Abstract
Members of the conserved small heat shock protein (sHSP) family, such as αB-crystallin and Hsp27, are constitutively expressed in diverse malignancies and have been linked to several hallmark features of cancer including apoptosis resistance. In contrast, the sHSP HspB2/MKBP, which shares an intergenic promoter with αB-crystallin, was discovered as a chaperone of the myotonic dystrophy protein kinase and has not been previously implicated in apoptosis regulation. Here we describe a new function for HspB2 as a novel inhibitor of apical caspase activation in the extrinsic apoptotic pathway. Specifically, we demonstrate that HspB2 is expressed in a subset of human breast cancer cell lines and that ectopic expression of HspB2 in breast cancer cells confers resistance to apoptosis induced by both TRAIL and TNF-α. We also show that HspB2 inhibits the extrinsic apoptotic pathway by suppressing apical caspases-8 and 10 activation, thereby blocking downstream apoptotic events, such as Bid cleavage and caspase-3 activation. Consistent with these in vitro effects, HspB2 attenuates the anti-tumor activity of TRAIL in an orthotopic xenograft model of breast cancer. Collectively, our results reveal a novel function of HspB2 as an anti-apoptotic protein that negatively regulates apical caspase activation in the extrinsic apoptotic pathway.
Keywords: Heat shock protein, HspB2, MKBP, Apoptosis, TRAIL, TNF-α
Introduction
Apoptosis is a genetically regulated cell suicide program that plays an essential role in development and in maintaining homeostasis, while defects in this process contribute to the pathogenesis of many diseases [1]. Indeed, genetic and epigenetic alterations that confer resistance to apoptosis are a defining “hallmark” of cancer that results in enhanced cell survival in the setting of oncogenic and/or oxidative stress [2]. As such, the apoptotic machinery represents an attractive therapeutic target to initiate cancer cell death [3].
Recently, pro-apoptotic receptor agonists such as TNF-related apoptosis-inducing ligand (TRAIL/Apo2L) have emerged as promising cancer therapies because they selectively induce apoptosis in cancer cells [4]. TRAIL is a pro-apoptotic member of the TNF-α cytokine family that bind to their death receptors, defined by the presence of a conserved intracellular death domain, to activate the extrinsic or death receptor pathway of apoptosis. TRAIL binds to the extracellular domain of its receptors, DR4/TRAIL-R1 and DR5/TRAIL-R2, to induce their trimerization on the cell surface and subsequent recruitment of the death domain-containing protein FADD to the cytoplasmic death domain of the receptor complex. FADD then recruits the apical procaspases-8 and 10 to the death receptor complex via their death effector domains, which results in the dimerization-dependent activation of apical caspases-8 and 10 by induced proximity [5–7]. Once activated, the apical caspases directly activate the effector caspase-3 or cleave the pro-apoptotic Bcl-2 family member Bid into truncated Bid (tBid). tBid translocates to the mitochondrial membrane where it activates Bax to release apoptotic mediators, such as cytochrome c, from the mitochondrial intermembrane space into the cytoplasm [8, 9]. The accumulation of cytochrome c in the cytoplasm leads to the Apaf-1-dependent assembly of the apoptosome, which activates caspase-9 and subsequently caspase-3 [10].
As with many cancer therapeutics, de novo or acquired resistance to TRAIL occurs via genetic and/or epigenetic alterations in one or more components of the TRAIL signaling pathway, a subset of which have been identified. For example, inactivating mutations or reduced surface expression of death receptors, deletion or epigenetic silencing of caspase-8 or Bax, and overexpression of FLIP, survivin and Bcl-2 have all been reported to confer resistance to TRAIL [11–18]. Moreover, we have demonstrated that the small heat shock protein (sHSP) αB-crystallin protects breast cancer cells from TRAIL-induced apoptosis by inhibiting caspase-3 activation [19]. αB-crystallin/HspB5 is a member of the sHSP family (named HspB1-B10) that plays a dual cytoprotective role by suppressing protein aggregation and inhibiting apoptosis [20]. Intriguingly, the αB-crystallin gene shares an intergenic promoter with the adjacent HspB2/MKBP gene [21, 22]. HspB2/MKBP (myotonic dystrophy protein kinase binding protein) is a sHSP that binds to the myotonic dystrophy protein kinase (DMPK), increasing its activity and conferring thermal protection [23]. HspB2 is not activated by heat induction and forms an oligomeric complex with another sHSP HspB3 (but not αB-crystallin) in muscle tissue [24]. It has also been shown to partly colocalize with mitochondria [25]. However, HspB2 has not previously been reported to regulate apoptosis.
Here we describe a new function for HspB2 as a novel negative regulator of apical caspase activation in response to activators of the extrinsic apoptotic pathway. Specifically, we demonstrate that ectopic expression of HspB2 in breast cancer cells confers resistance to apoptosis induced by both TRAIL and TNF-α. We also show that HspB2 inhibits the extrinsic apoptotic pathway by attenuating the activation of apical caspases-8 and 10 and subsequent events in the apoptotic cascade including Bid cleavage and effector caspase-3 activation. Consistent with these in vitro effects, HspB2 inhibits the anti-tumor efficacy of TRAIL in vivo in a xenograft model of breast cancer. Taken together, our findings indicate that HspB2 is a novel anti-apoptotic protein that negatively regulates apical caspase activation in the extrinsic apoptotic pathway.
Materials and methods
Cell culture and reagents
Human MDA-MB-231 cells were grown in DMEM (Invitrogen) supplemented with 10% fetal bovine serum (Invitrogen) and 1% penicillin–streptomycin–glutamine (Invitrogen). All cells were maintained at 37 °C in a 5% CO2 atmosphere. Mouse monoclonal antibodies against caspase-3, 7, MLH-1, HspB2, FADD, and cytochrome c were purchased from BD Biosciences. Polyclonal antibodies against caspase-10 and 9 were purchased from Cell Signaling and Cayman Chemical, respectively. Mouse monoclonal antibodies against α-tubulin and FLAG-M2 and a rabbit polyclonal FLAG antibody were purchased from Sigma. A mouse monoclonal antibody against caspase-8 was kindly provided by Dr. Marcus E. Peter (University of Chicago, Chicago, IL). A rat polyclonal antibody against Bid was kindly provided by Dr. Honglin Li (Northwestern University, Chicago, IL). A mouse monoclonal antibody against CoxIV was purchased from Molecular Probes. The horseradish peroxidase-conjugated goat anti mouse, rabbit, and rat antibodies were purchased from Southern Biotechnology. TNF-α was purchased from R&D Systems. A protease inhibitor cocktail was purchased from Sigma. Recombinant soluble TRAIL was made as described previously [19].
