Abstract
Central chemoreception, the highly sensitive ventilatory response to small changes in CO2/pH, involves many sites. Hypothalamic orexin neurons are CO2 sensitive in vitro, prepro-orexin knockout mice have a reduced CO2 response prominently in wakefulness, and focal antagonism of the orexin receptor 1 (OX1R) in two central chemoreceptor sites, the retrotrapezoid nucleus (RTN) or the medullary raphé, results in a reduction of the CO2 response predominately in wakefulness (−30% and −16%, respectively). Here we hypothesize that acute and selective inhibition of both orexin receptors (OX1R and OX2R) at all central locations by an orally administered dual orexin receptor antagonist, almorexant, will substantially attenuate the CO2 response in a vigilance-state- and diurnal-cycle-dependent manner. We found that almorexant attenuated the CO2 response by 26% only in wakefulness during the dark period of the diurnal cycle to a level observed during NREM sleep in the light period in controls suggesting that the sleep–wake difference in the CO2 response can be in large part attributed to orexin. Almorexant also decreased wakefulness and increased NREM and REM sleep during the dark period, as previously reported, and unexpectedly decreased the number of sighs and post-sigh apnoeas during wakefulness in both the light and the dark period and during both wakefulness and NREM sleep in the dark period. The results support our hypothesis that the orexin system participates importantly in central chemoreception in a vigilance-state- and diurnal-cycle-dependent manner and indicate a role for orexin in the important process of sighing.
Introduction
The orexins consist of two neuropeptides, orexin A (hypocretin-1) and orexin B (hypocretin-2), both derived from prepro-orexin, which is expressed in the orexin neurons (de Lecea et al. 1998; Sakurai et al. 1998) exclusively located in the lateral and dorsal hypothalamus and perifornical region (Peyron et al. 1998; Sakurai et al. 1998). Orexin neurons have extensive projections throughout the central nervous system including basal forebrain, limbic structures and brainstem (Nixon & Smale, 2007; Tsujino & Sakurai, 2009) and they receive cholinergic, serotonergic and GABAergic inputs from numerous brain nuclei (Peyron et al. 1998; Tsujino & Sakurai, 2009). The discharge of orexin neurons is synchronized with arousal states, being greater with increased arousal (Lee et al. 2005; Mileykovskiy et al. 2005), but the levels of orexin in cerebrospinal fluid (CSF) vary almost two-fold during the circadian cycle in rodents, monkeys and humans, with levels being highest at the end of the dark/active period and lowest toward the end of the light/inactive period (Yoshida et al. 2001; Desarnaud et al. 2004). There are two G-protein-coupled orexin receptors, orexin receptor 1 (OX1R) and 2 (OX2R) both of which are widely distributed in the central nervous system (Sakurai et al. 1998; Tsujino & Sakurai, 2009), which indicates orexin participation in a variety of functions including the sleep–wake cycle, feeding and neuroendocrine system.
Our focus is on the role of orexin in the control of breathing and central chemoreception in different arousal states examined in both the dark/active and light/inactive periods of the diurnal cycle. Recent work supports the hypothesis that orexin plays a role in the control of breathing and in chemoreception. Orexin projections and receptors are found in brainstem regions involved in the control of breathing including the rostral ventrolateral medulla (RVLM), medullary raphé, locus coeruleus, Kölliker–Fuse nucleus, nucleus of the solitary tract (NTS), dorsal vagal motor nucleus, hypoglossal motor nucleus and phrenic motor nuclei (Marcus et al. 2001; Young et al. 2005; Nixon & Smale, 2007; Kuwaki, 2008; Tsujino & Sakurai, 2009).
In anaesthetized and vagotomized rats, microinjection of orexin A into the RVLM or phrenic nuclei increased the amplitude of diaphragm EMG activity without affecting frequency (Young et al. 2005), while in a perfused rat brainstem preparation, injection of orexin B into the pontine Kölliker–Fuse nucleus increased respiratory frequency and augmented preinspiratory hypoglossal nerve activity (Gestreau et al. 2008). In vitro, orexin neurons increase their discharge when stimulated by CO2/H+ (Williams et al. 2007). In vivo, high CO2 (10% CO2 for 3 h) significantly increased c-fos expression in the orexin neurons in the perifornical region and the dorsomedial hypothalamus (Sunanaga et al. 2009). Also prepro-orexin knockout (ORX-KO) mice, with a deficiency of both orexin A and B, have a significantly attenuated hypercapnic ventilatory response (CO2 response) in wakefulness but not in sleep, a defect that is partially restored by injection of orexin A and B via the cerebral ventricles (Nakamura et al. 2007; Kuwaki, 2008). Orexin-deficient mice also showed frequent spontaneous apnoeas in both NREM and REM sleep (Nakamura et al. 2007), and lack of ventilatory long-term facilitation induced by repetitive intermittent hypoxia (Terada et al. 2008).
In previous studies we have shown that, in the conscious rat, focal antagonism of the OX1R in two central chemoreceptor regions, the RTN or the medullary raphé, by focal dialysis of an OX1R antagonist resulted in a reduction of the CO2 response predominately in wakefulness (Dias et al. 2009a,b;). In this study we hypothesize that acute inhibition of both OX1R and OX2R at all central locations by an oral dual orexin receptor antagonist (almorexant) will significantly attenuate the CO2 response in a vigilance-state- and diurnal-cycle-dependent manner. We administered either almorexant, a dual orexin receptor antagonist, or a vehicle control, in the same rats studied while breathing air and 7% CO2 during wakefulness, NREM and REM sleep in both the dark/active and light/inactive period of the diurnal cycle.
