Abstract
Intracellular recordings were made from motoneurones with axons in the intercostal nerves of T9 or T10 in adult rats, with neuromuscular blockade and artificial ventilation, under hypercapnia and under either anaesthesia or decerebration. In nearly all motoneurones, central respiratory drive potentials (CRDPs) were seen, which included an excitatory wave in inspiration, in expiration, or in both of these. This was the case both for motoneurones with axons in the internal intercostal nerve (n= 81) and for those with axons in the external intercostal nerve (n= 5). In the decerebrates, motoneurones with purely inspiratory CRDPs were rare (1/44), but those excited in both phases (showing biphasic CRDPs) were common (22/44). For about one-third of biphasic CRDPs (11/30), the inspiratory depolarization was seen to reverse to a hyperpolarization when the motoneurone was depolarized, which was interpreted as indicating concurrent inhibition and excitation during this phase. A few motoneurones were seen where depolarization revealed signs of inhibition in both phases. The results confirm the novel observations of biphasic excitation in individual intercostal nerve branches, EMG sites and motor units reported in a companion paper. They also provide new insights into the functional roles of inhibition in motoneurones physiologically activated in natural rhythmic behaviours.
Introduction
The central respiratory drive potential (CRDP; Sears, 1964) represents the synaptic input to motoneurones from the mammalian central pattern generator for respiration. The most important feature of the CRDP, as first recorded in cat thoracic motoneurones, is the alternation of phases of excitation and inhibition, which was subsequently also seen in phrenic motoneurones (Berger, 1979), as well as in numerous other categories of respiratory neurones. Descriptions of drive potentials from other mammalian central pattern generators followed those for respiration, most notably for locomotion, and most of these have also shown alternation of excitation and inhibition (for references see Endo & Kiehn, 2008). Such simple alternation is often regarded as a general principle (‘push–pull’). Thus, when a different behaviour, such as the balanced excitation and inhibition during the depolarizing phase of the drive potential for the scratch reflex in turtles was reported (Berg et al. 2007), a special explanation was thought necessary, such as an artificial bias in the preparation concerned (Endo & Kiehn, 2008). However, even for the apparently simple situation of phrenic motoneurones, measurements subsequent to those of Berger (1979) had already indicated that more complex patterns of excitation and inhibition could be present (Milano et al. 1992; Parkis et al. 1999), as was also reported for hypoglossal motoneurones (Saywell & Feldman, 2004). An alternative view to the absolute generality of push–pull organization, therefore, is that more complex patterns may frequently occur and that variation in this respect will be likely according to the particular motor act concerned, the species or the preparation.
The respiratory drive to motoneurones of the caudal thoracic spinal cord of the rat provides another variant of a central rhythmic drive (see accompanying paper, de Almeida et al. 2010). This includes two unique features that make it worthy of further investigation in this context. Firstly, there can be intermittent appearance of expiratory activity (‘quantal’ expiration), dependent on the anaesthetic status of the preparation, which allows for partial separation of different components of the central drive. Secondly, there is biphasic excitation of some of these motoneurones, i.e. excitation in both expiration and inspiration, which adds further complexity.
In some of the experiments described by de Almeida et al. (2010), intracellular motoneurone recordings were made for the purpose of measurements of connections from expiratory bulbospinal neurones. We have therefore taken advantage of these recordings, made under a variety of conditions of anaesthesia or decerebration, to analyse the CRDPs that underlie the complex patterns of intercostal nerve discharges. This study thus expands the known repertoire of central drive potentials present within a range of motoneurones, and it also complements the description of the spatial patterns of activity of the intercostal muscles from de Almeida et al. (2010) by revealing the origin of the corresponding temporal pattern, in terms of excitation and/or inhibition.
A preliminary account has appeared previously (de Almeida & Kirkwood, 2008).
Methods
The experiments were a subset of those already reported (de Almeida et al. 2010), which were performed on adult female Sprague–Dawley rats (Harlen, UK) weighing between 180 and 299 g, as approved by the Ethical Review Process of the Institute of Neurology, in accordance with UK legislation (Animals (Scientific Procedures) Act, 1986) and commensurate with the recommendations of Drummond (2009), involving a variety of different anaesthetic regimens. Table 1 lists numbers of animals from the original series that provided data for the analyses reported here.
Table 1.
Anaesthetic regimens employed
| Anaesthetic and dose | No. of animals |
|---|---|
| Ketamine/xylazine | |
| Induction, i.p., 100 mg kg−1, 10 mg kg−1, respectively, then supplements of ketamine/xylazine (same proportions), i.v., as required. | 4 |
| Ketamine only | |
| 100 mg kg−1, i.p., then supplements, i.v., as required. | 1 |
| Halothane then α-chloralose | |
| Halothane: induction 5%, maintenance 1–2% during surgery, titrated to α-chloralose (30–80 mg kg−1, i.v.) for recording. | 2 |
| Halothane then decerebration | |
| Induction 5%, maintenance 1–2% during surgery. Lowered to 0.5% at start of decerebration; turned off once decerebration completed. | 11 |
For more details see de Almeida et al. (2010).
Surgical procedures
These were identical to those described by de Almeida et al. (2010), including bilateral vagotomy, neuromuscular blockade subsequent to surgery (pancuronium bromide; 0.3 mg h−1i.v., Hospira UK Ltd), bilateral pneumothoraces, and artificial ventilation with CO2 added to the gas mixture to increase the end-tidal CO2 level to a value of around 7–8%. Table 1 describes the anaesthetic regimens used. Animals were suspended prone, with a laminectomy of four vertebrae (T7–T10) and the dura opened. In most of the experiments, which included medullary recordings, a partial occipital craniotomy and a partial laminectomy of C1 were made. In these experiments stimulating electrodes were placed in the spinal cord, usually in T11 segment, for antidromic identification of bulbospinal neurones. After a small patch of pia had been removed from the surface of the dorsal columns, a shaped pressure plate was lightly applied to the cord dorsum to help minimize cord movement.
Nerves prepared for recording or stimulation in these animals, were generally fewer than in many of the experiments reported by de Almeida et al. (2010), usually comprising (a) the external intercostal nerve of T6, used for monitoring the timing of the respiratory cycle and the presence or absence of expiratory discharges, and (b) the external intercostal nerve and the internal intercostal nerve (distal to its first filament) of T9 or T10, used for antidromic identification of motoneurones.
At the end of the experiment, the animal was killed with an anaesthetic overdose.
Recordings
Glass microelectrodes with tips broken back to a diameter of 1.2–1.4 μm, filled with 4 m potassium acetate, were introduced through the dorsal columns at an angle of 10–15 deg to the vertical in the transverse plane. Intracellular recordings from motoneurones were sought on the left side of T9 (16 animals) or T10 (2 animals), mostly in the region from the level of the most rostral dorsal root entry up to about 1 mm further rostral (cf. Smith & Hollyday, 1983; Meehan et al. 2004). Motoneurones were identified by antidromic activation from either the external or the internal intercostal nerve. Positions of electrode tracks and motoneurones were noted with respect to the most rostral dorsal root entry. An afferent volley was recorded by a platinum wire electrode on the cord dorsum, referenced to nearby muscle, and the tracking stimuli set to 10 times nerve threshold. A few recordings from unidentified cells are also included in the Results. These were believed to be motoneurones on account of their positions in the spinal cord relative to identified motoneurones and general properties of their recordings, such as synaptic noise, presence of a low-threshold monosynaptic EPSP, firing frequency and, particularly, their characteristic spike shapes. These cells are referred to as ‘unidentified motoneurones’.
