Abstract
The colonic migrating motor complex (CMMC) is a rhythmically occurring neurally mediated motor pattern. Although the CMMC spontaneously propagates along an empty colon it is responsible for faecal pellet propulsion in the murine large bowel. Unlike the peristaltic reflex, the CMMC is an ‘all or none’ event that appears to be dependent upon Dogiel Type II/AH neurons for its regenerative slow propagation down the colon. A reduction in the amplitude of CMMCs or an elongated colon have both been thought to underlie slow transit constipation, although whether these phenomena are related has not been considered. In this study we examined the mechanisms by which colonic elongation might affect the CMMC using video imaging of the colon, tension and electrophysiological recordings from the muscle and Ca2+ imaging of myenteric neurons. As faecal pellets were expelled from the murine colon, it shortened by up to ∼29%. Elongation of the colon resulted in a linear reduction in the velocity of a faecal pellet and the amplitude of spontaneous CMMCs. Elongation of the oral end of a colonic segment reduced the amplitude of CMMCs, whereas elongation of the anal end of the colon evoked a premature CMMC, and caused the majority of CMMCs to propagate in an anal to oral direction. Dogiel Type II/AH sensory neurons and most other myenteric neurons responded to oral elongation with reduced amplitude and frequency of spontaneous Ca2+ transients, whereas anal elongation increased their amplitude and frequency in most neurons. The inhibitory effects of colonic elongation were reduced by blocking nitric oxide (NO) production with l-NA (100 μm) and soluble guanylate cyclase with 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ; 10 μm); whereas, l-arginine (1–2 mm) enhanced the inhibitory effects of colonic elongation. In conclusion, polarized neural reflexes can be triggered by longitudinal stretch. The dominant effect of elongation is to reduce CMMCs primarily by inhibiting Dogiel Type II/AH neurons, thus facilitating colonic accommodation and slow transit.
Introduction
The peristaltic reflex, which can be evoked by radial distension and mucosal stimulation in both the small and large intestine of most species, underlies propulsion of gut contents in a distal direction (Bayliss & Starling, 1899, 1900; Hirst et al. 1975; Costa & Furness, 1976; Smith & Furness, 1988; Smith et al. 1991, 1992; Grider, 1994; Sims et al. 1995; Smith & Robertson, 1998; Hennig et al. 1999; Spencer et al. 1999; Spencer & Smith, 2001, 2002; D’Antona et al. 2001). Despite the presence of this reflex in both regions of bowel, transit through the human small intestine is much faster (7 m in 2–4 h) than transit through the shorter large intestine (1.5 m in 20–56 h; Hinton et al. 1969; Southwell et al. 2009).
One phenomenon that has generally been unappreciated and under-reported is that the large bowel undergoes substantial changes in length, as well as increases in diameter, as it fills with faecal matter (Dickson et al. 2007, 2008). The dominant effect of longitudinal stretch is to activate myenteric, mechanosensitive, descending interneurons that release nitric oxide (NO) to inhibit neurons in peristaltic nerve circuits rather than producing a direct output to the muscle; therefore we have called this reflex an ‘occult’ reflex (‘hidden from view’: Dickson et al. 2007, 2008; Smith et al. 2007a,b;).
In the current study we have examined the effects of longitudinal stretch on the colonic migrating motor complex (CMMC), which is a prominent motor pattern rather than a simple reflex, in the large bowel (Wood, 1973; Sarna et al. 1984; Bywater et al. 1989; Bayguinov et al. 2010a,b; Dickson et al. 2010a,b;). CMMCs have been observed in many species where they have been referred to as propulsive migrating spike bursts (MSBs; Cherbut & Ruckerbusch, 1985), giant migrating contractions (GMCs; Li et al. 2002) and propagating pressure sequences (PSs; Dinning et al. 2008). This neurally mediated event consists of a rhythmically occurring series of long-duration contractions or electrical activity of the circular muscle. Unlike the classic migrating motor complex (MMC) in the small intestine that occurs only in the fasted state, the CMMC in the large intestine occurs in both the fasted and the fed state (Sarna et al. 1984; Cherbut & Ruckerbusch, 1985). CMMCs appear as stationary contractions, or as contractions that migrate relatively slowly over the whole or partial lengths of colon, propagating in either an oral or an anal direction (Sarna et al. 1984; Bywater et al. 1989; Li et al. 2002; Heredia et al. 2009; Bayguinov et al. 2010a). CMMCs are thought to provide mixing of colonic contents and their slow aboral propagation (Sarna et al. 1984; Dinning et al. 2008). Moreover, the presence of a faecal pellet in the murine colon, which activates local reflexes, causes the CMMC to become more regular and propagate mainly in an oral to anal direction causing the propulsion of faecal pellets (Heredia et al. 2009). The CMMC occurs in isolated colons and is, therefore, independent of the CNS (Bywater et al. 1989; Spencer et al. 2005; Heredia et al. 2009) and is generated by the enteric nervous system or ‘little brain’ within the colonic wall (Bayguinov et al. 2010a,b;).
Electrical recordings from the muscle have shown that the CMMC consists of a brief hyperpolarization, which determines its direction of propagation (Spencer et al. 2005), followed by fast oscillations with action potentials superimposed on a slow depolarization of the circular muscle (Bywater et al. 1989; Spencer et al. 2005; Heredia et al. 2009; Dickson et al. 2010a,b;). The preceding inhibition is generated by activation of descending nervous pathways, whereas the fast oscillations and the slow depolarization are largely generated by the activation of ascending excitatory nervous pathways that release acetylcholine (ACh) and tachykinins (TKs) onto the circular muscle (Brierley et al. 2001; Dickson et al. 2010a), as well as a reduction in activity in inhibitory motor neurons (Bayguinov et al. 2010a,b;). The CMMC is initiated by 5-HT release from enterochromaffin (EC) cells in the mucosa that excites 5-HT3 receptors on the mucosal endings of Dogiel Type II/AH sensory neurons (Heredia et al. 2009; Bayguinov et al. 2010a,b; Dickson et al. 2010a,b;).
In contrast to the classic peristaltic reflex that is graded according to stimulus strength and conducts rapidly through the myenteric plexus (Smith & Furness, 1988; Smith et al. 1992; Spencer & Smith, 2001), the CMMC appears to be an all or none event that propagates relatively slowly (0.8 mm s−1) along the colon (Heredia et al. 2009) because it is likely to require the activation of slow excitatory postsynaptic potentials (SEPSPs) in rapidly adapting Dogiel Type II/AH neurons that appear necessary for its regeneration at different points along the colon (see below and Bayguinov et al. 2010a,b; Dickson et al. 2010b).
In the current study we show that the overall effect of colonic elongation is to suppress CMMCs by inhibiting Dogiel Type II/AH neurons.
Methods
Male C57/BL6 mice (28–42 days old) were killed by inhaling a 5% concentration of isofluorane, followed by cervical dislocation. A ventral midline incision was made and the whole colon (proximal colon and distal colon) was carefully excised. These procedures were in accordance with National Institutes of Health guidelines for the care and use of laboratory animals and approved by the Animal Ethics Committee at the University of Nevada, Reno. The animal experimentation policies and procedures comply with those in the UK, as outlined by Drummond (2009).
Preparations
Expulsion of pellets
The entire colon naturally full of faecal pellets was loosely pinned in a Sylgard (Dow-Corning)-lined organ bath (WPI, Sarosota, Fl, USA) continuously perfused with oxygenated Krebs–Ringer bicarbonate (KRB) solution (see drugs and solutions below) at 36.0 ± 0.5°C at a flow rate of 6.5 ml min−1 to observe spontaneous evacuation of the faecal pellets. The organ bath, which had a volume of 30 ml, was also continuously bubbled with 97% O2–3% CO2. The whole colon was pinned to the floor of the organ bath via the mesentery. The movement of faecal pellets and the length of the colon were viewed with a video camera (WV-BP330; Panasonic CCTV) and recorded directly to a computer (PowerMac G4, Apple, CA, USA) and analysed using previously described methods (Heredia et al. 2009; Dickson et al. 2010a,b;).