Construction of FLAG-tagged cDNAs and stable cell lines
To generate a cDNA encoding wild-type HspB2, human skeletal muscle cDNA was subjected to PCR amplification as described previously [26] with the following primers: MKBPECO (forward): 5′-GGCCGAATTCATGTCGGGC CGCTCAGTG-3′; MKBPXHO2 (reverse): 5′-GGCCCTCG AGTCAGGGCTCAACTATGGC-3′. MDA-MB-231 cells were transfected with the pcDNA3-NFLAG HspB2 or pcDNA3-NFLAG vector and G418-resistant clones stably expressing HspB2 or empty vector were created as described previously [26].
Apoptosis assays
MDA-MB-231 breast cancer cells stably expressing HspB2 or vector were plated onto 6-well plates at 0.4 × 106 cells/well. Cells were untreated or treated with 500 ng/ml TRAIL or 10 ng/ml TNF-α (R&D Systems) and 1 µg/ml cycloheximide (Sigma). Both floating and attached cells were collected with 0.05% Trypsin (Invitrogen) and fixed with glutaraldehyde (Sigma) for 15 min. Cell pellets were washed in PBS (Invitrogen) and cell nuclei were stained with 10 µg/ml bisbenzimide (Sigma) for 10 min at 37 °C. The percentage of cells with apoptotic nuclei was determined by fluorescence microscopy as previously described [26, 27]. At least 200 cells were scored in each experiment and experiments were performed three times. In other experiments, apoptosis was scored by flow cytometry using an Annexin V-PE apoptosis detection kit I (BD Biosciences) according to the manufacturer’s protocol.
Caspase substrate fluorogenic assays
MDA-MB-231 cells were plated at a concentration of 2 × 106 cells per plate and treated with TRAIL 200 ng/ml (DEVDase assays) or 500 ng/ml (IETD assays) for 0–4 h at 37 °C. Following treatment, the cells were collected using 0.05% Trypsin, and caspase-3-like and caspase-8-like activity were determined using the DEVD and IETD Fluorogenic Assay Kits (R&D Systems), respectively, according to the manufacturer’s recommendations. Fluorescence was measured by Spectra Max Gemini Microplate Spectrofluorimeter (IBB, excited at 405 nm and read at 500 nm). Fold caspase activity was determined by normalizing fluorescence to t = 0 levels.
Western blot analysis
MDA-MB-231 cells were plated at 2 × 106 cells per plate and were untreated or treated with 500 ng/ml TRAIL for 0–8 h. After 8 h, both floating and adherent cells were collected with 0.05% Trypsin and pelleted by microcentrifugation. Cells were washed with ice-cold PBS supplemented with 1 mM PMSF and pelleted by microcentrifugation. The cell pellets were resuspended in RIPA buffer (50 mM Tris–HCl pH 7.4, 0.1% SDS, 0.5% deoxycholic acid, 150 mM NaCl, and 1% NP40) and incubated on ice. At the end of 30 min, the samples were spun in a microcentrifuge at 13,200 rpm for 15 min. The supernatants were transferred to new tubes. Total protein concentrations were determined using the BCA Assay Kit (Pierce Biotechnology) following the manufacturer’s protocol. After completing protein assays, an equal volume of 2× sample buffer (100 mM Tris pH 6.8, 4% SDS, 200 mM DTT, 20% glycerol, and 0.01% Bromophenol blue) was added. Samples were then boiled for 5 min in a 100 °C heating block and subjected to SDS–PAGE analysis on 13.3% polyacrylimide gels as described. Proteins were detected by western blotting as described using the Perkin Elmer Western Lightning Chemiluminescence system.
Induction of apoptosis by truncated Bid
MDA-MB-231 cells stably expressing HspB2 or vector were transiently transfected with pEGFP-N1 (Clontech Laboratories) and either pCMV-5a empty vector or pCMV-5a-tBid (kindly provided by Honglin Li, Northwestern University). Both adherent and floating cells were collected using 0.05% Trypsin and fixed with 4% paraformaldehyde. After fixation, nuclei were stained with 10 µg/ml bisbenzimide and apoptotic nuclei were scored as described [26, 27].
Orthotopic xenograft model
Human MDA-MB-231 breast cancer cells stably expressing HspB2 or empty vector were injected into the mammary fat pads of 4- to 5-week-old female athymic nude mice (Harlan) and mammary tumors were established. Tumors were then excised, dissected into 1 mm3 pieces, and implanted subcutaneously into both mammary fat pads of 4- to 5-week-old female athymic nude mice (10 mice with MDA-MB-231 tumors stably expressing HspB2 and 10 mice with tumors expressing empty vector). Two weeks later, mice with established tumors (expressing vector or HspB2) were treated with PBS (vehicle) or TRAIL 5 mg/kg/day by intraperitoneal injection (four groups of five mice each). Tumor volume was measured weekly for a 5-week period.
Statistical analysis
Unless otherwise stated, all statistical analysis of the data was analyzed by two-way ANOVA followed by a Bonferroni post-test. Analysis was performed using GraphPad Prism software.