Methods
Ethical approval
All animal experimentation and surgical protocols were within the guidelines of the National Institutes of Health for animal use and care and the American Physiological Society's Guiding Principles in the Care and Use of Animals and approved by the Institutional Animal Care and Use Committee at the Dartmouth College Animal Resource Center. The authors have read and complied with guidelines for research in rodents outlined for The Journal of Physiology and UK regulations (Drummond, 2009).
Animals
A total of six adult male Sprague–Dawley rats (250–350 g) were used for the experiments and they were individually housed in a light- and temperature-controlled environment. Food and water were provided ad libitum. The general methods are those in common use in our lab (Nattie & Li, 2000; Li et al. 2006). Below they are described in brief. At the conclusion of the experiments, the rats were killed with an overdose of sodium pentobarbitone injected intraperitoneally (>75 mg kg−1).
Diurnal cycles
All the rats are housed in 12 h light–12 h dark cycle. In order to efficiently study the same rats in both the light/inactive cycle and the dark/active cycle during the investigator's daylight hours, two different light settings were used for this study: (1) 07:00 h lights on and 19:00 h lights off for study in the morning during the early period of the light cycle; and (2) 12:00 h lights off and 00:00 h lights on for study in the afternoon during the early period of the dark cycle. The same rats were randomly assigned to either light cycle first. When both the control and drug treatment experiments were completed in the 1st assigned light cycle, the rats were moved to the opposite light cycle and acclimatized for at least 7 days before the second set of the experiments was conducted.
Surgery
All the rats were surgically implanted with EEG and EMG electrodes along with a telemetric temperature probe. The detailed surgical procedures have been described in our earlier publications (Nattie & Li, 2000; Li et al. 2006). In brief, sterile surgery was performed under general anaesthesia induced with intramuscular administration of ketamine (100 mg kg−1) and xylazine (15 mg kg−1). Three EEG electrodes were screwed into the skull and two EMG electrodes were sutured onto the dorsal neck muscles, and all the electrode wires were connected to a six-prong plastic pedestal. A sterile telemetry temperature probe (TA-F20, Data Sciences, St Paul, MN, USA) was placed in the abdominal cavity. The incision was closed, and the animal was allowed to recover for at least 7 days. Following the surgery, the animals received two doses of buprenorphine (0.05 mg kg−1) for post-surgical discomfort. The first dose was give at the conclusion of the surgery and the second 6–12 h later.
Dual orexin receptor antagonist
Almorexant (ACT-078573, (2R)-2-{(1S)-6,7-dimethoxy-1-[2-(4-trifluoromethylphenyl)-ethyl]-3,4-dihydro-1H-isoquinolin-2-yl}-N-methyl-2-phenyl-acetamide), an OX1–OX2 receptor antagonist, is a tetrahydroisoquinoline derivative optimized by Actelion Pharmaceuticals Ltd. It is an orally available, potent, selective and competitive orexin receptor antagonist that is active at both rat and human OX1R and OX2R. It inhibited the increase in intracellular Ca2+ induced by 10 nm human orexin-A in Chinese hamster ovary cells overexpressing the rat or human OX1 and OX2 receptors (Brisbare-Roch et al. 2007). Almorexant was supplied generously by Actelion Pharmaceuticals, Ltd. An oral gavage was made by dissolving almorexant (300–400 mg kg−1) in 0.25% methylcellulose solution in water with 0.25% methylcellulose solution in water alone used as the vehicle control. Each rat received either almorexant gavage or vehicle control gavage 2 h prior to any experimental recording.
Ventilation, oxygen consumption and temperature measurement
The methods used to measure the ventilation, body temperature and oxygen consumption (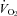 ) were those in common use in our lab (Nattie & Li, 2000; Li et al. 2006). In brief, to measure ventilation in conscious rats, we used the whole body plethysmograph technique. The volume of the plethysmograph was 7.6 l with a 3.5 l top to protect the head pedestal. It is connected to a similarly sized reference chamber by a high resistance port. Analog output from the pressure transducer was sampled at 150 Hz and converted into a digital signal by a computer using the DATAPAC 2K2 system (RUN Technologies, Laguna Hills, CA, USA). The rates of inflow and outflow to the plethysmograph were balanced to one another with a flow meter such that flow was maintained at ≥1.4 l min−1 and the plethysmograph remained at atmospheric pressure. CO2 and O2 fractions were sampled from the outflow line at ∼100 ml min−1 by the CO2 and O2 gas analyser (Applied Electrochemistry). To calibrate the plethysmograph before each experiment, we obtained pressure data from four 0.3 ml air injections made with a 1 ml syringe. Breath-by-breath analysis was performed with the pressure deflections and the respiratory cycle time for each breath. Tidal volume (VT) was calculated and breathing frequency (fR) per breath to estimate ventilation (
) were those in common use in our lab (Nattie & Li, 2000; Li et al. 2006). In brief, to measure ventilation in conscious rats, we used the whole body plethysmograph technique. The volume of the plethysmograph was 7.6 l with a 3.5 l top to protect the head pedestal. It is connected to a similarly sized reference chamber by a high resistance port. Analog output from the pressure transducer was sampled at 150 Hz and converted into a digital signal by a computer using the DATAPAC 2K2 system (RUN Technologies, Laguna Hills, CA, USA). The rates of inflow and outflow to the plethysmograph were balanced to one another with a flow meter such that flow was maintained at ≥1.4 l min−1 and the plethysmograph remained at atmospheric pressure. CO2 and O2 fractions were sampled from the outflow line at ∼100 ml min−1 by the CO2 and O2 gas analyser (Applied Electrochemistry). To calibrate the plethysmograph before each experiment, we obtained pressure data from four 0.3 ml air injections made with a 1 ml syringe. Breath-by-breath analysis was performed with the pressure deflections and the respiratory cycle time for each breath. Tidal volume (VT) was calculated and breathing frequency (fR) per breath to estimate ventilation (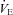 ) per breath. Ventilatory parameters are expressed as mean values for quiet wakefulness, NREM and REM sleep during room air or 7% CO2 conditions. Oxygen consumption (