Artificial pellet propulsion
To manipulate the length of the colon for elongation experiments, two pins were placed in the oral opening to allow epoxy-coated faecal pellet insertion and one pin in the anal opening to allow for adjustment of colonic length. Faecal pellet movements were monitored via video recordings.
To determine the effect of whole colonic elongation on CMMCs
A glass microelectrode pipette (1 mm OD; 6 cm long; Sutter Instrument Co., Novato, CA, USA) was inserted through and glued to an epoxy coated faecal pellet. The pipette was inserted through the lumen and secured to the floor of the organ bath using U-shaped pins at each end; these held the pipette in position with the fixed pellet located in the middle of the colon (Fig. 1A). Three isometric tension transducers (model TST125C; Biopac Systems Inc., Santa Barbara, CA, USA) were attached by suture silk at regular intervals to the colonic wall in order to measure the tension of the circular muscle; the silk was glued to the colon by a bead of Vetbond (n-butyl cyanoacrylate; 3M, St Paul, MN, USA) and the initial resting tension was set to 8 mN. Three tension transducers were placed at ∼10–15 mm from the oral end of the colon (TO), at the colonic flexure (TM) and at 10–15 mm from the anal end (TA). Tissues were equilibrated for 1–2 h, until regular spontaneous colonic migrating complexes (CMMCs) were observed. Elongation (longitudinal stretch) was applied to the whole colon by stretching and then repinning the colon at the anal end, and resetting the tension to its control resting level of 8 mN. Elongation of the colon while recording tension was achieved by removing tension from the transducers, unpinning a segment of the colon and repinning it at its new length. The tension was then restored to its original resting point.
Figure 1. Preparations.
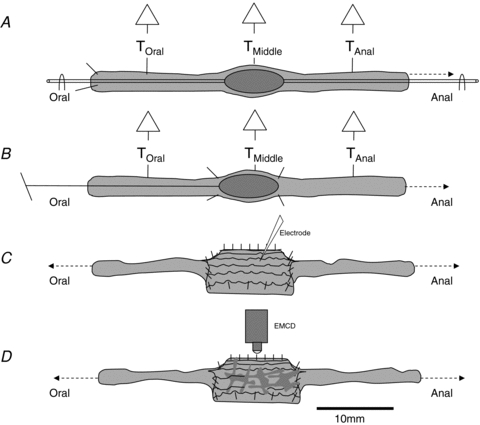
A, effect of colonic elongation on the CMMC. An epoxy coated faecal pellet was secured to a glass pipette that was attached at both ends, by U-shaped pins, to the floor of the organ bath. The colon was pinned at the oral end and elongated by repinning the anal end. Isometric tension transducers were attached at three sites along the colon. B, effect of oral and anal stretch. An epoxy coated faecal pellet was glued to the end of a silk thread. The pellet was allowed to propagate down to the middle of the colon and held fixed in position by pinning the colon around the pellet. Isometric tension transducers monitored circular muscle tension before and following insertion of the pellet. Longitudinal stretch was applied by elongating either the oral or the anal end of the colon. C, a 10 mm section of colon was cut open in the middle of the preparation and pinned with the mucosa down. Microelectrode impalements were made by advancing a microelectrode through the outer longitudinal muscle into the underlying circular muscle. Longitudinal stretch was then applied to either of the loose ends. D, to test the effects of longitudinal stretch on the activity in myenteric neurons the same preparation was used as in C, except that strips of longitudinal muscle were stripped away to reveal the myenteric plexus.
Polarized reflexes
Longitudinal stretch was applied by elongating either the oral or the anal end of the colon (Fig. 1B). An epoxy coated faecal pellet was glued to the end of a silk thread and allowed to propagate down to the middle of the colon and was held fixed in position by pinning the colon around the pellet. Isometric tension transducers were used to monitor circular muscle tension before and following insertion of the pellet.
Electrophysiological recordings
A 10 mm section of colon was cut open in the middle of the preparation and pinned with the mucosa against the base of the organ bath (Fig. 1C). Microelectrode impalements were made by advancing a microelectrode through the outer longitudinal muscle into the underlying circular muscle as previously described (Dickson et al. 2007, 2010a,b). Elongation (longitudinal stretch) was then applied to either of the loose ends of the colon.
Calcium imaging of myenteric neurons
To test the effects of longitudinal stretch on the activity in myenteric neurons, the same preparation was used as in Electrophysiological recordings above except strips of longitudinal muscle were peeled away to reveal the myenteric plexus (Fig. 1D). The preparation was then loaded with the calcium indicator Fluo-4 as described below and Bayguinov et al. (2010a). The capture and analysis of calcium transients in myenteric neurons is described below.
Fluorescent dye loading
Following tissue equilibration (∼1 h), perfusion was stopped, and the bath volume was minimized (∼5 ml). Tissues were incubated with 50 nm Fluo-4 AM (Molecular Probes, Eugene, OR, USA), 0.02% dimethyl sulfoxide (DMSO), and 0.01% of the non-toxic detergent Chremophor EL (Sigma, St Louis, MO, USA) for 15 min at 21°C. Following loading, tissues were perfused with oxygenated KRB solution at 37°C for another 15 min to allow for de-esterification and dye-trapping within cells of the tissues (see Lee et al. 2009).
Probenecid (0.5 μm; Molecular Probes) was added to the recording chamber immediately prior to dye loading to prevent dye expulsion from Dogiel Type II/AH neurons (Bayguinov et al. 2010a). Following incubation with Fluo-4, probenecid was further perfused through the system for the duration of the experiment at the same concentration.
Mito Tracker Red CMXRos (Molecular Probes) was used at the conclusion of each experiment to label mitochondria in myenteric neurons so that Dogiel Type II/AH sensory neurons that are rich in mitochondria could be identified (Pompolo & Furness, 1988; Vanden Berghe et al. 2002; Bayguinov et al. 2010a,b;). Mitotracker was dissolved using 0.02% DMSO and 0.01% Chremophor EL, and was used in concentrations ranging between 0.2 and 0.4 μm. Mitotracker was allowed to penetrate the tissue for 15 min at 21°C, followed by perfusion with oxygenated KRB solution at 37°C for another 20 min before visualization (Bayguinov et al. 2010a).
Image acquisition
Recordings were made using a Nikon Eclipse E600FN upright fluorescence microscope and Nikon Fluor® water-immersion lenses (10–60×; Nikon, USA). Fluo-4 was excited at 488 nm and Mitotracker Red was excited at 594 nm using a Lambda DG-5 illumination system (Sutter Instrument Co.), and image sequences were captured using an Andor IXON+897, 512 × 512 pixels EMCCD (Electron multiplied, charge couple device) camera (Andor, Belfast, UK). Image sequences (1000–2000 frames, captured at 30 Hz) were acquired on a Windows-based PC using the Andor Solis imaging software 4.14.
Analysis of data
Video imaging, tension recordings and microelectrode recordings were analysed as described previously (Heredia et al. 2009; Dickson et al. 2010a,b;). Tension was recorded with a Biopac MP100 (Santa Barbara, CA, USA) acquisition system and stored on a PC. Frequency, duration and amplitude of contractile complexes were measured using Acqknowledge 3.2.6 (Biopac Systems, Inc, Goleta, CA, USA), and tests for statistical significance were made using SigmaPlot 5.0 (Systat Software Inc., San Jose, CA, USA). Propagation velocity was determined by calculating the delay between the 50% amplitude points of CMMCs. Microelectrode electrophysiology was analysed using in-house algorithms as described previously (Heredia et al. 2009; Dickson et al. 2010b).
