Abstract
Nerve sprouting to reinnervate partially denervated muscles is important in several disease and injury states. To examine the effectiveness of sprouting of active and inactive motor units (MUs) and the basis for a limit to sprouting, one of three rat lumbar spinal roots was cut under normal conditions and when the spinal cord was hemisected at T12. Muscle and MU isometric contractile forces were recorded and muscle fibres in glycogen-depleted single muscle units enumerated 23 to 380 days after surgery. Enlargement of intact MUs by sprouting was effective in compensating for up to 80% loss of innervation. For injuries that removed >70–80% of the intact MUs, muscle contractile force and weight dropped sharply. For partial denervation of <70%, all MUs increased contractile force by the same factor in both normally active muscles and muscles whose activity was reduced by T12 hemisection. Direct measurements of MU size by counting glycogen-depleted muscle fibres in physiologically and histochemically defined muscle units, provided direct evidence for a limit in MU size, whether or not the activity of the muscles was reduced by spinal cord hemisection. Analysis of spatial distribution of muscle fibres within the outer boundaries of the muscle unit demonstrated a progressive increase in fibres within the territory to the limit of sprouting when most of the muscle unit fibres were adjacent to each other. We conclude that the upper limit of MU enlargement may be explained by the reinnervation of denervated muscle fibres by axon sprouts within the spatial territory of the muscle unit, formerly distributed in a mosaic pattern.
Introduction
Motor axonal sprouting is a well-recognized process that increases motor unit size to compensate for partial denervation of skeletal muscles (Son et al. 1996; Tam & Gordon, 2003a; Gordon et al. 2004a). Sprouts emanating from nodes and/or terminal regions of the intramuscular branches of remaining intact motor nerves reinnervate denervated endplates and are especially important when reinnervation by the injured or damaged nerves is impossible. Examples include motoneuron loss at spinal cord lesion sites and in motoneuron diseases of poliomyelitis, amyotrophic lateral sclerosis (ALS) and spinal muscular atrophy (Gordon et al. 2004a).
The capacity for motor unit enlargement remains unclear. Yang et al. (1990) reported up to 5-fold enlargement of human motor units after cervical spinal cord lesion. Whether this motor unit enlargement compares with that of reduced numbers of motoneurons in the intact spinal cord has yet to be determined. In ALS patients, motor units enlarge initially but the sprouting capacity wanes as the disease progresses and the remaining motor units fail to compensate for the progressive loss of functioning motor units (Dengler et al. 1990). In transgenic mouse models of ALS, the largest and fastest motor units are affected first with progressive loss and conversion of remaining intact motor units towards non-fatiguing types (Hegedus et al. 2007, 2008). The latter motor units survive longer and sustain their capacity to sprout (Frey et al. 2000; Pun et al. 2006; Hegedus et al. 2007).
The uncertainties of the extent of motor unit enlargement arise in part from discrepancies between measurements of motor unit size by electromyography (EMG) and isometric force in humans: EMG measurements indicated motor unit enlargement in ALS patients (Dengler et al. 1990; Pinelli et al. 1991; Vogt & Nix, 1997) but force measurements did not (Milner-Brown et al. 1974). In animal experiments, comparison of motor unit force distributions in partially denervated hindlimbs of rats and cats was consistent with enlargement of up to 5–8 times (Brown & Ironton, 1978; Yang et al. 1990; Rafuse et al. 1992; Gordon et al. 1993, 2004a; Tam & Gordon, 2003a,b;). However, comparison of single unit forces in partially denervated hindlimb muscles with those in normally innervated muscles indicated considerably higher limits of sprouting (Luff et al. 1988).
Neuromuscular activity plays a key role in sprouting capacity of motor nerves. Elevated daily neuromuscular activity by daily running exercise or functional electrical stimulation reduces motor unit enlargement in partially denervated rat hindlimb muscles (Tam et al. 2001). It does so by inhibiting the bridging of innervated and denervated endplates by perisynaptic Schwann cell processes (Tam & Gordon, 2003b). Perisynaptic Schwann cells at the neuromuscular junctions normally extend processes between innervated and denervated endplates, guiding axon sprouts to reinnervate denervated muscle endplates (Son & Thompson, 1995a,b;); outgrowth of processes and their bridging between endplates precede outgrowth of sprouts from remaining intact axons (Son & Thompson, 1995b). High daily neuromuscular activity reduces extension of processes from the perisynaptic Schwann cells at innervated but not denervated endplates and thereby prevents bridge formation and effective sprouting (Tam & Gordon, 2003b). Yet attempts to eliminate neuromuscular activity did not promote sprouting. Agents that include tetrodotoxin to block conduction of action potentials in the nerve and botulinum toxin and α-bungarotoxin to block neuromuscular transmission pre- and post-synaptically, reduce rather than promote axon sprouting (Connold & Vrbová, 1990, 1991; Tam et al. 2002b; Tam & Gordon, 2003a). The reduced incidence of bridging between innervated and denervated neuromuscular junctions suggested that bridge formation requires some synaptic transmission, possibly mediated by trophic factors such as glial growth factor or cell adhesion molecules (Love & Thompson, 1999).
In this study of partially denervated tibialis anterior (TA) muscle in the rat hindlimb under normal conditions and under conditions in which neuromuscular activity was dramatically reduced by hemisection of the thoracic spinal cord, we have (1) re-examined the capacity of normal motor units to sprout to compensate for reduced motor unit numbers and (2) determined whether reduced rather than eliminated neuromuscular activity affects this capacity. We cut either L4 or L5 spinal root simultaneously with hemisection of the lower thoracic spinal cord at T12 to dramatically reduce but not eliminate neuromuscular transmission during the first 10 days of axon sprouting when almost all the reinnervation of denervated endplates occurs (Tam & Gordon, 2003b). Neuromuscular activity is dramatically reduced immediately after thoracic hemisection with progressive recovery of activity over a period of a month (Celichowski et al. 2009). We will refer to the rats with spinal cord thoracic hemisection as the hemisected rats to distinguish them from the rats in which the spinal cord was intact.
It is the aim of this study to induce measurable extents of partial denervation in TA muscle in rats with intact and hemisected spinal cords in order to measure recovery of muscle function in association with enlargement of motor units. We examine the enlargement of motor units following partial denervation using force as an indirect measure of size in a large sample of motor units and using counts of the muscle fibres innervated by single motoneurons (muscle units) as a direct measure in a subset of motor units. Our findings demonstrate that the capacity for axon sprouting of flexor motoneurons is the same in normally active motoneurons and in those whose neuromuscular activity is dramatically diminished by spinal cord hemisection (Celichowski et al. 2009).
Methods
Animals
The experiments were performed on 90 young (4–5 months), female Sprague–Dawley rats with an initial body weight of 220–260 g. Surgery was performed on 67 animals. The remaining 23 age-matched rats were used in the final experiments for comparison with the rats in which hindlimb muscles were partially denervated. All surgical procedures were approved by the University of Alberta animal care committee and adhered strictly to the guidelines set by the Canadian Council on Animal Care.
Surgery for partial denervation and spinal hemisection
Partial denervation of tibialis anterior (TA) muscle in 67 rats was carried out under sterile conditions with sodium pentobarbitol (45 mg kg−1) anaesthesia injected intraperitoneally (i.p.), and atropine (0.1 mg kg−1) added to reduce respiratory congestion.
One hindlimb was partially denervated by sectioning either L4 or L5 of the contributing three spinal roots, L3, L4 and L5, that supply the TA muscle. In order to minimize the possibility of the cut axons regenerating (Kugelberg et al. 1970; Rafuse et al. 1992), a few millimeters of the root were excised. In a subgroup of 25 rats, a small laminectomy was carried out to expose the spinal cord at T12 and a no. 12 scalpel blade was used to hemisect the right side of the spinal cord at the T12 level in addition to partial denervation of the right TA muscle. Food pellets were placed on the bedding for the latter rats during the first week following the surgery to ensure their nutrition. Manuel bladder expression was rarely necessary. All the rats were allowed to move freely within their cages during the recovery periods of between 23 and 380 days after which acute experiments were performed to examine TA motor unit populations and to isolate and deplete single motor units for later histochemical analyses.
Recording of TA muscle and motor unit EMG and contractile forces
After 23–380 days following the transection of one of three spinal roots that supply the TA muscle with and without spinal cord hemisection at T12, a final acute experiment was performed with the rats maintained throughout in the surgical plane of anaesthesia using sodium pentobarbital that was diluted 1 in 5 from the dose given i.p. Identical experiments were performed on the 23 age-matched normal control animals. The body weights of the animals ranged from 250 to 650 g. Two days prior to each final acute experiment the rat was given glucose in the drinking water (5% solution). A venous cannula was used to administer anaesthesia, as well as 5% glucose in saline solution to maintain blood pressure and blood glucose levels (Tötösy de Zepetnek et al. 1992b; Fu & Gordon, 1995, 1997 for details). A laminectomy was performed to expose the spinal cord from T12 to L6 to allow isolation of the contributing roots to the motor innervation of TA muscle (Fig. 1). The muscles in both legs were exposed and all muscles except the TA were denervated. A pair of fine wires was inserted into muscle tissue on which the sciatic nerve lay to permit stimulation of the TA muscle via the nerve. EMG signals were recorded from the TA muscle using bipolar electrodes fixed 5 mm apart on a silastic sheet and sewn on to the fascia of the muscle. The skin was closed around the muscles and either clipped together or sewn, and a string (00 silk suture) attached to the distal tendon protruded at the level of the ankle. Using clamps to fix the knees and ankles, the rat was mounted rigidly to a steel table. The TA muscles from both hindlimbs were attached to Grass force transducers (FT03) by the strings attached to the distal tendons. The skin on the back was held stretched open with rubber bands that were fixed to posts on the table and a spinal pool of mineral oil was formed. The ventral roots L3, L4 and L5 on both sides were identified and cut from the spinal cord. A heating blanket and a lamp providing radiant heat were used to maintain core temperature of 37°C and muscle temperature of 34°C, which were monitored using a rectal probe and probes in the legs under the skin.
Figure 1. Recording in vivo of muscle and motor unit electromyographic (EMG) signals and isometric twitch forces from the tibialis anterior (TA) muscle.
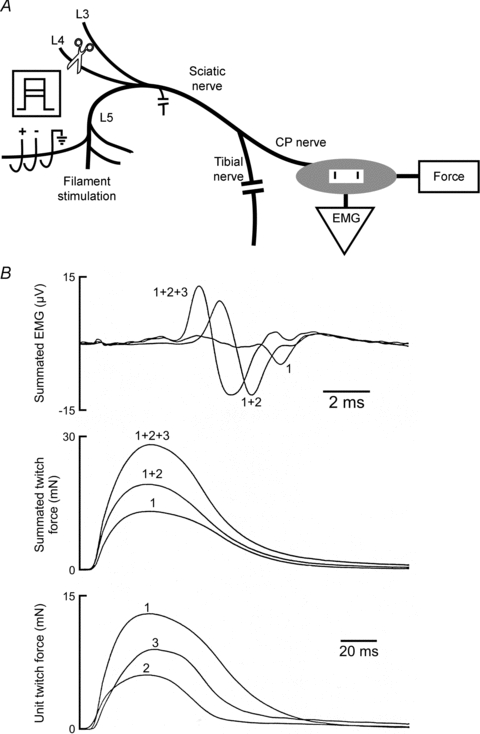
A. The innervation to the TA muscle was isolated by cutting all nerve branches in the hindlimb other than the common peroneal (CP) nerve supplying the TA muscle. Ventral root filaments were teased for stimulation via a triphasic array of electrodes to evoke all-or-none increments in EMG and twitch force. The array was used to isolate the stimulus to the ventral root filament that was contained within an oil pool around the open spinal cord after the laminectomy. B, all-or-none EMG and twitch contractile forces that were elicited by incremental stimulation of 3 different motor axons. The twitch contractile forces of the second and third motor units were obtained by subtraction of the first twitch contraction from the second and the combined force of the first and second motor units from the third increment in twitch contractile force.