Results
HspB2 inhibits the extrinsic apoptotic pathway
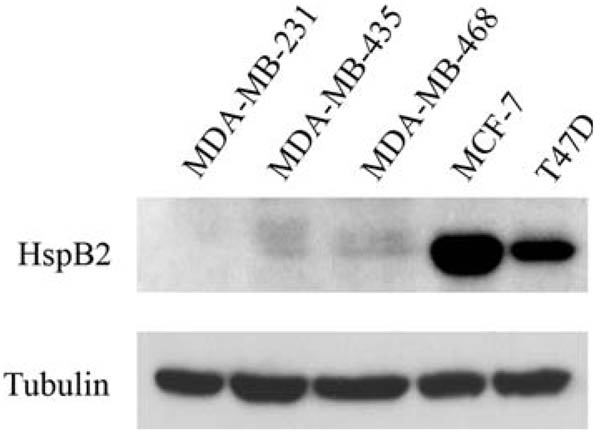
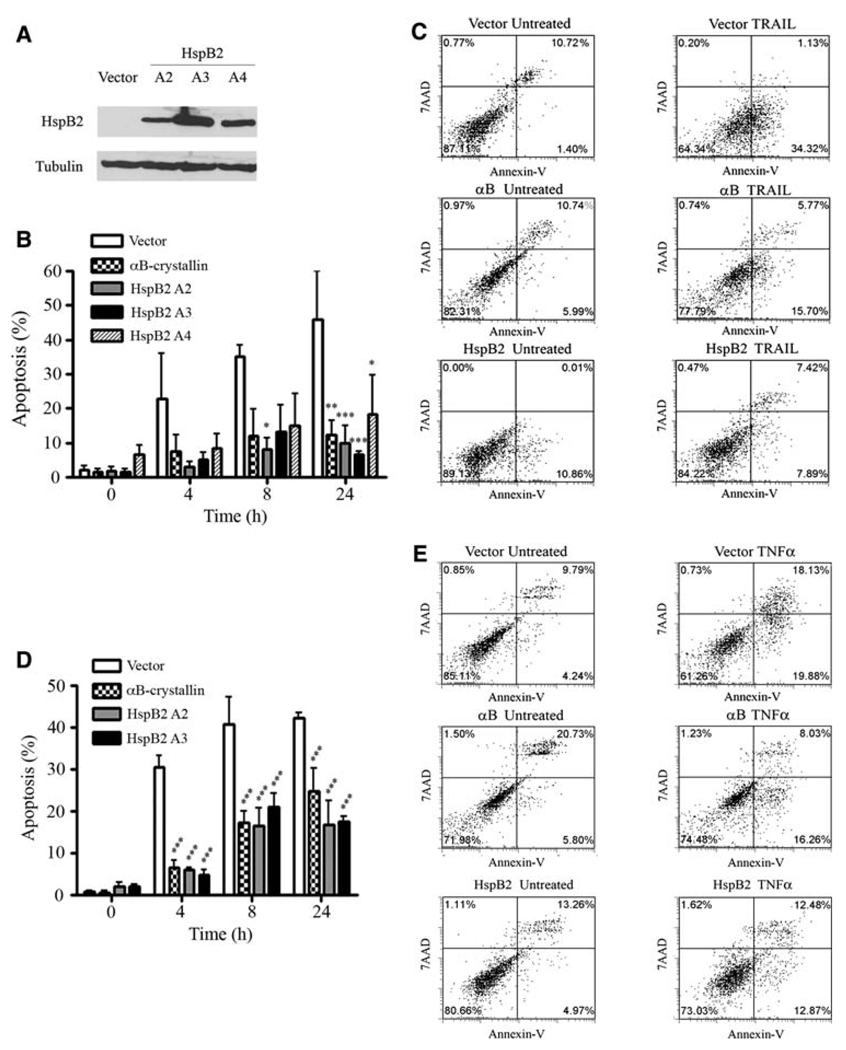
We observed that HspB2 was highly expressed in ER-positive MCF-7 and T47D breast cancer cells, expressed at low levels in ER-negative MDA-MB-435 and MDA-MB-468 breast cancer cells, and expressed at undetectable levels by immunoblotting in ER-negative MDA-MB-231 breast cancer cells (Fig. 1). Intriguingly, we have previously demonstrated that of these five breast cancer cell lines, only MDA-MB-231 cells are sensitive to TRAIL [19], indicating that HspB2 expression correlates with TRAIL-resistance. We selected MDA-MB-231 cells to overexpress HspB2 to avoid the potentially confounding effect of the endogenous protein. To determine whether HspB2 confers protection against apoptosis, we examined the sensitivity to TRAIL of MDA-MB-231 breast carcinoma cells stably expressing empty vector or a FLAG-tagged HspB2 cDNA (three independent clones, Fig. 2a). Stable expression of HspB2 in all three clones conferred robust protection against TRAIL-induced apoptosis compared to vector-expressing breast cancer cells (Fig. 2b and c). Consistent with our prior results [19], MDA-MB-231 cells stably expressing αB-crystallin were also protected against TRAIL, and the magnitude of protection was similar to that observed by overexpressing HspB2. We next examined whether HspB2 conferred protection against a second activator of the extrinsic apoptotic pathway, TNF-α. TNF-α binds to the TNF receptor-1 (TNFR-1) and assembles a receptor complex that includes TRADD and FADD to activate apical caspases and induce apoptosis [28]. Indeed, all three breast cancer clones stably expressing HspB2 were protected against TNF-α induced apoptosis to a comparable degree as breast cancer cells stably expressing αB-crystallin (Fig. 2d and e). Taken together, these results indicate that HspB2 inhibits the extrinsic apoptotic pathway initiated by TRAIL and TNF-α.
Fig. 1.
HspB2 expression in a panel of human breast cancer cell lines. Immunoblot analysis of HspB2 expression in ER-negative (MDA-MB-231, MDA-MB-435, and MDA-MB-468) and ER-positive (MCF-7 and T47D) breast cancer cell lines. Tubulin was used as a loading control
Fig. 2.
HspB2 inhibits TRAIL and TNF-α-induced apoptosis. a Immunoblot of MDA-MB-231 cells stably transfected with FLAG-tagged wild-type HspB2 cDNA or empty vector. The ectopically expressed proteins were detected using the FLAG M2 monoclonal antibody. b MDA-MB-231 breast cancer cells stably expressing HspB2 (three clones, A2, A3, and A4), αB-crystallin or empty vector were treated with 500 ng/ml TRAIL for 0–24 h, and the percentage of cells with apoptotic nuclei were scored. The data represent the mean ± SEM of three independent experiments (*P < 0.05, **P < 0.01, ***P < 0.001 versus vector control for each time point). c MDA-MB-231 stably expressing HspB2, αB-crystallin or empty vector were untreated or treated with 500 ng/ml TRAIL for 4 h. After 4 h, cells were analyzed by flow cytometry for Annexin V-PE and 7AAD fluorescence. The percentage of cells in each quadrant is indicated. d MDA-MB-231 breast cancer cells stably expressing HspB2, αB-crystallin or empty vector were treated with 10 ng/ml TNF-α and 1 µg/ml cycloheximide for 0–24 h, and the percentage of cells with apoptotic nuclei was scored. The data represent the mean ± SEM of three independent experiments (***P < 0.001 versus control at each time point). e MDA-MB-231 cells stably expressing HspB2, αB-crystallin or empty vector were untreated or treated with 10 ng/ml TNF-α and 1 µg/ml cycloheximide for 4 h, at which time cells were analyzed for Annexin V-PE and 7AAD fluorescence by flow cytometry
