Abstract
Sprint interval training (SIT) and traditional endurance training elicit similar physiological adaptations. From the perspective of metabolic function, superior glucose regulation is a common characteristic of endurance-trained adults. Accordingly, we have investigated the hypothesis that short-term SIT will increase insulin sensitivity in sedentary/recreationally active humans. Thirty one healthy adults were randomly assigned to one of three conditions: (1) SIT (n= 12): six sessions of repeated (4–7) 30 s bouts of very high-intensity cycle ergometer exercise over 14 days; (2) sedentary control (n= 10); (3) single-bout SIT (n= 9): one session of 4 × 30 s cycle ergometer sprints. Insulin sensitivity was determined (hyperinsulinaemic euglycaemic clamp) prior to and 72 h following each intervention. Compared with baseline, and sedentary and single-bout controls, SIT increased insulin sensitivity (glucose infusion rate: 6.3 ± 0.6 vs. 8.0 ± 0.8 mg kg−1 min−1; mean ±s.e.m.; P= 0.04). In a separate study, we investigated the effect of SIT on the thermogenic response to beta-adrenergic receptor (β-AR) stimulation, an important determinant of energy balance. Compared with baseline, and sedentary and single-bout control groups, SIT did not affect resting energy expenditure (EE: ventilated hood technique; 6274 ± 226 vs. 6079 ± 297 kJ day−1; P= 0.51) or the thermogenic response to isoproterenol (6, 12 and 24 ng (kg fat-free mass)−1 min−1: %ΔEE 11 ± 2, 14 ± 3, 23 ± 2 vs. 11 ± 1, 16 ± 2, 25 ± 3; P= 0.79). Combined data from both studies revealed no effect of SIT on fasted circulating concentrations of glucose, insulin, adiponectin, pigment epithelial-derived factor, non-esterified fatty acids or noradrenaline (all P > 0.05). Sixteen minutes of high-intensity exercise over 14 days augments insulin sensitivity but does not affect the thermogenic response to β-AR stimulation.
Introduction
Habitual endurance exercise training is associated with superior metabolic regulation in adult humans. Two important examples include increased insulin sensitivity, the principal determinant of blood glucose control (Mikines et al. 1989; Hardin et al. 1995; Takala et al. 1999; Goodpaster et al. 2001; Frosig et al. 2007; Wadley et al. 2007; Dube et al. 2008) and augmented thermogenic response to beta-adrenergic receptor (β-AR) stimulation (Bell et al. 2006a; Stob et al. 2007a,b;), a physiologically significant determinant of energy expenditure and thus a major regulator of energy balance and body mass/composition (van Baak, 2001). Despite the enormous potential health benefits of endurance exercise training, many adults choose not to participate, citing insufficient time as a perceived overriding obstacle (Stutts, 2002). Thus, an alternative program of exercise that may elicit similar favourable metabolic adaptations without requiring such an appreciable time commitment is highly attractive and worthy of investigation. Short-term sprint interval exercise training represents a very time-efficient mode of exercise training and stimulates many similar metabolic adaptations to regular endurance exercise training (Burgomaster et al. 2005, 2006, 2007, 2008; Gibala et al. 2006; Rakobowchuk et al. 2008; Babraj et al. 2009; Whyte et al. 2010). Accordingly, we have completed two separate studies to investigate the hypotheses that short-term sprint interval training will increase insulin sensitivity (Study 1) and augment β-AR metabolic function (Study 2).
Methods
Subjects – Studies 1 and 2
We studied 59 adult males and females. Selected characteristics from participating subjects are presented in Tables 1 and 2 (Studies 1 and 2, respectively). Inclusion criteria for both studies consisted of a sedentary or recreationally active lifestyle (less than 3 days per week of regular moderate-intensity exercise over the previous year), normal fasting blood glucose concentration (<5.5 mmol l−1 (<100 mg dl−1)), and normal blood pressure (<140/90 mmHg). Exclusion criteria included regular use of tobacco products or medications that might confound the interpretation of data, and contraindications to vigorous exercise (as determined by 12-lead beat-by-beat electrocardiogram and blood pressure measurements at rest and during incremental exercise). Consequently, subjects demonstrated physiological attributes typical of young sedentary/recreationally active adults. That is, on average they were normal to slightly overweight (based on body mass index and body composition), of low to average aerobic capacity (based on peak oxygen uptake (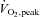 )), but otherwise healthy. The experimental protocol conformed to the standards set by the Declaration of Helsinki of 1975, as revised in 1983, and was approved by the Institutional Review Board at Colorado State University. The nature, purpose and risks of the study were explained to each subject before written informed consent was obtained.
)), but otherwise healthy. The experimental protocol conformed to the standards set by the Declaration of Helsinki of 1975, as revised in 1983, and was approved by the Institutional Review Board at Colorado State University. The nature, purpose and risks of the study were explained to each subject before written informed consent was obtained.
Table 1.
Selected subject characteristics from Study 1: sprint interval training and insulin sensitivity
| Sprint interval training | Single bout control | Sedentary control | P value | |
|---|---|---|---|---|
| Male/female | 5/7 | 2/7 | 2/8 | — |
| Age (years) | 29 ± 3 | 24 ± 1 | 23 ± 1 | 0.14 |
| Body mass (kg) | 75.8 ± 5.8 | 72.4 ± 5.1 | 80.3 ± 6.7 | 0.67 |
| Height (m) | 1.69 ± 0.03 | 1.66 ± 0.04 | 1.70 ± 0.04 | 0.75 |
| BMI (kg m−2) | 26.2 ± 1.3 | 26.0 ± 1.3 | 27.6 ± 1.7 | 0.70 |
| % Body fat | 29.6 ± 1.8 | 28.3 ± 2.5 | 31.0 ± 2.5 | 0.72 |
| Fat mass (kg) | 22.7 ± 2.8 | 20.8 ± 2.8 | 24.7 ± 3.2 | 0.67 |
| Fat-free mass (kg) | 52.8 ± 4.2 | 49.5 ± 4.3 | 53.9 ± 4.8 | 0.79 |
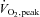 (ml kg−1 min−1) (ml kg−1 min−1) |
32.7 ± 2.1 | 38.0 ± 3.9 | 35.1 ± 3.0 | 0.45 |
| HRpeak (beats min−1) | 188 ± 3 | 188 ± 4 | 192 ± 2 | 0.59 |
| RERpeak | 1.16 ± 0.03 | 1.16 ± 0.03 | 1.16 ± 0.04 | 0.99 |
Data are mean ±s.e.m. BMI, body mass index; HRpeak, peak heart rate; 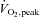 , peak oxygen uptake; RERpeak, peak respiratory exchange ratio.
, peak oxygen uptake; RERpeak, peak respiratory exchange ratio.
Table 2.
Selected subject characteristics from Study 2: sprint interval training and thermogenic response to beta-adrenergic receptor stimulation
| Sprint interval training | Single bout control | Sedentary control | P value | |
|---|---|---|---|---|
| Male/female | 3/8 | 5/3 | 6/3 | — |
| Age (years) | 25 ± 3 | 28 ± 2 | 28 ± 2 | 0.75 |
| Body mass (kg) | 66.0 ± 3.3 | 74.3 ± 2.9 | 73.9 ± 3.5 | 0.14 |
| Height (m) | 1.68 ± 0.03 | 1.74 ± 0.02 | 1.71 ± 0.03 | 0.30 |
| BMI (kg m−2) | 23.2 ± 0.8 | 24.5 ± 1.0 | 25.3 ± 1.2 | 0.32 |
| % Body fat | 27.6 + 2.2 | 21.7 ± 3.9 | 19.6 ± 2.6 | 0.13 |
| Fat mass (kg) | 18.9 ± 2.1 | 15.7 ± 3.0 | 14.2 ± 2.1 | 0.36 |
| Fat-free mass (kg) | 47.2 ± 2.9 | 56.9 ± 3.7* | 58.5 ± 3.6* | 0.05 |
| REEFFM (kJ day−1) | 6274 ± 226 | 6414 ± 256 | 6289 ± 285 | 0.51 |
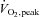 (ml kg−1 min−1) (ml kg−1 min−1) |
34.8 ± 3.1 | 42.7 ± 4.6 | 42.4 ± 5.0 | 0.25 |
| HRpeak (beats min−1) | 187 ± 3 | 185 ± 3 | 187 ± 4 | 0.87 |
| RERpeak | 1.17 ± 0.03 | 1.19 ± 0.04 | 1.20 ± 0.04 | 0.87 |
Data are mean ±s.e.m. BMI, body mass index; REEFFM, resting energy expenditure adjusted for fat-free mass; HRpeak, peak heart rate; 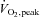 , peak oxygen uptake; RERpeak, peak respiratory exchange ratio.