The force and EMG signals were suitably amplified, recorded in milliNewtons and millivolts, respectively, and sampled by a computer at appropriate sampling rates (EMG, 12.5 kHz; twitch, 1250 Hz; tetanus, 500 Hz). Averages of 1 to 30 were made for each measurement. The averaging greatly reduced noise in the measurements especially when recording small force and EMG levels from single motor units. Springs in the force transducer were adjusted for 10 and 0.05 N maximum force ranges for whole muscle and motor unit recordings, respectively.
Left and right muscle comparison and root contributions to the TA muscle
Twitch and tetanic isometric forces developed by left and right hindlimb TA muscles were first recorded in response to supramaximal (2× threshold) stimulation of the sciatic nerve once the optimal muscle length was ascertained for maximal twitch and tetanic forces (Rafuse et al. 1992; Tam et al. 2001). Then each of the three intact ventral roots supplying the left intact hindlimb were placed on an array of three electrodes to stimulate each root supramaximally and record TA muscle twitch and tetanic forces (Fig. 1A). Thereby, their neural contribution to the TA muscle was determined. The two remaining ventral roots supplying the right partially denervated hindlimb were then stimulated and the isometric contractile forces recorded. The cut ventral root was also examined and occasionally this cut root(s) had regenerated despite the precautions taken. In this study, only those muscles that did not have regenerated axons were used. If the stimulation of the cut root on the right side did not produce any force in the TA muscle, there was no regeneration of the cut axons. Using the corresponding root on the left side, the extent of partial denervation was determined by the ratio of the maximal tetanic contractile force recorded in response to 100 Hz stimulation of that root and the sum of the forces elicited by the stimulation of all three ventral roots. The root contribution was expressed as a percentage as shown in Fig. 4 for each of the three ventral roots.
Figure 4. The frequency distributions of the percentage contributions of L3 (A), L4 (B) and L5 (C) ventral roots to the motor innervation of the tibialis anterior (TA) muscle.
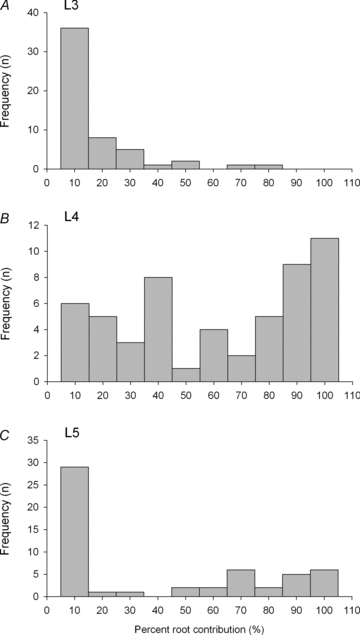
The distributions are skewed and demonstrate the relatively small contributions of the L3 and L5 ventral roots to the innervation of TA muscle with the majority of the contribution coming from the L4 ventral root.
TA motor units
Each of the contributing ventral roots in the control rats and which remained in the partially denervated experimental rats was dissected to selectively stimulate single motor units in the TA muscle. Nerve bundles were teased from the roots and placed on a 3-electrode array (Fig. 1A). An electrode at ground potential was placed distal to the cathode and anode to reduce accidental stimulation of other nerves by volume conduction. Motor unit sizes were estimated by recording the incremental force during graded stimulation of each nerve bundle (de Koning et al. 1989; Tam et al. 2001, 2002a,b). The technique of using incremental force to estimate motor unit size was described in detail previously and is shown in the example in Fig. 1B. If all the axons have distinct thresholds, by increasing the voltage of the stimulus pulse, different numbers of axons could be recruited with each new voltage level adding one more motor unit. Graphical subtraction of the twitch traces was used to estimate the contractile forces of the different motor units, i.e. subtracting the first trace from the second yields the force due to the second motor unit, subtracting the second trace from the third yields the twitch force of the third motor unit, etc. The groups of motor units were kept small, about three to four, so as to minimize the problem of alternation whereby the higher force levels will not be a monotonic increase in the number of recruited units but may have different combinations of units being stimulated due to slight variability in the stimulus thresholds (Major et al. 2007). At least 40% and up to 80% of the ∼120 motor units in the TA muscle was sampled on average from each muscle with this method.
Single motor and muscle units, glycogen depletion and histology
After the groups of motor units were sampled, a single axon supplying the TA muscle was isolated and the motor unit was physiologically characterized in terms of maximum tetanic force (a 200 ms train of pulses with a 10 ms interpulse interval), fatigability (100 pulses s−1 stimulation in 50-ms-long trains repeated every second for 2 min), and sag during an unfused tetanic contraction (800 ms train with an interpulse interval 1.25 times the twitch contraction time) (Tötösy de Zepetnek et al. 1991, 1992a,b).
The method of glycogen depletion of the muscle fibres of a single isolated motor unit by repetitive muscle contraction and periodic acid Schiff (PAS) staining has been described in detail previously (Tötösy de Zepetnek et al. 1991, 1992a,b). Briefly, an isolated motor unit was stimulated to deplete its glycogen content using intermittent trains of five pulses with a 10 ms interpulse interval at 1 Hz. A steady decline in force was produced and, if the decline in force reached a plateau, the train repetition rate was increased to 0.5 Hz in order to maintain the force decline. After this decline, motor unit force was encouraged to recover in response to 0.1 Hz stimulation. The fatiguing and recovery periods of stimulation were repeated until motor force failed to recover, about four repetitions for fatigable motor units and up to eight for fatigue-resistant motor units. This protocol ensured that the muscle unit fibres were well depleted of glycogen. It also allowed unequivocal identification of the depleted muscle unit fibres. Immediately after this depletion protocol, the muscle was quickly removed, blotted dry on filter paper for weighing, frozen in melting isopentane and stored at −70°C. The muscle was later cut into serial sections of 10 μm thickness, and using the PAS reaction, the glycogen-depleted muscle fibres were identified (Fig. 2A and C). Sequential muscle cross-sections were also stained for myosin ATPase following acid and alkaline preincubations for determination of type (Brooke & Kaiser, 1970; Guth & Samaha, 1970). Camara lucida drawings were made from the PAS-stained muscle cross-sections where glycogen-depleted fibres were outlined within the whole muscle cross-section (Fig. 2B and D). As described previously (Tötösy de Zepetnek et al. 1992a), such drawings were made from a number of sections that were taken at different proximo-distal levels along the muscle. The cross-sections yielding the largest number of muscle fibres were selected for territorial analysis (see also Wang & Kernell, 2000).
Figure 2. Photographs and camera lucida drawings of periodic-Schiff (PAS)-stained cross-sections of tibialis anterior (TA) muscles of normal (A and B) and partially denervated (C and D) hindlimbs showing glycogen-depleted muscle units.
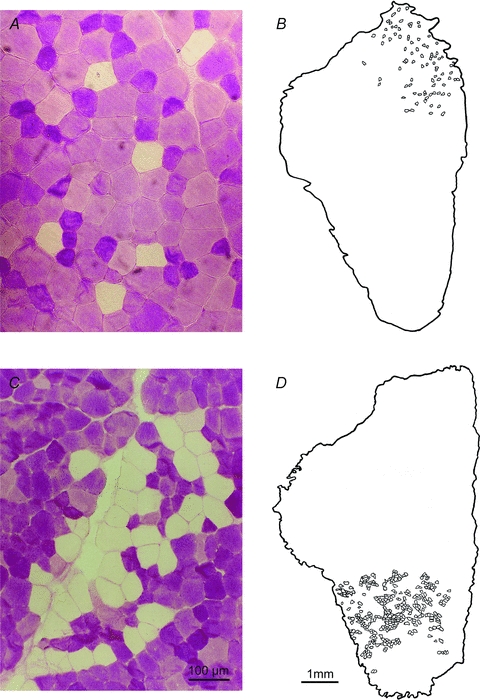
The 88 glycogen-depleted white muscle fibres in the normal muscle unit are intermingled with pink non-unit muscle fibres in a mosaic distribution (A); fibres occupy a defined area of territory within the cross-secction of the muscle (B). The territory area is contained within lines that connect the most outermost muscle unit fibres (not shown), see Methods for details). The 354 fibres in the muscle unit in the partially denervated muscle occupy a territory of similar size but their numbers are obviously higher and the fibres are distributed in clumps within the muscle unit territory (C and D). Note that the muscle unit fibres in both the normal and partially denervated muscles are contained in several fascicles (A and C).
Using photographs or digitized images of the muscle cross-section, the number of fibres in the depleted muscle unit, the muscle fibre areas and the territory containing the unit fibres were measured. The motor unit territory was defined as the area limited by lines connecting the outermost glycogen-depleted muscle fibres (Edstrom & Larsson, 1987).
Data analysis and statistics
The left and right TA muscles in normal rats were compared by regression analysis of the maximal isometric twitch and tetanic contractile forces (Fig. 3). Bilateral symmetry in the ventral root contributions was tested by examining the percentage contribution of each of the three roots on each side of the spinal cord to the respective TA muscles. The difference in contribution of corresponding roots was taken to be the deviation from perfect symmetry (for example, if the left L3 contributed 7% to the left TA muscle, while the right L3 contributed 5% to the right TA it was taken to be a deviation of 2%). The sum of the absolute deviations of the three pairs of roots is the total bilateral asymmetry and consequently, a measure of the inaccuracy in estimating partial denervation of one side using the root contributions of the other.
Figure 3. Bilateral symmetry of the lumbosacral motor axons that exit through the ventral roots on the left and right sides in normal unoperated control rats.
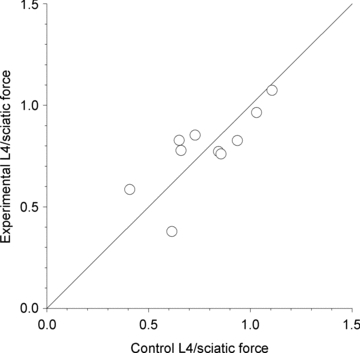
The left and right tibialis anterior (TA) muscle tetanic forces evoked by stimulation of the L4 ventral root at 100 Hz were expressed as a ratio of the tetanic forces evoked by suprathreshold stimulation of the sciatic nerve. These measurements showed that there was bilateral symmetry in the total muscle force as well as in the root contributions to the TA muscle.
Motor unit (MU) force production can be described by the equation, FMU=NAS where N is the number of muscle fibres innervated by a motoneuron, A is the mean cross-sectional area (CSA) of the constituent muscle fibres, and S is the specific force or the force per unit area that can be generated by these muscle fibres. As described previously, the three factors become additive by taking the logarithm of the equation. It is then possible to estimate their relative importance in determining motor unit force output, log F= log N+ log A+ log S (Tötösy de Zepetnek et al. 1992a). N and A were counted and calculated, respectively, and plotted as a function of motor unit tetanic force on double logarithmic scales (Figs 10C and D, and 11C and D) to determine the relative contributions of each factor to motor unit force. The slope of the regression line (m) gives the relative contribution of that factor to the observed variation in motor unit force. Regression lines were fitted to the data by the use of a least mean square criterion. The values of the slope and the standard error of the slope were obtained by minimizing the deviation of the y-values after plotting tetanic force, which shows the greatest range, on the x-axis. With this convention, each of the three possible contributing factors to unit force could be directly compared.
Figure 10. Frequency distributions of the number of muscle fibres in an isolated single motor unit (N) and the relationship between the muscle unit fibre number and the motor unit tetanic force in normally innervated and partially denervated tibialis anterior (TA) muscles under conditions of the spinal cord remaining intact or hemisected.