) per breath. Ventilatory parameters are expressed as mean values for quiet wakefulness, NREM and REM sleep during room air or 7% CO2 conditions. Oxygen consumption (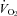 ) was calculated by application of the Fick principle, using the difference in O2 content between inflow air and outflow air and the flow rate as it was described previously (Nattie & Li, 2000; Li et al. 2006). It is important to note in the context of this study that our measure of
) was calculated by application of the Fick principle, using the difference in O2 content between inflow air and outflow air and the flow rate as it was described previously (Nattie & Li, 2000; Li et al. 2006). It is important to note in the context of this study that our measure of  is not a breath-by-breath measure but an average of many minutes of breathing as the time course of the expired gas measured reflects plethysmograph chamber size and the flow rate through the chamber as well as animal utilization. Core body temperature is measured continuously and recorded every 10 min using the signal from the telemetric temperature probe within the abdomen. The temperature of the experimental chamber is measured using a thermometer within the plethysmograph.
is not a breath-by-breath measure but an average of many minutes of breathing as the time course of the expired gas measured reflects plethysmograph chamber size and the flow rate through the chamber as well as animal utilization. Core body temperature is measured continuously and recorded every 10 min using the signal from the telemetric temperature probe within the abdomen. The temperature of the experimental chamber is measured using a thermometer within the plethysmograph.
Sighs, post-sigh apnoeas and spontaneous apnoeas
Apnoea is defined as a respiratory pause or cessation lasting greater than 800 ms which represents at least two missed breaths (Stephenson & Horner, 2006). Apnoeas are divided into two categories: post-sigh apnoeas, in which the apnoeic interval occurred within 5 s following a ‘sigh’ or augmented breath (breath with tidal volume (VT) more than 50% greater than those of preceding breaths), and spontaneous apnoeas in which the apnoea was not preceded by an augmented breath. Occasionally, sighs were observed without apnoea (Monti et al. 1995; Stephenson & Horner, 2006).
Determination of vigilance state
Raw EEG and EMG outputs from the skull and skeletal muscle electrodes were sampled at 150 Hz, filtered at 0.3–70 and 0.1–100 Hz, respectively, and were acquired continuously using the DATAPAC system. The vigilance states were determined by analysis of EEG and EMG records as previously described (Nattie & Li, 2000; Li et al. 2006). In brief, a fast Fourier transform was performed on the EEG and EMG signal at 4.0-s-long epochs using SleepSign software (Kissei Comtec Co. Ltd, Japan). The vigilance states were categorized as wakefulness, non-REM (NREM) and REM sleep. The duration and percentage of time the animal spent in each state during the experimental hours were also analysed by SleepSign.
Statistics
We applied SigmaStat software for our statistical analysis. For the evaluation of treatment effects on 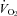 and body temperature we applied a repeated measures (RM) ANOVA separately to the light/inactive and dark/active period data with application of the Tukey post hoc test when appropriate. For the analysis of treatment effects on arousal we categorized vigilance states into wakefulness, NREM and REM sleep, and evaluated the treatment effect as a RM separately within the light and dark periods, applying a Tukey post hoc test as appropriate. For analysis of ventilation, we applied a RM ANOVA for a treatment effect separately in the light and dark periods with state and inspired gas as categorical variables, applying a Tukey post hoc test as appropriate. For the analysis of the treatment effect on the numbers of sighs, apnoeas following sighs and apnoeas unrelated to sighs we applied a RM ANOVA separately in the light and dark circadian periods only during air breathing with arousal state as the categorical variable. We ask if almorexant treatment affects these apnoea-related measures within each circadian period as a function of arousal state.
and body temperature we applied a repeated measures (RM) ANOVA separately to the light/inactive and dark/active period data with application of the Tukey post hoc test when appropriate. For the analysis of treatment effects on arousal we categorized vigilance states into wakefulness, NREM and REM sleep, and evaluated the treatment effect as a RM separately within the light and dark periods, applying a Tukey post hoc test as appropriate. For analysis of ventilation, we applied a RM ANOVA for a treatment effect separately in the light and dark periods with state and inspired gas as categorical variables, applying a Tukey post hoc test as appropriate. For the analysis of the treatment effect on the numbers of sighs, apnoeas following sighs and apnoeas unrelated to sighs we applied a RM ANOVA separately in the light and dark circadian periods only during air breathing with arousal state as the categorical variable. We ask if almorexant treatment affects these apnoea-related measures within each circadian period as a function of arousal state.
