Abstract
Objective
To assess the effectiveness of cotrimoxazole preventive therapy (CPT) among individuals with CD4 >200 cells/mm3 and varying WHO clinical stages in reducing mortality during combination antiretroviral therapy (cART).
Design
Cohort study.
Methods
Using proportional hazards modeling, we compared mortality during the first 12 months after cART initiation among patients receiving CPT with patients not receiving CPT. We adjusted for clinic level confounding throughout.
Results
We included 14,097 patients starting cART between January 2003 and January 2008, 62% of whom were men, the median CD4 count was 132 cells/mm3, and 1,289 died (11%). The baseline median CD4 count was lower (118 vs 153 cells/mm3) among the 7,508 who received CPT compared to the 6,589 who did not. In adjusted multivariate modeling, stratifying for baseline CD4 count and WHO stage, CPT reduced mortality overall (hazard ratio 0.64, p<0.001) and for all individuals with CD4 count <200 cells/mm3 or WHO clinical stage 3 or 4 conditions but did not reduce mortality for patients with both CD4 count >200 cells/mm3 and WHO clinical stage 1 or 2.
Conclusions
We demonstrated a 36% reduction in mortality extending to patients associated with CPT when used with cART that extended to patients with CD4 >350 cells/mm3 in a setting with minimal malaria and high rates of co-trimoxazole resistant bacteria. This provides important additional data toward efforts to increase CPT provision among all cART initiators in resource limited settings.
Keywords: HIV, antiretroviral therapy, mortality, co-trimoxazole, Africa
Background
Primary prophylaxis with co-trimoxazole (trimethoprim-sulfamethoxazole) is recommended for persons living with HIV to reduce morbidity and mortality. In industrialized countries co-trimoxazole preventive therapy (CPT) is principally used to prevent Pneumocystis jirovecii infection among individuals with CD4 counts <200 cells/mm3 and toxoplasmosis among individuals with CD4 counts <100 cells/mm3 [1]. In resource-limited settings, the main benefit from CPT arises less from prevention of the classic opportunistic infections, Pneumocystis pneumonia and toxoplasmosis, but primarily from prevention of severe disease from malaria, bacterial pneumonia and sepsis, and diarrhea [2, 3]. Thus the benefit among adults in settings where these diseases are common extends above a CD4 count threshold of 200 cells/mm3 [2–4]. The World Health Organization (WHO) guidelines for CPT use in resource-limited settings reflect the extended CD4 range of benefit and recommend CPT for adolescents and adults with CD4 count <350 cells/mm3 or WHO clinical stage 3 or 4 disease (or WHO clinical stage 2, 3, or 4 where CD4 assays are not available) [5]. The evidence driving these guidelines has primarily been derived from regions with high malaria burden and possibly lower bacterial resistance to co-trimoxazole [2–4]. Perhaps as a result, the WHO guidelines direct the recommendation for use of CPT “particularly in resource-limited settings where bacterial infections and malaria are prevalent among people living with HIV” [5]. In addition, the majority of studies assessing CPT in resource-limited settings were conducted prior to the availability of combination antiretroviral therapy (cART).
Uncertainty exists regarding the most effective use of CPT among patients receiving cART especially among those living in areas without prevalent malaria or with a high prevalence of co-trimoxazole resistance among common bacterial pathogens [5–9]. Because individuals often have rapid increases in CD4 count after cART initiation and because high initial early mortality may be attributed to conditions present at the time of cART initiation [10–13], clinicians may also place less emphasis on CPT in cART initiators. However, the high mortality early after cART initiation calls for optimizing management. Improved recognition of which patients may benefit from CPT, in all resource-limited regions, is needed to both focus effort where most beneficial and avoid providing an agent with potential toxicity to individuals who are unlikely to benefit. To extend the evidence base, we sought to evaluate the effectiveness of CPT at cART initiation on reducing mortality at CD4 counts above and below 200 cells/mm3 in a large South African cohort with minimal exposure to malaria but with high reported rates of co-trimoxazole resistant bacteria [8, 9, 14–16].
Methods
Patients
Patients included in this study were enrolled in a multisite community and workplace-based HIV cART treatment management programme in South Africa and met the following criteria: 1) initiated cART between January 2003 and January 2008, 2) were ≥18 years old, 3) were ART naive at cART initiation, 4) had a CD4 assay result within 6 months prior to cART initiation, and 5) started CPT <30 days before or <7 days after starting cART, or did not receive any CPT during the first 12 months of cART. We included all eligible patients in our analysis.
