same anaesthetic. The view we are promoting of the recruitment of the expiratory oscillator in the adult without a post-inspiratory component is also contrary to the predictions of the model of Wittmeier et al. (2008), which were extrapolated from the in vitro neonatal preparations, and for which a post-inspiratory component is a central feature. A possible explanation is that activation of this oscillator in the adult still involves a post-inspiratory component, but that this component is suppressed in the output, for instance at the level of the EBSN. Post-inspiratory inhibition has been shown to occur in EBSNs in the cat (Ballantyne & Richter, 1986) and in unidentified augmenting expiratory neurones in the rat (Shen et al. 2003). Verification awaits recordings from retrotrapezoid/parafacial expiratory neurones of the adult under conditions where the expiratory oscillator is shown to be active.
Arguments about which of the two oscillators is more fundamental (see discussion in Abdala et al. 2009 for references) can become rather artificial. It is well recognized that the respiratory oscillator can be reconfigured for different behaviours, such as emesis in the cat (Miller et al. 1987), or defaecation in the dog (Fukuda & Fukai, 1988) or presumably speech in humans. Moreover, all physiological preparations represent a somewhat abnormal state. In isolated neonatal brainstem–spinal cord preparations (Onimaru et al. 1988; Janczewski et al. 2002), or in neonatal and juvenile rats with opioids (Janczewski et al. 2002; Janczewski & Feldman, 2006), the expiratory oscillator may dominate; in the juvenile in situ preparation (Abdala et al. (2009) or in the decerebrate adults here, the inspiratory one does. In this vein, we would also disagree with the conclusion of Abdala et al. (2009) that the late expiratory bursts seen in their preparation are an expression of extreme conditions, or as they put it, ‘pathological’. This case cannot be directly argued from our results, because we used a high level of CO2, a similar condition to that of Abdala et al. (2009) when they recorded late expiratory bursts. However, the argument can be made from the observations of expiratory discharges in the external intercostal nerves, which in our experiments were associated with the individual cycles where the strong late expiratory activation occurred. If, therefore, these represent activation of the expiratory oscillator, then the similar discharges observed by Tian & Duffin (1996) indicate that the expiratory oscillator was most likely operating in their animals. These animals were maintained at eupnoeic levels of CO2. A possible reason why activation of this oscillator did not occur under eupnoeic conditions in the experiments of Abdala et al. (2009) might have been a general reduction in excitability in their preparation, resulting from the widespread deafferentation inherent to this preparation, as compared to in vivo (cf. discussion in Janczewski et al. 2002). In Abdala et al. (2009), the operation of this oscillator also needed the integrity of connections with the pons. We suggest that in intact animals there may be many, varied inputs that maintain or modulate the excitability of the respiratory oscillators, or reconfigure them. The input from the pons may be regarded as one of these.
In the generation of an expiratory output from the medulla, at least three stages may be defined. At the most basic, EBSNs can produce a rhythmic expiratory discharge at the apnoeic threshold, simply by the periodic inspiratory inhibition of a tonic CO2-dependent excitation (Bainton & Kirkwood, 1979). At slightly higher levels of CO2, a ramp of excitation occurs during expiration, e.g. such as may be clearly seen in the first and third respiratory cycles of Fig. 2B. The origin of this ramp is most likely to be a pattern generator in the pre-Bötzinger or Bötzinger regions, as proposed in several modelling studies (e.g. Rybak et al. 2008). With rather more excitation, the rostrally located oscillator should contribute. Under physiological conditions, one might assume that the transitions are not likely to be abrupt, as in many experimental preparations, but that the system should operate in a more seamless fashion, the two oscillators then contributing to the security and stability of the whole (Feldman & Del Negro, 2006). It is possible that the gradual (rather than ‘quantal’) appearance of expiratory discharges that we saw in most of the EMG experiments here also involved the rostral oscillator, but this time recruited in a seamless manner in the spontaneously breathing preparations. The same could apply to some of the ketamine/xylazine animals. Of course, without the occurrence of ‘quantal expiration’, the activation of this oscillator could not be identified. However, if this oscillator was activated in the EMG experiments, then the difference between these and the other experiments, where it was activated later and at a slower rate, could be assigned to the absence, in the spontaneously breathing animals, of the relative deafferentation that had been produced by neuromuscular blockade in the others, even though both groups were vagotomized.