, peak oxygen uptake; RERpeak, peak respiratory exchange ratio.
denotes different to sprint interval training group (P= 0.05).
Overall experimental design (Studies 1 and 2)
All hypotheses were addressed using a repeated-measures design with two control groups (single bout sprint interval training and sedentary control). Briefly, following pre-screening and habituation procedures, and initial determination of primary dependent variables, subjects completed an intervention: short-term sprint interval training, single bout sprint interval training, or sedentary control. Primary dependent variables were re-determined 72 h after completion of the assigned intervention in all subjects. Data collection occurred in the morning following a 12 h fast, 24 h abstention from vigorous exercise, 12 h abstention from caffeine, and 2 h abstention from water. Subjects were studied under quiet resting conditions in the semi-recumbent position. Measurements were performed in a dimly lit room at a comfortable temperature (∼23°C).
Short-term sprint interval training
Sprint interval training, as previously described (Burgomaster et al. 2005), entailed six sprint interval training sessions consisting of 4–7 × 30 s maximal efforts on a stationary cycle ergometer (Monark Ergomedic 874 E, Monark, Sweden) performed against a resistance equivalent to 0.075 kg (kg body mass)−1. Each 30 s bout was separated by 4 min; to facilitate recovery between training sessions, each session was separated by 1–2 days. Performance parameters during each training session (e.g. peak work rate, mean work rate, etc.) were measured/computed online via a hard-wire connection between the cycle ergometer and a personal computer, and using task-specific software (SMI Power 5.2.8, Delray Beach, FL, USA).
Single-bout sprint interval training (acute-effect control)
In order to determine whether changes in the primary dependent variables were due to an adaptation to short-term sprint interval training or an acute response to the most recent exercise bout, a subgroup of research participants were assigned to a single-bout of sprint interval training performed 72 h prior to ‘post-tests’. This single bout consisted of 4 × 30 s maximal efforts on a stationary cycle ergometer against a resistance equivalent to 0.075 kg (kg body mass)−1, and was identical to the final bout completed by the sprint interval training group. Each 30 s bout was separated by 4 min.
Sedentary control
To determine the day-to-day reliability of our measurements, the primary dependent variables were determined on two separate occasions in a subgroup of subjects separated by a minimum of 14 days; the subjects maintained their normal habitual physical activity and refrained from sprint interval training.
Study 1 – insulin sensitivity
Insulin sensitivity was determined using the hyperinsulinaemic euglycaemic clamp technique (DeFronzo et al. 1979; Rattarasarn et al. 2004); this is considered by many to be the gold-standard measurement of insulin sensitivity (Bloomgarden, 2006). Briefly, intravenous catheters were inserted into an antecubital vein for infusion of insulin and glucose, and into a contralateral dorsal hand vein, warmed via a heated blanket for arterialized-venous blood sampling. A descending dose (127–40 mU (m body surface area)−2 min−1) of regular insulin (Humulin, Eli Lilly and Co., Indianapolis, IN, USA) was administered over the first 10 min, followed by a continuous infusion (40 mU (m body surface area)−2 min−1) from 10 to 180 min. Administration of a 20% dextrose solution was initiated at 4 min (2 mg (kg body mass)−1 min−1) and adjusted as necessary to maintain blood glucose at a concentration of 5 mmol l−1 (90 mg dl−1) throughout the clamp period. Arterialized-venous blood samples (∼1 ml) were obtained every 5 min and blood glucose concentration was analysed immediately using an automated device (2300 STAT Plus Glucose Lactate Analyzer, YSI Inc., Yellow Springs, OH, USA). Insulin sensitivity was determined from the mean rate of glucose infusion during the last 30 min of the clamp and expressed as milligrams of glucose per kilogram body weight per minute.
Metabolic flexibility
Metabolic flexibility (the ability to transition between lipid and carbohydrate as a primary fuel source) is typically expressed as the increase in respiratory exchange ratio (RER: CO2 production/O2 uptake (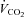 /
/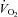 )) measured immediately prior to and during the final 5 min of a hyperinsulinaemic euglycaemic clamp. Compared with healthy lean adults the increase in RER during a clamp is attenuated in adults who are obese and/or insulin resistant (Kelley et al. 1999; Kelley & Mandarino, 2000; Storlien et al. 2004). To investigate the influence of sprint interval training on metabolic flexibility, breath-by-breath
)) measured immediately prior to and during the final 5 min of a hyperinsulinaemic euglycaemic clamp. Compared with healthy lean adults the increase in RER during a clamp is attenuated in adults who are obese and/or insulin resistant (Kelley et al. 1999; Kelley & Mandarino, 2000; Storlien et al. 2004). To investigate the influence of sprint interval training on metabolic flexibility, breath-by-breath 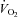 and
and 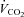 data were collected at the mouth using a respiratory mass spectrometer (Perkin Elmer MGA 1100, MA Tech Services, St Louis, MO, USA) and an ultrasonic flow sensor (ndd Medizintechnik AG, Zürich, Switzerland) and averaged over 5 min.
data were collected at the mouth using a respiratory mass spectrometer (Perkin Elmer MGA 1100, MA Tech Services, St Louis, MO, USA) and an ultrasonic flow sensor (ndd Medizintechnik AG, Zürich, Switzerland) and averaged over 5 min.
Study 2 – thermogenic response to β-AR stimulation
Resting energy expenditure was measured over 45 min. The first 15 min were considered a habituation period. 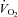 and
and 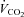 were averaged each minute for 30 min using a custom-built ventilated hood indirect calorimetry system (Nighthawk Design, Boulder, CO, USA). The system was calibrated daily with precision-mixed gases (Airgas, Denver, CO, USA). Energy expenditure was calculated using the Weir formula (Weir, 1949). In our laboratory the measurement of resting energy expenditure has a coefficient of variation of 3.3% and a test re-test r2 of 0.93 (Newsom et al. 2008).
were averaged each minute for 30 min using a custom-built ventilated hood indirect calorimetry system (Nighthawk Design, Boulder, CO, USA). The system was calibrated daily with precision-mixed gases (Airgas, Denver, CO, USA). Energy expenditure was calculated using the Weir formula (Weir, 1949). In our laboratory the measurement of resting energy expenditure has a coefficient of variation of 3.3% and a test re-test r2 of 0.93 (Newsom et al. 2008).
Immediately following determination of resting energy expenditure the thermogenic response to β-AR stimulation was quantified as the percentage increase in energy expenditure above rest during continuous and incremental intravenous (antecubital or dorsal hand) administration of the non-selective β-AR agonist isoproterenol (6, 12 and 24 ng (kg fat-free mass)−1 min−1), as previously described (Bell et al. 2006a,b; Stob et al. 2007a,b;). Each dose was administered over 30 min. Energy expenditure was calculated from the average of the final 25 min of each 30 min collection. Beat-by-beat heart rate (3-lead electrocardiogram) and blood pressure were determined throughout via an automated physiological monitor (Cardiocap 5, GE Datex-Ohmeda, Madison, WI, USA).
Procedures common to both studies
Fasting basal venous blood samples collected during Studies 1 and 2 (representing identical interventions) were combined and analysed for circulating concentrations of factors previously associated with insulin sensitivity and/or β-AR thermogenic function. Blood (∼20 ml preserved with K3 ethylenediaminetetraacetic acid, plus ∼5 ml preserved with ethylene glycol tetraacetic acid/glutathione) was collected in chilled tubes, placed immediately on ice and centrifuged within 60 min of collection to isolate plasma. Plasma samples were stored at –80°C until analysis. Plasma catecholamine concentrations were analysed in duplicate using high-performance liquid chromatography. Enzyme-linked immunosorbent assays (ELISA) were used to measure, in duplicate, plasma concentrations of insulin, adiponectin and PEDF (all Millipore Corporation, Billerica, MA, USA), and non-esterified fatty acids (NEFA; Wako Diagnostics, Richmond, VA, USA).
Fat mass and fat-free mass were measured using dual-energy x-ray absorptiometry (DXA-IQ; Lunar Radiation Corp., Madison, WI, USA; software version 4.1). 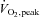 was determined with a metabolic cart (Parvo Medics, Sandy, UT, USA) during incremental cycle ergometer exercise (20–25 W min−1) to volitional fatigue, as previously described (Bell et al. 1999a,b, 2003a).
was determined with a metabolic cart (Parvo Medics, Sandy, UT, USA) during incremental cycle ergometer exercise (20–25 W min−1) to volitional fatigue, as previously described (Bell et al. 1999a,b, 2003a).