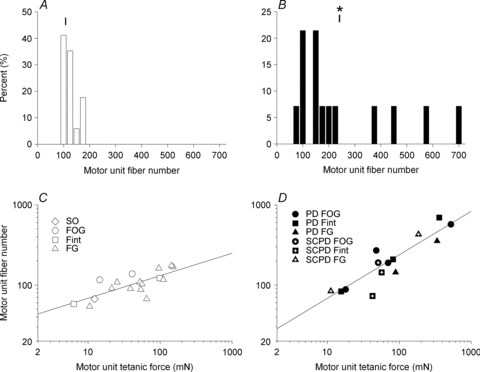
The frequency distribution of the number of muscle fibres in isolated single motor units of 17 normally innervated (A) and 14 partially denervated TA muscles, of which the neuromuscular activity was reduced in 5 TA muscles by spinal cord hemisection at T12 (B). The means values (±s.e.m.) for motor unit numbers of 118 ± 8 (A) and 263 ± 52 (B) for the normally and partially denervated muscles, respectively, were significantly different as indicated by the asterisk in B. The regression lines in the double logarithmic plots of the number of muscle fibres (N) and tetanic contractile force developed by the same motor unit in control (C), and partially denervated muscles (D) have slopes (±s.e.m.) of 0.26 ± 0.07 and 0.56 ± 0.08 that were significantly different from zero (P < 0.01). These demonstrated the linear relationship between the muscle fibre number and the force generated by those muscle fibres. The correlation coefficients were 0.51 and 0.80.
Figure 11. Frequency distributions of the mean cross-sectional area of the muscle fibres in isolated single motor units and the relationship between these areas and motor unit tetanic forces for the single muscle units in normally innervated tibialis anterior (TA) muscles and partially denervated. TA muscles in rats with intact and hemisected spinal cords.
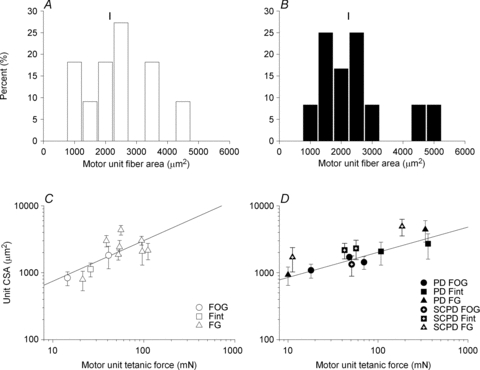
The frequency distribution of the mean cross-sectional areas of muscle fibres in isolated single motor units of 17 normally innervated (A) and 14 partially denervated TA muscles (B). The means ±s.e.m. for motor unit fibre area were 2138 ± 645 μm2 (A) and 2236 (± 645) μm2 (B) for the normally and partially denervated muscles, respectively. The regression lines in the double logarithmic plot of the cross-sectional areas of the muscle fibres (Unit CSA) and their tetanic contractile force in control (C) and partially denervated muscles (D) have slopes ±s.e.m. of 0.61 ± 0.19 and 0.34 ± 0.08 that were significantly different from zero (P < 0.01). These demonstrated the linear relationship between the mean muscle fibre cross-sectional area and the force generated by those muscle fibres. The correlation coefficients were 0.53 and 0.64, respectively. The vertical bars shown above the histograms are the mean values.
The difference between mean values (both arithmetic and geometric) was tested by the use of a standard Student's t test. Differences between distributions of unit variables were tested with the Kolmogorov–Smirnov test (Fisz, 1963). In all cases, statistical significance was taken at the 5% level of confidence.
Results
Measurement of relative motor unit loss by spinal root section
Maximal twitch and tetanic forces in the tibialis anterior (TA) muscles of left and right hindlimbs of intact control rats were evoked by supramaximal (2× threshold) stimulation of the sciatic nerve and of the contributing L3, L4 and L5 ventral roots. As shown in Fig. 3, isometric tetanic forces that were evoked by stimulation of L4 root relative to sciatic nerve stimulation were the same from both TA muscles demonstrating the bilateral symmetry of the root exit of motor nerves. In the experimental group of rats therefore, the extent of partial denervation of the right TA muscle after cutting one of the three contributing ventral roots was reasonably accurately determined by the contribution of the corresponding roots to the contractile force of the TA muscles in the left intact hindlimb. The differences between the two hindlimbs seen as a deviation from the line due to experimental and physiological variability is within acceptable limits (mean difference between right and left sides in the contributions of the three roots was 6.4 ± 5.2% of the total muscle force).
The motor axons that innervate muscle fibres in the TA exit the lumbosacral cord via L3, L4 and L5 ventral roots with the highest percent contribution of axons being from the L4 ventral root (Fig. 4). As described many times previously (Weiss & Edds, 1946; Greensmith et al. 1997; Tam et al. 2001), the relative contributions of the roots to the innervation of hindlimb muscles shows a wide extent of variability even within inbred strains of rats. The contributions of the L3 and L5 ventral roots to the rat TA muscle were both highly skewed and relatively minor as compared to the contribution of the L4 root (Fig. 4A and C). The contribution of the L4 ventral root to the TA motor innervation was widely variable (Fig. 4B). Consequently, our transection of either the L4 or L5 spinal roots partially denervated TA muscle as little as 5% and as much as 98%.
Behavioural observations after partial denervation with and without spinal cord hemisection
All the rats were monitored daily after partial denervation with and without spinal cord hemisection at T12. In the rats with intact spinal cords, the deficits depended on which spinal root was cut on the right side and the extent of remaining ankle flexion depended on whether L4 or L5 spinal root was cut and the variability in the root contribution to the innervation of the TA muscles (Fig. 4). In the first 3 days there was obvious weakness in the hindlimb in several animals. In some, the knee was hyperflexed, in others partial denervation of the lower hindlimb muscles including the ankle extensors and flexors was evident as flexion or extension of the ankle. Temporary weakness of the ankle with poor flexor and/or extension was relatively short-lived in line with the relatively rapid progress of sprouting with reinnervation of denervated endplates occurring within the first 7 days (Tam & Gordon, 2003b). By 7 to 10 days, there remained relatively little functional deficit unless the spinal cord was hemisected at the same time when the right hindlimb demonstrated little or no obvious movement and the foot lay on the plantar surface. Small bursts of low-amplitude electromyographic activity were observed in the TA muscle after a week (U. Slawinska, personal communication) but movement in the hip and knee joints were very limited with even less movement in the ankle joint (Celichowski et al. 2009). In the same manner as the rats with intact spinal cords, some rats with hemisected thoracic cords showed evidence of more limb movement within a month in line with data of Celichowski and colleagues that the extent of movement increased visibly within a month of thoracic spinal hemisection with less sliding of the limb on the ground during ambulation. Most rats regained stable locomotor pattern with wider base of support in hindlimbs although the locomotor patterns remain abnormal for up to 6 months (Celichowski et al. 2009). The rats were permitted free movement in their cages and demonstrated considerable improvement in their movement over the course of the 23 to 380 weeks of study, the majority of experiments being carried out within 6 months of initial surgeries (see Fig. 7).
Figure 7. Tetanic contractile forces and muscle wet weights as a function of days of recovery after partial denervation.
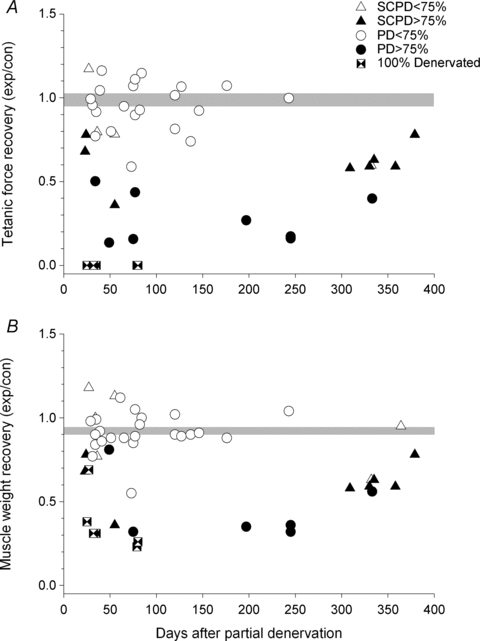
A, the tetanic contractile forces and B, muscle wet weights of partially denervated tibialis anterior (TA) muscles in the left hindlimb, expressed as a ratio of the forces and weights of the corresponding innervated TA muscles in the right hindlimb, are plotted as a function of days of recovery after partial denervation under conditions of intact (PD) and hemisected (SCPD) spinal cords. The muscles that suffered <75% and >75% partial denervation (PD) are shown, as well as those muscles that were completely denervated by the root section (100% Denervated). The points that fall below ∼1.0 are those that suffered partial denervation of >75% with complete recovery occurring as soon as 30 days after the root transection.
Muscle recovery after partial denervation with and without spinal cord hemisection
Maximal isometric tetanic forces were recorded in partially denervated and contralateral intact TA muscles in rats with intact spinal cords (shown as open and filled circles in Fig. 5) and in rats whose hindlimb activity was reduced by spinal cord hemisection (open and filled triangles). The contractile force of the partially denervated muscles would be expected to equal that in the contralateral muscles and fall on the same straight line in Fig. 5A only if sprouting from remaining intact motor units completely compensated for the reduced number of motor units after cutting one ventral root on the right experimental side of the rat. This was the case for 18 of the 28 rats with intact spinal cords and 4 of the 13 partially denervated muscles in rats in which the spinal cord was hemisected. In the rats in which the partial denervation removed less than 75% of the normal number of motor units, the tetanic forces of the partially denervated muscles in rats with intact or hemisected spinal cords, equaled those of the normally intact TA muscles in the contralateral left hindlimb, the contractile forces of the TA muscles from both hindlimbs falling on the line of unity slope. On the other hand, when the partial denervation exceeded 75% in ∼50% of the rats, almost all the partially denervated muscles failed to develop tetanic forces as high as those in the contralateral left intact TA muscles and the contractile forces fell to the right of the line of unity slope (Fig. 5A). These data demonstrate an upper limit to the sprouting capacity in partially denervated muscles that did not appear to be different in rats with intact or hemisected spinal cords. An upper limit to sprouting capacity is in agreement with data in the cat (Rafuse et al. 1992). It is important to note that the assumption that the contralateral side serves well as a reference for the experimental muscle is supported by the fact that the range of forces of the contralateral muscle was the same as that of muscles in normal intact control rats.
Figure 5. Tetanic isometric contractile forces (A) and muscle wet weights (B) of partially denervated tibialis anterior (TA) muscles in the left hindlimb plotted on double logarithmic scales as a function of the corresponding forces and weights of the intact control TA muscles in the intact right hindlimb under conditions of an intact and a hemisected spinal cord.
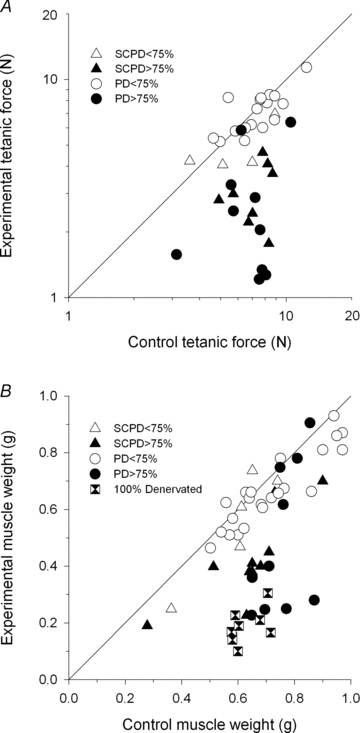
The points that fall on the line of unity slope are those in which the partially denervated (PD) muscles develop as much force and have the same wet weights as the intact contralateral muscles. The points falling to the right of the line demonstrate that the muscles do not develop as much force or are less heavy than the corresponding normally intact muscles on the contralateral left side of the rats. Muscles that were fully denervated by section of one ventral root are also shown. The total number of data points is 41 and 57 in A and B, respectively. Symbols are shown in the insets. SCPD, spinal cord partially denervated.