Finally in this section, we should summarize the links between our hypotheses and others in the literature. The presence of an expiratory oscillator comes directly from the observations. The suggested link to the parafacial/retrotrapezoid region stems from the similarity between the behaviour of our preparations and those described by Abdala et al. (2009). The idea that the convergence of many inputs may be needed to support the activity of the medullary neurones together with the depressant effects of anaesthesia on them, are very similar to those of Guyenet et al. (2009) for neurones of the retrotrapezoid nucleus. A possible divergence between our views and those of Guyenet et al. (2009) is that we are suggesting, following Feldman & Del Negro (2006), that this group of neurones could still act as an expiratory oscillator in the adult, whereas Guyenet et al. (2009) emphasize more their role in providing a tonic drive to inspiratory neurones. However, the full repertoire of neurones in this region in the adult has yet to be explored, including the presence of sub-groups with different properties, so there may not be any real contradiction.
Factors affecting expiratory motoneurone discharge
Why was there such a long delay before expiratory discharges appeared in our preparations? We do not have a certain explanation, but it is most likely to be simply a feature of the general depression of expiration by anaesthetics and its long-lasting effects, as in barbiturate-anaesthetized cats. This depression may be overcome by the use of relatively light anaesthesia, ‘including keeping the preparation in this state during the preparatory period’ (Saywell et al. 2007). For urethane or α-chloralose, where anaesthetic supplements were unnecessary after the first hour or so after induction or transfer from halothane, respectively, the anaesthesia almost certainly became somewhat lighter as time progressed. For the other anaesthetics, this was probably also the case. Although we were consistent in our criteria for administering supplements during recording periods (as described in Methods), during the surgery the anaesthesia was often somewhat deeper. For the decerebrates, many systems must be in flux in the hours immediately after the decerebration. The 2 h (median) at which we first saw expiratory discharges is about the same as the time traditionally allowed for such preparations to stabilize before physiological measurements are made. It is unlikely that the development of the expiratory discharges reflects an increase in level of CO2. Although we do not have complete records of the end-tidal level hour by hour, it was usually stable within a few tenths of a per cent once it had been set up at the start of the recording period. Moreover, one of the advantages of hypercapnia is that the relationship between respiratory output and the CO2 level is much less steep than in normocapnia (Bainton & Kirkwood, 1979), so variation in the CO2 level both within and between preparations is less important.
Is the activation of the expiratory oscillator required to evoke a significant expiratory motoneurone discharge? In most of the experiments here, just as for Iizuka & Fregosi (2007) or for Abdala et al. (2009), this was the case, but in the EMG experiments the situation is less clear: phasic expiratory discharges in the EMG recordings, often strong (Fig. 5C), were always recruited, even if only sometimes accompanied by an expiratory discharge in the external intercostal nerve. However, the question only becomes important in the situation that this oscillator is separated from the inspiratory one, which, as we have argued above, may be a consequence of the artificiality of the experimental conditions. One factor influencing the view of some authors, that the activation of this oscillator is an extreme occurrence, is the belief that phasic late expiratory motoneurone discharge generally does not occur in eupnoea (e.g. recently, Abdala et al. 2009; perhaps also Iizuka & Fregosi, 2007). In fact there is plentiful evidence that this can occur, in a variety of preparations, in the cat (Sears, 1964b; Bainton et al. 1978; Bainton & Kirkwood, 1979; Fregosi & Bartlett, 1989), the dog (Oliven & Kelsen, 1989; Legrand & De Troyer, 1999) and the rat (Tian & Duffin, 1996). In many situations the discharge may not be strong, the appearance of the activity being affected by factors such as the level of anaesthesia, posture (e.g. prone vs. supine) and the region of thorax or abdomen sampled (Abdala et al. 2009). It should be remembered, though, that the level of discharge in a motoneurone represents the depolarization above threshold, which may be only a fraction of the excitatory input it receives (Sears, 1977).