Most of the intracellular recordings were made in the context of connectivity measurements, in this case spike-triggered averaging from expiratory bulbospinal neurones (EBSNs). The procedure therefore consisted of simultaneous intracellular recordings from the motoneurones together with the EBSN discharges and the efferent discharges from an external intercostal nerve (T6 or, for one experiment, T9) as an indicator of respiratory phase. See de Almeida et al. (2010) for details of EBSN recordings. After motoneurone identification, all stimulators were turned off and the recordings continued, usually until the membrane potential deteriorated to an unacceptable level (see Results). Recordings used for analysis ranged from 20 s to 12 min in duration. Data acquisition and storage were as in de Almeida et al. (2010). The intracellular recordings were stored in two forms: low gain, DC to 3 kHz, for observations of membrane potential and respiratory drive potentials, and a higher gain, 3 Hz to 3 kHz, for observations of EPSPs or IPSPs and of synaptic noise.
Statistics
Means are quoted ±s.d. Significance was taken as P < 0.05.
Results
The recordings came from animals in a variety of states of anaesthesia or decerebration, including the whole range described by de Almeida et al. (2010). For any one recording, the state therefore depended not only on the anaesthetic regimen employed but also on the time after anaesthesia (or the time after switching from halothane to α-chloralose). The efferent discharges (usually from the T6 external intercostal nerve) provided a monitor for the state, by the presence and/or the intensity of expiratory discharges in this nerve, thus also indicating those states where a strong expiration occurred in a quantal fashion with respect to the inspiratory phases (de Almeida et al. 2010).
Intracellular recordings were examined in 105 motoneurones, 101 in T9 and 4 in T10. Eighty-one were antidromically identified from the internal intercostal nerve, 5 from the external intercostal nerve and 19 were unidentified motoneurones. It should be remembered that the external intercostal nerve motoneurones are likely to have included a small proportion innervating serratus muscle as well as those innervating external intercostal muscle (de Almeida et al. 2010). The internal intercostal nerve group would have included motoneurones innervating one of several muscles: internal intercostal muscle, including subcostalis (but not the most proximal part); abdominal muscles; interchondral muscles; possibly serratus. The motoneurones came from 18 rats; 11 decerebrates following halothane (55 motoneurones), 2 under α-chloralose following halothane (5 motoneurones), 1 under ketamine alone (9 motoneurones) and 4 under ketamine/xylazine (36 motoneurones).
Motoneurones were only accepted for analysis if the membrane potential was more negative than −30 mV near the start of the recording. This is a rather more generous inclusion criterion than used recently for cat data from this laboratory (Saywell et al. 2007), but was based directly on observations of synaptic potentials, particularly the CRDPs. Most motoneurones eventually showed a gradual depolarization. However, the excitatory phases of the CRDPs usually did not show major attenuation at least until the membrane potential reached around −30 mV, and often not until about −20 mV. For the cat, severe attenuation was seen for most membrane potentials more positive than −40 mV. The actual range of membrane potentials here (measured at the start of expiration) was −75 to −30 mV (mean 49 ± 10 mV).
All motoneurones but one showed a CRDP. The description of these CRDPs constitutes the main analysis of the intracellular recordings here. Amplitudes of the CRDPs were measured peak-to-peak and averaged over 3–6 respiratory cycles, usually during a period when the cell was at its most polarized, unless that period was a time of large movement artefact. In instances of residual movement artefact (ventilator or cardiac), interpolation was used so as to estimate the CRDP amplitude independently of the artefact (e.g. Fig. 2C). An exception to the averaging rule was made for periods when the respiratory drive showed all-or-none variations between expiratory drive in different cycles (see below). For these, the amplitudes were estimated from a few successive cycles from the series of cycles that included strong expirations. Table 2 lists the occurrence of CRDPs of different categories, as described below.
Figure 2. Inspiratory CRDPs.
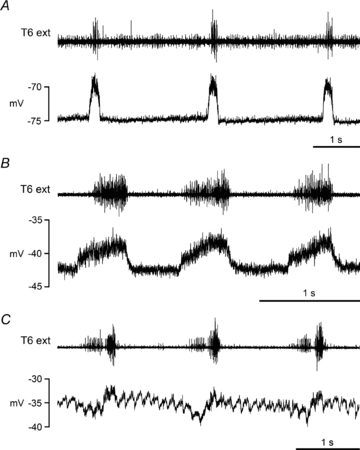
Examples are shown from motoneurones with different identifications and/or animals in different states. A, internal intercostal nerve motoneurone, α-chloralose following halothane; B, unidentified motoneurone, ketamine/xylazine; C; unidentified motoneurone, decerebrate following halothane. The upper trace (T6 external intercostal nerve recording) shows a weak inspiratory discharge in A, a strong inspiratory discharge in B, and inspiratory plus expiratory discharges in C (see de Almeida et al. 2010 for further descriptions). The fast periodic waveform in C (about 8 s−1) is a cardiac movement artefact. The CRDP amplitude was estimated, independently of the artefact, as 4 mV in this instance.
Table 2.
Numbers of CRDPs in each category, according to the motoneurone identity and anaesthetic regimen
| Biphasic |
||||||
|---|---|---|---|---|---|---|
| CRDP category | Inspiratory | Expiratory | Type 1 | Type 2 | Biphasic inhibitory | Total |
| External intercostal nerve motoneurones | ||||||
| Ketamine/xylazine | 1 | 1 | — | — | — | 2 |
| Ketamine | — | — | — | — | — | — |
| (Halothane) α-chloralose | — | — | — | — | — | — |
| (Halothane) decerebrate | 1 | — | 1 | 1 | — | 3 |
| Subtotal | 2 | 1 | 1 | 1 | — | 5 |
| Internal intercostal nerve motoneurones | ||||||
| Ketamine/xylazine | 3 | 20 | 3 | 1 | — | 27 |
| Ketamine | 3 | 5 | 1 | — | — | 9 |
| (Halothane) α-chloralose | 1 | 3 | — | — | — | 4 |
| (Halothane) decerebrate | — | 19 | 6 | 14 | 2 | 41 |
| Subtotal | 7 | 47 | 10 | 15 | 2 | 81 |
| Unidentified motoneurones | ||||||
| Ketamine/xylazine | 2 | 2 | 1 | 1 | — | 7* |
| Ketamine | — | — | — | — | — | — |
| (Halothane) α-chloralose | 1 | — | — | — | — | 1 |
| (Halothane) decerebrate | 5 | 4 | — | 1 | 1 | 11 |
| Subtotal | 8 | 6 | 1 | 2 | 1 | 19* |
| Total | 17 | 54 | 12 | 18 | 3 | 105* |
Totals include one unidentified motoneurone with ketamine/xylazine in which no CRDP could be detected.