Statistical analysis
These were controlled, repeated measures studies. Accordingly, in Study 1 the influence of sprint interval training on insulin sensitivity (glucose infusion rate) was examined via two-way (sprint training vs. single bout vs. sedentary control) repeated measures (before vs. after) analysis of variance (ANOVA). Similarly, in Study 2 the influence of sprint interval training on the thermogenic response to β-AR stimulation (% increase in energy expenditure above rest) was also examined by two-way repeated measures ANOVA. Resting energy expenditure was positively associated with fat-free mass (r= 0.95, P < 0.0001) thus, differences in resting energy expenditure were examined using analysis of co-variance (ANCOVA) with fat-free mass as the co-variant. Multiple comparisons of factor means were performed using the Newman–Keuls test. The level of statistical significance was set at P < 0.05. Data are expressed as mean ±s.e.m.
Results
Study 1 – sprint interval training and insulin sensitivity
Sprint interval training
Twelve subjects were prescribed 32 sprints over 2 weeks, equaling 384 sprints in total; 380 were successfully completed (>98%). No subject completed <30 sprints. Subjects who failed to complete the prescribed number of sprints in a given session attributed their failure to nausea; no injuries were sustained as a result of sprint interval training. The first and final sprint interval sessions each comprised four sprints. Peak and mean power outputs during these sessions are presented in Supplementary Table S1. There was a session–interval interaction for peak power (P= 0.03) in that peak power was greater in the final session for the 3rd and 4th intervals. The session–interval interaction for mean power failed to attain statistical significance (P= 0.08).
Insulin sensitivity
Insulin sensitivity, as indicated by the glucose infusion rate required to maintain a blood glucose concentration of 5 mmol l−1 during administration of insulin, was increased (P= 0.04) following sprint interval training (mean change: +1.66 ± 0.61 mg kg−1 min−1) compared with the single-bout (+0.82 ± 0.93 mg kg−1 min−1) and sedentary (+0.34 ± 0.40 mg kg−1 min−1) controls (Fig. 1). Sprint interval training increased insulin sensitivity in 10 subjects, decreased it in 1 (from 7.1 to 6.5 mg kg−1 min−1) and left it unchanged in another. Due to technical reasons, we were unable to determine plasma insulin concentration at the end of the hyperinsulinaemic euglycaemic clamp in all subjects. However, in a sub-sample of subjects, the end-clamp insulin concentrations appeared to be relatively constant between trials (sprint interval training (n= 5): 457 ± 40 vs. 403 ± 25 pmol l−1; single-bout (n= 6): 389 ± 83 vs. 397 ± 41 pmol l−1 sedentary control (n= 9): 462 ± 142 vs. 401 ± 167 pmol l−1). Although not a primary focus of this investigation, there was no sprint interval training/insulin sensitivity/sex interaction (P= 0.20).
Figure 1.
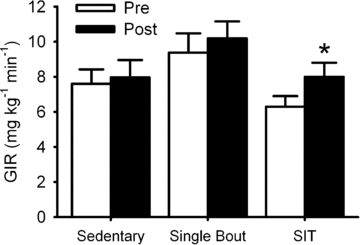
Short-term sprint interval training increases insulin sensitivity Short-term sprint interval training increases insulin sensitivity, as described by the intravenous glucose infusion rate required to maintain blood glucose concentration at 5 mmol l−1 (90 mg dl−1) during a standardized administration of insulin (hyperinsulinaemic euglycaemic clamp). Data are mean ±s.e.m. SIT, short-term sprint interval training. GIR, glucose infusion rate. * denotes difference between pre- and post-intervention (P= 0.04).
Unexpectedly, basal insulin sensitivity appeared to be greater in the single bout relative to the sprint interval training group; however, this difference did not attain statistical significance (P= 0.059). Moreover, the change in insulin sensitivity between interventions was unrelated to basal insulin sensitivity in any of the groups (sprint interval: r=−0.26, P= 0.42; single-bout: r=−0.55, P= 0.26; or sedentary: r= 0.18, P= 0.61).
Neither fasting nor end-clamp blood glucose concentrations were different between or within groups (all P > 0.64). Similarly, body mass did not differ between or within groups (P > 0.37).
RER increased in all subjects, in all groups and in all conditions during the final 5 min of the clamp compared with the pre-clamp measurement (Supplementary Data Fig. S1; P < 0.0001). However, there were no group/condition interactions (P= 0.45) indicating that metabolic flexibility was unaffected by sprint interval training relative to the control conditions.
In all subjects combined, basal insulin sensitivity was inversely associated with body mass index (r=−0.54, P= 0.002), %body fat (r=−0.40, P= 0.03) and fat mass (r=−0.53, P= 0.003), and was positively associated with 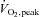 (r= 0.37, P= 0.045).
(r= 0.37, P= 0.045).
Study 2 – sprint interval training and thermogenic response to β-AR stimulation
Subjects
Unexpectedly, fat-free mass was slightly greater in the single bout and sedentary control groups compared with the sprint interval group (Table 2; P= 0.05). There were no other between group differences (all P > 0.12).
Sprint interval training
Over 98% (347/352) sprints were successfully completed. No subject completed <30 sprints. Peak and mean power outputs during these sessions are presented in Supplementary Table S2.
Basal data and responses to β-AR stimulation
Resting energy expenditure, adjusted for fat-free mass (Table 2), was unaffected by short-term sprint interval training relative to the control conditions (Supplementary data: Fig. S2; P= 0.51). Similarly, there was no impact of sprint-interval training on respiratory exchange ratio (Supplementary Table S3; P= 0.85). β-AR stimulation increased energy expenditure in all subjects (P < 0.001); however, there were no group-intervention interactions (P= 0.79), indicating that short-term sprint interval training did not affect the thermogenic response to β-AR stimulation (Fig. 2). Similarly, there were no sprint interval training/thermogenic response/sex interactions (P= 0.83). Furthermore, sprint interval training did not affect the cardiovascular (heart rate and blood pressure) responses to β-AR stimulation (Supplementary Table S3; all P > 0.43). Heart rate was unexpectedly greater in the sprint interval group at all doses of isoproterenol (P= 0.009) compared with the other groups; however, the magnitude of increase in heart rate above baseline during β-AR stimulation was not different between groups (P= 0.99).
Figure 2.
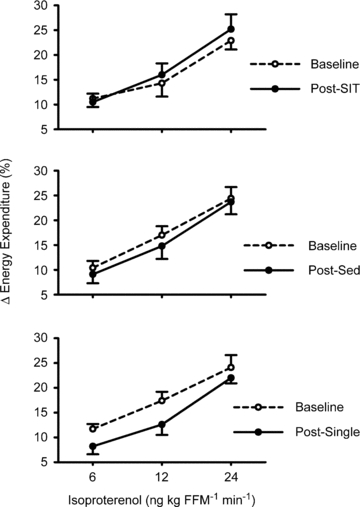
Short-term sprint interval training does not affect the thermogenic response to intravenous beta-adrenergic receptor (β-AR) stimulation β-AR stimulation increased energy expenditure in all subjects (P < 0.001); however, there were no group-intervention interactions (P= 0.79). Data are mean ±s.e.m. SIT, short-term sprint interval training. SED, sedentary control. Single, single-bout of sprint interval training. FFM, fat-free mass.
Combined blood/plasma data (Study 1 and Study 2)
Fasting plasma concentrations of glucose, insulin, noradrenaline, NEFA, adiponectin and PEDF are displayed in Table 3. Relative to the single-bout and sedentary control conditions, none of these variables were influenced by short-term sprint interval training (all P > 0.05).
Table 3.
Influence of short-term sprint interval training on selected blood parameters
| Sprint interval training |
Single bout |
Sedentary |
||||
|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | |
| Glucose (mmol l−1) | 4.44 ± 0.14 | 4.44 ± 0.11 | 4.05 ± 0.08 | 4.16 ± 0.06 | 4.22 ± 0.08 | 4.27 ± 0.06 |
| Insulin (pmol l−1) | 37.3 ± 7.9 | 32.1 ± 6.0 | 25.0 ± 4.8 | 22.8 ± 3.5 | 33.5 ± 8.1 | 33.1 ± 5.1 |
| NEFA (mmol l−1) | 0.51 ± 0.03 | 0.46 ± 0.04 | 0.50 ± 0.04 | 0.46 ± 0.04 | 0.45 ± 0.03 | 0.42 ± 0.03 |
| Noradrenaline (nmol l−1) | 0.97 ± 0.12 | 0.91 ± 0.09 | 0.86 ± 0.12 | 0.94 ± 0.12 | 0.94 ± 0.13 | 0.98 ± 0.10 |
| Adiponectin (μg ml−1) | 8.68 ± 0.97 | 8.72 ± 0.93 | 8.49 ± 0.62 | 8.52 ± 0.76 | 9.01 ± 0.47 | 8.59 ± 0.59 |
| PEDF (μg ml−1) | 4.28 ± 0.47 | 4.44 ± 0.62 | 4.32 ± 0.60 | 3.86 ± 0.48 | 4.74 ± 0.72 | 4.60 ± 0.74 |
Data are mean ±s.e.m. NEFA, non-esterified fatty acids; PEDF, pigment epithelial-derived factor.