ART programmes
The study cohort was recruited from 231 community and workplace-based ART clinics. Subjects from the workplace-based clinics started cART from January 2003 onwards while subjects in the community-based clinics started cART from January 2004 onwards. The number of patients meeting inclusion criteria from a given clinic varied between 1 and 1432 individuals. For the 67 workplace-based clinics, cART eligibility was based on CD4 count <250 cells/mm3; WHO stage 3 and CD4 count <350 cells/mm3; or WHO stage 4 condition. For the 164 community-based clinics, cART eligibility was CD4 count <200 cells/mm3 or WHO stage 4 condition. The first-line regimen was either zidovudine or stavudine, lamivudine, and either efavirenz or nevirapine. CPT use was initially recommended for patients with CD4 count <200 cells/mm3, for consistency with international guidelines. In 2005 this was changed, at all sites, to CD4 <350 cells/mm3 or any WHO stage 3 or 4 condition for consistency with WHO recommendations [5]. There was no specific policy at any time to discontinue CPT after reaching a certain CD4 count threshold. Actual clinical practice regarding CPT initiation and discontinuation varied considerably. Isoniazid preventive therapy for the treatment of latent tuberculosis (TB) infection was recommended for all individuals who had no symptoms suggestive of active TB and no treatment for TB within the prior two years. All clinics used similar monitoring schedules with HIV RNA and CD4 count determined before cART initiation, after six weeks on cART, and every six months.
Ethical approval for this study was obtained from the London School for Hygiene and Tropical Medicine and the University of KwaZulu-Natal.
Death Ascertainment
We used three methods of ascertaining death: (1) deregistration forms which record reasons for discontinuation of cART, including death, (2) active tracing of defaulters not returning within 6 months of the last clinic visit, and (3) in the workplace programmes, human resources data to identify workers who either died or were separated from employment. Workers who were terminally ill and were unable to report to work for 3 to 6 months and were not expected to return to work within another 12 months were medically separated from employment and were referred to community clinics for care. To minimize under-ascertainment of death as a result of work separations of terminally ill individuals, we included medical separations as a death surrogate for a combined mortality endpoint.
Definitions
cART
Combination antiretroviral therapy including two nucleoside reverse transcriptase inhibitors and either a non-nucleoside reverse transcriptase inhibitor or a protease inhibitor.
Co-trimoxazole preventive therapy (CPT)
The standard dose of co-trimoxazole used for CPT was daily sulfamethoxazole 800 mg and trimethoprim 160 mg. Individuals starting CPT <30 days before and <7 days after cART initiation were analyzed within the CPT group, even if they discontinued CPT prior to the end of the observation period. This approach was selected because (1) the goal of the analysis was to assess the effectiveness of including CPT at cART initiation and (2) because the risk for death declines during cART therapy and many individuals who initially received CPT discontinued use over time.
Loss to follow-up
Absence of any further clinic visits for >6 months after the last documented visit.
Laboratory
HIV RNA was assayed by polymerase chain reaction (Amplicor HIV-1 Monitor Test, Roche Diagnostics, Nutley, New Jersey, USA). Absolute CD4 counts were assayed by flow cytometry (Becton Dickinson, Mountain View, California, USA). All laboratory tests were performed at commercial certified laboratories in South Africa.
Statistical Analysis
We evaluated the following variables measured at the start of cART: age, sex, log10 HIV RNA, absolute CD4 count, WHO clinical stage, history of prior TB disease, isoniazid preventive therapy, and CPT use; and the following time updated variables: absolute CD4 count and HIV RNA suppression (defined as HIV RNA <400 copies/mL). If the CD4 count was not available from the last time period during evaluation, the latest available CD4 count was carried forward. CD4 at cART initiation was stratified as <200, 200–350, and >350 cells/mm3 to focus on the question of whether CPT reduces mortality at higher CD4 counts.