Experimental protocol
All six animals were studied in both the dark/active and light/inactive periods with both vehicle control and almorexant treatments. To study each animal in all these conditions two different diurnal cycles were used as described above: (1) for study in the light/inactive period, the animals were administered one dose of vehicle control or almorexant between 07:30–08:00 h, and the data collection started 2 h later; (2) for study in the dark/active period, the animals were administered one dose of vehicle control or almorexant between 11:30–12:00 h, and the data collection started 2 h later. Once both the vehicle control and almorexant treatment experiments for one light cycle were completed the rats were then moved into the other light cycle and acclimatized for at least 7 days before starting another set of the experiments. We allowed a minimum of 7 days recovery after almorexant and a minimum of 2 days for the vehicle control.
After gavage, the animals were placed into the whole body plethysmograph and allowed to recover from gavage and acclimatized for a 2 period prior to the experiment. All the data were collected continuously starting from 2 h post gavage. To obtain sufficient data from each vigilance state including wakefulness, NREM and REM sleep, all the animals were exposed to 1 h of air and followed by 1 h of 7% CO2 (CO2 response). We find that the 1 h time of exposure and measurement allows reasonable evaluation of ventilation in both sleep and wakefulness while minimizing the total exposure time to 7% CO2.
Results
Metabolic rate
Compared to control, almorexant-treated rats had a significantly lower 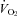 during air breathing in the dark, active period (P < 0.02, RM ANOVA applied within the dark period; P < 0.05; post hoc Tukey) (Fig. 1). There was no significant treatment effect within the light period. It is important to note that our measure of
during air breathing in the dark, active period (P < 0.02, RM ANOVA applied within the dark period; P < 0.05; post hoc Tukey) (Fig. 1). There was no significant treatment effect within the light period. It is important to note that our measure of  is averaged over one exposure period, air or CO2; our method does not allow detection of changes in
is averaged over one exposure period, air or CO2; our method does not allow detection of changes in 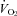 during rapid changes in arousal state.
during rapid changes in arousal state.
Figure 1. The effects of systemic treatment with almorexant or vehicle control on oxygen consumption and body temperature.
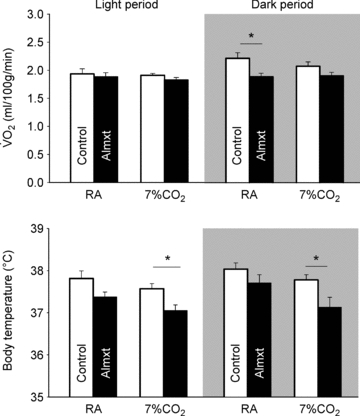
The effects of systemic treatment with almorexant (Almxt) or vehicle control on oxygen consumption (top panel) and body temperature (bottom panel) are shown as mean ±s.e.m. values during the period of exposure to air (RA) and 7% CO2 in both the light/inactive and dark/active periods. We evaluated these data using a RM ANOVA applied to all data separately within the light/inactive and dark/active periods with application of the Tukey post hoc test when appropriate. Within the dark period, there was a significant RM effect to increase 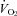 (P < 0.02) with post hoc analysis showing significance in RA (P < 0.05; Tukey). There was no significant effect within the light period. For body temperature, there was a significant overall RM effect to decrease body temperature in the light period (P < 0.01; Friedman Rank test) with no significant post hoc effects. In the dark period, there was a significant overall RM effect (P < 0.001) with a significant post hoc effect while breathing 7% CO2 (P < 0.05; post hoc Tukey).
(P < 0.02) with post hoc analysis showing significance in RA (P < 0.05; Tukey). There was no significant effect within the light period. For body temperature, there was a significant overall RM effect to decrease body temperature in the light period (P < 0.01; Friedman Rank test) with no significant post hoc effects. In the dark period, there was a significant overall RM effect (P < 0.001) with a significant post hoc effect while breathing 7% CO2 (P < 0.05; post hoc Tukey).
Body temperature
Almorexant treatment decreased body temperature within the light period (P < 0.01; Friedman rank test; no significant post hoc effect) and within the dark period (P < 0.001, RM ANOVA) that was significant during 7% CO2 breathing (P < 0.05, post hoc Tukey) (Fig. 1). Overall there was a tendency for body temperature to decrease during the 7% CO2 inhalation compared to room air breathing in both control and almorexant-treated animals (this was not tested statistically).
Vigilance states
We categorized vigilance states into wakefulness, NREM and REM sleep, and evaluated the almorexant treatment effect as a RM separately within the light and dark periods (Fig. 2). In the light period there is a significant state effect (P < 0.001) but no RM treatment effect or interaction. The rats spent more time in NREM sleep compared to wakefulness (P < 0.05; Tukey) and to REM sleep (P < 0.05; Tukey) and more time in wakefulness than in REM sleep (P < 0.05; Tukey). In the dark period, there was no RM effect but there was a significant state effect (P < 0.001) and a significant interactive effect (P < 0.01) with significant effects of almorexant to decrease the percentage time in wakefulness (−56%) (P < 0.05) while increasing the percentage time in NREM (+39%) (P < 0.05; Tukey) and REM (+146%) (P < 0.05; Tukey) sleep. Analysis of the number of bouts and duration of each state did not show any significant effects although the product of bout number and duration did. During the 7% CO2 exposure the rats continued to cycle through wakefulness and NREM sleep with similar distributions of bout number, mean bout duration and total percentage time as in room air and similar effects of almorexant (data not shown).
Figure 2. The effects of systemic treatment of Almxt or vehicle control on the percentage time spent in each arousal state in animals exposed to either the light/inactive or dark/active cycle.