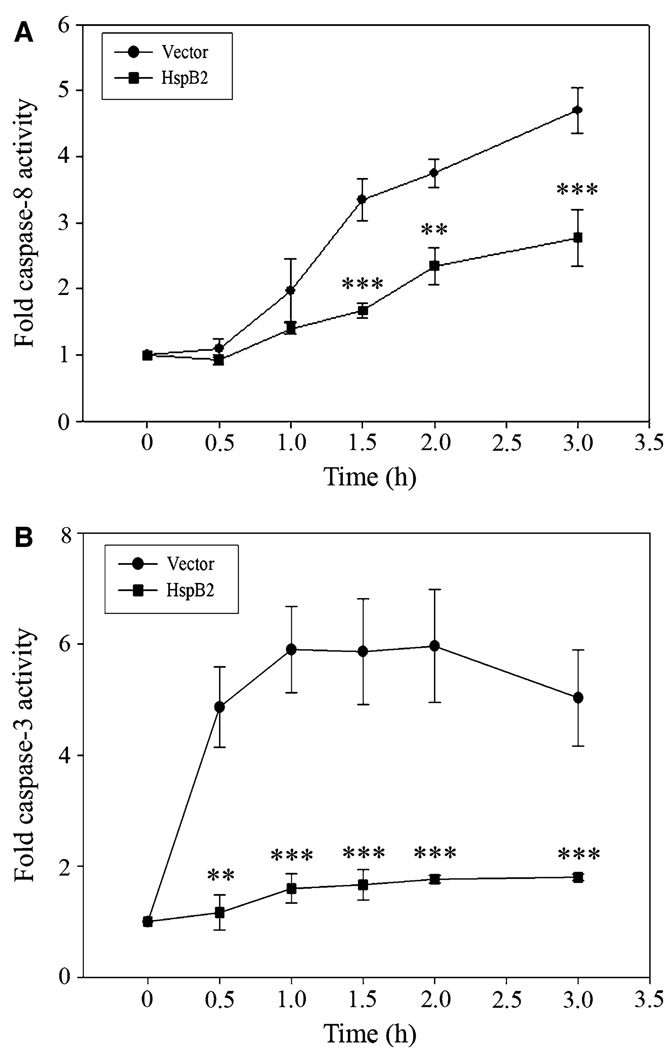
HspB2 inhibits TRAIL-induced caspase-8 and caspase-3 activity
To determine whether HspB2 inhibits TRAIL-induced activation of apical caspase-8 and effector caspase-3, we incubated breast cancer cells stably expressing HspB2 or empty vector with TRAIL for 0–4 h and measured caspase-8-like activity by incubating cell lysates with the fluorogenic caspase-8 substrate IETD-AFC. TRAIL treatment rapidly induced caspase-8-like (IETDase) activity in the vector-expressing cells, but HspB2 robustly suppressed TRAIL-induced caspase-8 activity (Fig. 3a). We next examined the effect of HspB2 on TRAIL-induced activation of the effector caspase-3 using a fluorogenic caspase-3 substrate DEVD-AFC. TRAIL treatment resulted in rapid and robust increase in caspase-3-like (DEVDase) activity in vector-expressing cells, which was dramatically attenuated by HspB2 expression (Fig. 3b). These results suggest that HspB2 inhibits the most proximal step in the extrinsic apoptotic pathway, namely, the activation of the apical caspase-8.
Fig. 3.
HspB2 inhibits TRAIL-induced caspase-8 and caspase-3 activity. a MDA-MB-231 breast cancer cells stably expressing HspB2 or empty vector were treated with 500 ng/ml TRAIL for 0–3 h. Caspase-8-like activity was determined by incubating cell lysates with the fluorogenic caspase-8 substrate IETD-AFC. Fold caspase-8 activity was determined by normalizing fluorescence to t = 0 levels. b MDA-MB-231 breast cancer cells stably expressing HspB2 or empty vector were treated with 200 ng/ml TRAIL for 0–3 h. Caspase-3-like activity was determined by incubating cell lysates with the fluorogenic caspase-3 substrate DEVD-AFC. Fold caspase-3 activity was determined by normalizing fluorescence to t = 0 levels. In both a and b, the data represent the mean ± SEM of three independent experiments (**P < 0.01, ***P < 0.001 versus vector control at each time point)
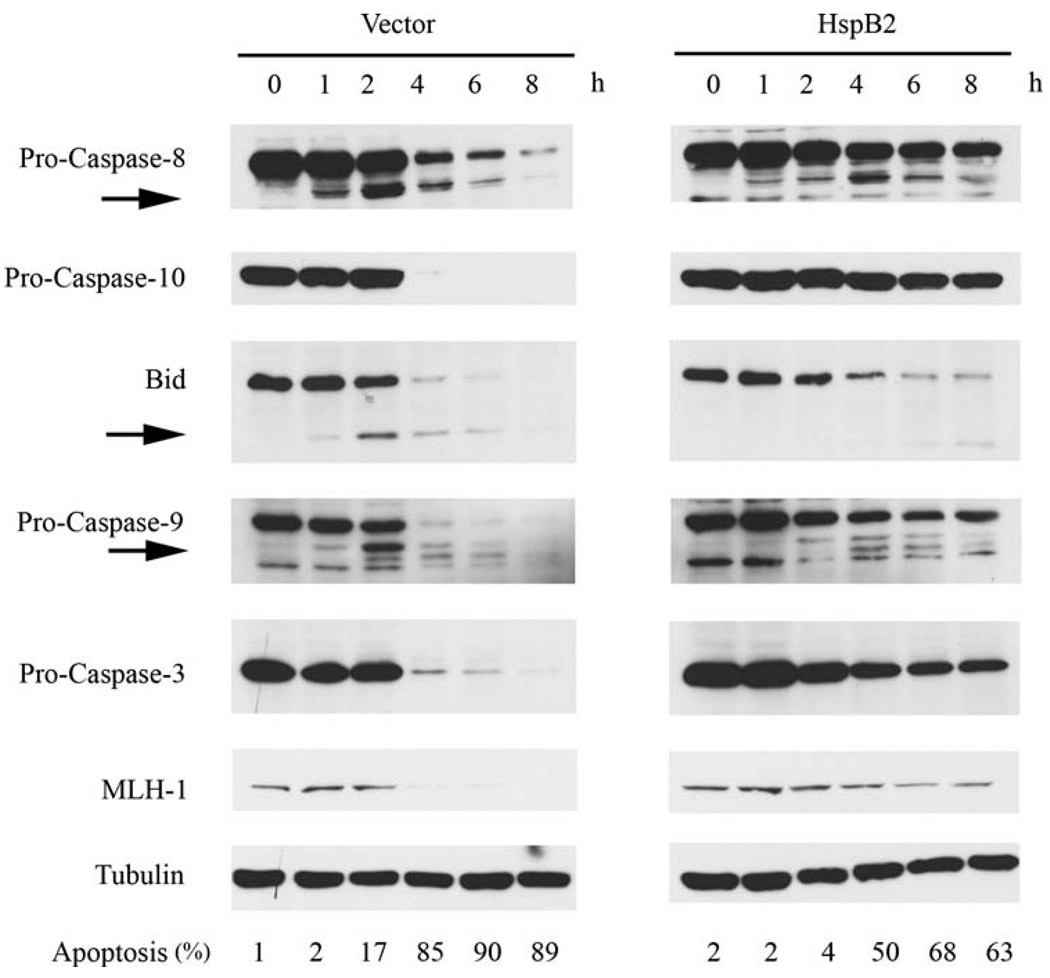
HspB2 inhibits TRAIL-induced apical caspase proteolysis and caspase substrate cleavage
To identify the specific caspases and caspase substrates that HspB2 protects against TRAIL-induced proteolytic cleavage, we treated MDA-MB-231 breast cancer cells stably expressing empty vector or HspB2 with TRAIL for 0–8 h and performed immunoblotting on whole cell lysates. HspB2 overexpression inhibited TRAIL-induced proteolytic processing of apical procaspases-8 and 10 (detected by stabilization of the proenzyme and/or diminished production of the cleavage product) and attenuated cleavage of the caspase-8 substrate Bid (Fig. 4). The inhibition of these apical events in the extrinsic apoptotic pathway by HspB2 was accompanied by a reduction in procaspase-9 and 3 cleavage and diminished proteolysis of the caspase-3 substrate MLH-1 [29]. The effects of HspB2 overexpression on the induction of apoptosis were determined in parallel with the immunoblotting experiments (indicated at the bottom of Fig. 4) and confirmed our earlier observation that HspB2 conferred protection against TRAIL. Collectively, these findings provide additional evidence that HspB2 inhibits the extrinsic apoptotic pathway by negatively regulating apical caspase activation.