Expiratory CRDPs
CRDPs were classified as expiratory if they showed a depolarizing wave in expiration and a hyperpolarizing wave in inspiration, or showed a hyperpolarizing wave in inspiration alone. The mean amplitude was 3.7 ± 2.3 mV (range 0.5–10 mV, n= 54). Examples are shown in Fig. 1, and may be seen to be generally similar to those in the cat (Sears, 1964; Saywell et al. 2007). For 46 of the group, the inspiratory hyperpolarizing wave appeared sufficiently distinct to be ascribed to phasic inhibition (Sears, 1964), as is the case for all of the examples in Fig. 1. The simplest criterion for an inhibitory phase was the sudden depolarization at the end of the phase, i.e. at the end of inspiration. Sometimes the start of this wave was not so obvious, but additional evidence for the presence of inhibition was often provided by increased synaptic noise accompanying the hyperpolarization.
Figure 1. Expiratory CRDPs.
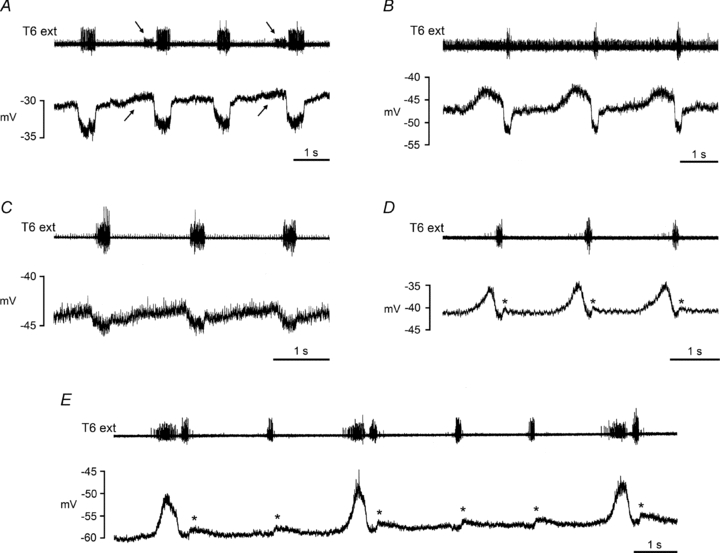
Examples are shown from motoneurones with different identifications and/or animals in different states. A, external intercostal nerve motoneurone, ketamine/xylazine; B, internal intercostal nerve motoneurone, α-chloralose following halothane; C, internal intercostal nerve motoneurone, ketamine; D and E, internal intercostal nerve motoneurones, decerebrates following halothane. The upper trace (T6 external intercostal nerve recording) shows an inspiratory discharge in A with a weak expiratory discharge in alternate cycles (note corresponding late expiratory depolarizing ramp in the CRDP, indicated by arrows), an inspiratory discharge with a strong expiratory discharge in approximately alternate cycles in E, only inspiratory discharges in B and C, and an inspiratory discharge with a weak expiratory discharge in each cycle in D (see de Almeida et al. 2010 for further descriptions). The asterisks in D and E indicate a small post-inspiratory depolarization, which followed a small inspiratory hyperpolarization for both these CRDPs.
Expiratory CRDPs were more frequent in anaesthetized animals than in decerebrates, but not significantly so (31/50 vs. 23/55, χ2, P > 0.05). Most of the expiratory CRDPs (46/54) showed a ramp of depolarization during expiration (amplitude up to 7.5 mV). The remaining nine, which were flat during expiration, were all recorded during a period when there were no expiratory discharges in the external intercostal nerve. Not only the occurrence, but also the amplitudes of the ramps varied with the state of the preparation. Considering CRDPs with ramps of amplitude ≥2 mV, a higher proportion occurred when an expiratory discharge was present (22/28) than when it was absent (5/26). Correspondingly, this proportion was higher in decerebrates (17/23) than in anaesthetized animals (10/31), both of these differences being significant (χ2, P < 0.001, P < 0.01, respectively).
Furthermore, the occurrence of the expiratory ramp frequently varied on a cycle-to-cycle basis, along with the expiratory discharges. Most notably, if this variation was all-or-none in the nerve, then the sub-threshold equivalent seen in the motoneurone was also all-or-none. This correspondence is very clear in Fig. 1A, comparing the 2nd and 4th cycles with the 1st and 3rd, and in Fig. 1E, comparing the 1st, 3rd and 6th cycle with the 2nd, 4th and 5th. The depolarization in panel A is no more than 1 mV, but is nevertheless made clear by the increased synaptic noise. Sixteen expiratory motoneurones were recorded at a time when there was all-or-none variation in the external intercostal nerve expiratory discharge. In 14 of these, an expiratory ramp was similarly present in an all-or-none fashion. In one of the others no ramp was visible, and in the remaining one, the ramp was no more than 1 mV in amplitude and rather obscured by pump artefact.
The CRDPs illustrated in Fig. 1D and E also show a small depolarization in post-inspiration (asterisks). This feature was relatively rare (seen in 8/54 CRDPs) and never large (amplitudes ≤1.5 mV). Figure 1B shows a CRDP with another unusual feature in that the maximum expiratory depolarization occurred before the end of expiration. This seemed to be a feature of the medullary output in this animal, since a simultaneously recorded EBSN (the only one in the experiment) showed a peak firing rate at the same point in the cycle.
Inspiratory CRDPs
CRDPs were classified as inspiratory if their peak depolarization was in inspiration and they had a constant membrane potential or a hyperpolarizing ramp in expiration, i.e. similar to those described by Sears (1964). Examples are shown in Fig. 2. The mean amplitude was 4.1 ± 2.1 mV (range 1–9 mV, n= 17). The occurrence of these CRDPs was higher in the anaesthetized animals (11/50) than in the decerebrates (6/55), but again not significantly so (χ2, P > 0.05). Most of the inspiratory CRDPs, as in Fig. 2A and B, were recorded at a time when an expiratory discharge was absent from the external intercostal nerve. Only two were recorded when an expiratory discharge was present, one of which is shown in Fig. 2C. These two were the only inspiratory CRDPs to show a hyperpolarizing component at the end of expiration that was sufficiently distinct to be readily ascribed to phasic inhibition (Sears, 1964) and both were recorded in unidentified motoneurones.