Even in hypercapnia in the relatively lightly anaesthetized cat, where the expiratory discharges from some intercostal nerves can be strong, Saywell et al. (2007) concluded that a tonic drive to expiratory motoneurones was needed in addition to the phasic drive from the medulla in order to evoke a discharge. The need for such an input should not mean that the expiratory activation represents a minor functional role for these motoneurones: it could be that the tonic input is a convenient way for the CNS to regulate specific respiratory functions, such as the spatial aspects of the activation of the thorax (De Troyer et al. 2005), but it could also represent integration between respiration and posture, as is necessary for all respiratory motoneurones. This consideration again raises the issue of deafferentation. This was discussed above with respect to effects on the rhythm generator. It is equally an issue for the spinal cord. We became aware of this in the EMG experiments, where nerve section was required to identify which nerve was responsible for a particular locally recorded activity pattern, but where the clear effects of nerve section on motoneurone excitability became a severe complication to an otherwise logically simple process. The conclusion from these considerations is that, if deafferentation is having a noticeable effect even in our in vivo preparation, how much more of an effect is it likely to have in the in situ preparation of Abdala et al. (2009)? Thus we suggest that conclusions about the functional role of a given activity (such as the late expiratory bursts) should not be made from observations in such a preparation. Of course, such conclusions should only be made with considerable caution even from in vivo experiments.
Like deafferentation, anaesthesia is a factor which may affect not only respiratory rhythm generation, but also transmission of the drive to motoneurones. The transmission could be affected at any level, from the synapses on the EBSNs, to those on the motoneurones (Saywell et al. 2007), including effects on any interposed interneurones. This issue will be revisited in a subsequent publication on the connectivity measurements from these same experiments (cf. de Almeida & Kirkwood, 2006).
α- or γ-motoneurones?
To properly assess the functional significance of the efferent discharges reported here we need to know whether they represent α- or γ-motoneurones, or both. However, we are not able to make an absolute distinction because, unlike for the intercostal nerve filament discharges in the cat (Sears, 1964b) the spikes here did not usually fall into two clear groups of amplitude. This is not surprising because the conduction velocities of α- and γ-motoneurones in the rat show considerable overlap (Andrew & Part, 1972; Andrew et al. 1978). Nevertheless, our conclusions should not be seriously affected, in that none of our observations of ‘anomalous’ activation can be explained by recording primarily γ-motoneurones alone. For the inspiratory activation of the internal intercostal layer, the activation was amply confirmed by the EMG recordings, and a similar pattern of excitation was shown in the intracellular recordings (de Almeida & Kirkwood, 2010), which are most unlikely to come from γ-motoneurones. For the expiratory activity in the external intercostal nerve, the wide range of spike amplitudes in most external intercostal nerve discharges during expiration makes it certain that α-motoneurones must also be included (e.g. Figs 3 and 4; also see illustrations in de Almedia & Kirkwood, 2010 and in Tian & Duffin, 1996).
The low level tonic discharge of small amplitude spikes that is visible in many of our recordings may well represent fusimotor activity, as in the cat (Sears, 1964b). Note that this may also be the case, though not acknowledged, in previous reports (e.g. Fregosi & Bartlett, 1989; Iizuka, 2003; Iizuka & Fregosi, 2007).
Comparative and functional considerations
Assuming the caveats expressed in the previous sections are accepted, the temporal aspects of the patterns of activity here are consistent with other reports from the rat. As for the spatial aspects, our results are also all consistent with earlier reports (Tian & Duffin, 1996; Lu et al. 2004), even though the observations of inspiratory activation of the proximal parts of the internal or subcostalis layer are entirely new. These observations are apparently discrepant with Tian & Duffin (1996), who reported that there was little activity in the internal intercostal nerves of caudal segments but, when present, it was expiratory. However, this discrepancy may not be real, because these authors did not record below T8 (our recordings were mostly from T9 or T10) and also they used eupnoeic levels of CO2, whereas we used hypercapnia. Importantly, Tian & Duffin (1996) reported biphasic discharges in the external intercostal nerves, which was confirmed here. Lu et al. (2004) saw only inspiratory discharges in these nerves, but they started recordings 4 h after decerebration in preparations previously anaesthetized with barbiturate, a time when our results might predict that such discharges were still suppressed by the anaesthetic.
The present results are also quite similar to the reported patterns of EMG activity in intercostal muscles of intact unanaesthetized rats from Megirian et al. (1987), where the activity of both internal and external layers was variable, and could be either inspiratory or expiratory. However, the degree of cross-talk revealed in the present study (also see Legrand & De Troyer, 1999) raises major doubts about the reliability of these earlier results, despite stated precautions.