Baseline plasma PEDF concentration was inversely associated with insulin sensitivity (r=−0.51; P= 0.02), and metabolic flexibility (r=−0.72; P= 0.001). Plasma PEDF concentration was decreased during β-AR stimulation (Fig. 3; P= 0.014).
Figure 3.
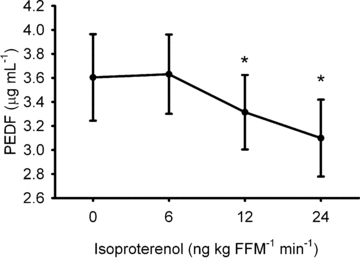
During acute, intravenous beta-adrenergic receptor stimulation circulating concentration of pigment epithelial-derived factor (PEDF) is decreased * denotes difference to baseline (0 ng kg FFM−1 min−1) value (P < 0.05). Data are mean ±s.e.m. FFM, fat-free mass.
Discussion
The novel findings of this investigation are: (1) short-term sprint interval training increased insulin sensitivity, as assessed using the gold-standard hyperinsulinaemic euglycaemic clamp technique. This improvement was not due to the acute effects of the most recent exercise bout, nor can it be attributed to changes in circulating concentrations of NEFA, adiponectin, PEDF or catecholamines; and (2) short-term sprint interval training did not affect resting energy expenditure or the thermogenic response to β-AR stimulation. We also report for the first time in humans on the inverse association between circulating PEDF concentration and a direct measure of insulin sensitivity, and on the decrease in circulating PEDF during β-AR stimulation, providing novel preliminary evidence for a role of the sympathetic nervous system in the regulation of PEDF in humans.
Based on several recent studies short-term sprint interval training appears to be a very time-efficient mode of exercise training that shares many of the same metabolic adaptations associated with traditional endurance exercise training (Burgomaster et al. 2005, 2006, 2007, 2008; Gibala et al. 2006; Rakobowchuk et al. 2008; Babraj et al. 2009; Whyte et al. 2010). In light of these observations, our hypotheses pertaining to increased insulin sensitivity and augmented β-AR thermogenic function are well supported. Increased aerobic enzyme capacities, mitochondrial biogenesis and increased glucose transporter 4 (GLUT4) expression have all been independently associated with improved insulin sensitivity (Hughes et al. 1993; Simoneau et al. 1995; Heilbronn et al. 2007) and short-term sprint interval training (Burgomaster et al. 2005, 2006, 2007, 2008; Gibala et al. 2006; Rakobowchuk et al. 2008; Babraj et al. 2009). Further, sprint interval training has been shown to decrease the magnitude and duration of the blood glucose response to oral consumption of a glucose-enriched beverage, providing indirect evidence of improved insulin sensitivity (Babraj et al. 2009).
In order to provide further insight into the influence of short-term sprint interval training on insulin sensitivity, we measured several circulating factors previously shown to be associated with insulin action. The link between plasma NEFA concentration and insulin sensitivity is clearly established (Steiner et al. 1980; Reaven et al. 1988; Byrne et al. 1994; Franks et al. 2002; Qvigstad et al. 2003; Gormsen et al. 2007; Schenk & Horowitz, 2007). In the current study, plasma NEFA was unaffected by short-term sprint interval training. This observation is supported by two previous studies demonstrating an unappreciable influence of identical programs of sprint interval training on circulating NEFA in young men (Babraj et al. 2009; Whyte et al. 2010).
Another circulating blood marker of potential significance to the current study is adiponectin; its insulin-sensitizing effects have been well described (Yamauchi et al. 2001; Wang et al. 2008). In the current study the relation between basal plasma adiponectin and insulin sensitivity did not attain statistical significance (r= 0.43, P= 0.095), nor did plasma adiponectin increase following short-term sprint interval training. The influence of exercise on plasma adiponectin concentration is unclear as some authors have reported an increase (Christiansen et al. 2010a; Okamoto et al. 2009), while others have either reported no change (Christiansen et al. 2010b; Ando et al. 2009) or a decrease (Moran et al. 2010; Van Berendoncks et al. 2010). These discrepancies may be attributed to differences in exercise intensity, duration and modality, and the pre-exercise training metabolic characteristics of the research participants.
PEDF is emerging as an important determinant of oxidative stress (Zhang et al. 2008; Banumathi et al. 2010); inflammation and angiogenesis (Jenkins et al. 2007; Zhang et al. 2008) is high in adults with diabetes (Jenkins et al. 2007; Ogata et al. 2007), and is positively associated with characteristics of the metabolic syndrome (Yamagishi et al. 2006). In the current investigation, basal plasma PEDF concentration was inversely associated with insulin sensitivity and metabolic flexibility; however, it was unaffected by short-term sprint interval training. We are unaware of any other study that has examined the influence of exercise training on PEDF in humans.
Other relevant, but not measured, circulating factors that may have contributed to the increased insulin sensitivity include, but are not limited to, tumour necrosis factor α (Hotamisligil et al. 1993, 1994; Plomgaard et al. 2005; Lambert et al. 2008), interleukin-6 (Carey et al. 2006; Lambert et al. 2008; Croft et al. 2009) and resistin (Pravenec et al. 2003; Singhal et al. 2007; Jones et al. 2009). Alternatively, it may be that the increase in insulin sensitivity was due to changes in the metabolic characteristics of skeletal muscle. This explanation is intuitively appealing as the hyperinsulinaemic euglycaemic clamp technique is thought to reflect skeletal muscle insulin sensitivity (Muniyappa et al. 2008). Potential skeletal muscle characteristics driving this response include, but are not limited to, increased GLUT 4, upregulated aerobic enzyme activity, increased mitochondrial biogenesis (all previously observed following short-term sprint interval training (Burgomaster et al. 2005, 2006, 2007, 2008; Gibala et al. 2006; Rakobowchuk et al. 2008; Babraj et al. 2009; Little et al. 2010)), and increased endogenous antioxidant defenses in response to exercise-induced production of reactive oxygen species (Marzatico et al. 1997; Ristow et al. 2009).
One potentially very important question pertinent to studies of exercise training and the resultant effects on insulin sensitivity is whether any change in insulin sensitivity is due to training, or to the most recent exercise bout. Effects of an acute exercise bout are detectable for up to 48 h (Cartee et al. 1989; Holloszy, 2005). In a recent investigation, insulin sensitivity (determined via an oral glucose tolerance test) was improved in overweight/obese men 24 h, but not 72 h, following short-term sprint interval training (Whyte et al. 2010). Differences between this and the current investigation include the technique for measurement of insulin sensitivity, subject characteristics (overweight/obese men vs. sedentary/recreationally active men and women) and the incorporation of control conditions (none vs. sedentary and single bout). We report that a single bout of sprint interval training, identical to the final bout of short-term sprint interval training, did not influence insulin sensitivity. Accordingly, we conclude that increased insulin sensitivity following short-term sprint interval training was indeed a training effect and not simply attributable to the acute influence of the final exercise bout.
Another important consideration, before prescribing short-term sprint interval training to improve insulin sensitivity, is the possibility that the insulin sensitizing effects of this type of exercise may be different, or even absent, in obese and/or insulin-resistant adults. A recent study showed that while sprint interval training may provide some health benefits to overweight/obese men, the improvement in insulin sensitivity was relatively short lasting (Whyte et al. 2010). Alternatively, exercise training has been shown to induce similar mitochondrial adaptations in adults with type II diabetes compared with healthy controls (Phielix et al. 2010).