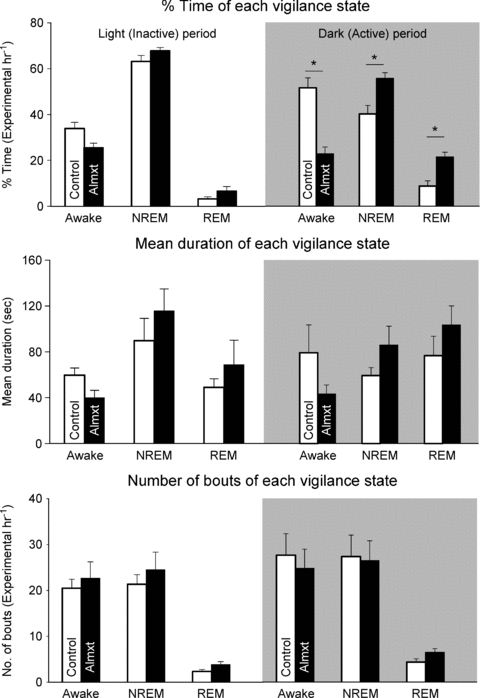
We show only the RA data and analyse the results separately by light and dark periods using Almxt treatment as the RM. In the dark period, there was no RM effect but there was a significant state effect (P < 0.001) and a significant interactive effect (P < 0.01) with significant post hoc effects (P < 0.05) in wakefulness, NREM and REM sleep. Analysis of the number of bouts and duration of each state did not show any significant effects.
Baseline ventilation and response to hypercapnia
For the analysis of the treatment effect on ventilation we applied a RM ANOVA separately for the light and dark periods with state (wakefulness, NREM, REM sleep) and inspired gas (room air, 7% CO2) as a categorical variables. In the light period, ventilation was significantly higher in CO2 in both groups in all three states but there were no significant effects between control and almorexant treatment (Fig. 3). In the dark period, ventilation was significantly higher in CO2 in both groups in all three states and there was a significant interaction between the RM and state. Post hoc analysis showed a significant difference between the ventilation breathing 7% CO2 in control compared to almorexant treatment in the awake state (P < 0.05; Tukey). This was a ∼ 26% decrease and was made up of decreases in both the tidal volume (P < 0.05; Tukey) and frequency (P < 0.05; Tukey) components of ventilation (data not shown). The ventilation breathing air in the dark period was slightly lower after almorexant treatment but was appropriate for the lower 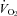 at that time, i.e. the
at that time, i.e. the 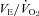 was unchanged.
was unchanged.
Figure 3. The effects of systemic treatment of Almxt or vehicle control on ventilation normalized to body weight.
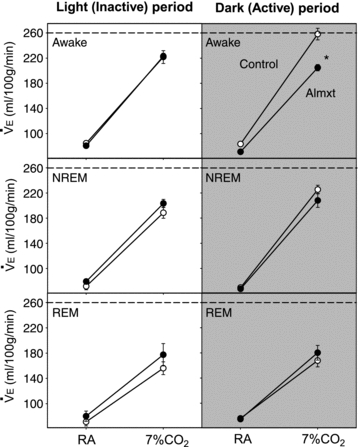
For the analysis of the treatment effect on ventilation we applied a RM ANOVA separately for the light and dark periods with state (wakefulness, NREM, REM sleep) and inspired gas (room air (RA); 7% CO2) as categorical variables. In the light and dark periods, ventilation was significantly higher in CO2 in both groups in all three states but there were no significant effects between control and Almxt treatment. In the dark period, there was a significant difference between the ventilation breathing 7% CO2 in control compared to Almxt treatment in the awake state (P < 0.05; Tukey). The dashed line shows the level of response to 7% CO2 in wakefulness in the dark period, the greatest response. Note that in controls the level of response during NREM sleep in the light period is much less and similar to the response after Almxt in the awake dark period.
Sigh frequency, post-sigh apnoeas and spontaneous apnoeas
The effects of almorexant on sigh frequency, post-sigh apnoea and spontaneous apnoea are shown in Fig. 4 separately for light and dark periods. We analysed only the data obtained in air breathing. A typical sigh and the accompanying post-sigh apnoea are shown in the middle panel (Fig. 4). In the light period, almorexant treatment significantly decreased the number of sighs and post-sigh apnoeas (P < 0.001, RM ANOVA) with post hoc significance in wakefulness (P < 0.05, Tukey). In the dark period, almorexant significantly decreased the number of sighs and post-sigh apnoeas (P < 0.001, RM ANOVA) with post hoc significance in both wakefulness and NREM sleep (P < 0.05, Tukey). There are not enough numbers of sighs and post-sigh apnoeas in the REM sleep in either circadian period to make any comparison in our current experimental design. In respect to spontaneous apnoeas, during either the light or the dark period there was no significant treatment effect nor was there any interaction by state. While there is a suggestion for increased spontaneous apnoeas in REM sleep after almorexant treatment this did not reach statistical significance in this protocol.
Figure 4. The effect of systemic Almxt treatment or vehicle control on the frequency of sighs and both post-sigh and spontaneous apnoeas.