Ca2+ imaging data files (16-bit tiff) were analysed on an Apple Powermac G5 desktop computer using in-house analysis software (Volumetry G6a, G. W. Hennig). Tissue movements were tracked to allow stable measurements from individual regions of interest (ROIs; Lee et al. 2009). Ca2+-induced fluorescence is reported as average intensity inside an ROI in 8-bit intensity units. Care was taken to avoid including fluorescence from nearby circular muscle cells in neuronal measurements.
Statistical methods
Statistical comparisons of data were performed using Student's (paired or unpaired) t test, ANOVA, or Wilcoxon's rank sum test, and P < 0.05 was considered statistically significant. n refers to the number of animals from which colons were taken. All data are presented as means ±s.e.m.
Drugs and solutions
Hexamethonium bromide, 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ), Chremophor, Nω-nitro-l-arginine (l-NA) and l-arginine were purchased from Sigma-Aldrich. DMSO and probenecid were purchased from Molecular Probes. The Krebs–Ringer bicarbonate (KRB) solution contained (mm): NaCl, 120.35; KCl, 5.9; NaHCO3, 15.5; NaH2PO4, 1.2; MgCl2, 1.2; CaCl2, 2.5; and glucose, 11.5 (continuously gassed with 3% CO2–97% O2, pH 7.3–7.4).
Results
Spontaneous faecal pellet propulsion
The isolated murine colon had an average length of 51.5 ± 2.0 mm (average 5 pellets, range 3–8 pellets) and took ∼30 min to empty its entire contents (1873.3 ± 20.2 s; n= 9). This is considerably longer than the time it takes to propagate a single epoxy coated faecal pellet through an empty colon (37–50 s). As faecal pellets were spontaneously expelled, the colon gradually reduced its length by up to 29% (full length: 52.0 ± 1.4 mm; empty length: 40.0 ± 1.2 mm; n= 20; Fig. 2A). As the colon shortened, the velocity of an expelled natural pellet increased from 0.31 ± 0.03 mm s−1 (when full) to a maximum of 0.85 ± 0.12 mm s−1 (last faecal pellet; Fig. 2B).
Figure 2. Emptying of faecal pellets from the colon.
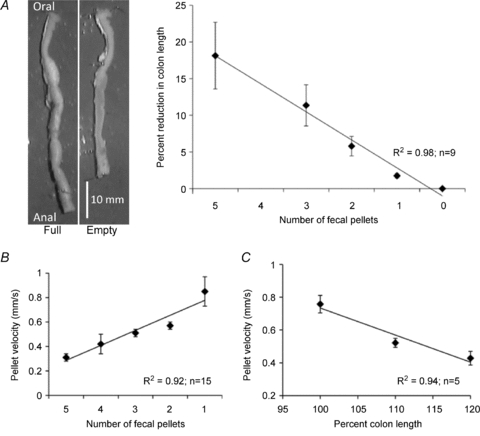
A, colon full of faecal pellets and following their spontaneous evacuation (left hand panels). Plot of the reduction in length of whole colon as each pellet is evacuated. B, velocity of faecal pellets as they are expelled from the colon. C, pellet velocity of an epoxy coated faecal pellet allowed to spontaneously propagate down the colon plotted against artificial increases in colonic length.
Increases in elongation of an empty colon led to a linear reduction in the velocity of an epoxy coated faecal pellet inserted into the oral end of the large bowel (Fig. 2C). The velocity of a faecal pellet went from 0.75 ± 0.05 mm s−1 in a flaccid colon to 0.40 ± 0.04 mm s−1 (n= 9) when the colon was elongated to 120% of its unstretched length.
Effect of elongating the whole colon on spontaneous CMMCs
The CMMC is necessary for faecal pellet propulsion along the murine colon; therefore, we examined the effect of colonic elongation on spontaneous CMMCs in preparations containing a fixed faecal pellet. This has been shown to produce regular CMMCs that propagate mainly in an oral to anal direction along the whole colon (see Heredia et al. 2009; Fig. 3A and B). Elongation of the whole colon, applied in a graded manner (5–20%), produced a significant reduction in the amplitude of spontaneous CMMCs, a 50% reduction in the amplitude of the CMMC at the proximal and distal recording site occurring between 10 and 15% increase in the slack length of the colon (Fig. 3C). At the lowest level of applied colonic elongation tested (5%), there was an overall increase in the amplitude of spontaneous CMMCs at the proximal and distal recording sites. This low level of colonic elongation reduced the amplitude of spontaneous CMMCs at the site over the pellet, which remained at similar level throughout further increases in colonic length (Fig. 3B and C). During colonic elongation, the frequency of CMMCs increased from 0.27 ± 0.05 to 0.33 ± 0.08 cycles min−1 (elongation 15–20%; P < 0.05, n= 10).
Figure 3. Effect of elongating the whole colon on spontaneous CMMCs.

A, tension recordings were used to record spontaneous CMMCs at three sites (TO= oral recording site; TM= middle recording site and TA= anal recording site) along the length of the entire colon. Gradually elongating the whole colon caused a graded reduction in the amplitude of CMMCs. Dotted rectangles indicate readjustment of tension following elongation of the colon. B, removing colonic elongation following 5% and 15% elongation restored CMMCs. Dotted rectangles indicate readjustment of tension following elongation and removal of elongation of the colon. C, percentage change in amplitude of CMMCs recorded at the three sites following graded elongation of whole colon (n= 10). D, amplitude of CMMCs at the 3 recording sites following elongation (15%) of whole colon and then following the removal of longitudinal stretch (n= 6). These preparations contained a fixed epoxy coated faecal pellet located in the centre of the large bowel. **P < 0.01.
Prior to colonic elongation the apparent propagation direction of most (88%; Fig. 4A) CMMCs was the oral to anal direction (Fig. 4D), which is expected when a fixed faecal pellet is present in the lumen (see Heredia et al. 2009). When the whole colon was elongated, most (62%) CMMCs were observed to travel in an anal to oral direction (Fig. 4D; Heredia et al. 2009), which is expected to inhibit the expulsion of faecal pellets. The remaining CMMCs (19%) appeared to originate in the middle of the colon and propagate in both an oral and an anal direction. Following the removal of colonic elongation, the amplitude of spontaneous CMMCs gradually returned to near control levels (Fig. 3B and D); however, the level of restoration varied between recording sites since spontaneous CMMCs in the mid colon rarely recovered to control levels (Fig. 3B). This suggests that the neuronal network underlying the generation and propagation of the CMMC is a dynamic metastable system that once perturbed doesn't always return to its starting condition (Bayguinov et al. 2010a).
Figure 4. Effect of oral and anal colonic elongation on CMMCs.
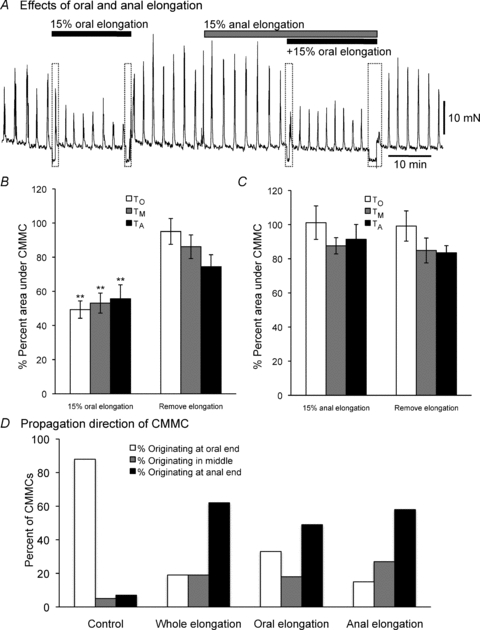
A, tension of the circular muscle was measured at 1 site along the colon. Elongating the oral end of the colon by 15% reduced the amplitude and area under CMMCs. Also, elongating the anal end of the colon by 15% had no obvious effect on CMMCs. Furthermore, elongating the anal end of the colon had no obvious effect on the inhibitory response to oral elongation. B and C, a summary of the effects of oral and anal elongation, respectively, on CMMCs (n= 6) measured at 3 sites along the colon. D, spontaneous CMMCs normally propagate in an oral to anal direction (see Control). Following elongation of the whole colon, oral elongation or anal elongation caused more CMMCs to propagate in an anal to oral direction or originate in the middle of the colon and propagate in both directions (data from 60 CMMCs; n= 6). These preparations contained a fixed epoxy coated faecal pellet located in the centre of the large bowel. **P < 0.01.