The second hypothesis of the current investigation (Study 2) pertained to the thermogenic response to β-AR stimulation. Tonic stimulation of β-ARs by the sympathoadrenal system is an important neuro-endocrine determinant of total daily energy expenditure and hence energy balance in humans (Tappy, 1996; Bell et al. 2001, 2003b, 2004; Monroe et al. 2001;van Baak, 2001). Further, habitual endurance exercise training is highly associated with augmented sympathoadrenal regulation of energy expenditure (Bell et al. 2001, 2004, 2006a; Jones et al. 2004; Stob et al. 2007b) and several studies have demonstrated favourable β-AR-mediated metabolic functioning in overweight and obese adults following endurance exercise training (van Aggel-Leijssen et al. 2001). In light of many of the metabolic adaptations common to both endurance and sprint interval training, we hypothesized that short-term sprint interval training would augment the magnitude of increase in energy expenditure during β-AR stimulation. Contrary to our hypothesis, we report that short-term sprint interval training does not affect resting energy expenditure or the thermogenic response to β-AR stimulation. Potential explanations for these observations include the perhaps trivial energetic cost of sprint interval exercise (∼600 kJ; Gibala et al. 2006) and the lack of influence of sprint interval training on basal sympathoadrenal tone, as reflected by plasma catecholamine concentration, and indirectly by resting heart rate and blood pressure.
An additional observation in Study 2 pertained to sympathoadrenal regulation of PEDF. Contrary to animal and cell culture data showing decreased PEDF following surgical sympatholysis, and increased PEDF following isoproterenol/noradrenaline administration (Lashbrook & Steinle, 2005; Steinle et al. 2008), we have demonstrated that β-AR stimulation decreased circulating PEDF. One caveat to this conclusion is that our data were collected without an acute control condition (e.g. vehicle administration). In light of multiple and strong associations in humans between PEDF and metabolic and cardiovascular disease risk factors, (Yamagishi et al. 2006; Crowe et al. 2009; Klaus et al. 2009), identification of the physiological mechanism responsible for the regulation of PEDF should be a high priority. Our preliminary data provide impetus for future studies of a regulatory role for β-ARs.
On first inspection, our two primary dependent variables from Studies 1 and 2 seem unrelated; however, a recent study in rats modified by selective breeding for poor aerobic capacity demonstrated an association between insulin resistance and attenuated lipolytic response to β-AR stimulation (Lessard et al. 2009). The rationale for the study pertained to the role of β-ARs in the regulation of skeletal muscle lipid storage: briefly, impaired ability of β-ARs to activate hormone-sensitive lipase and subsequently mobilize diacyglycerol and triacyglycerol may contribute to disrupted insulin signaling in skeletal muscle. Rats with a low aerobic exercise capacity demonstrated low insulin sensitivity and an unappreciable response to β-AR stimulation (Lessard et al. 2009). In the current investigation, we have provided indirect, dissociative data (albeit in separate populations) as short-term sprint interval training increased insulin sensitivity but did not affect β-AR metabolic function.
There are several potential limitations in the current investigation that warrant discussion. First, unexpectedly, the research participants assigned to the single-bout control group appeared to have greater basal insulin sensitivity than the short-term sprint interval group. A potential implication of this difference, and an alternative interpretation of our data, is that insulin sensitivity was already high in the single-bout group, thus the likelihood of an additional increase was low compared with the sprint interval participants. While plausible, we do not believe this to be the case as the difference in basal insulin sensitivity between the groups was not statistically significant (P= 0.06), and, more importantly, based on data from previous studies employing a similar hyperinsulinaemic euglycaemic clamp protocol to that utilized in the current investigation, our single-bout participants were far from an upper limit of insulin sensitivity (Goodpaster et al. 2001; Bruce et al. 2003; Bergman et al. 2007; Dube et al. 2008).
Another potential limitation was the absence of a measurement of body composition following short-term sprint interval training. The primary outcome variables of the current investigation are all influenced by fat-free mass, thus it is plausible that any changes (or lack thereof) in insulin sensitivity, resting energy expenditure and the thermogenic response to β-AR stimulation may be due in part to changes in fat-free mass. Given that body mass did not change following sprint interval training in the current and in previous studies (Babraj et al. 2009) we speculate, but cannot definitively state, that body composition is not affected by short-term sprint interval training.
The inclusion of both male and female study participants may be viewed as a strength of the current investigation, although the potential for sex differences in responses to exercise training with respect to insulin sensitivity (Hickey et al. 1997; Potteiger et al. 2003) and sympathetic/β-AR function (Crampes et al. 1989; Bell et al. 2001; Scott et al. 2007) may have introduced additional variability to our data. We did not observe an influence of sex on the response to sprint interval training for either of our primary outcomes, although our experimental design was not statistically powered a priori to address this issue.
Finally, in Study 1 we were unable to report plasma insulin concentration at the end of the hyperinsulinaemic euglycaemic clamp in all subjects. This limits our ability to rule out, with absolute certainty, the possibility that differences in glucose disposal were due to differences in end-clamp insulin concentration pre- and post-interventions. However, in the subset of subjects in whom we were able perform this analysis, the end-clamp insulin concentrations appeared to be relatively constant, thus rendering this potential alternative explanation as unlikely.
The perceived exertion associated with sprint interval training is extremely high and reports of nausea and light-headedness are not uncommon. Accordingly, successful completion of sprint interval training requires a high degree of motivation. In the current investigation, all sprint interval sessions were supervised and research participants received substantial verbal encouragement. While this combination of supervision and encouragement undoubtedly contributed to the high compliance rate, it raises the questions as to the feasibility of sprint interval training as a realistic alternative to traditional endurance exercise training. Future studies need to address whether similar benefits might be obtained from lower intensity exercise, and whether it is the absolute or relative load that determines the impact of short-term sprint interval training. For instance, a recent study has demonstrated that improvements in endurance performance and mitochondrial function are obtainable from low-volume (∼1 h per week), high relative-intensity (∼100% peak power) training (Little et al. 2010).
In summary, 16 min of very high-intensity, sprint interval exercise training distributed over 2 weeks, increased insulin sensitivity but did not impact resting energy expenditure or the thermogenic response to β-AR stimulation. The increased insulin sensitivity cannot be attributed to changes in plasma concentrations of NEFA, adiponectin and/or PEDF, nor can it be attributed to changes in body mass, and is unlikely to be due to changes in body composition. This implies that the increased insulin sensitivity may be due to adaptations within skeletal muscle. We also report for the first time in humans on the relation between plasma PEDF and a direct measure of insulin sensitivity, and on the potential regulatory role of β-ARs on plasma PEDF.
Acknowledgments
This study was supported by an award from the American Diabetes Association's Amaranth Diabetes Fund.
Glossary
Abbreviations
- β-AR
beta-adrenergic receptor
- EE
energy expenditure
- FFM
fat-free mass
- GLUT4
glucose transporter 4
- NEFA
non-esterified fatty acids
- PEDF
pigment epithelial-derived factor
- RER
respiratory exchange ratio
- SIT
sprint interval training
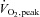
peak oxygen uptake
Author contributions
J.C.R., T.K.J., M.C.L. and C.B. were involved with the conception and design of the study. J.C.R., T.K.J., J.N.K., M.C.L., M.M.S. and C.B. were involved with analysis and interpretation of data. All authors were involved with drafting the article or revising it critically for important intellectual content and providing final approval of the version to be published. Experiments were performed in the Department of Health and Exercise Science, at Colorado State University, Fort Collins, CO, USA.