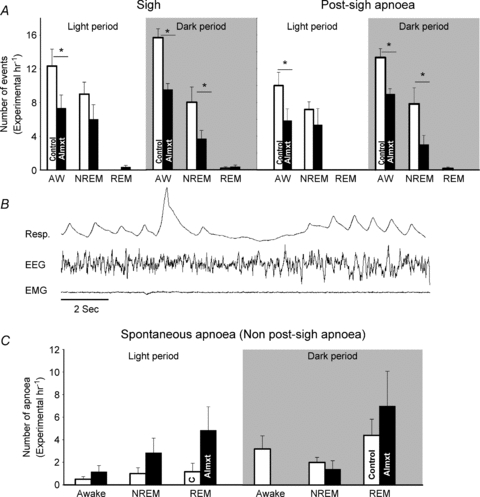
The effect of systemic Almxt treatment or vehicle control on the frequency of sighs and both post-sigh (A) and spontaneous (C) apnoeas are shown. A typical sigh with post-sigh apnoea is shown in B. In the light period, Almxt treatment significantly decreased the number of sighs and post-sigh apnoeas in wakefulness (P < 0.05, Tukey). In the dark period, Almxt significantly decreased number of sighs and post-sigh apnoeas in both wakefulness and NREM sleep (P < 0.05, Tukey). For spontaneous apnoeas, there was no significant treatment effect nor was there any interaction by state.
Discussion
We find that acute and specific antagonism of both orexin receptors by systemic administration of almorexant in unanaesthetized and freely moving adult rats: (1) attenuates the respiratory response to CO2 only in wakefulness and only during the dark period of the diurnal cycle, (2) decreases wakefulness and increases NREM and REM sleep during the dark period as reported previously (Brisbare-Roch et al. 2007), and (3) decreases the number of sighs and post-sigh apnoeas during wakefulness in both the light and the dark period and during both wakefulness and NREM sleep in the dark period. We interpret the data to show that the orexin system (1) participates importantly in central chemoreception in a vigilance-state- and diurnal-cycle-dependent manner, (2) contributes to the sleep–wake difference in the CO2 response, and (3) plays a role in the important process of sighing.
Orexin and breathing
Our almorexant treatment did not affect breathing at rest in any of our conditions, a finding that is congruent with observations in the ORX-KO mouse (Kuwaki, 2008). Resting ventilation in air exposure during wakefulness in the dark period is slightly decreased compared to the control (Fig. 3) but was appropriate for the lower 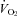 at that time (Fig. 1), i.e. the
at that time (Fig. 1), i.e. the 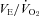 was unchanged. This lower VE and
was unchanged. This lower VE and 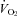 during wakefulness in the dark/active period may simply reflect less activity with dual orexin receptor antagonism. If so, this could contribute to the tendency for a lower body temperature as well.
during wakefulness in the dark/active period may simply reflect less activity with dual orexin receptor antagonism. If so, this could contribute to the tendency for a lower body temperature as well.
In respect to central chemoreception, our prior studies showed that focal inhibition of OX1R by microdialysis of an OX1R antagonist (SB-334867) in the RTN decreased the CO2 response substantially in wakefulness (−30%), with a smaller (−9%) but significant effect in sleep (Dias et al. 2009a), and in the medullary raphé region decreased the CO2 response in wakefulness (−16%) only during the dark/active period (Dias et al. 2009b). ORX-KO mice with no orexin exhibit an attenuated CO2 response only in wakefulness, with ∼15–20% reduction when tested with 5% CO2 and ∼50% reduction with 10% CO2 (Kuwaki, 2008). Here, with acute antagonism of both orexin receptors throughout the brain by systemic administration of almorexant, we observed a 26% reduction in ventilation with 7% CO2 but only during wakefulness in the dark/active period. The degree of inhibition that we observed is qualitatively comparable to that reported in the ORX-KO mice with absence of orexin. It is also comparable to that produced by the focal inhibition of OX1R only in the RTN region (Dias et al. 2009a). The relatively greater degree of CO2 response inhibition observed with focal OX1R antagonist (SB-334867) in the RTN may be explained by a greater concentration of drug being delivered to the RTN receptors by focal dialysis than by oral administration of almorexant. Using reported tissue almorexant data (Brisbare-Roch et al. 2007), we can estimate brain tissue values in our protocol as being in the range of 60 nm. The concentration of OX1R antagonist SB-33467 in the dialysate used for the RTN focal inhibition was 5 mm. Considering that in general the concentration delivered to the tissue is 10 times lower than the dialysate (Nattie & Li, 2000), then the concentration of antagonist in tissue adjacent to the dialysis probe would be in the range of 0.5 mm, quite a high value compared to the estimate for almorexant. We consider that the comparable inhibition of the CO2 response produced both by almorexant and by the absence of orexin in the knockout mouse suggests that the almorexant effect was at many sites involved in central chemoreception. The relatively large effect observed by focal inhibition of OX1R could reflect the higher dose delivered or perhaps a greater role for this single site in the overall orexin-mediated effect.
Orexin as a circadian modulator
The discharge rate of orexin neurons varies with arousal state, being highest during active waking, lower during quiet waking, and virtually absent during sleep, including REM sleep (Lee et al. 2005; Mileykovskiy et al. 2005; Takahashi et al. 2008). These arousal-state-related changes in firing rate seem to occur within both the light/inactive and dark/active circadian periods. In contrast, orexin levels in cerebrospinal fluid and in the extracellular fluid within the lateral hypothalamus vary almost two-fold during the circadian cycle in rodents, monkeys and humans, with levels being highest at the end of the dark/active period and lowest toward the end of the light/inactive period (Yoshida et al. 2001; Desarnaud et al. 2004). In conscious cat (Kiyashchenko et al. 2002) the orexin-A concentration in dialysate from the hypothalamus is significantly higher during active waking than NREM sleep but these state-related changes were much smaller in comparison to the circadian difference (Yoshida et al. 2001; Kiyashchenko et al. 2002). These data suggest that the orexin system is a powerful circadian modulator in addition to its arousal-state-related effects. Our observation that the effects of almorexant on the CO2 response are more potent during the dark period of the diurnal cycle when the level of orexin in the central nervous system is higher supports this view of orexin as a potent diurnal modulator. Studies of the diurnal variation of the ventilatory response to CO2 support this interpretation (Stephenson et al. 2000; Mortola, 2004).