Polarized reflexes activated by longitudinal stretch
Tension recordings from the circular muscle demonstrated that elongating the oral segment of the colon by 15–20% of its slack length resulted in a depression of spontaneous CMMC activity (Figs 1B and 4A and B). In contrast, elongating the anal segment of the colon by a similar amount resulted in no overall change in frequency, amplitude or area under the CMMC (Fig. 4A and C). We did find, however, that elongating the oral end of the colon or elongating the anal end of the colon increased the number of CMMCs that appeared to propagate in an anal to oral direction (Fig. 4D).
Intracellular microelectrode recordings from the circular muscle demonstrated that elongation (15–20%) applied to the oral end of the colon completely depressed the electrical activity (n= 4) associated with spontaneous CMMCs without a detectable change in the resting membrane potential (RMP; control: 61.2 ± 3.0 mV; 15–20% oral stretch 60.1 ± 2.0 mV, 10 oral stretches; P > 0.05, n= 5), an example of which is shown in Fig. 5A. In contrast, anal elongation (15–20%) produced a premature CMMC in preparations with spontaneous CMMCs. In 4 out of 10 of these preparations that showed only occasional spontaneous CMMCs, anal elongation (15–20%) of the colon evoked a robust, single CMMC after a latency of 4.90 ± 1.04 s (n= 4) regardless of the duration of the stimulus (15 pulls, n= 4; Fig. 5B). The premature evoked CMMC had a similar duration to spontaneous CMMCs (control: 38.6 ± 0.5 s; anal elongation: 36.27 ± 2.4 s; P > 0.05, n= 4). The premature CMMC presented fast oscillations (1.98 ± 0.2 Hz; n= 4) with several action potentials (5.6 ± 1.4; n= 4) superimposed on the slow depolarizing phase (amplitude of 8.7 ± 1.0 mV) (Figs 5B and 6A left hand panel). 16 out of 24 anally evoked CMMCs were preceded by hyperpolarization (duration: 5.4 ± 1.6 s; amplitude: 6.0 ± 2.0 mV; Fig. 6A), suggesting that anal elongation is not only stimulating interneurons in the ascending excitatory nervous pathway but also those interneurons in the descending inhibitory nervous pathway (Spencer et al. 2005; Dickson et al. 2008, 2010b; Smith et al. 2007a,b;).
Figure 5. Effect of oral and anal elongation on CMMC electrical activity.
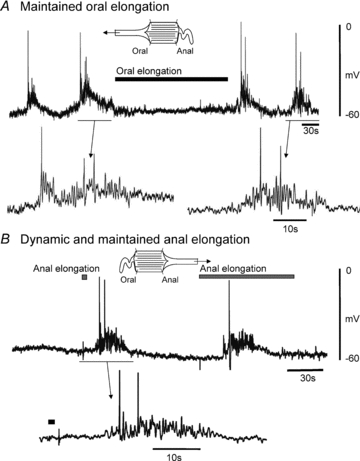
A, spontaneous CMMCs, which consisted of fast electrical oscillations and action potentials superimposed on a slow depolarization, were suppressed following oral elongation (15%). B, in contrast, a transient (1 s) or a prolonged (30 s) anal elongation to 15% evoked only a single CMMC, when the stimuli were applied more than 60 s apart.
Figure 6. Dominant effect of oral versus anal elongation on CMMC electrical activity.

A, left hand panel, in a preparation with infrequent spontaneous CMMCs, a brief (1 s) anal elongation (15%) evoked a CMMC that was identical in waveform to a spontaneous CMMC. Right hand panel, another brief anal elongation evoked a CMMC that was preceded by a hyperpolarization. B, in contrast, oral elongation (15%) had no detectable effect on the resting membrane potential. C, if oral elongation was maintained then anal elongation applied, there was no additional effect. D, a CMMC was evoked by anal elongation. When oral elongation was applied just after the onset of the evoked CMMC it was truncated.
Oral elongation produced no effect on the activity of the circular muscle between spontaneous CMMCs (Fig. 6B). When oral longitudinal stretch was administered and maintained, it prevented the generation of a CMMC evoked by subsequent anal stretch (Fig. 6C). When oral elongation was administered just after anal elongation it truncated the CMMC generated by anal elongation (Fig. 6D). These findings suggest that (1) the response to maintained oral stretch is powerful, ongoing and overrides anally evoked activity, and (2) the response to anal elongation is transient, regardless of the duration of the stimulus.
Effects of oral and anal elongation on activity in myenteric neurons
Myenteric Dogiel Type II/AH sensory neurons can be identified using a mitotracker dye, since they contain dense mitochondria compared to other myenteric neurons (Pompolo & Furness, 1988; Vanden Berghe et al. 2002; Bayguinov et al. 2010a,b;). Recently we have shown that 5-HT released from the enterochromaffin (EC) cells in the colonic mucosa is likely to first activate the mucosal processes of myenteric Dogiel Type II/AH sensory neurons that underlie the initiation of the CMMC, whereas CMMC generation and propagation involve interneurons that synapse with Dogiel Type II neurons (Bayguinov et al. 2010a,b; Dickson et al. 2010b).
An example of increases in Ca2+ transient activity in mitotracker positive (Dogiel Type II neurons; neurons 1–3) and mitotracker negative neurons (neurons 4, 5 and 6) leading to the generation of a CMMC-like contractile event (Δy-displacement/contraction of tissue) is shown in Fig. 7A. In this example, there was an increase in activity in all neurons densely stained with mitotracker prior to contraction of the muscle. These particular mitotracker negative neurons (4 and 6) are likely to be interneurons, since they show ongoing activity throughout the recording period (Dickson et al. 2007, 2010b; Bayguinov et al. 2010a).
Figure 7. Effects of oral elongation on calcium transients in myenteric neurons.
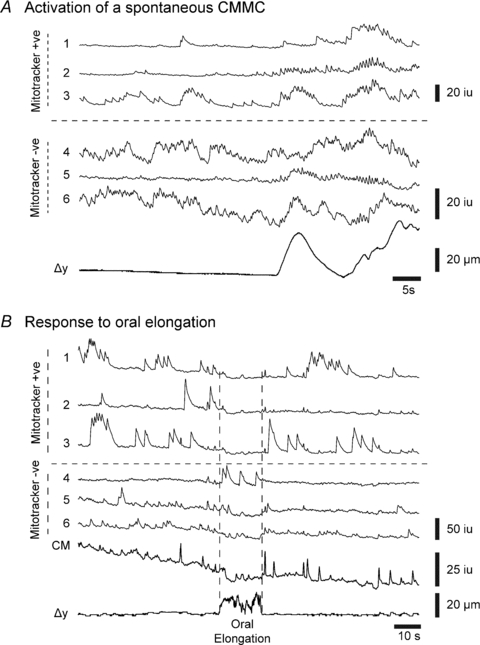
A, build up in spontaneous Ca2+ transient activity in both mitotracker positive (+ve; neurons 1–3) and mitotracker negative (−ve; neurons 4–6) neurons, leading to the initiation of a spontaneous CMMC like event. B, oral elongation (10%) inhibited ongoing Ca2+ transients in both mitotracker positive neurons (neurons 1–3) and mitotracker negative neurons (neurons 5 and 6) and the circular muscle. However, one mitotracker negative neuron (neuron 4) increased its activity for the duration of the stimulus. Δy= displacement or contraction of the tissue.