Biphasic CRDPs
This is a new category of CRDP. It is defined here as one that showed depolarizing phases in both expiration and in inspiration. Examples are shown in Fig. 3. For some of these CRDPs (e.g. Fig. 3A), the inspiratory phase was the strongest; in others (e.g. Fig. 3B), the expiratory phase was strongest, though for some CRDPs the latter was only the case during the cycles with a strong efferent expiratory discharge. The biphasic CRDPs were therefore divided into two sub-categories, type 1 (n= 12) and type 2 (n= 18), corresponding to inspiratory or expiratory dominance, respectively. The mean amplitude of biphasic CRDPs was 4.4 ± 2.3 mV (range 1.0–10 mV, n= 30). The occurrence of these CRDPs was much greater in decerebrates than in anaesthetized animals (23/55 vs. 7/50, significant, χ2, P < 0.01). Similarly, in common with the general increased prevalence of an expiratory output in the decerebrates (de Almeida et al. 2010), the proportion of type 2 within the biphasic category was higher for the decerebrates than for the anaesthetized animals (16/23 vs. 2/7), though not quite significantly so (Fisher exact test, P= 0.059).
Figure 3. Biphasic CRDPs.
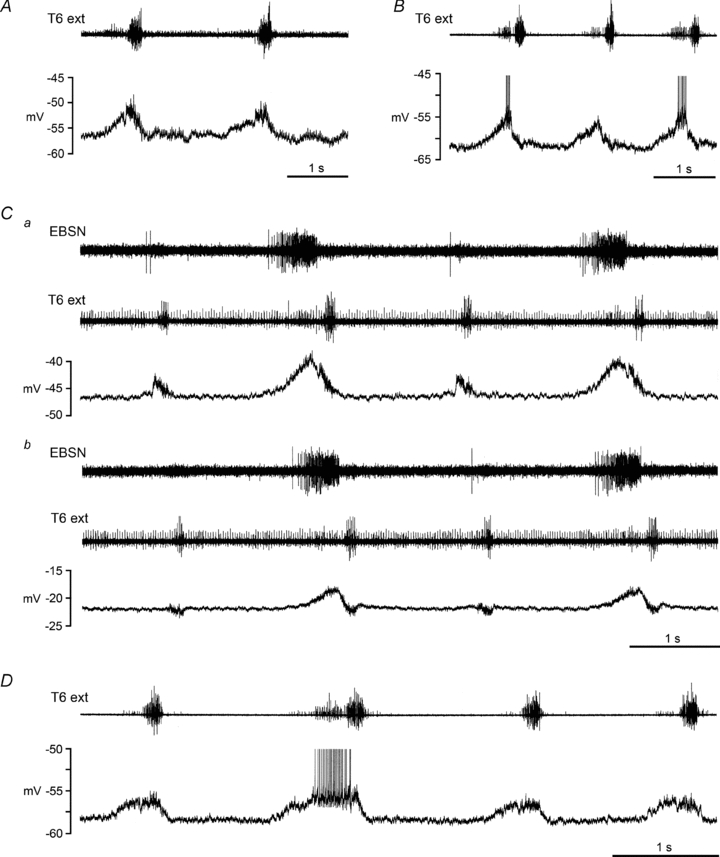
Examples are shown from 4 different internal intercostal nerve motoneurones in 3 different decerebrate rats, following halothane. A, type 1 biphasic CRDP, depolarization largest during inspiration; B–D, type 2 biphasic CRDPs, depolarization largest during expiration. The upper trace (T6 external intercostal nerve recording) shows an inspiratory discharge plus an expiratory discharge for all the examples illustrated, though the expiratory component is weak in A, is only present in alternate cycles in C, and is only strong for one of the cycles in D (see de Almeida et al. 2010 for further descriptions). An EBSN recording is included in C to make clear the expiratory excitation in alternate cycles, since the expiratory discharge in the external intercostal nerve is hard to detect. Both panels of C (a and b) come from the same recording, with b starting 167 s after the end of a, when the cell had depolarized from a membrane potential of −47 mV to −22 mV. Spikes are truncated in B and D.
In type 2 biphasic CRDPs, the inspiratory phase was most often short in duration and decrementing. Indeed, in some instances, the overall appearance was rather like a declining ‘tail’ on the expiratory depolarization. However, close inspection always showed a clear transition between the two phases. All four of the examples in Fig. 3 (one type 1, three type 2) show this transition as a brief hyperpolarizing notch. Furthermore, in a number of instances, the cycle-to-cycle variation in the occurrence of an expiratory drive also revealed the two separate phases, as in Fig. 3Ca. Note that the 1st and 3rd cycles here show no expiratory discharge in the external intercostal nerve (or, more clearly, no more than 1 or 2 spikes for the EBSN in this recording) and, correspondingly, no expiratory depolarization in the motoneurone, just as was the case for the expiratory CRDPs. Nevertheless, a distinct, purely inspiratory depolarization is clearly evident for this cycle. Finally, note that in many cases the transition to the inspiratory phase (for either type 1 or type 2) was accompanied by an increase in the synaptic noise, as is evident for at least three of the examples in Fig. 3.
For most of the type 2 CRDPs (14/18) and for one of the type 1 CRDPs, the inspiratory wave appeared to be depolarizing at the start of inspiration, but hyperpolarizing at the end of inspiration, suggesting that inhibition was present during inspiration, at least at the end of this phase. In fact, there is evidence that inhibition was probably present in these motoneurones throughout inspiration, in parallel with the excitation. The evidence comes from cells such as that in Fig. 3C, in which the membrane potential gradually declined towards the end of the recording. It is particularly clear in this example that, for the cycles without an expiratory depolarization, the inspiratory wave, initially seen as depolarizing, had almost entirely reversed to a hyperpolarizing wave when the motoneurone was sufficiently depolarized (Fig. 3Cb). The same was the case at the end of inspiration for the cycles which included an expiratory wave. Support for the interpretation of such hyperpolarizations as resulting from inhibition again comes from the synaptic noise. Note in particular that in Fig. 3Ca the synaptic noise is greater during inspiration than at the equivalent level of depolarization during expiration. Reversal of the inspiratory wave with motoneurone depolarization was seen in 11 of the type 2 CRDPs. For one of these, the expiratory wave also reversed, suggesting simultaneous excitation and inhibition in both respiratory phases for this motoneurone. For comparison, we also checked the inspiratory CRDPs. Two of these, both in internal intercostal nerve motoneurones, both in anaesthetized animals, also showed CRDPs reversing with depolarization (to membrane potentials of −35 mV and −25 mV).
It should also be noted that the depolarizing wave during expiration in Fig. 3C is typical of the majority of the depolarizing phases of the CRDPs of all categories in that it showed a relatively minor attenuation, from an amplitude of 6 mV to one of 4 mV, as the motoneurone depolarized from −46 to −22 mV. Examples such as this provide the justification (cited in the Methods section) for accepting data from cells with relatively low membrane potentials.
Biphasic inhibitory CRDPs
Three CRDPs were recorded that consisted of a hyperpolarization in inspiration (amplitudes 3–1.5 mV), and a somewhat smaller hyperpolarization during late expiration (amplitudes 1–0.5 mV). All of the motoneurones concerned were recorded when the expiratory discharge was intermittent in the T6 external intercostal nerve (3 different decerebrate animals) and the expiratory wave in each was present only for cycles with an efferent expiratory discharge (Fig. 4A). Both hyperpolarizations in all three motoneurones were characterized by the presence of synaptic noise and the CRDPs were therefore designated biphasic inhibitory.