Orexin and the sleep–wake differences in the CO2 response
The largest CO2 response that we observed in these experiments was in controls in wakefulness during the dark/active period (open symbol; dashed line; top panel of Fig. 3). We have placed a dashed line at this ventilation value in the other panels. This shows that the CO2 response during NREM sleep in the light/inactive period, that of deepest sleep, is the same after vehicle and almorexant and is much lower than observed in wakefulness in the dark/active period in controls (the dashed line). We interpret this to indicate that (1) the sleep–wake difference in the CO2 response may be optimally examined by comparison of wake in the dark/active period and NREM sleep in the light/inactive period, and (2) this sleep–wake difference in the CO2 response may be largely attributable to orexin.
Sighs, apnoeas and sleep disorders
Almorexant significantly decreased the number of sighs and post-sigh apnoeas in both parts of the circadian cycle. Sighs or augmented deep breaths serve to prevent atelectasis and enhance surfactant turnover, which maintains normal pulmonary compliance and prevents hypoxaemia (Bartlett, 1973; Nicholas et al. 1982; Patroniti et al. 2002). Sighs can be abolished by vagotomy or carotid body ablation in anaesthetized rats (Bartlett, 1973). Our results suggest a role for orexin in these sigh-inducing reflexes perhaps at the NTS.
We also observed a trend towards an increase in spontaneous apnoeas after almorexant in all vigilance states in the light period and in REM sleep in the dark period.
We note that: (1) the dose used in this study was much higher (300–400 mg kg−1) than that used in the original studies of almorexant effects on sleep and wakefulness (20–100 mg kg−1) (Brisbare-Roch et al. 2007); and (2) our study was conducted for 1 h of room air breathing in each diurnal cycle. To make any definitive conclusion on apnoeas requires study with more hours of sleep–wake cycle recording. It is noteworthy to mention, however, that the ORX-KO mice did have a 2–3 times higher incidence of spontaneous apnoeas in both NREM and REM sleep than that in the wild-type control mice but no significant decrease in number of sighs or post-sigh apnoeas (Nakamura et al. 2007). It is possible that a certain level of orexin is necessary to maintain the excitability of respiratory neurons and prohibit the genesis of sleep apnoea during both NREM and REM sleep as well as promote reflexes that contribute to sighs.
Acknowledgments
This work was supported by a grant from the National Heart, Lung and Blood Institute (NHLBI), R37 HL 28066. The authors thank Actelion Pharmaceuticals Ltd for almorexant.
Glossary
Abbreviations
- aCSF
artificial cerebrospinal fluid
- Almxt
almorexant
- EEG
electroencephalogram
- EMG
electromyogram
- f
breathing frequency
- NREM
non-rapid eye movement
- NTS
nucleus of the solitary tract
- ORX-KO
prepro-orexin knockout
- OX1R
orexin receptor-1
- OX2R
orexin receptor-2
- REM
rapid eye movement
- RTN
retrotrapezoid nucleus
- RVLM
rostral ventrolateral medulla
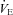
ventilation
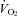
oxygen consumption
- VT
tidal volume
- Tb
body temperature
Author contributions
Both authors contributed to the conception and design of the experiments and data interpretation, to the drafting and revision of the article and to the final approval of the paper.
References
- Bartlett DJ. Origin and regulation of spontaneous deep breaths. Respir Physiol. 1973;12:230–238. doi: 10.1016/0034-5687(71)90055-7. [DOI] [PubMed] [Google Scholar]
- Brisbare-Roch C, Dingemanse J, Koberstein R, Hoever P, Aissaoui H, Flores S, et al. Promotion of sleep by targeting the orexin system in rats, dogs and humans. Nat Med. 2007;13:150–155. doi: 10.1038/nm1544. [DOI] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desarnaud F, Murillo-Rodriguez E, Lin L, Xu M, Gerashchenko D, Shiromani SN, et al. The diurnal rhythm of hypocretin in young and old F344 rats. Sleep. 2004;27:851–856. doi: 10.1093/sleep/27.5.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias MB, Li A, Nattie E. The orexin receptor 1 (OX1R) in the rostral medullary raphe contributes to the hypercapnic chemoreflex in wakefulness, during the active period of the diurnal cycle. Respir Physiol Neurobiol. 2009a;170:96–102. doi: 10.1016/j.resp.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias MB, Li A, Nattie EE. Antagonism of orexin receptor-1 in the retrotrapezoid nucleus inhibits the ventilatory response to hypercapnia predominantly in wakefulness. J Physiol. 2009b;587:2059–2067. doi: 10.1113/jphysiol.2008.168260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gestreau C, Bevengut M, Dutschmann M. The dual role of the orexin/hypocretin system in modulating wakefulness and respiratory drive. Curr Opin Pulm Med. 2008;14:512–518. doi: 10.1097/MCP.0b013e32831311d3. [DOI] [PubMed] [Google Scholar]