Fig. 4.
HspB2 inhibits TRAIL-induced apical caspase proteolysis and caspase substrate cleavage. MDA-MB-231 breast cancer cells stably expressing HspB2 or empty vector were treated with 500 ng/ml TRAIL for 0–8 h. At each time point, whole cell lysates were analyzed by western blotting for caspase-8, caspase-10, Bid, caspase-9, caspase-3, and MLH-1 expression. Tubulin was used as a loading control. Arrows indicate cleavage fragments. The percentage of cells with apoptotic nuclei in each treatment condition was determined in parallel experiments
High levels of HspB2 inhibit apoptosis downstream of Bid activation
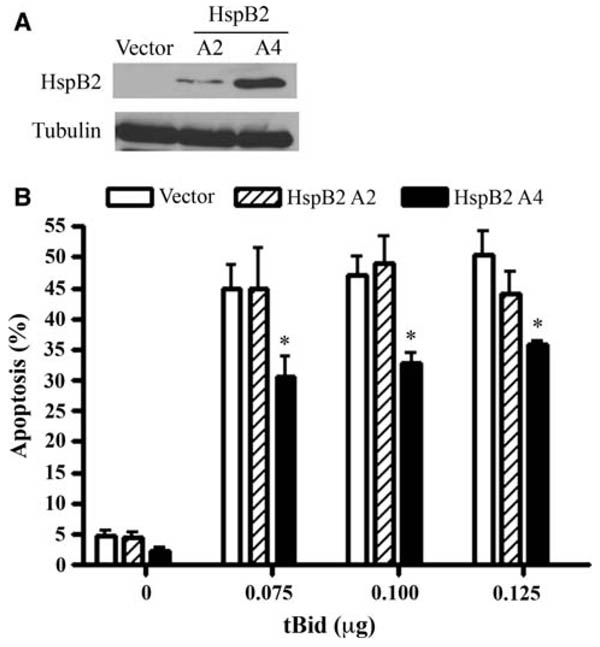
To further delineate the cytoprotective mechanisms of HspB2, we transiently co-transfected breast cancer cells stably expressing empty vector or HspB2 (low or high levels) with tBid and GFP. Transfected (GFP-positive) cells were then scored for apoptosis. We postulated that tBid would bypass the proximal apoptotic blockade conferred by HspB2 and initiate apoptosis by triggering the release of pro-apoptotic mitochondrial proteins such as cytochrome c. Although low levels of HspB2 (clone A2) did not protect against tBid-induced apoptosis, higher levels of HspB2 (clone A4) unexpectedly conferred partial protection against tBid (Fig. 5). These results suggest that higher levels of HspB2 may negatively regulate mitochondrial and/or post-mitochondrial apoptotic events such as cytochrome c release and/or apoptosome-induced activation of caspase-9, respectively.
Fig. 5.
HspB2 inhibits apoptosis downstream of Bid activation. a Whole cell lysates of MDA-MB-231 cells stably expressing HspB2 or empty were subjected to western analysis and probed for HspB2 and tubulin expression. b MDA-MB-231 breast cancer cells stably expressing HspB2 or empty vector were transiently co-transfected with pEGFPN1 and increasing amounts of or pCMV5a-tBid or pCMV5a vector. The percentage of GFP-positive cells with apoptotic nuclei was determined. The data represent the mean ±SEM of three independent experiments (*P < 0.05 versus empty vector at each time point)
HspB2 inhibits the anti-tumor effects of TRAIL in vivo
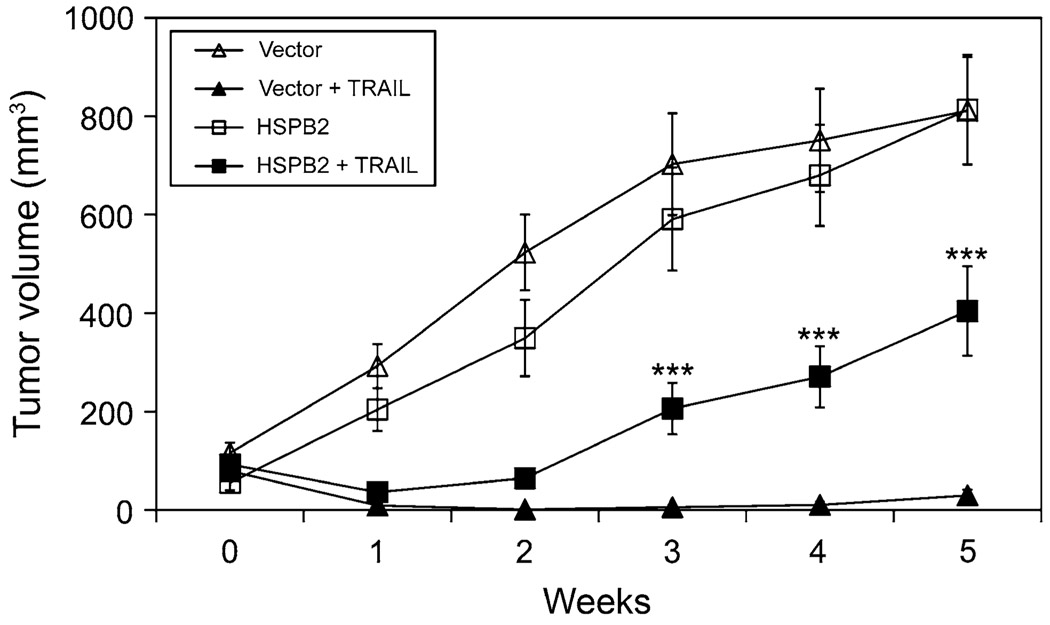
To determine whether HspB2 inhibited the anti-tumor effects of TRAIL in vivo, we treated female athymic nude mice with established orthotopic MDA-MB-231 tumors stably expressing vector or HspB2 with vehicle or 5 mg/kg/day of TRAIL for 5 weeks. Mammary tumors stably expressing vector or HspB2 that were treated with vehicle grew throughout the 5-week treatment period (Fig. 6). However, mammary tumors stably expressing vector were very sensitive to TRAIL and many tumors regressed. In contrast, mammary tumors stably expressing HspB2 were partially resistant to TRAIL and grew rapidly from weeks 2–5 of the study. These results indicate that HspB2 confers protection against the anti-tumor effects of TRAIL in vivo.