Figure 4. Evidence for more complex inhibitory inputs.

Two internal intercostal nerve motoneurones are illustrated. A, decerebrate following halothane: a CRDP defined as biphasic inhibitory: note the small hyperpolarizing potentials that precede the larger (2 mV) hyperpolarizations in inspiration. The former occur simultaneously with the EBSN discharge (top 2 traces) and the expiratory discharge in the T6 external nerve discharge (3rd trace). Note absence of all 3 in the second respiratory cycle. B, ketamine anaesthetized: a CRDP that was defined as expiratory. When initially recorded at a membrane potential of −42 mV, it showed a depolarizing ramp of amplitude 1.5 mV during expiration. Note that the synaptic noise increases in both respiratory cycles as expiration progresses. C, extract from B (at the time of the arrow) shown on an expanded time scale, illustrating that this noise is dominated by IPSP-like waveforms.
The membrane potentials for these CRDPs were low, initially −30 to −36 mV, declining thereafter. The expiratory hyperpolarizations were hardly detectable initially, only becoming obvious as the motoneurone depolarized further, the appearance then being very similar to the one biphasic hyperpolarizing CRDP that had appeared by reversal from a biphasic depolarizing waveform (previous section). It is quite likely therefore that, at more physiological membrane potentials, these CRDPs also would have been classified as biphasic (depolarizing) or perhaps as expiratory. With respect to the latter, none of the 53 expiratory CRDPs described above showed convincing reversals with depolarization of the motoneurone, though two of them, when depolarized, did show IPSP-dominated synaptic noise which increased as expiration progressed (Fig. 4B and C).
CRDPs according to motoneurone identity
Table 2 summarizes the CRDPs according to motoneurone identity. The majority were identified from the internal intercostal nerve. Not surprisingly these showed a distribution of CRDP types rather similar to the overall population. However, it is worth noting that the distribution of inspiratory CRDPs for the internal intercostal nerve group was even more strongly biased towards the anaesthetized animals than it was for the overall population, the difference between the anaesthetized and the decerebrates being significant (7/40 vs. 0/41, Fisher exact test, P= 0.0054).
Only five external intercostal nerve motoneurones were recorded. Nevertheless, three were depolarized in expiration (1 expiratory, 2 biphasic), consistent with the recordings of nerve discharges previously reported (Tian & Duffin, 1996; de Almeida et al. 2010).
For the relatively small group of unidentified motoneurones (19), the most notable feature was the relatively high number of inspiratory CRDPs (8), including a high proportion in the decerebrates (5/11), i.e. the reverse of the situation for the internal intercostal nerve group. The inspiratory CRDPs here had a mean amplitude of 5.0 ± 2.0 mV, including the two largest amplitude inspiratory CRDPs recorded (7.5 and 9 mV).
Discussion
There are two important new observations in this study, firstly the common occurrence of biphasic excitation of individual motoneurones and secondly, for a significant proportion of motoneurones, simultaneous excitation and inhibition. Neither of these chacteristics has been identified before in thoracic motoneurones. They have general relevance for the mechanisms of motoneurone excitation by rhythmic central pattern generators as well as raising new questions with regard to the respiratory actions of the muscles involved.
Inspiratory and expiratory activation of different categories of motoneurones
Both the nerve recordings and EMGs reported in the preceding paper (de Almeida et al. 2010) indicated that the proximal parts of the internal intercostal muscle and/or subcostalis in caudal intercostal spaces of the rat are nearly always activated in inspiration. This conclusion was amply confirmed in the intracellular recordings here, by the frequent observation of individual motoneurones showing excitation in both phases, i.e. biphasic CRDPs. These observations do not identify the particular region of the intercostal space (or abdomen) innervated by any one motoneurone, but because of the certainty in the observations of biphasic excitation, there is now no need to postulate unusual innervation patterns to explain the biphasic activity in a given nerve branch or at a given EMG site. Extensive controls to exclude such innervation are therefore not really necessary. Similarly, the assumption of expiratory activation of external intercostal muscle, though unproven in the EMG experiments, is strongly supported by the intracellular recording experiments. Three out of five of the external intercostal nerve motoneurones showed expiratory excitation, which, we suggest, would be most unlikely if the expiratory activity was only destined for the few motor axons innervating serratus (de Almeida et al. 2010).
It is worth considering which muscles are innervated by motoneurones with different CRDP types. We start with the category with the largest number, the internal intercostal nerve motoneurones, and from these, first, the inspiratory group. The most natural assumption might be to assign these to the innervation of interchondral muscle, since in other species investigated interchondral muscle has always been reported as inspiratory. However, it is noteworthy that all of the seven inspiratory CRDPs here were from anaesthetized animals, none from decerebrates, whereas for biphasic CRDPs, only 5/40 were from the anaesthetized animals versus 20/41 from the decerebrates (a significant difference, χ2, P < 0.001). We suggest that the most likely explanation for this is that the inspiratory CRDPs in the anaesthetized group came from the same motoneurones that would show biphasic CRDPs in decerebrates, but that the expiratory drive to make them biphasic was suppressed by the anaesthesia. Consistent with this, in two of the inspiratory CRDPs there was evidence for simultaneous excitation and inhibition during inspiration, similar to that seen for biphasic CRDPs. If the suggestion is correct, then there would be no special group here to be assigned to interchondral muscle. Perhaps this might have been expected. This part of the muscle is very small in T9 (absent in T10), comprising a narrow triangle between the last two costal cartilages, and quite distant from the sternebra (Greene, 1968). As far as we know, neither the electrical activity nor the mechanical properties of this part of the thorax have been investigated in any species.
The expiratory CRDPs in the internal intercostal nerve motoneurones seem to form a more definitive group in that a reasonable proportion occurred in both the anaesthetized and decerebrate animals. Given the obvious association between the biphasic CRDPs and the biphasic activation of internal intercostal muscle (de Almeida et al. 2010), we suggest that the biphasic CRDPs belonged to motoneurones innervating intercostal muscle, while the purely expiratory motoneurones innervated abdominal muscles. Indeed, descriptions to date of respiratory activation of abdominal muscles in the rat, either from abdominal nerves or from EMG, have mentioned only expiratory phasing (Sherrey et al. 1988; Iizuka, 2003; Iizuka & Fregosi, 2007; Zoccal et al. 2008; Abdala et al. 2009), although some of the illustrations in Iizuka (2003) and in Zoccal et al. (2008) appear to include some inspiratory activation. In Figs 2 and 5 of Zoccal et al. (2008), the integrated version of the abdominal nerve record from the control population shows a discharge starting well within inspiration. However, this nerve could have been in a thoracic segment and therefore included some fibres innervating internal intercostal muscle. In contrast, the recordings from Iizuka (2003), for internal oblique muscle, were specific, being EMG recordings. His Fig. 3 for this recording (low pH) shows a very brief discharge at the start of inspiration (also present in the recordings from a distal internal intercostal muscle and from T13 ventral root), apparently very like the brief burst seen in some of our internal intercostal nerve recordings (e.g. de Almeida et al. 2010, their Fig. 4B, T9 int). If so, this could have interesting implications for the role of inhibition in these motoneurones (see below).