- Kiyashchenko LI, Mileykovskiy BY, Maidment N, Lam HA, Wu MF, John J, Peever J, Siegel JM. Release of hypocretin (orexin) during waking and sleep states. J Neurosci. 2002;22:5282–5286. doi: 10.1523/JNEUROSCI.22-13-05282.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwaki T. Orexinergic modulation of breathing across vigilance states. Respir Physiol Neurobiol. 2008;164:204–212. doi: 10.1016/j.resp.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Lee MG, Hassani OK, Jones BE. Discharge of identified orexin/hypocretin neurons across the sleep-waking cycle. J Neurosci. 2005;25:6716–6720. doi: 10.1523/JNEUROSCI.1887-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Zhou S, Nattie E. Simultaneous inhibition of caudal medullary raphe and retrotrapezoid nucleus decreases breathing and the CO2 response in conscious rats. J Physiol. 2006;577:307–318. doi: 10.1113/jphysiol.2006.114504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435:6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- Mileykovskiy BY, Kiyashchenko LI, Siegel JM. Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron. 2005;46:787–798. doi: 10.1016/j.neuron.2005.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti D, Carley DW, Radulovacki M. Adenosine analogues modulate the incidence of sleep apneas in rats. Pharmacol Biochem Behav. 1995;51:125–131. doi: 10.1016/0091-3057(94)00395-y. [DOI] [PubMed] [Google Scholar]
- Mortola JP. Breathing around the clock: an overview of the circadian pattern of respiration. Eur J Appl Physiol. 2004;91:119–129. doi: 10.1007/s00421-003-0978-0. [DOI] [PubMed] [Google Scholar]
- Nakamura A, Zhang W, Yanagisawa M, Fukuda Y, Kuwaki T. Vigilance state-dependent attenuation of hypercapnic chemoreflex and exaggerated sleep apnea in orexin knockout mice. J Appl Physiol. 2007;102:241–248. doi: 10.1152/japplphysiol.00679.2006. [DOI] [PubMed] [Google Scholar]
- Nattie E, Li A. Muscimol dialysis in the retrotrapezoid nucleus region inhibits breathing in the awake rat. J Appl Physiol. 2000;89:153–162. doi: 10.1152/jappl.2000.89.1.153. [DOI] [PubMed] [Google Scholar]
- Nicholas TE, Power JH, Barr HA. The pulmonary consequences of a deep breath. Respir Physiol. 1982;49:315–324. doi: 10.1016/0034-5687(82)90119-0. [DOI] [PubMed] [Google Scholar]
- Nixon JP, Smale L. A comparative analysis of the distribution of immunoreactive orexin A and B in the brains of nocturnal and diurnal rodents. Behav Brain Funct. 2007;3:28. doi: 10.1186/1744-9081-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patroniti N, Foti G, Cortinovis B, Maggioni E, Bigatello LM, Cereda M, Pesenti A. Sigh improves gas exchange and lung volume in patients with acute respiratory distress syndrome undergoing pressure support ventilation. Anesthesiology. 2002;96:788–794. doi: 10.1097/00000542-200204000-00004. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, Van Den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Stephenson R, Horner RL. The effect of time of day on apnoea index in the sleeping rat. Respir Physiol Neurobiol. 2006;154:351–355. doi: 10.1016/j.resp.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Stephenson R, Mohan RM, Duffin J, Jarsky TM. Circadian rhythms in the chemoreflex control of breathing. Am J Physiol Regul Integr Comp Physiol. 2000;278:R282–R286. doi: 10.1152/ajpregu.2000.278.1.R282. [DOI] [PubMed] [Google Scholar]
- Sunanaga J, Deng BS, Zhang W, Kanmura Y, Kuwaki T. CO2 activates orexin-containing neurons in mice. Respir Physiol Neurobiol. 2009;166:184–186. doi: 10.1016/j.resp.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Lin JS, Sakai K. Neuronal activity of orexin and non-orexin waking-active neurons during wake-sleep states in the mouse. Neuroscience. 2008;153:860–870. doi: 10.1016/j.neuroscience.2008.02.058. [DOI] [PubMed] [Google Scholar]
- Terada J, Nakamura A, Zhang W, Yanagisawa M, Kuriyama T, Fukuda Y, Kuwaki T. Ventilatory long-term facilitation in mice can be observed during both sleep and wake periods and depends on orexin. J Appl Physiol. 2008;104:499–507. doi: 10.1152/japplphysiol.00919.2007. [DOI] [PubMed] [Google Scholar]
- Tsujino N, Sakurai T. Orexin/hypocretin: a neuropeptide at the interface of sleep, energy homeostasis, and reward system. Pharmacol Rev. 2009;61:162–176. doi: 10.1124/pr.109.001321. [DOI] [PubMed] [Google Scholar]
- Williams RH, Jensen LT, Verkhratsky A, Fugger L, Burdakov D. Control of hypothalamic orexin neurons by acid and CO2. Proc Natl Acad Sci U S A. 2007;104:10685–10690. doi: 10.1073/pnas.0702676104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida Y, Fujiki N, Nakajima T, Ripley B, Matsumura H, Yoneda H, Mignot E, Nishino S. Fluctuation of extracellular hypocretin-1 (orexin A) levels in the rat in relation to the light-dark cycle and sleep-wake activities. Eur J Neurosci. 2001;14:1075–1081. doi: 10.1046/j.0953-816x.2001.01725.x. [DOI] [PubMed] [Google Scholar]
- Young JK, Wu M, Manaye KF, Kc P, Allard JS, Mack SO, Haxhiu MA. Orexin stimulates breathing via medullary and spinal pathways. J Appl Physiol. 2005;98:1387–1395. doi: 10.1152/japplphysiol.00914.2004. [DOI] [PubMed] [Google Scholar]