Fig. 6.
HspB2 inhibits the anti-tumor effects of TRAIL in vivo. Female athymic nude mice with established orthotopic MDA-MB-231 mammary tumors expressing HspB2 or vector were treated with PBS (vehicle) or 5 mg/kg/day TRAIL by intraperitoneal injection (four groups of five mice each) for 5 weeks. Tumor volume was measured weekly. The data represent the mean ± SEM of tumors from five mice/group. (***P < 0.001 versus mammary tumors stably expressing vector treated with TRAIL)
Discussion
HspB2 is a member of the sHSP family that functions as a chaperone for the myotonic dystrophy protein kinase (DMPK), thereby enhancing its kinase activity [23]. We have reported a new function for HspB2 as a negative regulator of the extrinsic apoptotic pathway. Specifically, we have demonstrated that breast cancer cells stably expressing HspB2 are resistant to apoptosis induced by TRAIL and TNF-α. Moreover, we have shown that HspB2 inhibits TRAIL-induced proteolytic activation of initiator caspases-8 and 10 and effector caspase-3. These results strongly suggest that HspB2 inhibits the most proximal step in the extrinsic apoptotic, namely, activation of the initiator caspases-8 and 10. Because apical caspase activation is dependent on recruitment of both the adaptor protein FADD and procaspases-8 and 10 to ligand-bound death receptor complexes [6,7], it is tempting to speculate that HspB2 may disrupt the recruitment of one or more of these proteins to the death receptor complex. Intriguingly, at higher levels of expression, HspB2 also inhibits tBid-induced apoptosis, suggesting that HspB2 may weakly inhibit mitochondrial or post-mitochondrial apoptotic events. This latter function is consistent with the reported mitochondrial localization of HspB2 [25]. However, modest levels of HspB2 sufficient to inhibit TRAIL-induced apoptosis do not inhibit tBid-induced apoptosis, indicating that suppression of these mitochondrial/post-mitochondrial events is not required for HspB2’s anti-apoptotic function. Collectively, our results identify HspB2 as a novel anti-apoptotic protein that inhibits apical caspase activation in the extrinsic pathway.
Our lab has previously shown that the structurally related sHSP αB-crystallin confers protection against a broad range of apoptosis-inducing agents, including TRAIL, TNF-α, chemotherapy and growth factor deprivation [19, 26, 30]. αB-crystallin suppresses apoptosis by specifically inhibiting the activation of caspase-3 by binding to the pro-enzyme and the p24 processing intermediate of caspase-3 and preventing its maturation to the proteolytically active enzyme [26, 31]. Moreover, αB-crystallin has also been reported to inhibit apoptosis by raising intracellular glutathione levels, providing a buffer against reactive oxygen species, and by sequestering pro-apoptotic molecules such as p53, Bax and Bcl-xs in the cytoplasm [32–34]. Interestingly, the sHSP Hsp27 has also been shown to negatively regulate apoptosis by inhibiting caspase-3 activation [35]. Hsp27 has also been reported to bind to cytochrome c and suppress caspase-9 activation [36]. Together with the results presented here indicating that HspB2 inhibits activation of apical caspases-8 and 10, these findings suggest that sHSPs may work in concert to inhibit apoptosis by acting on partly unique and partly overlapping molecular targets. As such, this network of anti-apoptotic sHSPs represents an intricate and multilayered cytoprotective function in normal cells, while deregulated expression of these sHSPs in cancer results in profound apoptosis resistance in tumor cells.
Consistent with their anti-apoptotic function, sHSPs have been recently implicated in the pathogenesis of cancer. Indeed, αB-crystallin is expressed in a variety of highly aggressive solid tumors, including breast cancer, malignant gliomas (glioblastoma multiforme), renal cell carcinomas, and non-small cell lung cancers [31, 37–40]. In breast cancer, αB-crystallin is predominantly expressed in triple (ER/PR/HER2) negative breast tumors with a basal-like gene expression profile and is associated with poor clinical outcomes, including shorter disease-specific survival and chemotherapy resistance [39, 41, 42]. Moreover, Hsp27 has been implicated in the pathogenesis of androgen-independent prostate cancer [43, 44]. In contrast, the potential role of HspB2 in cancer has not been explored. Because HspB2 and αB-crystallin share an intergenic promoter [21, 22], it will be interesting to determine whether HspB2 and αB-crystallin are co-expressed in triple negative breast tumors and other neoplasms. Given the robust anti-apoptotic activity of HspB2, it is tempting to speculate that this sHSP may play a pathogenic role in diverse malignancies, an hypothesis we are exploring.
Acknowledgments
We are indebted to Drs. Honglin Li (Northwestern University) and Marcus Peter (University of Chicago) for providing reagents. These studies were supported by Department of Defense CDMRP grant DAMD17-03-1-0426 (VLC), NIH grants R01CA097198 (VLC) and T32DK007169 (SO), and by the Breast Cancer Research Foundation (VLC).