We examined the effects of oral and anal elongation (see Fig. 1D) on spontaneous Ca2+ transients in myenteric neurons either between spontaneous CMMCs or in preparations with only occasional CMMC activity. Oral elongation by ∼10% depressed ongoing Ca2+ transients in both myenteric mitotracker positive and mitotracker negative neurons at the same time, after a latency of 1.83 ± 0.31 s (9 neurons; n= 4) and 2.36 ± 0.21 s (18 neurons; n= 4), respectively, as well as suppressing activity in the circular muscle (Fig. 7B). This decrease in activity lasted for the duration of the stimulus (tested up to 30 s). Occasional mitotracker negative neurons increased, rather than decreased, their activity in response to oral elongation (see neuron 4, Fig. 7B).
In contrast, anal elongation by ∼10% increased Ca2+ transients in both mitotracker positive neurons and mitotracker negative neurons after a much longer latency of 4.01 ± 0.29 s (24 neurons; n= 4) and 5.62 ± 0.39 s, respectively (15 neurons; n= 5), which correlated with the onset of Ca2+ waves in the muscle (5.25 ± 0.75 s; n= 4; Fig. 8). Interestingly, some mitotracker negative neurons decreased their activity following anal elongation (see neurons 9 and 10; Fig. 8); these neurons are likely to be NOS positive inhibitory motor neurons that are inhibited to allow full excitation of the muscle (Bayguinov et al. 2010a,b;). Anal elongation generated a CMMC response in the muscle. When a second anal elongation stimulus was applied within ∼30 s of the first stimulus, or following a spontaneous CMMC, there was always a reduced response in both mitotracker positive neurons and mitotracker negative neurons suggesting that there was refractoriness within the neural circuit (Fig. 8; see Bayguinov et al. 2010a,b;).
Figure 8. Effects of anal elongation on calcium transients in myenteric neurons.
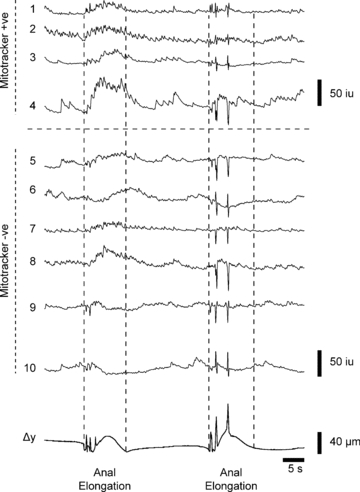
Anal elongation (10%) produced prolonged Ca2+ transients in 4 mitotracker positive neurons at the same time (neurons 1–4), and increased activity in 4 mitotracker negative neurons (neurons 5–8). Two neurons (neurons 9 and 10) were inhibited following the stimulus. Note that the second anal elongation stimulus applied ∼20 s later gave a diminished response in all neurons. Downward deflections in Ca2+ transient traces were artifacts (seen as upward transients in displacement traces (Δy)) caused by anal elongation.
Role of nitric oxide in the inhibitory responses to elongation
We examined whether nitric oxide (NO) was involved in mediating the inhibitory responses to colonic elongation (see Dickson et al. 2007, 2008). When NO production was inhibited by l-NA (100 μm; n= 6), the inhibitory effects of colonic elongation on the CMMC were abolished (Fig. 9A and B). Following l-NA, the addition of hexamethonium (100 μm; n= 4) completely abolished CMMCs (Fig. 9A), suggesting that nicotinic neurotransmission in interneuronal nerve pathways was essential for the generation of the CMMC (see Heredia et al. 2009; Bayguinov et al. 2010a,b;). Previous studies have shown that myenteric neurons that release NO are unlikely to be the targets of NO, since following sodium nitroprusside or electric field stimulation, the neurons that respond with increases in cGMP are likely to be cholinergic neurons and not NOS neurons (Shuttleworth et al. 1993). We also found that the soluble guanylate cyclase inhibitor ODQ (10 μm; n= 6) also reversed the inhibitory effects of colonic elongation (Fig. 9C and D; see Smith & McCarron, 1998). Increasing l-arginine (1 mm; n= 3), which is the amino acid required for the production of NO (Esplugues, 2002), enhanced the inhibitory effects of colonic elongation further by reducing the amplitude and frequency of CMMCs, an example of which is shown in Fig. 4E. Increased concentrations of l-arginine (2 mm; n= 3) abolished CMMCs inhibited by longitudinal stretch. Furthermore, in four experiments oral elongation in the presence of ODQ had no detectable effect on Ca2+ transients in mitotracker positive and mitotracker negative neurons.
Figure 9. Nitric oxide is involved in the inhibitory response to colon elongation.

A, spontaneous CMMCs were measured at 3 sites along the length of the colon. Blocking nitric oxide (NO) synthesis with l-NA (100 μm) reduced the inhibitory effects of colonic elongation on spontaneous CMMCs. B, summary of effects of l-NA on spontaneous CMMCs inhibited by elongating the whole colon (n= 6). Note that following l-NA the further addition of hexamethonium blocked the CMMCs. C, ODQ (10 μm) reduced the inhibitory effects of whole colon elongation on spontaneous CMMCs. D, summary of the effects of ODQ on CMMCs inhibited by elongating the whole colon (n= 6). E, elongating the whole colon by 10% reduced spontaneous CMMCs. The addition of l-arginine (200 μm) further reduced the amplitude of CMMCs and slowed their frequency. These preparations contained a fixed epoxy coated faecal pellet located in the centre of the large bowel. **P < 0.01.
Discussion
In this study we make a number of important observations concerning the effects of colonic elongation on CMMCs, which drive faecal pellet propulsion in the murine colon (Heredia et al. 2009). The CMMC in the murine colon is a complex motor event generated by an interaction between the mucosa, myenteric AH/Dogiel Type II sensory neurons and ascending and descending interneurons (Bayguinov et al. 2010a,b; Dickson et al. 2010a,b;). The isolated murine large intestine is naturally elongated when full of faecal pellets and emptying is slowed, apparently because the amplitude of CMMCs and their propagation along the colon is depressed. This conclusion is supported by the observation that elongating the colon caused a graded decrease in the velocity of a faecal pellet.
The suppression of the amplitude of CMMCs by colonic elongation is consistent with the novel idea that stretching the colon in the longitudinal axis predominantly activates an inhibitory ‘occult’ neural reflex that suppresses activity in myenteric neurons involved in the more complex neural circuitry underlying the CMMC in all regions (proximal to distal colon) of the murine large bowel (Fig. 10), thus facilitating accommodation and slow transit through the murine large bowel.
Figure 10. Polarized elongation reflexes control the circuitry involved in generating the CMMC.
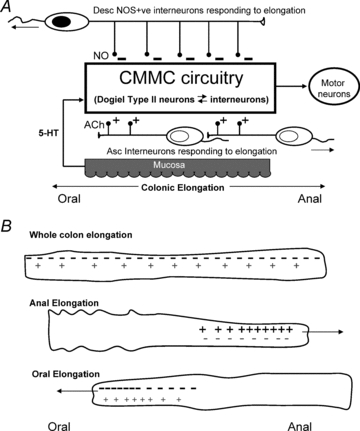
A, release of 5-HT from EC cells excites the mucosal endings of Dogiel Type II/AH neurons, bringing them to threshold. AH neurons then further excite both ascending interneurons and descending interneurons, which activate excitatory and inhibitory motor neurons to the muscle, respectively. Elongation activates long mechanosensitive, descending (NOS positive) interneurons that release NO to inhibit interneurons and Dogiel Type II neurons underlying the generation and propagation of the CMMC. Elongation also activates shorter, mechanosensitive ascending cholinergic interneurons that are likely to release ACh to excite interneurons and Dogiel type II neurons, leading to the generation of the CMMC. B, during elongation of the whole colon the suppression of the CMMC circuit by NO (−) is likely to be more powerful than the excitation of the circuit by ACh (+). When elongation is applied only to the anal end of the colon, mechanosensitive ascending cholinergic interneurons are activated preferentially (+) leading to net excitation (+) of the CMMC circuit. Conversely, when elongation is applied only to the oral end of the colon, mechanosensitive, descending (NOS positive) interneurons are activated preferentially, leading to a net inhibition (−) of the CMMC circuit.