Despite the above caveats, the assumption that the expiratory CRDPs represent mostly abdominal motoneurones, at least in the decerebrates, still seems likely. If so, then it would seem likely that the CRDPs in the remainder of the internal intercostal nerve motoneurones, which would innervate the T9 internal intercostal or subcostalis muscles (perhaps also including a few supplying interchondral muscle), all have an inspiratory component. It is of interest that the ventral root recordings of Smith et al. (1990) for T1–T13, Iizuka (2003) for T9 or Giraudin et al. (2008) for T11 all included an inspiratory component, which could correspond to this excitation or to the excitation of external intercostal or levator costae muscle.
For the external intercostal nerve motoneurones, the sample here is very small. As discussed above it is unlikely that all of the biphasic excitation was destined for serratus, rather than for external intercostal muscle. It remains to be determined how much of the external intercostal muscle receives expiratory excitation.
For the unidentified motoneurones, the simplest hypothesis as to the identity of some of these is that they belonged to an adjacent segment. In fact this is very likely for 8 of the 19 unidentified motoneurones, since all of these were recorded at the most rostral position for that experiment, more rostral than (in one case as rostral as) any identified motoneurone. It is of interest that only 1 out of the 8 inspiratory CRDPs in the unidentified motoneurones was in such a rostral position. Of the remaining 7 inspiratory CRDPs, the 3 that were in anaesthetized animals could have been in motoneurones with axons in the proximal internal intercostal nerve filament, which was not included for antidromic stimulation. However, the most likely identity for the other 4 (and perhaps also for these 3) is that they were levator costae motoneurones. Interestingly, the two inspiratory motoneurones with prominent hyperpolarizations in expiration were in this group (Fig. 1C), consistent with Hilaire et al. (1983), for whom strong expiratory inhibition of levator costae motoneurones was ‘a consistent finding’. The other unidentified motoneurones (2 expiratory CRDPs, 1 biphasic, 1 biphasic inhibitory) could also have had axons in the internal intercostal nerve filament, but equally well could have had axons in the dorsal ramus (other than supplying levator costae). Dorsal ramus motoneurones in the cat showed a variety of CRDP types (Saywell et al. 2007).
Excitation and inhibition
A prominent inspiratory hyperpolarization in expiratory motoneurones was very common in the present experiments, corresponding to a similar waveform in expiratory motoneurones in the cat (Sears, 1964; Saywell et al. 2007). This results from postsynaptic inhibition (Sears, 1964). The important new observation in this respect was the common occurrence of this inhibition simultaneous with a wave of excitation, particularly in the biphasic CRDPs. We may deduce that this simultaneous occurrence in inspiration is very frequent, not only because of the number of motoneurones in which the effects were detected, but also because of some features of the nerve discharges reported by de Almeida et al. (2010). Although the evidence can only be indirect from these recordings, the decrementing, or very brief bursts frequently observed in the internal intercostal nerves at the start of inspiration appear to correspond very well to the type 2 biphasic CRDPs, which most often showed a decrementing (or reversing) depolarization during inspiration (Fig. 3). Neither concurrent excitation and inhibition nor biphasic CRDPs have been seen in cat thoracic motoneurones, despite a good sample of recordings with a similarly strong respiratory drive (Saywell et al. 2007). A possible difference is that all of these cat recordings were in anaesthetized animals, although, additionally, neither phenomenon was seen in thoracic motoneurones in decerebrate cats (P. A. Kirkwood, M. Enríquez Denton, J. Wienecke & H. Hultborn, unpublished observations). However, concurrent excitation and inhibition of internal intercostal motoneurones during inspiration could also underlie some of the effects of strychnine on the internal intercostal EMG discharge (neonatal rat, in vitro) seen by Iizuka (1999).
The observations here of concurrent excitation and inhibition largely rely on the acceptance of data from motoneurones depolarized, presumably by injury, to membrane potentials close to –20 mV. Whereas it might be unwise to rely on quantitative measurements from cells in this condition, the justification of accepting the qualitative data here comes from cells such as that illustrated in Fig. 3C. There were numerous examples such as this, where the time course of excitatory potentials appeared largely unchanged as the cells depolarized over similar ranges and where the amplitude changed only in proportion to the presumed driving potential. In the cat, it is well recognized that K+ channel activation (Barrett et al. 1980) causes a steep increase in membrane conductance, which typically takes effect at around −40 mV (e.g. Lee & Heckman, 2000). One might presume from our observations that such an increase should not take place until nearer −20 mV in rat motoneurones. However, although equivalent studies on adult rat spinal motoneurones in vivo do not seem to exist, published in vitro studies in the rat to date do not give much support for this expectation. For hypoglossal motoneurones from juvenile rats, Powers & Binder (2003) described a ‘sharp increase in outward current’ at about −30 mV, but Li & Bennett (2007) showed a very steep conductance increase at membrane potentials as negative as −45 mV in adult sacrocaudal motoneurones. Despite this lack of support, we argue that the empirical observations are sufficient. The typical shunting of synaptic potentials at membrane potentials more positive than −40 mV, which is such an obvious and familiar feature of recordings in cat motoneurones in this laboratory, just did not take place in a high percentage of the rat motoneurones here. One possible explanation is that Na+ entry, in our experiments as a result of injury, inhibited the K+ channel activation (Van Damme et al. 2002).
The second component of expiratory CRDPs, the ramp in expiration, showed a wide variation in amplitude (including zero) and was apparently independent of the inhibitory component. Making the same observation in the cat, Saywell et al. (2007) suggested that a major determinant of the ramp amplitude was the state of the preparation, in particular the depth of anaesthesia. The differences between the anaesthetized and decerebrate animals here support that view. Furthermore, the cycle-to-cycle variation, either in the expiratory CRDPs (Fig. 1E) or in the biphasic ones (Fig. 4C) shows the independence between the two components rather directly, in that the inhibition appeared to have a rather constant amplitude despite all-or-nothing variation in the expiratory drive. The inhibitory phase is therefore likely to be derived from the activity of the presumed inspiratory oscillator, not the expiratory one. The third component of biphasic CRDPs, the inspiratory depolarization, would then most logically be considered as an independent addition (e.g. not present in the expiratory CRDPs).