References
- 1.Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. doi:10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2002;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. doi:10.1016/S0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 3.Johnstone RW, Ruefli AA, Lowe SW. Apoptosis: a link between cancer genetics and chemotherapy. Cell. 2002;108:153–164. doi: 10.1016/s0092-8674(02)00625-6. doi:10.1016/S0092-8674(02)00625-6. [DOI] [PubMed] [Google Scholar]
- 4.Ashkenazi A, Herbst RS. To kill a tumor cell: the potential of proapoptotic receptor agonists. J Clin Invest. 2008;118:1979–1990. doi: 10.1172/JCI34359. doi:10.1172/JCI34359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boatright KM, Renatus M, Scott FL, et al. A unified model for apical caspase activation. Molecular Cell. 2003;11:529–541. doi: 10.1016/s1097-2765(03)00051-0. doi:10.1016/S1097-2765(03)00051-0. [DOI] [PubMed] [Google Scholar]
- 6.Kischkel FC, Lawrence DA, Tinel A, et al. Death receptor recruitment of endogenous caspase-10 and apoptosis initiation in the absence of caspase-8. J Biol Chem. 2001;276:46639–46646. doi: 10.1074/jbc.M105102200. doi:10.1074/jbc.M105102200. [DOI] [PubMed] [Google Scholar]
- 7.Sprick MR, Weigand MA, Rieser E, et al. FADD/MORT1 and caspase-8 are recruited to TRAIL receptors 1 and 2 and are essential for apoptosis mediated by TRAIL receptor 2. Immunity. 2000;12:599–609. doi: 10.1016/s1074-7613(00)80211-3. doi:10.1016/S1074-7613(00)80211-3. [DOI] [PubMed] [Google Scholar]
- 8.Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. doi:10.1016/S0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- 9.Scaffidi C, Fulda S, Srinivasan A, et al. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998;17:1675–1687. doi: 10.1093/emboj/17.6.1675. doi:10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Srinivasula SM, Ahmad M, Fernandes-Alnemri T, Alnemri ES. Autoactivation of procaspase-9 by Apaf-1-mediated oligomerization. Mol Cell. 1998;1:949–957. doi: 10.1016/s1097-2765(00)80095-7. doi:10.1016/S1097-2765(00)80095-7. [DOI] [PubMed] [Google Scholar]
- 11.Burns TF, El-Deiry WS. Identification of inhibitors of TRAIL-induced death (ITIDs) in the TRAIL-sensitive colon carcinoma cell line SW480 using a genetic approach. J Biol Chem. 2001;276:37879–37886. doi: 10.1074/jbc.M103516200. doi:10.1074/jbc.M103516200. [DOI] [PubMed] [Google Scholar]
- 12.Fisher MJ, Virmani AK, Wu L, et al. Nucleotide substitution in the ectodomain of trail receptor DR4 is associated with lung cancer and head and neck cancer. Clin Cancer Res. 2001;7:1688–1697. [PubMed] [Google Scholar]
- 13.Fulda S, Meyer E, Debatin KM. Inhibition of TRAIL-induced apoptosis by Bcl-2 overexpression. Oncogene. 2002;21:2283–2294. doi: 10.1038/sj.onc.1205258. doi:10.1038/sj/onc/1205258. [DOI] [PubMed] [Google Scholar]
- 14.LeBlanc H, Lawrence D, Varfolomeev E, et al. Tumor-cell resistance to death receptor-induced apoptosis through mutational inactivation of the proapoptotic Bcl-2 homolog Bax. Nat Med. 2002;8:274–281. doi: 10.1038/nm0302-274. doi:10.1038/nm0302-274. [DOI] [PubMed] [Google Scholar]
- 15.Lu M, Strohecker A, Chen F, et al. Aspirin sensitizes cancer cells to TRAIL-induced apoptosis by reducing survivin levels. Clin Cancer Res. 2008;14:3168–3176. doi: 10.1158/1078-0432.CCR-07-4362. doi:10.1158/1078-0432.CCR-07-4362. [DOI] [PubMed] [Google Scholar]
- 16.Pai SI, Wu GS, Ozoren N, et al. Rare loss-of-function mutation of a death receptor gene in head and neck cancer. Cancer Res. 1998;58:3513–3518. [PubMed] [Google Scholar]
- 17.Teitz T, Wei T, Valentine MB, et al. Caspase 8 is deleted or silenced preferentially in childhood neuroblastomas with amplification of MYCN. Nat Med. 2000;6:529–535. doi: 10.1038/75007. doi:10.1038/75007. [DOI] [PubMed] [Google Scholar]
- 18.Yoshida T, Zhang Y, Rivera Rosado LA, Zhang B. Repeated treatment with subtoxic doses of TRAIL induces resistance to apoptosis through its death receptors in MDA-MB-231 breast cancer cells. Mol Cancer Res. 2009;7:1835–1844. doi: 10.1158/1541-7786.MCR-09-0244. doi:10.1158/1541-7786.MCR-09-0244. [DOI] [PubMed] [Google Scholar]
- 19.Kamradt MC, Lu M, Werner ME, et al. The small heat shock protein αB-crystallin is a novel inhibitor of TRAIL-induced apoptosis that suppresses the activation of caspase-3. J Biol Chem. 2005;280:11059–11066. doi: 10.1074/jbc.M413382200. doi:10.1074/jbc.M413382200. [DOI] [PubMed] [Google Scholar]
- 20.Parcellier A, Schmitt E, Brunet M, et al. Small heat shock proteins HSP27 and alphaB-crystallin: cytoprotective and oncogenic functions. Antioxid Redox Signal. 2005;7:404–413. doi: 10.1089/ars.2005.7.404. doi:10.1089/ars.2005.7.404. [DOI] [PubMed] [Google Scholar]
- 21.Iwaki A, Nagano T, Nakagawa M, Iwaki T, Fukumaki Y. Identification and characterization of the gene encoding a new member of the α-crystallin/small hsp family, closely linked to the αB-crystallin gene in a head-to-head manner. Genomics. 1997;45:386–394. doi: 10.1006/geno.1997.4956. doi:10.1006/geno.1997.4956. [DOI] [PubMed] [Google Scholar]
- 22.Swamynathan SK, Piatigorsky J. Orientation-dependent influence of an intergenic enhancer on the promoter activity of the divergently transcribed mouse Shsp/alpha B-crystallin and Mkbp/HspB2 genes. J Biol Chem. 2002;277:49700–49706. doi: 10.1074/jbc.M209700200. doi:10.1074/jbc.M209700200. [DOI] [PubMed] [Google Scholar]
- 23.Suzuki A, Sugiyama Y, Hayashi Y, et al. MKBP, a novel member of the small heat shock protein family, binds and activates the myotonic dystrophy protein kinase. J Cell Biol. 1998;140:1113–1124. doi: 10.1083/jcb.140.5.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sugiyama Y, Suzuki A, Kishikawa M, et al. Muscle develops a specific form of small heat shock protein complex composed of MKBP/HSPB2 and HSPB3 during myogenic differentiation. J Biol Chem. 2000;275:1095–1104. doi: 10.1074/jbc.275.2.1095. doi:10.1074/jbc.275.2.1095. [DOI] [PubMed] [Google Scholar]