Interneurons are clearly involved in the generation and spread of the CMMC (Spencer et al. 2005; Dickson et al. 2010b). The large bowel contains three classes of ascending interneuron and four classes of descending interneuron (Lomax & Furness, 2000) making it difficult to discriminate their individual roles. In the guinea-pig distal colon some ascending and descending interneurons are sensory since they are mechanosensitive, responding either to circumferential stretch to generate ongoing peristaltic activity (Spencer & Smith, 2002, 2004) or to longitudinal stretch to generate ‘occult’ reflexes (Smith et al. 2007a,b; Dickson et al. 2007, 2008). Dogiel Type II neurons and most interneurons contain acetylcholine (ACh), except one class of long descending interneurons that contain only NOS (Lomax & Furness, 2000). It is these particular NOS-positive interneurons that respond to longitudinal stretch by releasing nitric oxide (NO) to inhibit interneurons in peristaltic nerve circuits (Dickson et al. 2007, 2008; Smith et al. 2007a,b;).
CMMCs were also suppressed by colonic elongation and this was also dependent upon NO since they were inhibited by blocking NO production with l-NA and cGMP production in neurons with ODQ (see Shuttleworth et al. 1993; Smith & McCarron, 1998). Moreover, the inhibitory effect of elongation on CMMCs was enhanced by l-arginine, which is converted by NOS to NO and l-citrulline (Esplugues, 2002). This suggests that the dominant reflex pathway activated directly by colonic elongation in the murine colon also involves descending NOS positive, mechanosensitive interneurons that respond specifically to oral longitudinal stretch by releasing NO. NO appears to suppress activity in interneurons and Dogiel Type II/AH neurons that are critical for the generation and propagation of the CMMC (Dickson et al. 2007, 2010b; Bayguinov et al. 2010a,b;). Presumably, cGMP is suppressing activity by opening potassium channels to produce postsynaptic inhibitory potentials in these particular interneurons (see Dickson et al. 2007, 2008, 2010b; Smith et al. 2007a,b;) and possibly also Dogiel Type II neurons. It has been previously shown that both nerve stimulation and sodium nitroprusside increase cGMP in some colonic myenteric neurons that contain ACh but not in neurons that contain NOS, implying that NOS-positive interneurons and inhibitory motor neurons are not direct targets for NO (Shuttleworth et al. 1993; Bayguinov et al. 2010a). Therefore NO does not suppress activity in inhibitory (or excitatory) motor neurons directly, but only the neurons that synapse with them (Dickson et al. 2007, 2008; Smith et al. 2007a,b;).
Anal stretch applied to the colon had no significant effect on the amplitude of CMMCs measured with tension transducers; it did however produce a single premature CMMC when electrophysiological recordings were made from the circular muscle. Clearly, neural reflexes generated by colonic elongation are polarized, as they are in the guinea-pig distal colon (Dickson et al. 2007, 2008). Therefore, there is a dominant descending inhibitory nerve pathway and an ascending excitatory nerve pathway triggered by colonic elongation that likely involves the activation of mechanosensitive, descending (NOS positive) interneurons and mechanosensitive ascending (cholinergic) interneurons, respectively (Dickson et al. 2007, 2008; Smith et al. 2007a,b;). Presumably, the mechanosensitive dendrites of these neurons are in the myenteric plexus since removal of the longitudinal muscle does not affect the ‘occult’ reflex (Dickson et al. 2007).
What is surprising is that any form of colonic elongation causes more CMMCs to propagate in an anal to oral direction, as occurs in patients with severe constipation (Dinning et al. 2008), thus facilitating accommodation. Furthermore, ∼66% of anal elongations generated a hyperpolarization of the circular muscle that just preceded the CMMC, suggesting that mechanosensitive ascending interneurons activated by elongation not only trigger ascending excitatory nerve pathways but can also activate interneurons in descending inhibitory nerve pathways.
Descending inhibitory and ascending excitatory reflexes triggered by colonic elongation are ‘occult’ reflexes since they don't directly produce an output to the muscle layer, but either suppress activity or enhance activity in myenteric neurons, respectively, that underlie the CMMC (Fig. 10; see Dickson et al. 2007, 2008). When the colon is elongated and both reflex pathways are activated there is likely to be a balance between the two (Fig. 10).
The activation of mechanosensitive ascending interneurons that respond to longitudinal stretch trigger only a single CMMC, suggesting that these interneurons could be rapidly adapting rather than slowly adapting, as they are in the guinea-pig colon (Dickson et al. 2008); or alternatively, once activated Dogiel Type II neurons become refractory. NOS-positive, mechanosensitive descending interneurons appear to be slowly adapting since the inhibition of myenteric neurons lasts for as long as oral elongation persists. Interestingly, the latency of onset of excitation in myenteric neurons was much longer than that required to inhibit myenteric neurons. This suggests, that the mechanosensitive ascending interneurons triggered by elongation are probably short and that the excitatory responses they initiate are conducting slowly through the myenteric plexus, probably because propagation involves the sequential activation of Dogiel Type II/AH sensory neurons by interneurons along the bowel (see Bayguinov et al. 2010a,b;). Ascending interneurons are likely to be much shorter (<10 mm in the guinea-pig colon; Spencer & Smith, 2002) than descending NOS positive interneurons (∼50 mm long in the guinea-pig colon; McConologue & Furness, 1993) that probably inhibit Dogiel Type II/AH neurons and interneurons directly.
Compared to normal subjects, patients with severe slow transit constipation (STC) exhibit CMMCs (called propagating pressure sequences) that are reduced in amplitude, propagate more often in an anal to oral direction, and are less coupled between different regions of colon (Dinning et al. 2009b; 2008;). Historically, an elongated colon (called dolichocolon or redundant colon) was believed to be associated with STC (Kantor 1931a,b; Brummer et al. 1962; Davis 1960; McMahon et al. 1999; Dinning et al. 2009a). ‘The most common related cause of constipation after ARM (anorectal myectomy) was a redundant colon’ (Tomita & Howard, 2008). The idea of an elongated colon underlying slow transit constipation has been recently questioned (Muller-Lissner et al. 2005), presumably because a mechanism by which it might affect colonic transit was unclear (see Dinning et al. 2009b). Although, we don't understand what mechanical, neuronal, or tissue abnormalities produce an elongated colon it is certainly possible that an increase in NOS positive (inter)neurons or their activity, or a decrease in cholinergic (inter)neurons or their activity, could enhance the inhibitory occult reflex and slow transit (Tomita & Howard, 2002, 2008).
Changes in the length of the colon, which have been observed in a variety of motor disorders, have dramatic effects on colonic motility that may exacerbate or compound various disorders ranging from constipation to diarrhoea. Severe constipation and colitis may be opposite extremes of processes that involve the ‘occult’ reflex: STC being triggered by excessive elongation and colitis by colonic shortening. Following the induction of colitis there is a marked shortening of the large bowel in both rats and mice that is associated with soft and watery stools (Kimball et al. 2005; Mizuta et al. 2000; Aoi et al. 2008). This shortening and diarrhoea-like symptoms appears to result from reduced nNOS synthesis and NO release in myenteric neurons (Mizuta et al. 2000; Aoi et al. 2008). Following the termination of Dextran Sulfate Sodium (DSS) induced colitis; the rat colon gradually restores its length, unless recovery is prevented by drugs that block NO synthesis (Aoi et al. 2008).