Consistent with the incomplete recovery of contractile muscle force in the muscles that were partially denervated by more than 75%, the muscles were atrophic relative to contralateral control muscles (Fig. 5B). However, the effects on muscle weight were smaller than on muscle force even in those muscles that were completely denervated and which maintained some muscle bulk. When tetanic forces in the partially denervated TA muscles and their muscle weights were expressed relative to contralateral control muscles for the rats with intact and hemisected spinal cords, and were plotted as a function of the extent of partial denervation (the inverse of the number of remaining motor units expressed as a percentage, as described in Methods), it becomes clear that recovery of muscle force and muscle weight in partially denervated muscles fell from unity when more than 70–75% of the innervation was removed (Fig. 6A and B). When motor unit losses were less severe, motor unit enlargement by sprouting led to full recovery, irrespective of whether the muscles were normally active or when activity was reduced by spinal cord hemisection at T12: there is no obvious difference in the ability of active or less active motor units to enlarge by sprouting for any level of partial denervation. The reduced muscle forces and weights for partial denervations of >70% were not different for data collected from the rats with intact and hemisected spinal cords: the means (±s.e.m.s) of the muscle force recovery (experimental (exp)/control (con)) were 0.40 (± 0.07) and 0.40 (± 0.5) and for the muscle weights (exp/con) were 0.63 (± 0.09) and 0.66 (± 0.05) (Fig. 6). Note that these data of the recovery of the denervated muscles do not include muscles in which cut ventral roots regenerated and made functional nerve–muscle connections as those would confound discussion of sprouting limits.
Figure 6. Tetanic contractile forces and muscle wet weights as a function of the per cent partial denervation.
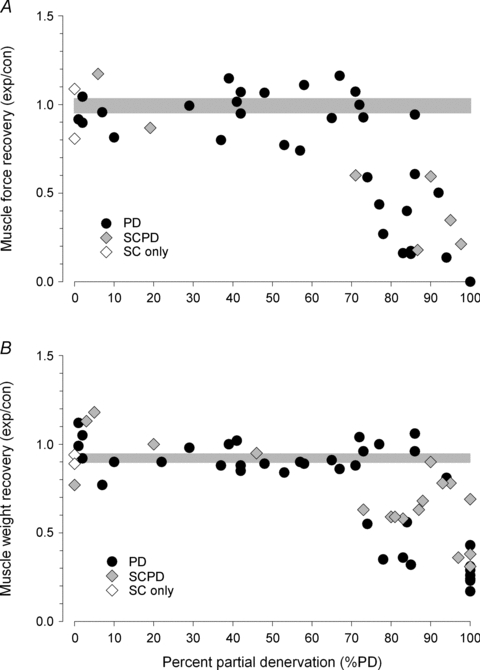
The tetanic contractile forces (A) and muscle wet weights (B) of partially denervated tibialis anterior (TA) muscles in the left hindlimb expressed as a ratio of the forces and weights of the corresponding innervated TA muscles in the right hindlimb, are plotted as a function of the per cent partial denervation under conditions of intact (•) and hemisected (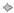 spinal cords. The points that fall on the line of slope unity are those in which the partially denervated muscles develop as much force and are as heavy as those in the intact muscles. The points in Fig. 5 were averaged and the ±s.e.m. values are shown as grey lines in the plots. Full muscle force and weight recoveries extended as far as 80–85% partial denervation but the majority of the data fell sharply in muscles that were partially denervated by >70–75%. Muscle forces and weights were not different for data collected for the rats with intact and hemisected spinal cords: the mean ±s.e.m.s for muscle forces were 0.40 ± 0.07 and 0.40 ± 0.5 and for muscle weights were 0.63 ± 0.09 and 0.66 ± 0.05. The total number of data points is 41 and 57 in A and B, respectively.
spinal cords. The points that fall on the line of slope unity are those in which the partially denervated muscles develop as much force and are as heavy as those in the intact muscles. The points in Fig. 5 were averaged and the ±s.e.m. values are shown as grey lines in the plots. Full muscle force and weight recoveries extended as far as 80–85% partial denervation but the majority of the data fell sharply in muscles that were partially denervated by >70–75%. Muscle forces and weights were not different for data collected for the rats with intact and hemisected spinal cords: the mean ±s.e.m.s for muscle forces were 0.40 ± 0.07 and 0.40 ± 0.5 and for muscle weights were 0.63 ± 0.09 and 0.66 ± 0.05. The total number of data points is 41 and 57 in A and B, respectively.
Sprouting of axons from intact motor units in partially denervated muscles reinnervate denervated muscle fibres within 1 to 2 weeks (Tam et al. 2001; Tam & Gordon, 2003b). This was confirmed in this study of motor unit contractile forces in partially denervated rat muscles. As shown in Fig. 7A and B, the recovery or lack of recovery was the same when contractile forces were recorded 23 to 380 days after partial denervation. Our previous findings of muscles recovering their normal contractile forces within ∼110 days of nerve transection and repair (Tötösy de Zepetnek et al. l992a), indicate that muscle atrophy is reversed if denervated muscles are reinnervated within 90 days. The relative short time-span of 7–14 days for reinnervation of partially denervated muscles is expected for the short distance for sprouting axons to reinnervate denervated fibres in the partially denervated muscles. This compares with the longer time-span for reinnervation after transection of the nerve ∼15 mm from the TA muscle where transected axons regenerate their axons over longer distances to make functional contact with denervated muscle. In addition, long delays for regenerating axons to cross a suture site contribute to the longer time-span of reinnervation after nerve section and repair (Brushart et al. 2002; Gordon et al. 2008).
Motor unit contractile force as an indirect measure of sprouting
In the rat TA muscle there is normally a ∼100-fold range in motor unit tetanic contractile forces, which are logarithmically distributed with the distribution on linear scales being skewed to the right with many more smaller as compared to large motor unit contractile forces (Tötösy de Zepetnek et al. l992a). Twitch motor unit forces are similarly distributed and provide a reasonable measure of force if measured under comparable conditions (Fig. 8A). In the present experiments, twitch forces were measured under non-potentiated conditions and provided comparable values from animal to animal. After partial denervation, motor unit twitch contractile force distributions moved to the right to larger motor unit force values for partial denervation that removed 35 to 70% of the total ventral root innervation of the TA muscle as seen in comparisons of frequency histograms on semi-logarithmic scales (Fig. 8A–C). When the distributions are plotted as cumulative frequency histograms on semi-logarithmic scales, the force distributions were shifted to the right for progressively higher levels of partial denervation (Fig. 8D). The shifts to the right were both significant (Kolmogorov–Smirnov test, P < 0.01). There was a trend for the larger forces of the larger motor units to increase more than those in the smaller motor units. It may be assumed that the increase in force was due to an increase in the number of muscle fibres innervated per motoneuron (N; see below) and that the low force motor units retain their lower N relative to larger motor units, consistent with size-dependent branching of motoneurons (Tötösy de Zepetnek et al. 1992a; Rafuse et al. 1997; Tam et al. 2001, 2002a; Gordon et al. 2004b).
Figure 8. Frequency histograms and cumulative frequency histograms of the twitch contractile forces of single motor units.
A–C, frequency histograms and D, cumulative frequency histograms of the twitch contractile forces of single motor units recorded from tibialis anterior (TA) muscles in intact hindlimbs and hindlimbs partially denervated by cutting 1 of 3 ventral roots, are plotted on semi-logarithmic scales. The mean (±s.e.m.) of the twitch forces increased significantly from 7.6 (± 0.42) mN in control intact muscles (A), to 13.5 (± 1.25) mN for 35% partial denervation (PD) (B) and 33.1 (± 3.6) mN for 70% partial denervation (C). The force distributions are progressively moved to the right with partial denervation that progressively removes 35% and 70% of the contributing ventral root axons that supply the TA muscle. In D, the rightward shifts of the cumulative frequency histograms were significant (Kolmogorov test, P < 0.01) although there was a clear trend for there to be greater motor enlargement for the larger than the smaller motor units. The vertical bars shown above the histograms are the mean values. The same bars are in Figures 10, 11, and 12.
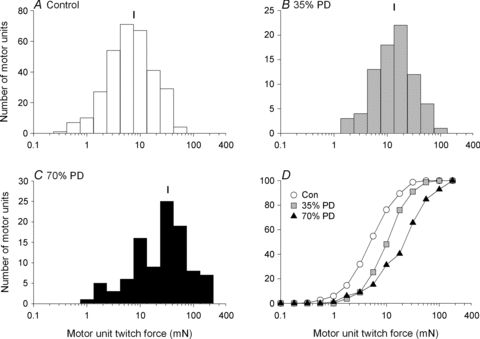
Since the characteristics of the force distributions were similar qualitatively, it is reasonable to compare mean values of motor unit contractile forces in partially denervated muscles with different numbers of remaining intact motor units. In Fig. 9A, average motor unit force of normal and partially denervated muscles with and without simultaneous spinal cord hemisection, is plotted as a function of the per cent partial denervation. The values are compared with the theoretical curve that was calculated from the expected values of twitch force if motor units enlarged in direct proportion to the per cent partial denervation. This being a power function, the average motor unit forces would be expected to increase by relatively small factors for partial denervations of up to 80%, namely 1–4 times, and show a very sharp increase for spinal root sections that partially denervated the muscles by >80%. The experimental data fit the theoretical curve reasonably well for partial denervations that removed fewer than 75% but not for more extensive denervations. The standard deviations are well below the theoretical curve in all the three cases of partial denervation that exceeded 75%. These data indicate that the enlargement of motor units by axonal sprouting in extensively denervated muscles is insufficient to reinnervate all denervated muscle fibres and therefore accounts for the very sharp decline in the recovery of the muscle contractile force and muscle weight shown in Fig. 5. The number of muscle fibres in single glycogen-depleted motor units also fit the theoretic curve in Fig. 9B for partial denervations of up to 75%. For partial denervation of ∼85%, one muscle unit did and one muscle unit did not fit the curve, with the fibre numbers falling below the curve for larger percentage partial denervation of the TA muscles. These data points being single and not mean values, the variability is to be expected (see below).
Figure 9. Motor unit twitch contractile force and fibre number as a functional of per cent partial denervation of the muscle.
A, the mean (±s.d.) of the twitch forces of single motor units recorded from rats (A) and the number of glycogen-depleted muscle fibres of single isolated muscle units, the motor unit fibre number (N) plotted as a function of the per cent partial denervation of the tibialis anterior (TA) in rats with intact and unilaterally transected spinal cords (B). Theoretical exponential lines are drawn to represent the exponential increase expected if there were no limit to the extent of enlargement of motor units with respect to either motor unit twitch forces or motor unit fibre numbers after partial denervation. Motor units: SO, slow oxidative; FOG, fast oxidative glycolytic; Fint, fatigue intermediate; FG, fast glycolytic.
Number of muscle fibres per motoneuron (N) as a direct measure of sprouting
In order to assert that the recovery of the contractile force of the partially denervated muscles was due to motor axon sprouting the feature of interest is the number of muscle fibres per motoneuron (N). N can be directly determined by counting the number of fibres in a muscle unit (the muscle fibres innervated by one motoneuron) that has been tagged by glycogen depletion and then stained for glycogen. This method, however, only allows measurement from one muscle unit per muscle and therefore is statistically a poor technique. Though the motor unit contractile force is an easily measured feature and can be measured for several units in each experimental muscle, the force is specified by the numbers of muscle fibres, their cross-sectional area and their specific force (Tötösy de Zepetnek et al. 1992a) as follows: F=NAS, where N is the number of muscle fibres in each motor unit, A is the mean cross-sectional area of these fibres and S is the specific force or the force per unit area that can be generated by these muscle fibres. The relative contribution of each of these three factors can be obtained by plotting them in a log–log graph as log F=m log N+c, where the coefficient ‘m’ gives the relative contribution of the muscle unit fibre number N to the force F (i.e. if m= 0.7, 70% of the variation in force among units is accounted for by differences in the number of muscle fibres, with the rest of the 30% of the variation being due to mean fibre cross-section area and specific force of fibres) (Tötösy de Zepetnek et al. 1992a).