These considerations are of interest in comparisons with other reports of concurrent excitation and inhibition. There are several such reports: for medullary respiratory neurones (Schmid et al. 1996; McCrimmon et al. 1997) or for inspiratory motoneurones (phrenic, Parkis et al. 1999; hypoglossal, Saywell & Feldman, 2004). More recently, Berg et al. (2007) described a similar phenomenon in turtle motoneurones during fictive scratch, interpreting this in terms of the generalized theoretical benefits of balanced excitation and inhibition in the computational properties of large scale neuronal networks, but without reference to the previous respiratory neuronal observations. For Schmid et al. (1996) phasic inhibition concurrent with excitation was part of the pattern-shaping network, whereas for McCrimmon et al. (1997) and for Parkis et al. (1999), it was suggested to act as a gain control mechanism. This was specifically so for Parkis et al. (1999), because the time course of the excitation and inhibition were identical. In hypoglossal motoneurones, Saywell & Feldman (2004) found ‘much stronger inhibition’ than in phrenic motoneurones, and with a variety of time courses in different subgroups of motoneurones, presumably with different functions. Similarly, here, within the category of internal intercostal nerve motoneurones, the concurrent excitation and inhibition was most common in one group of motoneurones, those with biphasic CRDPs, which most likely innervate one particular muscle, internal intercostal/subcostalis. Thus, we suggest that the inhibition here could be considered as one of several independently controlled components of the CRDP. Its functional role then is much more likely to be specific, such as modulation of the excitatory inputs in different circuit reconfigurations for various different motor acts (Parkis et al. 1999), than it is to represent a general computational property, as proposed by Berg et al. (2007). This is clearly worthy of further investigation, such as via further separation of the components by variation of the chemical drive for respiration, or by more controlled variation of the membrane potential. Neither of these was possible in the present experiments, where the priority, when the cells were in their best condition, was spike-triggered averaging.
In this regard it is intriguing that the abdominal recordings of Iizuka (2003) might show the same phenomenon (see earlier), in this case the presumed inhibition being strong enough to almost completely gate out the presumed excitation. If the end-result then is an expiratory CRDP (in the neonate, this would be an early expiratory depolarization), what would the function of the inspiratory excitation be? Any answer is likely to be guesswork. However, this is not the only CRDP component with a widespread distribution and unknown function. Another example is the expiratory decrementing CRDP in the cat, which appears in motoneurones innervating a wide variety of muscles (Ford & Kirkwood 2006). It was suggested by Saywell et al. (2007) that such an input (either expiratory decrementing or inspiratory) might really represent a non-respiratory function, but made respiratory in the unnatural circumstances of an experimental preparation with an exaggerated respiratory drive. Perhaps the inspiratory excitation in the rat in our experiments was similar, and actually had an even wider distribution than was observed. It could have been present to some extent in many of the expiratory CRDPs, but unseen because of their strong inhibition during inspiration. Indeed defining the borderline between expiratory and biphasic was quite fine (cf. Figs 1D and 3B and C). On the other hand, one could argue against this rather non-specific role for the inspiratory excitation of internal intercostal motoneurones, by noting the opposite effect, i.e. expiratory excitation for the external intercostal motoneurones. This would rather suggest an alternative, specific, reciprocally organized respiratory function for both of these ‘anomalous’ components.
The possible analogy with the expiratory decrementing input described by Ford & Kirkwood (2006) is also of interest because of the earlier evidence from Kirkwood et al. (2002) that this input was particularly susceptible to amplification via the activation of persistent inward currents. If the inspiratory excitation in thoracic motoneurones in the rat can similarly access such amplification, then inhibition might be needed as a control mechanism, perhaps to prevent the unwanted occurrence of plateau potentials and thus to allow rhythmic patterns to proceed. Inhibition is a potent mechanism for regulating motoneurone firing in the presence of persistent inward currents (Hultborn et al. 2003). Was there any evidence from our recordings for the activation of persistent inward currents? One might suppose that some of the differences between the anaesthetized and decerebrate animals might be assigned to such an effect, since these currents are well known to be suppressed by anaesthesia (Hultborn, 1999). We performed no procedures aimed specifically at revealing their actions, such as passing depolarizing current via the recording electrodes. These procedures too often brought out movement artefacts in our preparations, though this could be tested better in dedicated experiments. An alternative item of evidence for the activation of plateau potentials is the occurrence of all-or-nothing depolarizations, for instance as in Kirkwood et al. (2002). All-or-nothing depolarizations were indeed present in our recordings, but always had the better explanation of an all-or-nothing variation in the descending drive. Of course we cannot rule out a contribution from persistent inward currents in generating the CRDPs, but we have no evidence for such a contribution. It should also be pointed out that the depolarizations in individual respiratory cycles are relatively short for the activation of plateaux, making their detection even harder.
Implications for experimental spinal cord repair
One way of testing for specificity of restored connections to these segments following spinal cord repair could be to look for normal patterns of inputs to the motoneurones. However, our results have shown that there is not a simple relationship between the activation profile of an intercostal motoneurone and the muscle layer that it innervates. Thus, there is now no easy way of identifying inspiratory or expiratory activation of any individual motoneurone as being specific. Such identification now must rest on more detailed measurements of connections from the brainstem, for which some specificity is apparent, as will be shown in a forthcoming report, based on the same experiments as reported here.
Acknowledgments
Roseanna Smither, Victor Baller and Ed Bye are thanked for technical assistance. The work was supported by the International Spinal Research Trust and the Brain Research Trust. A. T. R. de Almeida held an MRC studentship.
Glossary
Abbreviations
- CRDP
central respiratory drive potential
- EBSN
expiratory bulbospinal neurone
Author contributions
Both authors contributed to all aspects of the study.