The spatial distribution of activity across the surface of the thorax has been studied most fully in the dog and the cat, and to a certain degree in the human (De Troyer et al. 2005). At least one aspect is similar here, the rostrocaudal gradient of the inspiratory discharges in the external intercostal nerve, with stronger activity rostrally (here seen in comparing T6 and T9). We do not have a sufficient spread of recordings to confirm the reverse gradient for expiratory discharges in the internal intercostal nerve. Interestingly, though, the expiratory discharges in the external intercostal nerve did show this reverse difference. Note that, although the inspiratory gradient might be explained by a difference in thickness of the nerves, the external intercostal nerves being thinner caudally than rostrally (Tian & Duffin, 1996), the expiratory gradient for these nerves cannot.
In contrast, the inspiratory activity in the caudal dorsal area of the internal layer and the biphasic activity (for any region) are completely different from the activity in the other three species mentioned above. Note that the earlier claim for biphasic activity from Le Bars & Duron (1984) was not supported by critical nerve section experiments (Legrand & De Troyer, 1999). For the interosseus intercostals in the other species, activity is strictly segregated (inspiratory in the external layer, expiratory in the internal). Further, in the dog and the human, the strength of activation for a given location in an individual layer is well matched to the mechanical advantage of that location (De Troyer et al. 2005). The general mechanical arrangement of the muscles in the rat appears very similar to the other species. Thus, prima facie, it seems unlikely that the mechanical advantage of the internal layer in the dorsal caudal area in the rat would be inspiratory, as would be required for appropriate matching in this species. Note that inspiratory activation of the internal or subcostalis layers in the present EMG experiments was seen as far rostral as T6.
It would clearly be of great interest to measure the distribution of mechanical advantage for the rat intercostal muscles, to see whether this does match the distribution of any of the components of the activation shown here, but the biggest challenge to the general principle set out in De Troyer et al. (2005) is the predominance of biphasic excitation. This inevitably introduces a contradiction, since this would need the mechanical advantage to be both inspiratory and expiratory at the same location. One possible explanation could be that the mechanical advantage of the internal intercostal layer might become inspiratory when the diaphragm and levator costae are contracting, particularly for the most caudal spaces where the attachments of the diaphragm are strongest. Then both phases of the biphasic excitation could be matched to mechanical advantage. However, the addition of an expiratory component to the more common inspiratory burst in the external intercostal nerve is harder to reconcile. Although the EMG measurements here failed to properly verify that this activity was destined for external intercostal muscle, it does seem unlikely that it should all be destined for the only other candidate, serratus. The segmental nerve branches to this muscle are very fine, probably no more than 1/3 the diameter of the external intercostal nerve. By comparison with other fine nerve branches (Sears, 1964a), similarly dissected in this laboratory, we estimate them to contain no more than five α-motoneurone axons, yet it was frequently obvious that more than five large axons were recruited into the expiratory bursts in the whole external intercostal nerve.
A further unanswered question is whether the inspiratory (or biphasic) excitation in the internal intercostal nerve is specifically destined for subcostalis, since this muscle is so much more prominent in the rat than in the dog or cat. However, even before asking this, we should ask how separate subcostalis is from the internal layer. For instance, do individual motor units include muscle fibres in both layers? This is unknown, as is the functional consequence of the bisegmental arrangement we have observed here.
Acknowledgments
Roseanna Smither, Victor Baller and Ed Bye are thanked for technical assistance, as are Phil Waite and Jim Duffin for advice on rat electrophysiology. The work was supported by the International Spinal Research Trust and the Brain Research Trust. A. T. R. de Almeida and S. Al-Izki both held MRC studentships.
Glossary
Abbreviation
- EBSN
expiratory bulbospinal neurone
Author contributions
S.A. and M.E.D. contributed to the study design, participated in early experiments and provided critical revisions of the manuscript. A.T.R.deA. and P.A.K. contributed to all aspects of the study. All authors approved the final version of the manuscript. The experiments were done at UCL Institute of Neurology.
Authors’ present addresses
S. Al-Izki: Centre for Neuroscience and Trauma, Blizard Institute of Cell and Molecular Science, Barts and The London School of Medicine and Dentistry, 4 Newark Street, London E1 2AT, UK.
M. Enríquez Denton: Institute of Biomedical and Life Sciences, West Medical Building, University of Glasgow, Glasgow G11 1QQ, UK.