- 25.Nakagawa M, Tsujimoto N, Nakagawa H, et al. Association of HSPB2, a member of the small heat shock protein family, with mitochondria. Exp Cell Res. 2001;271:161–168. doi: 10.1006/excr.2001.5362. doi:10.1006/excr.2001.5362. [DOI] [PubMed] [Google Scholar]
- 26.Kamradt MC, Chen F, Cryns VL. The small heat shock protein αB-crystallin negatively regulates cytochrome c- and caspase-8-dependent activation of caspase-3 by inhibiting its autoproteolytic maturation. J Biol Chem. 2001;276:16059–16063. doi: 10.1074/jbc.C100107200. doi:10.1074/jbc.C100107200. [DOI] [PubMed] [Google Scholar]
- 27.Byun Y, Chen F, Chang R, et al. Caspase cleavage of vimentin disrupts intermediate filaments and promotes apoptosis. Cell Death Differ. 2001;8:443–450. doi: 10.1038/sj.cdd.4400840. [DOI] [PubMed] [Google Scholar]
- 28.Hsu H, Shu H-B, Pan M-P, Goeddel DV. TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF receptor-1 signal transduction pathways. Cell. 1996;84:299–308. doi: 10.1016/s0092-8674(00)80984-8. doi:10.1016/S0092-8674(00)80984-8. [DOI] [PubMed] [Google Scholar]
- 29.Chen F, Arseven OK, Cryns VL. Proteolysis of the mismatch repair protein MLH1 by caspase-3 promotes DNA damage-induced apoptosis. J Biol Chem. 2004;279:27542–27548. doi: 10.1074/jbc.M400971200. doi:10.1074/jbc.M400971200. [DOI] [PubMed] [Google Scholar]
- 30.Kamradt MC, Chen F, Sam S, Cryns VL. The small heat shock protein αB-crystallin negatively regulates apoptosis during myogenic differentiation by inhibiting caspase-3 activation. J Biol Chem. 2002;277:38731–38736. doi: 10.1074/jbc.M201770200. doi:10.1074/jbc.M201770200. [DOI] [PubMed] [Google Scholar]
- 31.Stegh AH, Kesari S, Mahoney JE, et al. Bcl2L12-mediated inhibition of effector caspase-3 and caspase-7 via distinct mechanisms in glioblastoma. Proc Natl Acad Sci USA. 2008;105:10703–10708. doi: 10.1073/pnas.0712034105. doi:10.1073/pnas.0712034105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mao YW, Liu JP, Xiang H, Li DW. Human αA- and αB-crystallins bind to Bax and Bcl-X(S) to sequester their translocation during staurosporine-induced apoptosis. Cell Death Differ. 2004;11:512–526. doi: 10.1038/sj.cdd.4401384. doi:10.1038/sj.cdd.4401384. [DOI] [PubMed] [Google Scholar]
- 33.Mehlen P, Kretz-Remy C, Preville X, Arrigo AP. Human hsp27, Drosophila hsp27 and human αB-crystallin expression-mediated increase in glutathione is essential for the protective activity of these proteins against TNFα-induced cell death. EMBO J. 1996;1996(15):2695–2706. [PMC free article] [PubMed] [Google Scholar]
- 34.Liu S, Li J, Tao Y, Xiao X. Small heat shock protein αB-crystallin binds to p53 to sequester its translocation to mitochondria during hydrogen peroxide-induced apoptosis. Biochem Biophys Res Commun. 2007;354:109–114. doi: 10.1016/j.bbrc.2006.12.152. doi:10.1016/j.bbrc.2006.12.152. [DOI] [PubMed] [Google Scholar]
- 35.Pandey P, Farber R, Nakazawa A, et al. Hsp27 functions as a negative regulator of cytochrome c-dependent activation of procaspase-3. Oncogene. 2000;19:1975–1981. doi: 10.1038/sj.onc.1203531. [DOI] [PubMed] [Google Scholar]
- 36.Bruey JM, Ducasse C, Bonniaud P, et al. Hsp27 negatively regulates cell death by interacting with cytochrome c. Nat Cell Biol. 2000;2:645–652. doi: 10.1038/35023595. doi:10.1038/35023595. [DOI] [PubMed] [Google Scholar]
- 37.Chelouche-Lev D, Kluger HM, Berger AJ, Rimm DL, Price JE. αB-crystallin as a marker of lymph node involvement in breast carcinoma. Cancer. 2004;100:2543–2548. doi: 10.1002/cncr.20304. doi:10.1002/cncr.20304. [DOI] [PubMed] [Google Scholar]
- 38.Cherneva R, Petrov D, Georgiev O, et al. Expression profile of the small heat-shock protein αB-crystallin in operated-on non-small-cell lung cancer patients: clinical implication. Eur J Cardiothorac Surg. 2010;37:44–50. doi: 10.1016/j.ejcts.2009.06.038. doi:10.1016/j.ejcts.2009.06.038. [DOI] [PubMed] [Google Scholar]
- 39.Moyano JV, Evans JR, Chen F, et al. αB-Crystallin is a novel oncoprotein that predicts poor clinical outcome in breast cancer. J Clin Invest. 2006;116:261–270. doi: 10.1172/JCI25888. doi:10.1172/JCI25888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pinder SE, Balsitis M, Ellis IO. The expression of alpha B-crystallin in epithelial tumours: a useful tumour marker? J Pathol. 1994;174:209–215. doi: 10.1002/path.1711740310. [DOI] [PubMed] [Google Scholar]
- 41.Ivanov O, Chen F, Wiley EL, et al. αB-crystallin is a novel predictor of resistance to neoadjuvant chemotherapy in breast cancer. Breast Cancer Res Treat. 2008;111:411–417. doi: 10.1007/s10549-007-9796-0. doi:10.1007/s10549-007-9796-0. [DOI] [PubMed] [Google Scholar]
- 42.Sitterding SM, Wiseman WR, Schiller CL, et al. αB-crystallin: a novel marker of invasive basal-like and metaplastic breast carcinomas. Ann Diagn Pathol. 2008;12:33–40. doi: 10.1016/j.anndiagpath.2007.02.004. doi:10.1016/j.anndiagpath.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 43.Bubendorf L, Kolmer M, Kononen J, et al. Hormone therapy failure in human prostate cancer: analysis by complementary DNA and tissue microarrays. J Natl Cancer Institute. 1999;91:1758–1764. doi: 10.1093/jnci/91.20.1758. doi:10.1093/jnci/91.20.1758. [DOI] [PubMed] [Google Scholar]
- 44.Cornford PA, Dodson AR, Parsons KF, et al. Heat shock protein expression independently predicts clinical outcome in prostate cancer. Cancer Res. 2000;60:7099–7105. [PubMed] [Google Scholar]