References
- Abdala AP, Rybak IA, Smith JC, Paton JF. Abdominal expiratory activity in the rat brainstem–spinal cord in situ: patterns, origins and implications for respiratory rhythm generation. J Physiol. 2009;587:3539–3559. doi: 10.1113/jphysiol.2008.167502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett EF, Barrett JN, Barrett Crill WE. Voltage-sensitive outward currents in cat motoneurones. J Physiol. 1980;304:251–276. doi: 10.1113/jphysiol.1980.sp013323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg RW, Alaburda A, Hounsgaard J. Balanced inhibition and excitation drive spike activity in spinal half-centers. Science. 2007;315:390–393. doi: 10.1126/science.1134960. [DOI] [PubMed] [Google Scholar]
- Berger AJ. Phrenic motoneurons in the cat: subpopulations and nature of respiratory drive potentials. J Neurophysiol. 1979;42:76–90. doi: 10.1152/jn.1979.42.1.76. [DOI] [PubMed] [Google Scholar]
- de Almeida A, Kirkwood PA. Patterns of respiratory activation for intercostal muscles in the rat. Proc Physiol Soc. 2008;10:C11, PC60. [Google Scholar]
- de Almeida ATR, Al-Izki S, Enríquez Denton M, Kirkwood PA. Patterns of expiratory and inspiratory activation for thoracic motoneurones in the anaesthetized and the decerebrate rat. J Physiol. 2010;588:2707–2729. doi: 10.1113/jphysiol.2010.192518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo T, Kiehn O. Asymmetric operation of the locomotor central pattern generator in the neonatal mouse spinal cord. J Neurophysiol. 2008;100:3043–3054. doi: 10.1152/jn.90729.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford TW, Kirkwood PA. Respiratory drive in hindlimb motoneurones of the anaesthetized female cat. Brain Res Bull. 2006;70:450–456. doi: 10.1016/j.brainresbull.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Giraudin A, Cabirol-Pol MJ, Simmers J, Morin D. Intercostal and abdominal respiratory motoneurons in the neonatal rat spinal cord: spatiotemporal organization and responses to limb afferent stimulation. J Neurophysiol. 2008;99:2626–2640. doi: 10.1152/jn.01298.2007. [DOI] [PubMed] [Google Scholar]
- Greene E. Anatomy of the Rat. USA: Hafner Publishing Company Inc.; 1968. [Google Scholar]
- Hilaire GG, Nicholls JG, Sears TA. Central and proprioceptive influences on the activity of levator costae motoneurones in the cat. J Physiol. 1983;342:527–548. doi: 10.1113/jphysiol.1983.sp014867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H. Plateau potentials and their role in regulating motoneuronal firing. Prog Brain Res. 1999;123:39–48. doi: 10.1016/s0079-6123(08)62842-3. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Enríquez Denton M, Wienecke J, Nielsen JB. Variable amplification of synaptic input to cat spinal motoneurones by dendritic persistent inward current. J Physiol. 2003;552:945–952. doi: 10.1113/jphysiol.2003.050971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iizuka M. Intercostal expiratory activity in an in vitro brainstem–spinal cord–rib preparation from the neonatal rat. J Physiol. 1999;520:293–302. doi: 10.1111/j.1469-7793.1999.00293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iizuka M. GABAA and glycine receptors in regulation of intercostal and abdominal expiratory activity in vitro in neonatal rat. J Physiol. 2003;551:617–633. doi: 10.1113/jphysiol.2003.042689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iizuka M, Fregosi RF. Influence of hypercapnic acidosis and hypoxia on abdominal expiratory nerve activity in the rat. Respir Physiol Neurobiol. 2007;157:196–205. doi: 10.1016/j.resp.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Kirkwood PA, Lawton M, Ford TW. Plateau potentials in hindlimb motoneurones of female cats under anaesthesia. Exp Brain Res. 2002;146:399–403. doi: 10.1007/s00221-002-1163-0. [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Adjustable amplification of synaptic input in the dendrites of spinal motoneurons in vivo. J Neurosci. 2000;20:6734–6740. doi: 10.1523/JNEUROSCI.20-17-06734.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Bennett DJ. Apamin-sensitive calcium-activated potassium currents (SK) are activated by persistent calcium currents in rat motoneurons. J Neurophysiol. 2007;97:3314–3330. doi: 10.1152/jn.01068.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrimmon DR, Zuperku EJ, Hayashi F, Dogas Z, Hinrichsen CF, Stuth EA, Tonkovic-Capin M, Krolo M, Hopp FA. Modulation of the synaptic drive to respiratory premotor and motor neurons. Respir Physiol. 1997;110:161–176. doi: 10.1016/s0034-5687(97)00081-9. [DOI] [PubMed] [Google Scholar]
- Meehan CF, Ford TW, Road JD, Donga R, Saywell SA, Anissimova NP, Kirkwood PA. Rostrocaudal distribution of motoneurones and variation in ventral horn area within a segment of the feline thoracic spinal cord. J Comp Neurol. 2004;472:281–291. doi: 10.1002/cne.20096. [DOI] [PubMed] [Google Scholar]
- Milano S, Miller AD, Grélot L. Multi-phase expiratory inhibition of phrenic motoneurons in the decerebrate cat. NeuroReport. 1992;3:307–310. doi: 10.1097/00001756-199204000-00004. [DOI] [PubMed] [Google Scholar]
- Parkis MA, Dong X, Feldman JL, Funk GD. Concurrent inhibition and excitation of phrenic motoneurons during inspiration: phase-specific control of excitability. J Neurosci. 1999;19:2368–2380. doi: 10.1523/JNEUROSCI.19-06-02368.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers RK, Binder MD. Persistent sodium and calcium currents in rat hypoglossal motoneurons. J Neurophysiol. 2003;89:615–624. doi: 10.1152/jn.00241.2002. [DOI] [PubMed] [Google Scholar]
- Saywell SA, Anissimova NP, Ford TW, Meehan CF, Kirkwood PA. The respiratory drive to thoracic motoneurones in the cat and its relation to the connections from expiratory bulbospinal neurones. J Physiol. 2007;579:765–782. doi: 10.1113/jphysiol.2006.122481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saywell SA, Feldman JL. Dynamic interactions of excitatory and inhibitory inputs in hypoglossal motoneurones: respiratory phasing and modulation by PKA. J Physiol. 2004;554:879–889. doi: 10.1113/jphysiol.2003.054528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid K, Foutz AS, Denavit-Saubié M. Inhibitions mediated by glycine and GABAA receptors shape the discharge pattern of bulbar respiratory neurons. Brain Res. 1996;710:150–160. doi: 10.1016/0006-8993(95)01380-6. [DOI] [PubMed] [Google Scholar]
- Sears TA. The slow potentials of thoracic respiratory motoneurones and their relation to breathing. J Physiol. 1964;175:404–424. doi: 10.1113/jphysiol.1964.sp007524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrey JH, Pollard MJ, Megirian D. Proprioceptive, chemoreceptive and sleep state modulation of expiratory muscle activity in the rat. Exp Neurol. 1988;101:50–62. doi: 10.1016/0014-4886(88)90064-7. [DOI] [PubMed] [Google Scholar]
- Smith JC, Greer JJ, Liu G, Feldman JL. Neural mechanisms generating respiratory pattern in mammalian brain stem–spinal cord in vitro. I. Spatiotemporal patterns of motor and medullary neuron activity. J Neurophysiol. 1990;64:1149–1169. doi: 10.1152/jn.1990.64.4.1149. [DOI] [PubMed] [Google Scholar]
- Smith CL, Hollyday M. The development and postnatal organization of motor nuclei in the rat thoracic spinal cord. J Comp Neurol. 1983;220:16–28. doi: 10.1002/cne.902200104. [DOI] [PubMed] [Google Scholar]
- Tian GF, Duffin J. Connections from upper cervical inspiratory neurons to phrenic and intercostal motoneurons studied with cross-correlation in the decerebrate rat. Exp Brain Res. 1996;110:196–204. doi: 10.1007/BF00228551. [DOI] [PubMed] [Google Scholar]
- Van Damme P, Van Den Bosch L, Van Houtte E, Eggermont J, Callewaert G, Robberecht W. Na+ entry through AMPA receptors results in voltage-gated K+ channel blockade in cultured rat spinal cord motoneurons. J Neurophysiol. 2002;88:965–972. doi: 10.1152/jn.2002.88.2.965. [DOI] [PubMed] [Google Scholar]
- Zoccal DB, Simms AE, Bonagamba LG, Braga VA, Pickering AE, Paton JF, Machado BH. Increased sympathetic outflow in juvenile rats submitted to chronic intermittent hypoxia correlates with enhanced expiratory activity. J Physiol. 2008;586:3253–3265. doi: 10.1113/jphysiol.2008.154187. [DOI] [PMC free article] [PubMed] [Google Scholar]