In conclusion, in this study we have demonstrated that colonic elongation occurs naturally during colonic filling or accommodation. Elongation leads to a slowed emptying of faecal pellets by depressing activity in interneurons and Dogiel Type II neurons responsible for initiating, generating and propagating the CMMC, which is a major motor behaviour in the colon, rather than a simple reflex. Although the circuitry for the CMMC and the peristaltic reflex appears to exhibit some fundamental differences, the occult reflex inhibits both of these activities by similar mechanisms, since nitric oxide suppresses their neural activity. These data show that the occult reflex provides a general mechanism for suppression of propulsive motility patterns in the colon.
Acknowledgments
This study was funded by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases: RO1 DK45713 (T.K.S.). Calcium imaging was performed in a Core facility funded by NIH grant P20 RR-1875. There are no conflicts of interest to disclose.
Glossary
Abbreviations
- CMMC
colonic migrating motor complex
- RMP
resting membrane potential
Author contributions
D.J.H. and E.J.D. were responsible for video imaging of colonic contents, tension recordings and electrophysiological recordings, data analysis, figure preparation and helpful suggestions. P.O.B. was responsible for the calcium imaging of myenteric neurons and figures. G.W.H. was responsible for writing the algorithms used for video imaging, motion stabilization and image analysis of Ca2+ transients and proofing the manuscript. T.K.S. was responsible for much of the overall study design and manuscript preparation. All authors approved the final version of the paper for publication.
References
- Aoi Y, Terashima S, Ogura M, Nishio H, Kato S, Takeuchi K. Roles of nitric oxide (NO) and NO synthases in healing of dextran sulphate sodium-induced rat colitis. J Physiol Pharmacol. 2008;59:315–336. [PubMed] [Google Scholar]
- Bayguinov PO, Hennig GW, Smith TK. Calcium activity in different classes of myenteric neurons underlying the migrating motor complex in the murine colon. J Physiol. 2010a;588:399–421. doi: 10.1113/jphysiol.2009.181172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayguinov PO, Hennig GW, Smith TK. Generation of complex neuronal behavior in a mammalian nervous system. Physiology News. 2010b in press. [Google Scholar]
- Bayliss WM, Starling EH. The movements and innervation of the small intestine. J Physiol. 1899;24:99–143. doi: 10.1113/jphysiol.1899.sp000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss WM, Starling EH. The movements and innervation of the large intestine. J Physiol. 1900;26:107–118. doi: 10.1113/jphysiol.1900.sp000825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brierley SM, Nichols K, Grasby DJ, Waterman SA. Neural mechanisms underlying migrating motor complex formation in mouse isolated colon. Br J Pharmacol. 2001;132:507–517. doi: 10.1038/sj.bjp.0703814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummer P, Seppala P, Wegelius U. Redundant colon as a cause of constipation. Gut. 1962;3:140–141. doi: 10.1136/gut.3.2.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bywater RA, Small RC, Taylor GS. Neurogenic slow depolarisations and rapid oscillations in circular muscle of mouse colon. J Physiol. 1989;413:505–519. doi: 10.1113/jphysiol.1989.sp017666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherbut C, Ruckebusch Y. The effect of indigestible particles on digestive transit time and colonic motility in dogs and pigs. Br J Nutr. 1985;53:549–557. doi: 10.1079/bjn19850064. [DOI] [PubMed] [Google Scholar]
- Costa M, Furness JB. The peristaltic reflex: an analysis of nerve pathways and their pharmacology. Naunyn Schmiedebergs Arch Pharmacol. 1976;294:47–60. doi: 10.1007/BF00692784. [DOI] [PubMed] [Google Scholar]
- D’Antona G, Hennig GW, Costa M, Humphreys CM, Brookes SJ. Analysis of motor patterns in the isolated guinea-pig large intestine by spatio-temporal maps. Neurogastroenterol Motil. 2001;13:483–492. doi: 10.1046/j.1365-2982.2001.00282.x. [DOI] [PubMed] [Google Scholar]
- Davis DA. A new concept in the treatment of symptomatic redundant colon or dolichocolon. West J Surg Obstet Gynecol. 1960;68:101–106. [PubMed] [Google Scholar]
- Dickson EJ, Hennig GW, Heredia DJ, Lee HT, Bayguinov PO, Spencer NJ, Smith TK. Polarized intrinsic neural reflexes in response to colonic elongation. J Physiol. 2008;586:4225–4240. doi: 10.1113/jphysiol.2008.155630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson EJ, Heredia DJ, Hennig GW, Smith TK. Mechanisms underlying the colonic migrating motor complex in both wild-type and nNOS knockout mice. Am J Physiol Gastrointest Liver Physiol. 2010a;298:G222–232. doi: 10.1152/ajpgi.00399.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson EJ, Heredia DJ, Smith TK. Critical role of 5-HT1A, 5-HT3 and 5-HT7 receptor subtypes in the initiation, generation and propagation of the murine colonic migrating motor complex. Am J Physiol Gastrointest Liver Physiol. 2010b;299:G144–157. doi: 10.1152/ajpgi.00496.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson EJ, Spencer NJ, Hennig GW, Bayguinov PO, Ren J, Heredia DJ, Smith TK. An enteric occult reflex underlies accommodation and slow transit in the distal large bowel. Gastroenterology. 2007;132:1912–1924. doi: 10.1053/j.gastro.2007.02.047. [DOI] [PubMed] [Google Scholar]
- Dinning PG, Smith TK, Scott SM. Pathophysiology of colonic causes of chronic constipation. Neurogastroenterol Motil. 2009a;21(Suppl 2):20–30. doi: 10.1111/j.1365-2982.2009.01401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinning PG, Szczesniak MM, Cook IJ. Twenty-four hour spatiotemporal mapping of colonic propagating sequences provides pathophysiological insight into constipation. Neurogastroenterol Motil. 2008;20:1017–1021. doi: 10.1111/j.1365-2982.2008.01147.x. [DOI] [PubMed] [Google Scholar]
- Dinning PG, Szczesniak MM, Cook IJ. Spatio-temporal analysis reveals aberrant linkage among sequential propagating pressure wave sequences in patients with symptomatically defined obstructed defecation. Neurogastroenterol Motil. 2009b;21:945–975. doi: 10.1111/j.1365-2982.2009.01323.x. [DOI] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esplugues JV. NO as a signalling molecule in the nervous system. Br J Pharmacol. 2002;135:1079–1095. doi: 10.1038/sj.bjp.0704569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grider JR. CGRP as a transmitter in the sensory pathway mediating peristaltic reflex. Am J Physiol Gastrointest Liver Physiol. 1994;266:G1139–1145. doi: 10.1152/ajpgi.1994.266.6.G1139. [DOI] [PubMed] [Google Scholar]
- Hennig GW, Costa M, Chen BN, Brookes SJ. Quantitative analysis of peristalsis in the guinea-pig small intestine using spatio-temporal maps. J Physiol. 1999;517:575–590. doi: 10.1111/j.1469-7793.1999.0575t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heredia DJ, Dickson EJ, Bayguinov PO, Hennig GW, Smith TK. Localized release of serotonin (5-hydroxytryptamine) by a fecal pellet regulates migrating motor complexes in murine colon. Gastroenterology. 2009;136:1328–1338. doi: 10.1053/j.gastro.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinton JM, Lennard-Jones JE, Young AC. A new method for studying gut transit times using radioopaque markers. Gut. 1969;10:842–847. doi: 10.1136/gut.10.10.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst GD, Holman ME, McKirdy HC. Two descending nerve pathways activated by distension of guinea-pig small intestine. J Physiol. 1975;244:113–127. doi: 10.1113/jphysiol.1975.sp010786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantor JL. Common anomalies of duodenum and colon. Their practical significance. JAMA. 1931a;97:1785–1790a. [Google Scholar]