Supplemental material
Supplementary Data: FIGURE S1
Supplementary Data: FIGURE S2
SUPPLEMENTARY DATA: TABLE S1
SUPPLEMENTARY DATA: TABLE S2
SUPPLEMENTARY DATA: TABLE S3
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors
References
- Ando D, Hosaka Y, Suzuki K, Yamagata Z. Effects of exercise training on circulating high molecular weight adiponectin and adiponectin oligomer composition: a randomized controlled trial. J Atheroscler Thromb. 2009;16:733–739. doi: 10.5551/jat.2089. [DOI] [PubMed] [Google Scholar]
- Babraj JA, Vollaard NB, Keast C, Guppy FM, Cottrell G, Timmons JA. Extremely short duration high intensity interval training substantially improves insulin action in young healthy males. BMC Endocr Disord. 2009;9:3. doi: 10.1186/1472-6823-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banumathi E, Sheikpranbabu S, Haribalaganesh R, Gurunathan S. PEDF prevents reactive oxygen species generation and retinal endothelial cell damage at high glucose levels. Exp Eye Res. 2010;90:89–96. doi: 10.1016/j.exer.2009.09.014. [DOI] [PubMed] [Google Scholar]
- Bell C, Day DS, Jones PP, Christou DD, Petitt DS, Osterberg K, et al. High energy flux mediates the tonically augmented beta-adrenergic support of resting metabolic rate in habitually exercising older adults. J Clin Endocrinol Metab. 2004;89:3573–3578. doi: 10.1210/jc.2003-032146. [DOI] [PubMed] [Google Scholar]
- Bell C, Kowalchuk JM, Paterson DH, Scheuermann BW, Cunningham DA. The effects of caffeine on the kinetics of O2 uptake, CO2 production and expiratory ventilation in humans during the on-transient of moderate and heavy intensity exercise. Exp Physiol. 1999a;84:761–774. [PubMed] [Google Scholar]
- Bell C, Monahan KD, Donato AJ, Hunt BE, Seals DR, Beck KC. Use of acetylene breathing to determine cardiac output in young and older adults. Med Sci Sports Exerc. 2003a;35:58–64. doi: 10.1097/00005768-200301000-00010. [DOI] [PubMed] [Google Scholar]
- Bell C, Paterson DH, Kowalchuk JM, Cunningham DA. Oxygen uptake kinetics of older humans are slowed with age but are unaffected by hyperoxia. Exp Physiol. 1999b;84:747–759. [PubMed] [Google Scholar]
- Bell C, Petitt DS, Jones PP, Seals DR. Influence of adiposity on tonic sympathetic support of resting metabolism in healthy adults. Int J Obes Relat Metab Disord. 2003b;27:1315–1318. doi: 10.1038/sj.ijo.0802413. [DOI] [PubMed] [Google Scholar]
- Bell C, Seals DR, Monroe MB, Day DS, Shapiro LF, Johnson DG, Jones PP. Tonic sympathetic support of metabolic rate is attenuated with age, sedentary lifestyle, and female sex in healthy adults. J Clin Endocrinol Metab. 2001;86:4440–4444. doi: 10.1210/jcem.86.9.7855. [DOI] [PubMed] [Google Scholar]
- Bell C, Stob NR, Seals DR. Thermogenic responsiveness to β-adrenergic stimulation is augmented in exercising vs. sedentary adults: role of oxidative stress. J Physiol. 2006a;570:629–653. doi: 10.1113/jphysiol.2005.098756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell C, Stob NR, Seals DR. Thermogenic responsiveness to nonspecific β-adrenergic stimulation is not related to genetic variation in codon 16 of the β2-adrenergic receptor. Am J Physiol Endocrinol Metab. 2006b;290:E703–E707. doi: 10.1152/ajpendo.00411.2005. [DOI] [PubMed] [Google Scholar]
- Bergman BC, Cornier MA, Horton TJ, Bessesen DH. Effects of fasting on insulin action and glucose kinetics in lean and obese men and women. Am J Physiol Endocrinol Metab. 2007;293:E1103–E1111. doi: 10.1152/ajpendo.00613.2006. [DOI] [PubMed] [Google Scholar]
- Bloomgarden ZT. Measures of insulin sensitivity. Clin Lab Med. 2006;26:611–633. doi: 10.1016/j.cll.2006.06.007. vi. [DOI] [PubMed] [Google Scholar]
- Bruce CR, Anderson MJ, Carey AL, Newman DG, Bonen A, Kriketos AD, et al. Muscle oxidative capacity is a better predictor of insulin sensitivity than lipid status. J Clin Endocrinol Metab. 2003;88:5444–5451. doi: 10.1210/jc.2003-030791. [DOI] [PubMed] [Google Scholar]
- Burgomaster KA, Cermak NM, Phillips SM, Benton CR, Bonen A, Gibala MJ. Divergent response of metabolite transport proteins in human skeletal muscle after sprint interval training and detraining. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1970–R1976. doi: 10.1152/ajpregu.00503.2006. [DOI] [PubMed] [Google Scholar]
- Burgomaster KA, Heigenhauser GJ, Gibala MJ. Effect of short-term sprint interval training on human skeletal muscle carbohydrate metabolism during exercise and time-trial performance. J Appl Physiol. 2006;100:2041–2047. doi: 10.1152/japplphysiol.01220.2005. [DOI] [PubMed] [Google Scholar]
- Burgomaster KA, Howarth KR, Phillips SM, Rakobowchuk M, Macdonald MJ, McGee SL, Gibala MJ. Similar metabolic adaptations during exercise after low volume sprint interval and traditional endurance training in humans. J Physiol. 2008;586:151–160. doi: 10.1113/jphysiol.2007.142109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgomaster KA, Hughes SC, Heigenhauser GJ, Bradwell SN, Gibala MJ. Six sessions of sprint interval training increases muscle oxidative potential and cycle endurance capacity in humans. J Appl Physiol. 2005;98:1985–1990. doi: 10.1152/japplphysiol.01095.2004. [DOI] [PubMed] [Google Scholar]
- Byrne CD, Wareham NJ, Brown DC, Clark PM, Cox LJ, Day NE, et al. Hypertriglyceridaemia in subjects with normal and abnormal glucose tolerance: relative contributions of insulin secretion, insulin resistance and suppression of plasma non-esterified fatty acids. Diabetologia. 1994;37:889–896. doi: 10.1007/BF00400944. [DOI] [PubMed] [Google Scholar]
- Carey AL, Steinberg GR, Macaulay SL, Thomas WG, Holmes AG, et al. Interleukin-6 increases insulin-stimulated glucose disposal in humans and glucose uptake and fatty acid oxidation in vitro via AMP-activated protein kinase. Diabetes. 2006;55:2688–2697. doi: 10.2337/db05-1404. [DOI] [PubMed] [Google Scholar]
- Cartee GD, Young DA, Sleeper MD, Zierath J, Wallberg-Henriksson H, Holloszy JO. Prolonged increase in insulin-stimulated glucose transport in muscle after exercise. Am J Physiol Endocrinol Metab. 1989;256:E494–E499. doi: 10.1152/ajpendo.1989.256.4.E494. [DOI] [PubMed] [Google Scholar]
- Christiansen T, Paulsen SK, Bruun JM, Pedersen SB, Richelsen B. Exercise-training versus diet-induced weight-loss on metabolic risk factors and inflammatory markers in obese subjects. A 12-week randomized intervention-study. Am J Physiol Endocrinol Metab. 2010a;298:E824–E831. doi: 10.1152/ajpendo.00574.2009. [DOI] [PubMed] [Google Scholar]
- Christiansen T, Paulsen SK, Bruun JM, Ploug T, Pedersen SB, Richelsen B. Diet-induced weight loss and exercise alone and in combination enhance the expression of adiponectin receptors in adipose tissue and skeletal muscle, but only diet-induced weight loss enhanced circulating adiponectin. J Clin Endocrinol Metab. 2010b;95:911–919. doi: 10.1210/jc.2008-2505. [DOI] [PubMed] [Google Scholar]
- Crampes F, Riviere D, Beauville M, Marceron M, Garrigues M. Lipolytic response of adipocytes to epinephrine in sedentary and exercise-trained subjects: sex-related differences. Eur J Appl Physiol Occup Physiol. 1989;59:249–255. doi: 10.1007/BF02388324. [DOI] [PubMed] [Google Scholar]
- Croft L, Bartlett JD, MacLaren DP, Reilly T, Evans L, Mattey DL, et al. High-intensity interval training attenuates the exercise-induced increase in plasma IL-6 in response to acute exercise. Appl Physiol Nutr Metab. 2009;34:1098–1107. doi: 10.1139/H09-117. [DOI] [PubMed] [Google Scholar]
- Crowe S, Wu LE, Economou C, Turpin SM, Matzaris M, Hoehn KL, et al. Pigment epithelium-derived factor contributes to insulin resistance in obesity. Cell Metab. 2009;10:40–47. doi: 10.1016/j.cmet.2009.06.001. [DOI] [PubMed] [Google Scholar]
- DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol Endocrinol Metab. 1979;237:E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- Dube JJ, Amati F, Stefanovic-Racic M, Toledo FG, Sauers SE, Goodpaster BH. Exercise-induced alterations in intramyocellular lipids and insulin resistance: the athlete's paradox revisited. Am J Physiol Endocrinol Metab. 2008;294:E882–E888. doi: 10.1152/ajpendo.00769.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks PW, Wong MY, Luan J, Mitchell J, Hennings S, Wareham NJ. Non-esterified fatty acid levels and physical inactivity: the relative importance of low habitual energy expenditure and cardio-respiratory fitness. Br J Nutr. 2002;88:307–313. doi: 10.1079/BJN2002663. [DOI] [PubMed] [Google Scholar]
- Frosig C, Rose AJ, Treebak JT, Kiens B, Richter EA, Wojtaszewski JF. Effects of endurance exercise training on insulin signaling in human skeletal muscle: interactions at the level of phosphatidylinositol 3-kinase, Akt, and AS160. Diabetes. 2007;56:2093–2102. doi: 10.2337/db06-1698. [DOI] [PubMed] [Google Scholar]
- Gibala MJ, Little JP, van Essen M, Wilkin GP, Burgomaster KA, Safdar A, et al. Short-term sprint interval versus traditional endurance training: similar initial adaptations in human skeletal muscle and exercise performance. J Physiol. 2006;575:901–911. doi: 10.1113/jphysiol.2006.112094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodpaster BH, He J, Watkins S, Kelley DE. Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes. J Clin Endocrinol Metab. 2001;86:5755–5761. doi: 10.1210/jcem.86.12.8075. [DOI] [PubMed] [Google Scholar]
- Gormsen LC, Jessen N, Gjedsted J, Gjedde S, Norrelund H, Lund S, et al. Dose-response effects of free fatty acids on glucose and lipid metabolism during somatostatin blockade of growth hormone and insulin in humans. J Clin Endocrinol Metab. 2007;92:1834–1842. doi: 10.1210/jc.2006-2659. [DOI] [PubMed] [Google Scholar]
- Hardin DS, Azzarelli B, Edwards J, Wigglesworth J, Maianu L, Brechtel G, et al. Mechanisms of enhanced insulin sensitivity in endurance-trained athletes: effects on blood flow and differential expression of GLUT 4 in skeletal muscles. J Clin Endocrinol Metab. 1995;80:2437–2446. doi: 10.1210/jcem.80.8.7629239. [DOI] [PubMed] [Google Scholar]
- Heilbronn LK, Gan SK, Turner N, Campbell LV, Chisholm DJ. Markers of mitochondrial biogenesis and metabolism are lower in overweight and obese insulin-resistant subjects. J Clin Endocrinol Metab. 2007;92:1467–1473. doi: 10.1210/jc.2006-2210. [DOI] [PubMed] [Google Scholar]
- Hickey MS, Houmard JA, Considine RV, Tyndall GL, Midgette JB, Gavigan KE, et al. Gender-dependent effects of exercise training on serum leptin levels in humans. Am J Physiol Endocrinol Metab. 1997;272:E562–E566. doi: 10.1152/ajpendo.1997.272.4.E562. [DOI] [PubMed] [Google Scholar]
- Holloszy JO. Exercise-induced increase in muscle insulin sensitivity. J Appl Physiol. 2005;99:338–343. doi: 10.1152/japplphysiol.00123.2005. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS, Budavari A, Murray D, Spiegelman BM. Reduced tyrosine kinase activity of the insulin receptor in obesity-diabetesCentral role of tumor necrosis factor-α. J Clin Invest. 1994;94:1543–1549. doi: 10.1172/JCI117495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-α: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- Hughes VA, Fiatarone MA, Fielding RA, Kahn BB, Ferrara CM, Shepherd P, et al. Exercise increases muscle GLUT-4 levels and insulin action in subjects with impaired glucose tolerance. Am J Physiol Endocrinol Metab. 1993;264:E855–E862. doi: 10.1152/ajpendo.1993.264.6.E855. [DOI] [PubMed] [Google Scholar]
- Jenkins AJ, Zhang SX, Rowley KG, Karschimkus CS, Nelson CL, Chung JS, et al. Increased serum pigment epithelium-derived factor is associated with microvascular complications, vascular stiffness and inflammation in Type 1 diabetes. Diabet Med. 2007;24:1345–1351. doi: 10.1111/j.1464-5491.2007.02281.x. [DOI] [PubMed] [Google Scholar]
- Jones PP, Van Pelt RE, Johnson DG, Seals DR. Role of sympathetic neural activation in age- and habitual exercise-related differences in the thermic effect of food. J Clin Endocrinol Metab. 2004;89:5138–5144. doi: 10.1210/jc.2004-0101. [DOI] [PubMed] [Google Scholar]
- Jones TE, Basilio JL, Brophy PM, McCammon MR, Hickner RC. Long-term exercise training in overweight adolescents improves plasma peptide YY and resistin. Obesity (Silver Spring) 2009;17:1189–1195. doi: 10.1038/oby.2009.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley DE, Goodpaster B, Wing RR, Simoneau JA. Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity, and weight loss. Am J Physiol Endocrinol Metab. 1999;277:E1130–E1141. doi: 10.1152/ajpendo.1999.277.6.E1130. [DOI] [PubMed] [Google Scholar]
- Kelley DE, Mandarino LJ. Fuel selection in human skeletal muscle in insulin resistance: a reexamination. Diabetes. 2000;49:677–683. doi: 10.2337/diabetes.49.5.677. [DOI] [PubMed] [Google Scholar]
- Klaus JR, Hurwitz BE, Llabre MM, Skyler JS, Goldberg RB, Marks JB, et al. Central obesity and insulin resistance in the cardiometabolic syndrome: pathways to preclinical cardiovascular structure and function. J Cardiometab Syndr. 2009;4:63–71. doi: 10.1111/j.1559-4572.2008.00038.x. [DOI] [PubMed] [Google Scholar]
- Lambert CP, Wright NR, Finck BN, Villareal DT. Exercise but not diet-induced weight loss decreases skeletal muscle inflammatory gene expression in frail obese elderly persons. J Appl Physiol. 2008;105:473–478. doi: 10.1152/japplphysiol.00006.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lashbrook BL, Steinle JJ. Beta-adrenergic receptor regulation of pigment epithelial-derived factor expression in rat retina. Auton Neurosci. 2005;121:33–39. doi: 10.1016/j.autneu.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Lessard SJ, Rivas DA, Chen ZP, van Denderen BJ, Watt MJ, Koch LG, et al. Impaired skeletal muscle β-adrenergic activation and lipolysis are associated with whole-body insulin resistance in rats bred for low intrinsic exercise capacity. Endocrinology. 2009;150:4883–4891. doi: 10.1210/en.2009-0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little JP, Safdar AS, Wilkin GP, Tarnopolsky MA, Gibala MJ. A practical model of low-volume high-intensity interval training induces mitochondrial biogenesis in human skeletal muscle: potential mechanisms. J Physiol. 2010;588:1011–1022. doi: 10.1113/jphysiol.2009.181743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzatico F, Pansarasa O, Bertorelli L, Somenzini L, Della Valle G. Blood free radical antioxidant enzymes and lipid peroxides following long-distance and lactacidemic performances in highly trained aerobic and sprint athletes. J Sports Med Phys Fitness. 1997;37:235–239. [PubMed] [Google Scholar]
- Mikines KJ, Sonne B, Farrell PA, Tronier B, Galbo H. Effect of training on the dose-response relationship for insulin action in men. J Appl Physiol. 1989;66:695–703. doi: 10.1152/jappl.1989.66.2.695. [DOI] [PubMed] [Google Scholar]
- Monroe MB, Seals DR, Shapiro LF, Bell C, Johnson D, Jones PP. Direct evidence for tonic sympathetic support of resting metabolic rate in healthy adult humans. Am J Physiol Endocrinol Metab. 2001;280:E740–E744. doi: 10.1152/ajpendo.2001.280.5.E740. [DOI] [PubMed] [Google Scholar]
- Moran CN, Barwell ND, Malkova D, Cleland SJ, McPhee I, Packard CJ, et al. Effects of diabetes family history and exercise training on the expression of adiponectin and leptin and their receptors. Metabolism. 2010 doi: 10.1016/j.metabol.2009.12.026. Epub ahead of print), doi 10.1016/j.metabol.2009.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab. 2008;294:E15–E26. doi: 10.1152/ajpendo.00645.2007. [DOI] [PubMed] [Google Scholar]
- Newsom SA, Paxton RJ, Rynn GM, Bell C. Influence of ascorbic acid on the thermic effect of feeding in overweight and obese adult humans. Obesity (Silver Spring) 2008;16:1749–1754. doi: 10.1038/oby.2008.304. [DOI] [PubMed] [Google Scholar]
- Ogata N, Matsuoka M, Matsuyama K, Shima C, Tajika A, Nishiyama T, et al. Plasma concentration of pigment epithelium-derived factor in patients with diabetic retinopathy. J Clin Endocrinol Metab. 2007;92:1176–1179. doi: 10.1210/jc.2006-2249. [DOI] [PubMed] [Google Scholar]