References
- Abdala AP, Rybak IA, Smith JC, Paton JF. Abdominal expiratory activity in the rat brainstem–spinal cord in situ: patterns, origins and implications for respiratory rhythm generation. J Physiol. 2009;587:3539–3559. doi: 10.1113/jphysiol.2008.167502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew BL, Leslie GC, Part NJ. Some observations on the efferent innervation of rat soleus muscle spindles. Exp Brain Res. 1978;31:433–443. doi: 10.1007/BF00237300. [DOI] [PubMed] [Google Scholar]
- Andrew BL, Part NJ. Properties of fast and slow motor units in hind limb and tail muscles of the rat. Q J Exp Physiol. 1972;57:213–225. doi: 10.1113/expphysiol.1972.sp002151. [DOI] [PubMed] [Google Scholar]
- Bainton CR, Kirkwood PA. The effect of carbon dioxide on the tonic and the rhythmic discharges of expiratory bulbospinal neurones. J Physiol. 1979;296:291–314. doi: 10.1113/jphysiol.1979.sp013006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainton CR, Kirkwood PA, Sears TA. On the transmission of the stimulating effects of carbon dioxide to the muscles of respiration. J Physiol. 1978;280:249–272. doi: 10.1113/jphysiol.1978.sp012383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballantyne D, Richter DW. The non-uniform character of expiratory synaptic activity in expiratory bulbospinal neurones of the cat. J Physiol. 1986;370:433–456. doi: 10.1113/jphysiol.1986.sp015943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MI, Feldman JL, Sommer D. Caudal medullary expiratory neurone and internal intercostal nerve discharges in the cat: effects of lung inflation. J Physiol. 1985;368:147–178. doi: 10.1113/jphysiol.1985.sp015851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida A, Kirkwood PA. 2006 Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience; 2006. Duel descending pathways to thoracic motoneurones: Substrates for assessing experimental spinal cord repair. Programme No. 88.15. [Google Scholar]
- de Almeida A, Kirkwood PA. Patterns of respiratory activation for intercostal muscles in the rat. Proc Physiol Soc. 2008;10:C11, PC60. [Google Scholar]
- de Almeida ATR, Kirkwood PA. Multiple phases of excitation and inhibition in central respiratory drive potentials of thoracic motoneurones in the rat. J Physiol. 2010;588:2731–2744. doi: 10.1113/jphysiol.2009.186346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis MJ, Ziskind-Conhaim L, Harris AJ. Development of neuromuscular junctions in rat embryos. Dev Biol. 1981;81:266–279. doi: 10.1016/0012-1606(81)90290-6. [DOI] [PubMed] [Google Scholar]
- De Troyer A, Farkas GA. Inspiratory function of the levator costae and external intercostal muscles in the dog. J Appl Physiol. 1989;67:2614–2621. doi: 10.1152/jappl.1989.67.6.2614. [DOI] [PubMed] [Google Scholar]
- De Troyer A, Kirkwood PA, Wilson TA. Respiratory action of the intercostal muscles. Physiol Rev. 2005;85:717–756. doi: 10.1152/physrev.00007.2004. [DOI] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett J. Repair of spinal cord injuries: where are we, where are we going? Spinal Cord. 2002;40:615–623. doi: 10.1038/sj.sc.3101328. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Del Negro CA. Looking for inspiration: new perspectives on respiratory rhythm. Nat Rev Neurosci. 2006;7:232–241. doi: 10.1038/nrn1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortuna MG, West GH, Stornetta RL, Guyenet PG. Bötzinger expiratory-augmenting neurons and the parafacial respiratory group. J Neurosci. 2008;28:2506–2515. doi: 10.1523/JNEUROSCI.5595-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fregosi RF, Bartlett D., Jr Internal intercostal nerve discharges in the cat: influence of chemical stimuli. J Appl Physiol. 1989;66:687–694. doi: 10.1152/jappl.1989.66.2.687. [DOI] [PubMed] [Google Scholar]
- Fukuda H, Fukai K. Discharges of bulbar respiratory neurons during rhythmic straining evoked by activation of pelvic afferent fibers in dogs. Brain Res. 1988;449:157–166. doi: 10.1016/0006-8993(88)91034-7. [DOI] [PubMed] [Google Scholar]