There was a significant shift to the right of the per cent frequency histograms of muscle unit fibre numbers of one glycogen-depleted motor unit per muscle in the partially denervated muscles in rats with intact and hemisected spinal cords as compared to the intact muscles; the mean values (±s.e.m.) of 263 (± 52) and 118 (± 8) were significantly different (P < 0.01) (Fig. 10A and B). These unit muscle fibre numbers were plotted against the motor unit tetanic force developed by the muscle fibres on double logarithimic axes with symbols identifying fibre types in the partially denervated muscles of intact and hemisected spinal cords in Fig. 10C and D. Significant relationships between the two variables were observed as described earlier for normal and reinnervated TA muscles after common peroneal nerve transection and surgical repair (Tötösy de Zepetnek et al. 1992a). Regression lines fitted to data for the partially denervated muscles in the rats with intact and hemisected spinal cords had slopes that were not significantly different (0.57 and 0.59, respectively). One regression line was therefore fitted to both sets of data in Fig. 10D. The slope of 0.56 ± 0.08 was significantly different from zero, demonstrating that 56% of the variation in force amongst the motor units in the partially denervated muscles in the rats with intact and hemisected spinal cords is accounted for by differences in the number of muscle unit fibres. This compares with a slope of 0.26 ± 0.07 for normal units in intact hindlimbs and hence a 26% of the variation of force in these normally innervated muscles. As found previously in reinnervated TA muscles (Tötösy de Zepetnek et al. 1992a), the normal trend for unit force and muscle fibre number to increase from the small to large from the slow oxidative (SO) to fatigue intermediate (Fint) and fast glycolytic (FG) muscle unit types in normally innervated muscles (Fig. 10C) was less clear in the partially denervated muscles, where, for example, both the muscle unit fibre number and motor unit tetanic forces of muscle units whose muscle fibre type was FG or Fint spanned the entire range to a greater extent than in the normally innervated TA muscle (Fig. 10C and D). The trend for the slopes of the regression lines to be lower than the slopes of 0.65 ± 0.06 and 0.39 ± 0.08 for the contribution of N (number of unit muscle fibres) to self-reinnervated and normal TA muscle tetanic forces that we had reported previously (Tötösy de Zepetnek et al. 1992a), was not significant. Hence, the relative contributions of muscle unit fibre number to the variation of contractile force in the muscle units in reinnervated TA muscles after partial and complete denervation are similar.
The mean values of 2138 ± 645 μm2 and 2236 ± 645 μm2 for the muscle unit fibre cross-sectional areas were not statistically significant although the range of the areas was slightly less in the partially denervated muscles than in normally innervated muscles (Fig. 11A and B). The slopes of the regression lines (0.37 and 0.34) for the partially denervated muscles in the intact and hemisected spinal cords were significantly different from zero but not significantly different from one another. One regression line was therefore fitted to the data in Fig. 11D, the slope being 0.35 ± 0.08 for the enlarged muscle units after partial denervation of the TA muscles in rats with intact and hemisected spinal cords, as compared to a slope of 0.61 ± 0.19 for the intact TA muscle. These slopes compare with slopes of 0.19 ± 0.08 and 0.49 ± 0.10 for normal and self-reinnervated TA muscles (Tötösy de Zepetnek et al. 1992a). Again, the relative contributions of muscle fibre cross-sectional area to contractile forces of reinnervated motor units were similar for reinnervated muscles after partial denervation and after nerve transection and repair. The reduction from 61% to 35% contribution of muscle unit cross-sectional area to the variation in force in the partially denervated muscles demonstrates that, as in reinnervated muscles after nerve section and surgical repair, the range in motor unit forces depends less on the cross-sectional area of the muscle unit fibres than on numbers of muscle unit fibres. This contrasts with normally innervated muscles where cross-sectional area is the major determinant of the range in motor unit force. The explanation for this transition is, at least in part, the relatively poor re-specification of muscle properties after muscle reinnervation: fibres of the same type in reinnervated muscles show a wider range in size than normal with the result that differences in muscle fibre size between unit types are reduced (Gordon et al. 1988; Rafuse et al. 1992; Tötösy de Zepetnek et al. 1992a; Rafuse & Gordon, 1996a).
The product of the number (N) and cross-sectional area (A) of the muscle unit fibres gives the total cross-sectional area of all the muscle fibres of the muscle unit, accounting for 91% and 87% of the muscle unit contractile force of partially denervated and normally innervated TA muscles, respectively. The remaining determinant, specific force, S, has a mean (±s.e.m.) value of 2.17 ± 0.47 kg/cm2. for the motor units in partially denervated muscles and of 2.83 ± 0.25 kg/cm2 in normally innervated muscles; it does not vary systematically with muscle unit force in either the partially denervated or the normally innervated muscles, the slope of the relationships between specific force and tetanic force of the muscle units having slopes of 2.17 in the partially denervated muscles and 2.83 in the normally innervated intact muscles that are not significantly different from zero (Fig. 12C and D). These data are quite consistent with data in reinnervated muscles or normally intact tibialis anterior muscles (Tötösy de Zepetnek et al. 1992a).
Figure 12. Frequency distributions of the specific force of isolated single motor units and the relationship between the specific force and the motor unit tetanic force in normally innervated and partially denervated tibialis anterior (TA) muscles under conditions of the spinal cord remaining intact or hemisected.
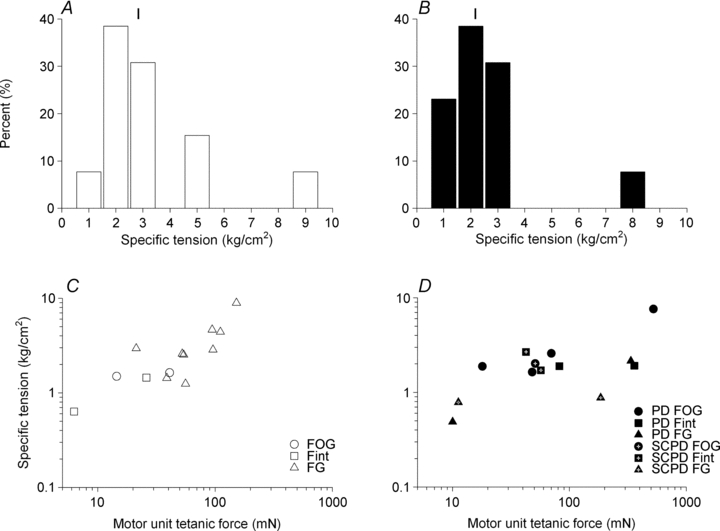
The frequency distribution of the specific force of isolated single motor units of 13 normally innervated (A) and 13 partially denervated TA muscles, of which the neuromuscular activity was reduced in 5 TA muscles by spinal cord hemisection at T12 (B). The mean values ±s.e.m. for specific force of 2.83 ± 0.58 and 2.17 ± 0.47 cm2 for the normally and partially denervated muscles, respectively, were not significantly different. The regression lines in double logarithmic plots of the specific force and tetanic contractile force developed by the same motor unit in control (C) and partially denervated muscles (D) had slopes ±s.e.m. of 0.55 ± 0.25 and 0.33 ± 0.12 that were not significantly different from zero (P > 0.05). Hence the specific force of all motor units did not vary systematically with tetanic force as demonstrated for the number and cross-sectional area of muscle unit fibres.
Conversion of muscle units to more oxidative phenotype in partially denervated muscles in rats with intact or hemisected spinal cords
Muscle units in the normally intact (n= 17) and partially denervated TA muscles in rats with intact (n= 9) and hemisected spinal cords (n= 5) were glycogen depleted and characterized as fast glycolytic (FG), fatigue intermediate (Fint), fast oxidative glycolytic (FOG), and slow oxidative (SO), based on their mATPase, oxidative and glycolytic histochemical reactivity (see Methods). These normally correspond well with physiologically classified motor units (Tötösy de Zepetnek et al. 1992b). The normally intact TA muscles demonstrated the typical distribution of muscle fibre types with a majority of FG muscle units (69%) and relative small proportions of Fint (12%), FOG (12%) and SO (7%) muscle units. In the partially denervated muscles of rats with intact spinal cords, there was a clear shift of muscle units to more fatigue-resistant FOG (36%) and Fint (36%) muscle units with the proportion of FGs reduced to 28% (Figs 10 and 11). This shift is consistent with the increased neuromuscular activity of the reduced population of motor units after partial denervation of the TA muscle previously described (Rosenblatt & Parry, 1993). Whilst there was also the same trend for the muscles in the rats with spinal hemisection, the shift was more subtle for the same numbers of the glycogen-depleted motor units (20% FOG, 40% Fint and 40% FG), partly because two of the five identified motor units were characterized within a month of partial denervation and hemisection, the other three being characterized at least 310 days after the operation when the neuromuscular activity of the motor units is likely to have been similar to those of the rats with intact spinal cords (U. Slawinska, personal communication).
Progressive clumping of reinnervated muscle unit fibres parallels fibre-type clumping in partially denervated muscles of both rats with intact and hemisected spinal cords
Typical muscle unit territories, defined as the area confined within the boundaries of the outermost muscle fibres of the unit (see Methods), occupied ∼30% of the total area of the whole muscle cross-sectional area in partially denervated muscles in rats with intact (Fig. 13A and C) and hemisected (Fig. 13B and D) spinal cords. Typically, these territories are located either in the lateral, middle or deep locations of the TA muscle. In the lateral and middle locations, fibres are exclusively type II fast twitch fibres and both type I and II fibres in the deep location (Edstrom & Kugelberg, 1968; Tötösy de Zepetnek et al. 1992b). The clumping of muscle unit fibres within unit territories after extensive partial denervation (Figs 13C and D, and 14D, G and H) is accompanied by progressive fibre-type clumping of non-unit muscle fibres (Fig. 14B, E and F). The typical mosaic distribution of fibre types in the normally innervated muscles (Fig. 14A) was progressively replaced by fibre-type clumping (Fig. 14B, E and F) that occurred in parallel with clumping of muscle unit fibres within the territory boundaries (Figs 2, and 14D, G and H). There was a gradual progression from a mosaic distribution of muscle unit fibres in normally innervated muscles (Fig. 14A) through a trend towards clumping in mildly denervated muscles (∼20%: Fig. 13A and B, 20%: Fig. 14D, 40%) to overt clumping of muscle unit fibres (Fig. 14G and H) accompanied by parallel fibre-type clumping (Fig. 14E and F) for extensively denervated muscles. The progressive clumping of muscle unit fibres as their numbers increase with progressively greater losses of motor units after partial denervation is illustrated: fibre number increase from 109 in intact muscles to 209, 572 and 696 unit fibres after 40, 80 and 85% loss of motor units in partially denervated muscles (Fig. 14C, D, G and H).
Figure 13. Camera lucida drawings of glycogen-depleted muscle fibres.
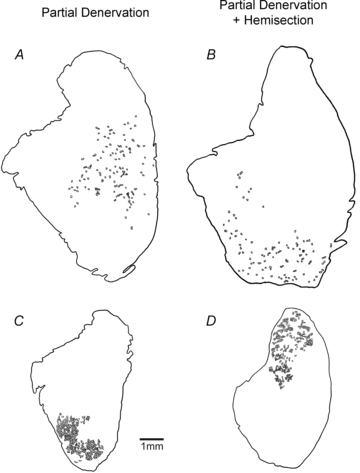
Camera lucida drawings of glycogen-depleted muscle fibres identified by absence of periodic acid Schiff (PAS) staining in muscles with 80% (A and B) and 10% (C and D) remaining motor units in partially denervated tibialis anterior (TA) muscles of rats with intact (A and C) and hemisected (B and D) spinal cords. The same progressive clumping of muscle fibre types (not shown) and of glycogen-depleted muscle fibres occurs with progressive reduction in numbers of intact motor units, i.e. progressive partial muscle denervation. The numbers of muscle unit fibres are 189, 144, 696 and 430 in A, B, C and D. The corresponding tetanic forces of the muscle units were 70, 57, 360 and 185 mN. The right hand side of the muscle cross-sections corresponds with the most superficial portion of the tibialis anterior muscle. The left hand side corresponds with the deep portion of the muscle.
Figure 14. Photographs of muscle cross-sections stained for acid mATPase and camera lucida drawings of glycogen-depleted muscle fibres.