- Kantor JL. Anomalies of the colon: Their roentgen diagnosis and clinical significance. Radiology. 1931b;23:651–662b. [Google Scholar]
- Kimball ES, Palmer JM, D’Andrea MR, Hornby PJ, Wade PR. Acute colitis induction by oil of mustard results in later development of an IBS-like accelerated upper GI transit in mice. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1266–G1273. doi: 10.1152/ajpgi.00444.2004. [DOI] [PubMed] [Google Scholar]
- Lee HT, Hennig GW, Park KJ, Bayguinov PO, Ward SM, Sanders KM, Smith TK. Heterogeneities in ICC Ca2+ activity within canine large intestine. Gastroenterology. 2009;136:2226–2236. doi: 10.1053/j.gastro.2009.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Johnson CP, Adams MB, Sarna SK. Cholinergic and nitrergic regulation of in vivo giant migrating contractions in rat colon. Am J Physiol Gastrointest Liver Physiol. 2002;283:G544–552. doi: 10.1152/ajpgi.00114.2001. [DOI] [PubMed] [Google Scholar]
- Lomax AE, Furness JB. Neurochemical classification of enteric neurons in the guinea-pig distal colon. Cell Tissue Res. 2000;302:59–72. doi: 10.1007/s004410000260. [DOI] [PubMed] [Google Scholar]
- McConologue K, Furness JB. Projections of nitric oxide synthesizing neurons in the guinea-pig colon. Cell Tissue Res. 1993;271:545–553. doi: 10.1007/BF02913739. [DOI] [PubMed] [Google Scholar]
- McMahon JM, Underwood ES, Kirby WE. Colonic spasm and pseudo-obstruction in an elongated colon secondary to physical exertion: Diagnosis by stress barium enema. Am J Gastroenterol. 1999;94:3362–3364. doi: 10.1111/j.1572-0241.1999.01554.x. [DOI] [PubMed] [Google Scholar]
- Mizuta Y, Isomoto H, Takahashi T. Impaired nitrergic innervation in rat colitis induced by dextran sulfate sodium. Gastroenterology. 2000;118:714–723. doi: 10.1016/s0016-5085(00)70141-7. [DOI] [PubMed] [Google Scholar]
- Muller-Lissner SA, Kamm MA, Scarpignato C, Wald A. Myths and misconceptions about chronic constipation. Am J Gastroenterol Rev. 2005;100:232–242. doi: 10.1111/j.1572-0241.2005.40885.x. [DOI] [PubMed] [Google Scholar]
- Pompolo S, Furness JB. Ultrastructure and synaptic relationships of calbindin-reactive, Dogiel type II neurons, in myenteric ganglia of guinea-pig small intestine. J Neurocytol. 1988;17:771–782. doi: 10.1007/BF01216705. [DOI] [PubMed] [Google Scholar]
- Sarna SK, Condon R, Cowles V. Colonic migrating and nonmigrating motor complexes in dogs. Am J Physiol Gastrointest Liver Physiol. 1984;246:G355–360. doi: 10.1152/ajpgi.1984.246.4.G355. [DOI] [PubMed] [Google Scholar]
- Shuttleworth CW, Xue C, Ward SM, de Vente J, Sanders KM. Immunohistochemical localization of 3′,5′-cyclic guanosine monophosphate in the canine proximal colon: responses to nitric oxide and electrical stimulation of enteric inhibitory neurons. Neuroscience. 1993;56:513–522. doi: 10.1016/0306-4522(93)90350-o. [DOI] [PubMed] [Google Scholar]
- Sims MA, Hasler WL, Chey WD, Kim MS, Owyang C. Hyperglycemia inhibits mechanoreceptor-mediated gastrocolonic responses and colonic peristaltic reflexes in healthy humans. Gastroenterology. 1995;108:350–359. doi: 10.1016/0016-5085(95)90060-8. [DOI] [PubMed] [Google Scholar]
- Smith TK, Dickson EJ, Hennig GW, Bayguinov PO, Spencer NJ. Colonic elongation activates an intrinsic reflex that underlies slow transit and accommodation. Physiology News. 2007a;69:33–35. [Google Scholar]
- Smith TK, Bornstein JC, Furness JB. Interaction between reflexes evoked by distension and by stimulation of the mucosa of the guinea-pig ileum. J Auton Nerv Syst. 1991;34:69–76. doi: 10.1016/0165-1838(91)90009-r. [DOI] [PubMed] [Google Scholar]
- Smith TK, Bywater RAR, Holman ME, Taylor GS. Electrical responses of the muscularis externa to distension of the isolated guinea-pig distal colon. J Gastrointest Motil. 1992;4:145–156. [Google Scholar]
- Smith TK, Furness JB. Reflex changes in the circular muscle activity elicited by stroking the mucosa: an electrophysiological analysis in the isolated guinea-pig ileum. J Auton Nerv Syst. 1988;25:205–218. doi: 10.1016/0165-1838(88)90025-2. [DOI] [PubMed] [Google Scholar]
- Smith TK, McCarron S. Nitric oxide modulates cholinergic reflex pathways to the longitudinal and circular muscle in the isolated guinea-pig distal colon. J Physiol. 1998;512:893–906. doi: 10.1111/j.1469-7793.1998.893bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TK, Robertson WJ. Synchronous movements of the longitudinal and circular muscle during peristalsis in the isolated guinea-pig distal colon. J Physiol. 1998;506:563–577. doi: 10.1111/j.1469-7793.1998.563bw.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TK, Spencer NJ, Hennig GW, Dickson EJ. Recent advances in enteric neurobiology: mechanosensitive interneurons. Neurogastroenterol Motil. 2007b;19:869–878. doi: 10.1111/j.1365-2982.2007.01019.x. [DOI] [PubMed] [Google Scholar]
- Southwell BR, Clarke MC, Sutcliffe J, Hutson JM. Colonic transit studies: normal values for adults and children with comparison of radiological and scintigraphic methods. Pediatr Surg Int. 2009;25:559–572. doi: 10.1007/s00383-009-2387-x. [DOI] [PubMed] [Google Scholar]
- Spencer NJ, Hennig GW, Dickson E, Smith TK. Synchronization of enteric neural firing during the murine colonic MMC. J Physiol. 2005;564:829–847. doi: 10.1113/jphysiol.2005.083600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer NJ, Smith TK. Simultaneous intracellular recordings from longitudinal and circular muscle during the peristaltic reflex in guinea-pig distal colon. J Physiol. 2001;533:787–799. doi: 10.1111/j.1469-7793.2001.00787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer NJ, Smith TK. A rhythmic motor pattern activated by circumferential stretch in guinea-pig distal colon. J Physiol. 2002;545:629–648. doi: 10.1113/jphysiol.2002.028647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer NJ, Smith TK. Mechanosensory S-neurons rather than AH-neurons appear to generate a rhythmic motor pattern in guinea-pig distal colon. J Physiol. 2004;558:577–596. doi: 10.1113/jphysiol.2004.063586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer N, Walsh M, Smith TK. Is the “Law of the Intestine” obeyed in the guinea-pig small intestine. J Physiol. 1999;517:889–898. doi: 10.1111/j.1469-7793.1999.0889s.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita R, Howard ER. Role of nitric oxide in the colon of patients with slow-transit constipation. Dis Colon Rectum. 2002;45:593–600. doi: 10.1007/s10350-004-6251-8. [DOI] [PubMed] [Google Scholar]
- Tomita R, Howard ER. Clinical studies on anorectal myectomy for chronically constipated patients with outlet obstruction in childhood. Hepatogastroenterology. 2008;55:1600–1605. [PubMed] [Google Scholar]
- Vanden Berghe P, Kenyon JL, Smith TK. Mitochondrial Ca2+ uptake regulates the excitability of myenteric neurons. J Neurosci. 2002;22:6962–6971. doi: 10.1523/JNEUROSCI.22-16-06962.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JD. Electrical activity of the intestine of mice with hereditary megacolon and absence of enteric ganglion cells. Dig Dis. 1973;18:477–487. doi: 10.1007/BF01076598. [DOI] [PubMed] [Google Scholar]