- Giraudin A, Cabirol-Pol MJ, Simmers J, Morin D. Intercostal and abdominal respiratory motoneurons in the neonatal rat spinal cord: spatiotemporal organization and responses to limb afferent stimulation. J Neurophysiol. 2008;99:2626–2640. doi: 10.1152/jn.01298.2007. [DOI] [PubMed] [Google Scholar]
- Goldman MD, Loh L, Sears TA. The respiratory activity of human levator costae muscles and its modification by posture. J Physiol. 1985;362:189–204. doi: 10.1113/jphysiol.1985.sp015670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene E. Anatomy of the Rat. USA: Hafner Publishing Company Inc.; 1968. [Google Scholar]
- Guyenet PC, Bayliss DA, Stornetta RL, Fortuna MG, Abbott SBG, DePuy SD. Retrotrapezoid nucleus, respiratory chemosensitivity and breathing automaticity. Resp Physiol Neurobiol. 2009;168:59–68. doi: 10.1016/j.resp.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilaire GG, Nicholls JG, Sears TA. Central and proprioceptive influences on the activity of levator costae motoneurones in the cat. J Physiol. 1983;342:527–548. doi: 10.1113/jphysiol.1983.sp014867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iizuka M. GABAA and glycine receptors in regulation of intercostal and abdominal expiratory activity in vitro in neonatal rat. J Physiol. 2003;551:617–633. doi: 10.1113/jphysiol.2003.042689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iizuka M, Fregosi RF. Influence of hypercapnic acidosis and hypoxia on abdominal expiratory nerve activity in the rat. Respir Physiol Neurobiol. 2007;157:196–205. doi: 10.1016/j.resp.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Iscoe S. Control of abdominal muscles. Progr Neurobiol. 1998;56:433–506. doi: 10.1016/s0301-0082(98)00046-x. [DOI] [PubMed] [Google Scholar]
- Janczewski WA, Feldman JL. Distinct rhythm generators for inspiration and expiration in the juvenile rat. J Physiol. 2006;570:407–420. doi: 10.1113/jphysiol.2005.098848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janczewski WA, Onimaru H, Homma I, Feldman JL. Opioid-resistant respiratory pathway from the preinspiratory neurones to abdominal muscles: in vivo and in vitro study in the newborn rat. J Physiol. 2002;545:1017–1026. doi: 10.1113/jphysiol.2002.023408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood PA. Synaptic excitation in the thoracic spinal cord from expiratory bulbospinal neurones in the cat. J Physiol. 1995;484:201–225. doi: 10.1113/jphysiol.1995.sp020659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bars P, Duron B. Are the external and internal intercostal muscles synergist or antagonist in the cat? Neurosci Lett. 1984;51:383–386. doi: 10.1016/0304-3940(84)90407-5. [DOI] [PubMed] [Google Scholar]
- Legrand A, De Troyer A. Spatial distribution of external and internal intercostal activity in dogs. J Physiol. 1999;518:291–300. doi: 10.1111/j.1469-7793.1999.0291r.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu F, Qin C, Foreman RD, Farber JP. Chemical activation of C1-C2 spinal neurons modulates intercostal and phrenic activity in rats. Am J Physiol Regul Integr Comp Physiol. 2004;286:R1069–R1076. doi: 10.1152/ajpregu.00427.2003. [DOI] [PubMed] [Google Scholar]
- Meehan CF, Ford TW, Road JD, Donga R, Saywell SA, Anissimova NP, Kirkwood PA. Rostrocaudal distribution of motoneurones and variation in ventral horn area within a segment of the feline thoracic spinal cord. J Comp Neurol. 2004;472:281–291. doi: 10.1002/cne.20096. [DOI] [PubMed] [Google Scholar]
- Megirian D, Pollard MJ, Sherrey JH. The labile respiratory activity of ribcage muscles of the rat during sleep. J Physiol. 1987;389:99–110. doi: 10.1113/jphysiol.1987.sp016648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellen NM, Janczewski WA, Bocchiaro CM, Feldman JL. Opioid-induced quantal slowing reveals dual networks for respiratory rhythm generation. Neuron. 2003;37:821–826. doi: 10.1016/s0896-6273(03)00092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AD, Tan LK, Suzuki I. Control of abdominal and expiratory intercostal muscle activity during vomiting: role of ventral respiratory group expiratory neurons. J Neurophysiol. 1987;57:1854–1866. doi: 10.1152/jn.1987.57.6.1854. [DOI] [PubMed] [Google Scholar]