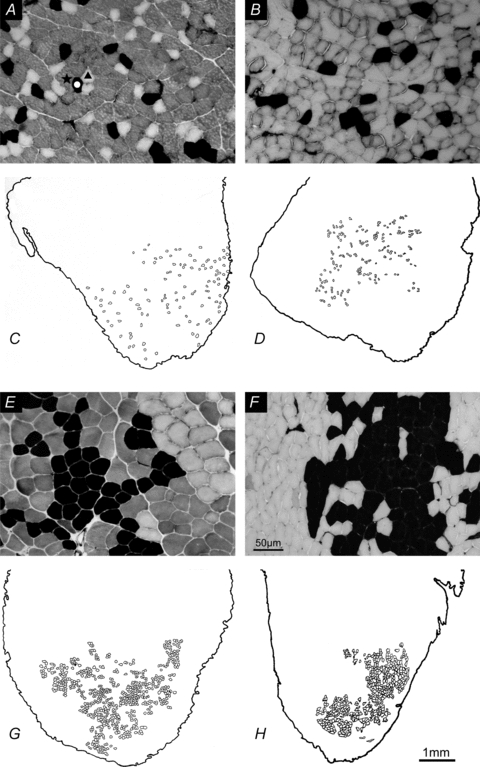
Photographs of muscle cross-sections stained for acid mATPase (A, B, E and F) and camera lucida drawings of glycogen-depleted muscle fibres identified by absence of periodic acid Schiff (PAS) staining (C, D, G and H) in tibialis anterior (TA) muscles with 100% remaining motor units (intact control) (A and C), 60% (B and D), 20% (E and G), and 15% (F and H) remaining intact motor units active cutting one ventral root in the lumbosacral spinal cord. Progressive clumping of muscle fibre types and of glycogen-depleted muscle fibres occurs with progressive reduction in numbers of intact motor units, i.e. progressive partial muscle denervation. The numbers of muscle unit fibres are 109, 209, 572 and 696 in C, D, G and H. The corresponding tetanic forces of the muscle units were 52.5, 82, 360 and 524 mN.
The size or area of the muscle unit territories was not altered as increasing numbers of muscle unit fibres progressively clumped within the territories in partially denervated muscles: the mean (±s.e.m.) area of 6.6 (± 1.05) mm2 for the territories in normally innervated muscles was not statistically different from 6.10 (± 1.06) mm2 in the partially denervated muscles. The same territory area contained ∼2 times the number of muscle unit fibres, increasing from 118 (± 8) in the intact muscles to 263 (± 52) in partially denervated muscles. In parallel with the increased density of muscle fibres within unit territories, numbers of non-glycogen-depleted (non-unit) muscle fibres surrounding each depleted muscle unit muscle fibre declined from 5.56 ± 0.06 (range of 3–8) in intact muscles to 1.54 ± 0.6 (range of 0–7) in ∼70% partially denervated muscles. The relative decline in the ratio of non-muscle unit to muscle unit fibres of ∼3.6-fold corresponds reasonably well with the upper limit of ∼4-fold for motor unit enlargement by sprouting.
Discussion
Our direct and indirect methods of determining motor unit size in the rat tibialis anterior (TA) muscle demonstrate an upper limit in the sprouting capacity of motor nerves and that this limit is the same whether or not the spinal cord is injured. The methods included: (1) two independent measures of motor unit size, motor unit contractile force and the number of muscle fibres innervated by a single motoneuron, (2) quantification of the extent of partial denervation, and (3) estimates of the accuracy of these measurements. Our analysis of the spatial distribution of muscle units revealed that the upper limit in the sprouting capacity corresponds with the number of non-unit muscle fibres that surrounds each muscle unit fibre that is within a muscle unit (i.e. innervated by a single motoneuron).
We used a popular technique of studying motoneuron sprouting. We partially denervated a muscle by cutting one spinal root, the remaining axons sprouting to innervate the denervated muscle fibres within 7 to 14 days (Tam & Gordon, 2003a). It is impossible to determine the extent of sprouting directly as this would require resolving the number of muscle fibres innervated by a given axon, N, before and after sprouting. The simplest measure of muscle reinnervation by axon sprouts is valuation of whether muscle denervation remains as was done here in the rats with intact or hemisected spinal cords by simply weighing the partially denervated muscles and/or recording their contractile forces for comparisons with contralateral intact muscles. Early observations in the 1800s of there being no degenerating muscle fibres in partially denervated muscles (Exner, 1884) were confirmed in the next century (Van Harreveld, 1945). The latter study also pointed out that hypertrophy of some of the muscle fibres was not of sufficient magnitude to explain the recovery of function of the partially denervated muscles. Exner (1884) correctly surmised that intramuscular (collateral) nerve growth by the remaining intact nerves accounted for the recovery. Our comparisons of muscle wet weights and contractile forces of left partially denervated and right intact TA muscles demonstrated equal weights and forces for partial denervations <75–80%, indicating an upper limit of sprouting capacity of the remaining motor axons (Figs 5, 6, and 7). This capacity was the same whether or not the rat spinal cord was intact or hemisected. This was shown too by measuring contractile forces generated by single motor units and by counting number of muscle fibres of single motor units, N. Our data showed that axon sprouting increased N to account for the increased muscle unit forces recorded from the partially denervated muscles (Fig. 10). That the muscle fibre cross-sectional areas were not different in intact and partially denervated muscles (Fig. 11) further supports the conclusion that axon sprouting to enlarge motor units is the basis for the full recovery of muscle function as Exner had surmised.
Up to the upper limit of 75–80% partial denervation, motor unit enlargement was proportional to the extent of the partial denervation, the distribution of motor unit contractile forces shifting progressively to the right as the extent of partial denervation increased (Fig. 8). Both motor unit contractile force and the number of muscle unit fibres increased progressively until partial denervation exceeded 75–80%, when both motor unit forces and fibre numbers did not increase further (Fig. 9). This accounts for the decline in muscle weights and contractile forces in the partially denervated muscles as compared with those of muscles in the intact contralateral hindlimbs (Fig. 6) and establishes the upper limit of sprouting to 4- to 5-fold in muscles supplied by motoneurons in the intact or hemisected spinal cords.
The validity of determining axon sprouting by muscle unit contractile forces and fibre counts
The most direct way of measuring number of muscle unit fibres is to tag all the muscle unit fibres by repeatedly stimulating the motoneuron to deplete glycogen in the muscle fibres and then to count these glycogen-depleted fibres (Kugelberg et al. 1970; Tötösy de Zepetnek et al. 1992b). The principal drawback of this method is that it allows the identification of only one motor unit per muscle. In addition, the method does not resolve the number of muscle fibres per motor unit prior to sprouting. Alternatively, the motor unit size can be determined indirectly by measuring motor unit contractile force. Although this latter method relies on the assumption that any variations in the size of the muscle fibres amongst the motor units can be taken into account and will not confound the data, it has the distinct advantage of being able to study a large number of motor units in each muscle. Therefore, by obtaining a sample of motor units from a muscle with sprouted axons, the motor unit forces were compared to a similar sample from a normal control muscle to estimate the extent of sprouting. The accuracy of this estimate is directly related to the fraction of the total number of motor units in the muscle that are sampled. We sampled at least 40% and up to 60% of motor units in the extensively denervated muscles so as to obtain representative samples of motor unit forces for comparison of their distributions with progressive partial denervation (Fig. 8).
A difficulty in comparing motor unit forces amongst muscles is that muscles are usually not identical in terms of numbers of fibres, the number of innervating axons, the distribution of fibre sizes and the distribution of motor unit sizes. Since reasonable bilateral symmetry within each rat was seen in this study (Fig. 3), the whole muscle force of partially denervated muscles was always compared with the control side and the motor unit forces were expressed in absolute force units of milliNewtons. Unless the two yield contradictory results, normalization is and was not warranted a priori in this and other studies (see also Rafuse et al. 1992; Rafuse & Gordon, 1996a; Tam et al. 2001). There are potential problems using the twitch as a measure of unit force, with variations in the tetanic force/twitch peak force ratios due to mechanical and biochemical factors that can be considerable in different motor units. These could distort the unit size distributions especially in a large muscle like the rat TA where there is a ∼100-fold range (Fig. 8). In the sample of motor units in which single axons were isolated to record twitch and tetanic muscle forces and to deplete the muscle fibres of glycogen by tetanic stimulation, we found that the mean twitch/tetanic ratio was 0.18 ± 0.04. As this was not significantly different from the mean of 0.18 ± 0.02 for all the motor units that were recorded, the potential problems of using twitch contractile forces appeared to be relatively small. Moreover, any problems associated with using only twitch contractile forces as the measure of motor unit size are minimized by the large sample of motor units of 40–60% of the total in each rat.
The enumeration of muscle unit fibres depends critically on the pinnation angle of the fibres as discussed in detail elsewhere (Tötösy de Zepetnek et al. 1992a). The pinnation angle being relatively small in the TA muscle, this muscle provides a good model to examine both the number and location of muscle unit fibres. In normal and reinnervated muscles, these numbers may be examined in a reasonable sample as described previously (Tötösy de Zepetnek et al. 1992a). The indirect evidence of the limit to sprouting was supported by both our sample of motor unit forces and our counts of muscle fibres in depleted single muscle units in partially denervated TA muscles with and without spinal transection (Fig. 9).
Upper limit of motor unit enlargement by sprouting
Our study shows that with intermediate denervation (<75%), motor units over the entire range of unit contractile forces enlarged, the enlargement being roughly proportional to the size of the motor units, although there was a trend for small motor units to enlarge less than the larger motor units (Fig. 8). The proportional increase in the contractile forces of the partially denervated muscles in intact rats and those with a hemisected lumbosacral spinal cord, concurs with our previous findings in several different rat muscles, including medial gastrocnemius, extensor digitorum longus and soleus (Tam et al. 2001). Likewise, in the small peroneus tertius muscle, regardless of whether regeneration of cut spinal axons occurred, there was proportional enlargement of motor units such that the size principle was sustained, the size of the motor units and their axons being linearly related (Brown & Ironton, 1978).
Motor unit enlargement by sprouting was the same whether or not neuromuscular activity in the TA muscle was reduced. Thoracic hemisection profoundly reduces neuromuscular activity in the TA muscle during the first 10 days when the majority of denervated endplates are reinnervated (Tam & Gordon, 2003b). Activity recovers slowly over the next 6 months (Celichowski et al. 2009). These data are consistent with the increased motor unit forces in paralyzed hand muscles of C5 to C6 spinal-injured patients where EMG activity is also profoundly reduced and there was also a trend for the small motor units to enlarge less than the larger units (Yang et al. 1990). Hence, minimal neuromuscular activity remaining after spinal hemisection in the rat or complete injury in spinal-injured human subjects does not interfere with the maximal sprouting capacity of remaining motoneurons. Sprouting and motor unit enlargement are, however, reduced by elimination of neuromuscular activity with TTX blockade of neural action potentials, botulinum toxin block of acetylcholine release from nerve terminals, and α-bungarotoxin block of acetylcholine binding to receptors on the postjunctional muscle membranes (Connold & Vrbová, 1990, 1991; Tam et al. 2002b). It is possible that sufficient calcium influx supports axon outgrowth for sprouting after spinal hemisection in contrast to insufficient calcium influx into inactive nerve terminals that does not (Kater & Mills, 1991; Rehder et al. 1992; Tosney, 2002). Together with evidence for a strong inhibitory effect of increased neuromuscular activity on sprouting and motor unit enlargement in partially denervated muscles (Tam et al. 2001), these data demonstrate that minimal but not excessive neuromuscular activity is required for axon sprouts to form nerve–muscle contacts in partially denervated muscles.
There is a limit to the sprouting capacity of motoneurons because, in the more extensive partially denervated TA muscles where >70–80% of the motor innervation was removed, muscle recovery was incomplete with motor unit enlargement not sufficing to reinnervate all the denervated muscle fibres. This upper limit of sprouting capacity was the same for the normally active partially denervated muscles and those whose activity was reduced by spinal cord hemisection at T12 (Fig. 6). Where partial denervation of the TA muscles exceeded 90%, the required enlargement of motor units for complete recovery rose rapidly, increasing to a factor of 20 at 95% partial denervation (Fig. 9). The increase in both motor unit force and muscle unit fibre number did not attain these large increases, the maximal enlargement being 4.4-fold and 4.6-fold, respectively, these calculations having mean values at ∼70–80% partial denervation. Taking into consideration that the sampling of muscle fibre number was much smaller than the sampling of motor unit forces, the agreement between the maximal motor unit enlargements is very good. Hence, we conclude that the maximum sprouting capacity of the motoneuron is the limit on complete recovery of muscle contractile force. What is the basis for the limit?