- Okamoto T, Masuhara M, Ikuta K. Home-based resistance training improves arterial stiffness in healthy premenopausal women. Eur J Appl Physiol. 2009;107:113–117. doi: 10.1007/s00421-009-1102-x. [DOI] [PubMed] [Google Scholar]
- Phielix E, Meex R, Moonen-Kornips E, Hesselink MK, Schrauwen P. Exercise training increases mitochondrial content and ex vivo mitochondrial function similarly in patients with type 2 diabetes and in control individuals. Diabetologia. 2010;53(8):1714–1721. doi: 10.1007/s00125-010-1764-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plomgaard P, Bouzakri K, Krogh-Madsen R, Mittendorfer B, Zierath JR, Pedersen BK. Tumor necrosis factor-α induces skeletal muscle insulin resistance in healthy human subjects via inhibition of Akt substrate 160 phosphorylation. Diabetes. 2005;54:2939–2945. doi: 10.2337/diabetes.54.10.2939. [DOI] [PubMed] [Google Scholar]
- Potteiger JA, Jacobsen DJ, Donnelly JE, Hill JO. Glucose and insulin responses following 16 months of exercise training in overweight adults: the Midwest Exercise Trial. Metabolism. 2003;52:1175–1181. doi: 10.1016/s0026-0495(03)00146-x. [DOI] [PubMed] [Google Scholar]
- Pravenec M, Kazdova L, Landa V, Zidek V, Mlejnek P, Jansa P, et al. Transgenic and recombinant resistin impair skeletal muscle glucose metabolism in the spontaneously hypertensive rat. J Biol Chem. 2003;278:45209–45215. doi: 10.1074/jbc.M304869200. [DOI] [PubMed] [Google Scholar]
- Qvigstad E, Mostad IL, Bjerve KS, Grill VE. Acute lowering of circulating fatty acids improves insulin secretion in a subset of type 2 diabetes subjects. Am J Physiol Endocrinol Metab. 2003;284:E129–E137. doi: 10.1152/ajpendo.00114.2002. [DOI] [PubMed] [Google Scholar]
- Rakobowchuk M, Tanguay S, Burgomaster KA, Howarth KR, Gibala MJ, Macdonald MJ. Sprint interval and traditional endurance training induce similar improvements in peripheral arterial stiffness and flow mediated dilation in healthy humans. Am J Physiol Regul Integr Comp Physiol. 2008;295:R236–R242. doi: 10.1152/ajpregu.00069.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattarasarn C, Leelawattana R, Soonthornpun S, Setasuban W, Thamprasit A. Gender differences of regional abdominal fat distribution and their relationships with insulin sensitivity in healthy and glucose-intolerant Thais. J Clin Endocrinol Metab. 2004;89:6266–6270. doi: 10.1210/jc.2004-0209. [DOI] [PubMed] [Google Scholar]
- Reaven GM, Hollenbeck C, Jeng CY, Wu MS, Chen YD. Measurement of plasma glucose, free fatty acid, lactate, and insulin for 24 h in patients with NIDDM. Diabetes. 1988;37:1020–1024. doi: 10.2337/diab.37.8.1020. [DOI] [PubMed] [Google Scholar]
- Ristow M, Zarse K, Oberbach A, Kloting N, Birringer M, Kiehntopf M, et al. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci U S A. 2009;106:8665–8670. doi: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk S, Horowitz JF. Acute exercise increases triglyceride synthesis in skeletal muscle and prevents fatty acid-induced insulin resistance. J Clin Invest. 2007;117:1690–1698. doi: 10.1172/JCI30566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JM, Esch BT, Haykowsky MJ, Isserow S, Koehle MS, Hughes BG, et al. Sex differences in left ventricular function and β-receptor responsiveness following prolonged strenuous exercise. J Appl Physiol. 2007;102:681–687. doi: 10.1152/japplphysiol.00641.2006. [DOI] [PubMed] [Google Scholar]
- Simoneau JA, Colberg SR, Thaete FL, Kelley DE. Skeletal muscle glycolytic and oxidative enzyme capacities are determinants of insulin sensitivity and muscle composition in obese women. FASEB J. 1995;9:273–278. [PubMed] [Google Scholar]
- Singhal NS, Lazar MA, Ahima RS. Central resistin induces hepatic insulin resistance via neuropeptide Y. J Neurosci. 2007;27:12924–12932. doi: 10.1523/JNEUROSCI.2443-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner G, Morita S, Vranic M. Resistance to insulin but not to glucagon in lean human hypertriglyceridemics. Diabetes. 1980;29:899–905. doi: 10.2337/diab.29.11.899. [DOI] [PubMed] [Google Scholar]
- Steinle JJ, Cappocia FC, Jr, Jiang Y. Beta-adrenergic receptor regulation of growth factor protein levels in human choroidal endothelial cells. Growth Factors. 2008;26:325–330. doi: 10.1080/08977190802442070. [DOI] [PubMed] [Google Scholar]
- Stob NR, Bell C, van Baak MA, Seals DR. Thermic effect of food and β-adrenergic thermogenic responsiveness in habitually exercising and sedentary healthy adult humans. J Appl Physiol. 2007a;103:616–622. doi: 10.1152/japplphysiol.01434.2006. [DOI] [PubMed] [Google Scholar]
- Stob NR, Seals DR, Jorgen J, van Baak MA, Steig AJ, Lindstrom RC, et al. Increased thermogenic responsiveness to intravenous beta-adrenergic stimulation in habitually exercising humans is not related to skeletal muscle β2-adrenergic receptor density. Exp Physiol. 2007b;92:823–830. doi: 10.1113/expphysiol.2007.038174. [DOI] [PubMed] [Google Scholar]
- Storlien L, Oakes ND, Kelley DE. Metabolic flexibility. Proc Nutr Soc. 2004;63:363–368. doi: 10.1079/PNS2004349. [DOI] [PubMed] [Google Scholar]
- Stutts WC. Physical activity determinants in adults. Perceived benefits, barriers, and self efficacy. AAOHN J. 2002;50:499–507. [PubMed] [Google Scholar]
- Takala TO, Nuutila P, Knuuti J, Luotolahti M, Yki-Jarvinen H. Insulin action on heart and skeletal muscle glucose uptake in weight lifters and endurance athletes. Am J Physiol Endocrinol Metab. 1999;276:E706–E711. doi: 10.1152/ajpendo.1999.276.4.E706. [DOI] [PubMed] [Google Scholar]
- Tappy L. Thermic effect of food and sympathetic nervous system activity in humans. Reprod Nutr Dev. 1996;36:391–397. doi: 10.1051/rnd:19960405. [DOI] [PubMed] [Google Scholar]
- van Aggel-Leijssen DP, Saris WH, Homan M, van Baak MA. The effect of exercise training on β-adrenergic stimulation of fat metabolism in obese men. Int J Obes Relat Metab Disord. 2001;25:16–23. doi: 10.1038/sj.ijo.0801470. [DOI] [PubMed] [Google Scholar]
- van Baak MA. The peripheral sympathetic nervous system in human obesity. Obesity Reviews. 2001;2:3–14. doi: 10.1046/j.1467-789x.2001.00010.x. [DOI] [PubMed] [Google Scholar]
- Van Berendoncks AM, Beckers P, Hoymans VY, Possemiers N, Wuyts FL, Vrints CJ, Conraads VM. Exercise training reduces circulating adiponectin levels in patients with chronic heart failure. Clin Sci (Lond) 2010;118:281–289. doi: 10.1042/CS20090213. [DOI] [PubMed] [Google Scholar]
- Wadley GD, Konstantopoulos N, Macaulay L, Howlett KF, Garnham A, Hargreaves M, Cameron-Smith D. Increased insulin-stimulated Akt pSer473 and cytosolic SHP2 protein abundance in human skeletal muscle following acute exercise and short-term training. J Appl Physiol. 2007;102:1624–1631. doi: 10.1152/japplphysiol.00821.2006. [DOI] [PubMed] [Google Scholar]
- Wang Y, Lam KS, Yau MH, Xu A. Post-translational modifications of adiponectin: mechanisms and functional implications. Biochem J. 2008;409:623–633. doi: 10.1042/BJ20071492. [DOI] [PubMed] [Google Scholar]
- Weir J. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol. 1949;109:1–9. doi: 10.1113/jphysiol.1949.sp004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte LJ, Gill JM, Cathcart AJ. Effect of 2 weeks of sprint interval training on health-related outcomes in sedentary overweight/obese men. Metabolism. 2010 doi: 10.1016/j.metabol.2010.01.002. doi 10.1016/j.metabol.2010.01.002. [DOI] [PubMed] [Google Scholar]
- Yamagishi S, Adachi H, Abe A, Yashiro T, Enomoto M, Furuki K, et al. Elevated serum levels of pigment epithelium-derived factor in the metabolic syndrome. J Clin Endocrinol Metab. 2006;91:2447–2450. doi: 10.1210/jc.2005-2654. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- Zhang SX, Wang JJ, Dashti A, Wilson K, Zou MH, Szweda L, et al. Pigment epithelium-derived factor mitigates inflammation and oxidative stress in retinal pericytes exposed to oxidized low-density lipoprotein. J Mol Endocrinol. 2008;41:135–143. doi: 10.1677/JME-08-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