- Oliven A, Kelsen SG. Effect of hypercapnia and PEEP on expiratory muscle EMG and shortening. J Appl Physiol. 1989;66:1408–1413. doi: 10.1152/jappl.1989.66.3.1408. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Arata A, Homma I. Primary respiratory rhythm generator in the medulla of brainstem–spinal cord preparation from newborn rat. Brain Res. 1988;445:314–324. doi: 10.1016/0006-8993(88)91194-8. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Kumagawa Y, Homma I. Respiration-related rhythmic activity in the rostral medulla of newborn rats. J Neurophysiol. 2006;96:55–61. doi: 10.1152/jn.01175.2005. [DOI] [PubMed] [Google Scholar]
- Rybak IA, O’Connor R, Ross A, Shevtsova NA, Nuding SC, Segers LS, Shannon R, Dick TE, Dunin-Barkowski WL, Orem JM, Solomon IC, Morris KF, Lindsey BG. Reconfiguration of the pontomedullary respiratory network: a computational modeling study with coordinated in vivo experiments. J Neurophysiol. 2008;100:1770–1799. doi: 10.1152/jn.90416.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto H, Akita K, Sato T. Ramification of the intercostal nerves in the rhesus monkey (Macaca mulatta) with special reference to the innervation of the intercostal muscles. Acta Anat (Basel) 1993;146:62–70. doi: 10.1159/000147422. [DOI] [PubMed] [Google Scholar]
- Sakamoto H, Akita K, Sato T. An anatomical analysis of the relationships between the intercostal nerves and the thoracic and abdominal muscles in man. I. Ramification of the intercostal nerves. Acta Anat (Basel) 1996;156:132–142. doi: 10.1159/000147838. [DOI] [PubMed] [Google Scholar]
- Saywell SA, Anissimova NP, Ford TW, Meehan CF, Kirkwood PA. The respiratory drive to thoracic motoneurones in the cat and its relation to the connections from expiratory bulbospinal neurones. J Physiol. 2007;579:765–782. doi: 10.1113/jphysiol.2006.122481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears TA. The fibre calibre spectra of sensory and motor fibres in the intercostal nerves of the cat. J Physiol. 1964a;172:150–161. doi: 10.1113/jphysiol.1964.sp007409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears TA. Efferent discharges in alpha and fusimotor fibres of intercostal nerves of the cat. J Physiol. 1964b;174:295–315. doi: 10.1113/jphysiol.1964.sp007488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears TA. The respiratory motoneuron and apneusis. Fed Proc. 1977;36:2412–2420. [PubMed] [Google Scholar]
- Shen LL, Duffin J. Caudal expiratory neurones in the rat. Pflugers Arch. 2002;444:405–410. doi: 10.1007/s00424-002-0817-x. [DOI] [PubMed] [Google Scholar]
- Shen LL, Li YM, Duffin J. Inhibitory connections among rostral medullary expiratory neurones detected with cross-correlation in the decerebrate rat. Pflugers Arch. 2003;446:365–372. doi: 10.1007/s00424-003-1024-0. [DOI] [PubMed] [Google Scholar]
- Smith CL, Hollyday M. The development and postnatal organization of motor nuclei in the rat thoracic spinal cord. J Comp Neurol. 1983;220:16–28. doi: 10.1002/cne.902200104. [DOI] [PubMed] [Google Scholar]
- Suzue T. Respiratory rhythm generation in the in vitro brain stem–spinal cord preparation of the neonatal rat. J Physiol. 1984;354:173–183. doi: 10.1113/jphysiol.1984.sp015370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tani M, Kida MY, Akita K. Relationship between the arrangement of motoneuron pools in the ventral horn and ramification pattern of the spinal nerve innervating trunk muscles in the cat (Felis domestica) Exp Neurol. 1994;128:290–300. doi: 10.1006/exnr.1994.1139. [DOI] [PubMed] [Google Scholar]
- Tian GF, Duffin J. Connections from upper cervical inspiratory neurons to phrenic and intercostal motoneurons studied with cross-correlation in the decerebrate rat. Exp Brain Res. 1996;110:196–204. doi: 10.1007/BF00228551. [DOI] [PubMed] [Google Scholar]
- Walmsley T. The costal musculature. J Anat Physiol. 1916;50:165–171. [PMC free article] [PubMed] [Google Scholar]
- Wittmeier S, Song G, Duffin J, Poon CS. Pacemakers handshake synchronization mechanism of mammalian respiratory rhythmogenesis. Proc Natl Acad Sci U S A. 2008;105:18000–18005. doi: 10.1073/pnas.0809377105. [DOI] [PMC free article] [PubMed] [Google Scholar]