Sprouting limits – mechanisms
The upper limit of sprouting has not been adequately explained as yet (Tam & Gordon, 2003a). A parsimonious explanation is suggested by analyses of the spatial distributions of muscle unit and non-muscle unit fibres in normally innervated and partially denervated muscles (Figs 13–15). In normally innervated muscles, muscle unit fibres are distributed in the same mosaic pattern as the non-unit fibres of any one muscle fibre type (Fig. 14A and C) (Venema, 1988). The muscle unit fibres are contained within defined territories whose area is defined by the outermost muscle fibres (Edstrom & Kugelberg, 1968; Bodine-Fowler et al. 1990; Tötösy de Zepetnek et al. 1992a; Weijs et al. 1993; Rafuse & Gordon, 1996b). The size of the territories does not appear to change after partial denervation, each muscle unit occupying ∼30% of the muscle cross-section but the number of non-motor unit muscle fibres that surrounds each muscle unit fibre declines from 5.56 ± 0.06 (mean ±s.e.m.) to 1.54 ± 0.6 in extensively denervated muscles, a 4-fold decline that corresponds well with maximum sprouting capacity of motor nerves.
Figure 15. Model of the distribution of muscle fibres in intact and partially denervated hindlimb skeletal muscles that are innervated by single motoneurons whose axons exit the spinal cord via L5 and/or L4 ventral roots.
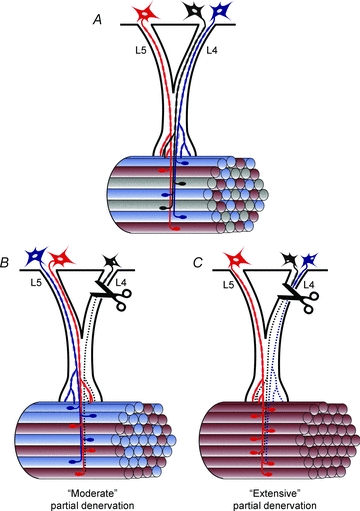
A, in normally innervated tibialis anterior (TA) muscle, the muscle fibres innervated by single axons (red, blue and black) in either L4 or L5 ventral roots show the typical mosaic distribution with muscle unit fibres intermingled with non-unit muscle fibres within a muscle unit territory that encloses all the muscle fibres innervated by intramuscular branching of motor nerves. B, after ‘moderate’ partial denervation by cutting 1 of the 2 ventral roots to remove 1 of 3 motor units, there are more muscle unit fibres that are adjacent to each other. C, after ‘extensive’ partial denervation, illustrated as the cutting of 2 of 3 axons, many more, here illustrated as all the muscle unit fibres, are adjacent to one another. Further details are provided in the text of the Discussion.
Since axon branching of motor nerves normally occurs intramuscularly as illustrated figuratively in Fig. 15, the spatial extent of the nerve branching must define the territories. Similarities between the relative size of these territories in the relatively small rat tibialis anterior muscle, and in the large tibialis anterior and medial gastrocnemius muscles in the cat (Edstrom & Kugelberg, 1968; Bodine-Fowler et al. 1990; Tötösy de Zepetnek et al. 1992a; Weijs et al. 1993; Rafuse & Gordon, 1996b) indicate similarities in intramuscular branching patterns that are indicated by the mosaic distributions of muscle unit fibres within the territories (Fig. 15A). Moreover, because axon sprouting in partially denervated muscles is a localized event with the majority of axon sprouts, the nodal sprouts, emerging from the last node of Ranvier before unmyelinated nerve terminals innervate the neuromuscular junctions (Tam et al. 2001; Tam & Gordon, 2003a), the progressive enlargement of muscle units (with increased motor unit forces) would be expected to result in progressive clumping of the muscle unit fibres within the territories (Fig. 14A and B). Axon sprouts follow perisynaptic Schwann cell processes from both innervated and denervated endplates after partial denervation and appear to innervate nearby denervated muscle fibres (Son & Thompson, 1995a,b; Love & Thompson, 1998; Tam & Gordon, 2003a). This model could therefore explain the observed upper limit of sprouting observed in our experiments.
This explanation of the limit of motor unit enlargement certainly does not exclude others. One possibility is that highly atrophic denervated muscle fibres within a muscle unit territory disappear so that the denervated muscle fibres included into an enlarged remaining motor unit will lie adjacent to the normally innervated muscle fibres of the same motor unit. Whilst this possibility cannot be excluded without examination of muscle unit territories within weeks of partial denervation, this model predicts a decline in size of the muscle unit territories that was not seen in this or other studies of enlarged motor units (Rafuse & Gordon, 1996b).
Whilst clumping is dramatic in extensively denervated muscles, findings that all muscle unit fibres are not always adjacent to one another in the unit territories also argue that other factors must be considered in the explanation of the limit of sprouting. These include fascicular boundaries that would prevent axon sprouts from reinnervating muscle fibres in adjacent fascicles. As described more than 30 years ago by Kugelberg and colleagues and confirmed repeatedly in studies of glycogen-depleted motor units in rats and cats (Edstrom & Kugelberg, 1968; Bodine-Fowler et al. 1990; Tötösy de Zepetnek et al. 1992a; Weijs et al. 1993; Rafuse & Gordon, 1996b), the intramuscular branching of individual motor nerves accounts for the distribution of muscle unit fibres across several muscle fascicles. This fascicular connective tissue acts as a physical barrier to the axons that sprout within fascicular boundaries and would prevent reinnervation of denervated endplates of muscle fibres enclosed by other fascicles. This explanation would also hold for larger muscle unit territories in larger gastrocnemius muscles, for example, where similar numbers of non-unit to unit fibre ratios were observed and the relative size of the unit territories are similar to smaller muscles of the rat (Rafuse et al. 1992; Rafuse & Gordon, 1996b).
When partial denervation is extensive, spatial competition among sprouting units to innervate denervated muscle fibres is also likely and the location of the denervated muscle fibres relative to the sprouting motoneurons will determine the success of sprouting in each specific case (Karpati & Engel, 1968a,b; Slack et al. 1979). For instance, when the partial denervation is extensive with few remaining intact motor units, there will be a large number of sprouting axons with few available denervated fibres in some regions, while in other areas there will be denervated fibres that are not sufficiently close to an intracellular motor nerve branch to supply enough sprouts.
Conclusions
The results of this study on the extent and limits of axon sprouting in partially denervated TA muscles of the rat with intact and hemisected thoracic spinal cords, demonstrate the same sprouting capacities for normally active motor units and those units whose neuromuscular activity is severely curtailed during the first 10 days when the sprouting response and muscle reinnervation occurs. With progressively more severe partial denervation, muscle fibres innervated by single motoneurons become progressively clumped within the muscle unit territories that normally occupy ∼30% of the cross-sectional area of the muscles. Our findings indicate that axon sprouts grow over relatively short distances to reinnervate denervated muscle fibres within the unit territory and that the retained relative territory size may explain, at least in part, the ultimate limit of motor unit enlargement by sprouting.
Acknowledgments
We are grateful to the Amyotrophic Lateral Sclerosis Society of Canada for their financial support of this research work on axon sprouting. T.G. is a Professor Emeritus at the University of Alberta and Senior Scientist at the Hospital for Sick Children, University of Toronto, Ontario, Canada.
Glossary
Abbreviations
- ALS
amyotrophic lateral sclerosis
- FG
fast glycolytic
- Fint
fatigue intermediate
- FOG
fast oxidative glycolytic
- MU
motor unit
- SO
slow oxidative
- TA
tibialis anterior
Author contributions
All experiments were performed at the Divisions of Physical Medicine and Rehabilitation and of Neuroscience at the University of Alberta in Edmonton, Alberta, Canada. Both authors contributed to all aspects of the study, and both approved the final version of the manuscript.
References
- Bodine-Fowler SC, Garfinkel A, Roy RR, Edgerton VR. Spatial distribution of muscle fibres within the territory of a motor unit. Muscle Nerve. 1990;13:1133–1145. doi: 10.1002/mus.880131208. [DOI] [PubMed] [Google Scholar]
- Brooke MH, Kaiser KK. Muscle fibre types: how many and what kind. Arch Neurol. 1970;23:369–379. doi: 10.1001/archneur.1970.00480280083010. [DOI] [PubMed] [Google Scholar]
- Brown MC, Ironton R. Sprouting and regression of neuromuscular synapses in partially denervated mammalian muscles. J Physiol. 1978;278:325–348. doi: 10.1113/jphysiol.1978.sp012307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brushart TM, Hoffman PN, Royall RM, Murinson BB, Witzel C, Gordon T. Electrical stimulation promotes motoneuron regeneration without increasing its speed or conditioning the neuron. J Neurosci. 2002;22:6631–6638. doi: 10.1523/JNEUROSCI.22-15-06631.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celichowski J, Krysciak K, Krutki P, Majczynski H, Gorska T, Slawinska U. Time-related changes of motor unit properties in the rat medial gastrocnemius muscle after the spinal cord injury. II. Effects of a spinal cord hemisection. J Electromyogr Kinesiol. 2009;20:532–541. doi: 10.1016/j.jelekin.2009.07.003. [DOI] [PubMed] [Google Scholar]
- Connold AL, Vrbová G. The effect of muscle activity on motor unit size in partially denervated rat soleus muscles. Neuroscience. 1990;34:525–532. doi: 10.1016/0306-4522(90)90161-v. [DOI] [PubMed] [Google Scholar]
- Connold AL, Vrbová G. Temporary loss of activity prevents the increase of motor unit size in partially denervated rat soleus muscles. J Physiol. 1991;434:107–119. doi: 10.1113/jphysiol.1991.sp018461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Koning P, Verhaagen J, Sloot W, Jennekens FG, Gispen WH. Org.2766 stimulates collateral sprouting in the soleus muscle of the rat following partial denervation. Muscle Nerve. 1989;12:353–359. doi: 10.1002/mus.880120503. [DOI] [PubMed] [Google Scholar]
- Dengler R, Konstanzer A, Kuther G, Hesse S, Wolf W, Struppler A. Amyotrophic lateral sclerosis: macro-EMG and twitch forces of single motor units. Muscle Nerve. 1990;13:545–550. doi: 10.1002/mus.880130612. [DOI] [PubMed] [Google Scholar]
- Edstrom L, Kugelberg E. Histochemical composition, distribution of fibres and fatiguability of single motor units. Anterior tibial muscle of the rat. J Neurol Neurosurg Psychiatry. 1968;31:424–433. doi: 10.1136/jnnp.31.5.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edstrom L, Larsson L. Effects of age on contractile and enzyme-histochemical properties of fast- and slow-twitch single motor units in the rat. J Physiol. 1987;392:129–145. doi: 10.1113/jphysiol.1987.sp016773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exner S. Die innervation des Kehlkopfes. Sitzungsb d k Akad d Wissench Math -naturw Cl. 1884;89:63–118. [Google Scholar]
- Fisz M. Probability Theory and Mathematical Statistics. New York: Wiley; 1963. [Google Scholar]
- Frey D, Schneider C, Xu L, Borg J, Spooren W, Caroni P. Early and selective loss of neuromuscular synapse subtypes with low sprouting competence in motoneuron diseases. J Neurosci. 2000;20:2534–2542. doi: 10.1523/JNEUROSCI.20-07-02534.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu SY, Gordon T. Contributing factors to poor functional recovery after delayed nerve repair: prolonged axotomy. J Neurosci. 1995;15:3876–3885. doi: 10.1523/JNEUROSCI.15-05-03876.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu SY, Gordon T. The cellular and molecular basis of peripheral nerve regeneration. Mol Neurobiol. 1997;14:67–116. doi: 10.1007/BF02740621. [DOI] [PubMed] [Google Scholar]
- Gordon T, Brushart TM, Chan KM. Augmenting nerve regeneration with electrical stimulation. Neurol Res. 2008;30:1012–1022. doi: 10.1179/174313208X362488. [DOI] [PubMed] [Google Scholar]
- Gordon T, Hegedus J, Tam SL. Adaptive and maladaptive motor axonal sprouting in aging and motoneuron disease. Neurol Res. 2004a;26:174–185. doi: 10.1179/016164104225013806. [DOI] [PubMed] [Google Scholar]
- Gordon T, Thomas CK, Munson JB, Stein RB. The resilience of the size principle in the organization of motor unit properties in normal and reinnervated adult skeletal muscles. Can J Physiol Pharmacol. 2004b;82:645–661. doi: 10.1139/y04-081. [DOI] [PubMed] [Google Scholar]
- Gordon T, Thomas CK, Stein RB, Erdebil S. Comparison of physiological and histochemical properties of motor units after cross-reinnervation of antagonistic muscles in the cat hindlimb. J Neurophysiol. 1988;60:365–378. doi: 10.1152/jn.1988.60.1.365. [DOI] [PubMed] [Google Scholar]
- Gordon T, Yang JF, Ayer K, Stein RB, Tyreman N. Recovery potential of muscle after partial denervation: a comparison between rats and humans. Brain Res Bull. 1993;30:477–482. doi: 10.1016/0361-9230(93)90281-f. [DOI] [PubMed] [Google Scholar]
- Greensmith L, Hind A, Vrbova G. Neonatal paralysis of the rat soleus muscle selectively affects motoneurones from more caudal segments of the spinal cord. Brain Res Dev Brain Res. 1997;98:281–286. doi: 10.1016/s0165-3806(96)00200-3. [DOI] [PubMed] [Google Scholar]
- Guth L, Samaha FJ. Procedure for the histochemical demonstration of actomyosin ATPase. Exp Neurol. 1970;28:365–367. [PubMed] [Google Scholar]
- Hegedus J, Putman CT, Gordon T. Time course of preferential motor unit loss in the SOD1(G93A) mouse model of amyotrophic lateral sclerosis. Neurobiol Dis. 2007;28:154–164. doi: 10.1016/j.nbd.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Hegedus J, Putman CT, Tyreman N, Gordon T. Preferential motor unit loss in the SOD1G93A transgenic mouse model of amyotrophic lateral sclerosis. J Physiol. 2008;586:3337–3351. doi: 10.1113/jphysiol.2007.149286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpati G, Engel WK. ‘Type grouping’ in skeletal muscles after experimental reinnervation. Neurol. 1968a;18:447–455. doi: 10.1212/wnl.18.5.447. [DOI] [PubMed] [Google Scholar]
- Karpati G, Engel WK. Correlative histochemical study of skeletal muscle after suprasegmental denervation, peripheral nerve section, and skeletal fixation. Neurol. 1968b;18:681–692. doi: 10.1212/wnl.18.7.681. [DOI] [PubMed] [Google Scholar]
- Kater SB, Mills LR. Regulation of growth cone behaviour by calcium. J Neurosci. 1991;11:891–899. doi: 10.1523/JNEUROSCI.11-04-00891.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugelberg E, Edstrom L, Abbruzzese M. Mapping of motor units in experimentally reinnervated rat muscle. Interpretation of histochemical and atrophic fibre patterns in neurogenic lesions. J Neurol Neurosurg Psychiatry. 1970;33:319–329. doi: 10.1136/jnnp.33.3.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love FM, Thompson WJ. Schwann cells proliferate at rat neuromuscular junctions during development and regeneration. J Neurosci. 1998;18:9376–9385. doi: 10.1523/JNEUROSCI.18-22-09376.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love FM, Thompson WJ. Glial cells promote muscle reinnervation by responding to activity-dependent postsynaptic signals. J Neurosci. 1999;19:10390–10396. doi: 10.1523/JNEUROSCI.19-23-10390.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luff AR, Hatcher DD, Torkko K. Enlarged motor units resulting from partial denervation of cat hindlimb muscles. J Neurophysiol. 1988;59:1377–1394. doi: 10.1152/jn.1988.59.5.1377. [DOI] [PubMed] [Google Scholar]
- Major LA, Hegedus J, Weber DJ, Gordon T, Jones KE. Method for counting motor units in mice and validation using a mathematical model. J Neurophysiol. 2007;97:1846–1856. doi: 10.1152/jn.00904.2006. [DOI] [PubMed] [Google Scholar]
- Milner-Brown HS, Stein RB, Lee RG. Pattern of recruiting human motor units in neuropathies and motor neurone disease. J Neurol Neurosurg Psychiatry. 1974;37:665–669. doi: 10.1136/jnnp.37.6.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinelli P, Pisano F, Ceriani F, Miscio G. EMG evaluation of motor neuron sprouting in amyotrophic lateral sclerosis. Ital J Neurol Sci. 1991;12:359–367. doi: 10.1007/BF02335775. [DOI] [PubMed] [Google Scholar]
- Pun S, Santos AF, Saxena S, Xu L, Caroni P. Selective vulnerability and pruning of phasic motoneuron axons in motoneuron disease alleviated by CNTF. Nat Neurosci. 2006;9:408–419. doi: 10.1038/nn1653. [DOI] [PubMed] [Google Scholar]
- Rafuse VF, Gordon T. Self-reinnervated cat medial gastrocnemius muscles. I. Comparisons of the capacity of regenerating nerves to form enlarged motor units after extensive peripheral nerve injuries. J Neurophysiol. 1996a;75:268–281. doi: 10.1152/jn.1996.75.1.268. [DOI] [PubMed] [Google Scholar]
- Rafuse VF, Gordon T. Self-reinnervated cat medial gastrocnemius muscles. II. Analysis of the mechanisms and significance of fibre type grouping in reinnervated muscles. J Neurophysiol. 1996b;75:282–297. doi: 10.1152/jn.1996.75.1.282. [DOI] [PubMed] [Google Scholar]
- Rafuse VF, Gordon T, Orozco R. Proportional enlargement of motor units after partial denervation of cat triceps surae muscles. J Neurophysiol. 1992;68:1261–1275. doi: 10.1152/jn.1992.68.4.1261. [DOI] [PubMed] [Google Scholar]
- Rafuse VF, Pattullo MC, Gordon T. Innervation ratio and motor unit force in large muscles: A study of chronically stimulated cat medial gastrocnemius. J Physiol. 1997;499:809–823. doi: 10.1113/jphysiol.1997.sp021970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehder V, Jensen JR, Kater SB. The initial stages of neural regeneration are dependent upon intracellular calcium levels. Neurosci. 1992;51:565–574. doi: 10.1016/0306-4522(92)90296-e. [DOI] [PubMed] [Google Scholar]
- Rosenblatt JD, Parry DJ. Adaptation of rat extensor digitorum longus muscle to gamma irradiation and overload. Pflugers Arch. 1993;423:255–264. doi: 10.1007/BF00374404. [DOI] [PubMed] [Google Scholar]
- Slack JR, Hopkins WG, Williams MN. Nerve sheaths and motoneurone collateral sprouting. Nature. 1979;282:506–507. doi: 10.1038/282506a0. [DOI] [PubMed] [Google Scholar]
- Son YJ, Thompson WJ. Schwann cell processes guide regeneration of peripheral axons. Neuron. 1995a;14:125–132. doi: 10.1016/0896-6273(95)90246-5. [DOI] [PubMed] [Google Scholar]
- Son YJ, Thompson WJ. Nerve sprouting in muscle is induced and guided by processes extended by Schwann cells. Neuron. 1995b;14:133–141. doi: 10.1016/0896-6273(95)90247-3. [DOI] [PubMed] [Google Scholar]
- Son YJ, Trachtenberg JT, Thompson WJ. Schwann cells induce and guide sprouting and reinnervation of neuromuscular junctions. Trends Neurosci. 1996;19:280–285. doi: 10.1016/S0166-2236(96)10032-1. [DOI] [PubMed] [Google Scholar]
- Tam SL, Archibald V, Tyreman N, Gordon T. Effect of exercise on stability of chronically enlarged motor units. Muscle Nerve. 2002a;25:359–369. doi: 10.1002/mus.10057. [DOI] [PubMed] [Google Scholar]
- Tam SL, Archibald V, Tyreman N, Gordon T. Tetrodotoxin prevents motor unit enlargement after partial denervation in rat hindlimb muscles. J Physiol. 2002b;543:655–663. doi: 10.1113/jphysiol.2001.012982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam SL, Archibald V, Tyreman N, Jassar B, Gordon T. Increased neuromuscular activity reduces sprouting in partially denervated muscles. J Neurosci. 2001;21:654–667. doi: 10.1523/JNEUROSCI.21-02-00654.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam SL, Gordon T. Mechanisms controlling axonal sprouting at neuromuscular junction. J Neurocyto. 2003a;32:961–974. doi: 10.1023/B:NEUR.0000020635.41233.0f. [DOI] [PubMed] [Google Scholar]
- Tam SL, Gordon T. Neuromuscular activity impairs axonal sprouting in partially denervated muscles by inhibiting bridge formation of perisynaptic Schwann cells. J Neurobiol. 2003b;57:221–234. doi: 10.1002/neu.10276. [DOI] [PubMed] [Google Scholar]
- Tosney KM. Growth cone navigation in the proximal environment of the chick embryo. In: Letourneau PC, Kater SB, Macagno ER, editors. The Nerve Growth Cone. New York: Raven Press, Ltd; 2002. pp. 387–403. [Google Scholar]
- Tötösy de Zepetnek JE, Gordon T, Stein RB, Zung HV. Comparison of force and EMG measures in normal and reinnervated tibialis anterior muscles of the rat. Can J Physiol Pharmacol. 1991;69:1774–1783. doi: 10.1139/y91-262. [DOI] [PubMed] [Google Scholar]
- Tötösy de Zepetnek JE, Zung HV, Erdebil S, Gordon T. Innervation ratio is an important determinant of force in normal and reinnervated rat tibialis anterior muscles. J Neurophysiol. 1992a;67:1385–1403. doi: 10.1152/jn.1992.67.5.1385. [DOI] [PubMed] [Google Scholar]
- Tötösy de Zepetnek JE, Zung HV, Erdebil S, Gordon T. Motor-unit categorization based on contractile and histochemical properties: a glycogen depletion analysis of normal and reinnervated rat tibialis anterior muscle. J Neurophysiol. 1992b;67:1404–1415. doi: 10.1152/jn.1992.67.5.1404. [DOI] [PubMed] [Google Scholar]
- Van Harreveld A. Re-innervation of denervated muscle fibres by adjacent functioning motor units. Am J Physiol. 1945;144:477–493. [Google Scholar]
- Venema HW. Spatial distribution of fibre types in skeletal muscle: test for a random distribution. Muscle Nerve. 1988;11:301–311. doi: 10.1002/mus.880110405. [DOI] [PubMed] [Google Scholar]
- Vogt T, Nix WA. Functional properties of motor units in motor neuron diseases and neuropathies. Electroencephalogr Clin Neurophysiol. 1997;105:328–332. doi: 10.1016/s0924-980x(97)00028-3. [DOI] [PubMed] [Google Scholar]
- Wang LC, Kernell D. Proximo-distal organization and fibre type regionalization in rat hindlimb muscles. J Muscle Res Cell Motil. 2000;21:587–598. doi: 10.1023/a:1026584307999. [DOI] [PubMed] [Google Scholar]
- Weijs WA, Juch PJW, Kwa SHS, Korfage JAM. Motor unit territories and fibre types in rabbit masseter muscle. J Dent Res. 1993;72:1491–1498. doi: 10.1177/00220345930720110601. [DOI] [PubMed] [Google Scholar]
- Weiss P, Edds MV., Jr Spontaneous recovery of muscle following partial denervation. Am J Physiol. 1946;145:587–607. doi: 10.1152/ajplegacy.1946.145.4.587. [DOI] [PubMed] [Google Scholar]
- Yang JF, Stein RB, Jhamandas J, Gordon T. Motor unit numbers and contractile properties after spinal cord injury. Ann Neurol. 1990;28:496–502. doi: 10.1002/ana.410280405. [DOI] [PubMed] [Google Scholar]