Baseline factors were compared by CPT group, using either the percentage or median with the chi-square or Wilcoxon rank sum test, as appropriate. Kaplan-Meier survival functions were used to calculate survival probabilities for the first 12 months of cART. Cox proportional hazards modeling was used to evaluate the effect of CPT receipt on survival during cART. CPT use was defined as a fixed (not time updated) variable during the entire observation period. Univariable and multivariable associations were assessed with the combined mortality endpoint over the observation period that started at cART initiation and continued until the earlier of 12 months, discontinuation of cART, last follow-up visit, or death or termination of employment due to terminal illness. We initially assessed for association between factors and mortality in univariable analysis. Factors that either affected the hazard ratio for CPT (confounders) or were indications for CPT use were included in an adjusted multivariable model, in addition, we included age and sex, a priori, in the model. Factors statistically associated with mortality but that neither affected the association between CPT and mortality nor were indications for CPT use were not included in the adjusted analysis as the objective of this analysis was to describe the effect of CPT on mortality. The proportional hazards assumption was assessed and a p-value of less than 0.1 used to define failure to meet the proportional hazards assumption. To assess for association between CPT and mortality at differing baseline CD4 counts, we modeled an interaction between CPT and baseline CD4 strata. We further restricted analysis to patients with WHO stage 1 or 2 at cART initiation to better identify patients most likely to benefit from CPT. We also performed a sensitivity analysis only using known deaths (excluding medical separations). All univariable and multivariable modeling included clinic site as a random effect (proportional hazards model with frailty) to control for residual clinic level confounding and potential clustering at clinic level.
Results
Subjects
Out of a total of 16,068 cART initiators, we included 14,097 with a total of 10,751 person years of observation and a mean duration of follow-up of 9.2 months. Of the excluded patients, 401 were excluded because they started CPT after cART initiation, 462 were excluded because they started CPT >30 days before cART initiation, and 1108 were excluded because they lacked CD4 data within 6 months prior to cART initiation. CPT receipt among subjects included and those excluded due to missing CD4 data was similar: 55% and 57%, respectively. Of the included subjects, 8,696 (62%) were men, the median age was 39 (interquartile range, IQR: 33, 46), and median CD4 count was 132 (IQR: 60, 206) at cART initiation (Table 1). CPT was started among 7,508 (53%) subjects. The median CD4 at baseline was lower among CPT recipients (118 cells/mm3, IQR 53–184) than those who did not receive CPT (153 cells/mm3, IQR 70–236; p<0.001). The mean time on CPT was 9 months (95% confidence interval (CI): 8.8–9.2) with a ratio of time receiving CPT to follow-up time of 0.62. 1,186 (18%) individuals not receiving CPT compared to 901 (12%) receiving CPT discontinued cART, left care, or were lost to follow-up (censored) before the end of the observation period (p<0.001).
Table 1.
Characteristics at cART initiation
| Characteristic | No co-trimoxazole n or median (% or IQR) | Received co-trimoxazole n or median (% or IQR) | p |
|---|---|---|---|
| Number | 6,589 | 7,508 | |
| Sex, proportion of patients | <0.001 | ||
| Men | 4,641 (70) | 4,055 (54) | |
| Women | 1,948 (30) | 3,453 (46) | |
| Age (median, years) | 40 (34–47) | 38 (32–45) | <0.001 |
| Age (years) | <0.001 | ||
| ≤30 | 990 (15) | 1,451 (19) | |
| 31–35 | 1,225 (18) | 1,771 (24) | |
| 36–40 | 1,381 (21) | 1,702 (23) | |
| 41–45 | 1,318 (20) | 1,311 (17) | |
| >45 | 1,976 (30) | 1,698 (23) | |
| WHO stage | <0.001 | ||
| Stage 1 or 2 | 2,286 (35) | 3,072 (41) | |
| Stage 3 | 1,921 (29) | 1,896 (25) | |
| Stage 4 | 2,382 (36) | 2,540 (34) | |
| CD4 (median) cells/mm3 | 153 (70–236) | 118 (53–184) | <0.001 |
| CD4 cells/mm3 | <0.001 | ||
| <200 | 4,252 (64) | 6,052 (81) | |
| 200–350 | 1,744 (26) | 1,132 (15) | |
| >350 | 593 (9) | 324 (4) | |
| Weight (kg) | <0.001 | ||
| ≤60 | 937 (14) | 1255 (17) | |
| 61–75 | 5,249 (80) | 5,868 (78) | |
| >75 | 403 (6) | 385 (5) | |
| Tuberculosis prior to cART | 1,755 (27) | 1,756 (23) | <0.001 |
| Log10 HIV RNA (median) | 4.7 (4.5–5.1) | 4.7 (4.6–5.1) | 0.9 |
| Received isoniazid | 395 (6) | 1189 (16) | <0.001 |
Mortality
During the observation period, 1137 patients died and 152 were medically separated (1,289 total events in combined endpoint). In univariable analysis the following were associated with decreased mortality (Table 2): receipt of CPT (hazard ratio, HR: 0.60, 95% CI: 0.53–0.68), younger age (p=0.03), female sex (p<0.001), higher baseline CD4 count (p<0.001), receipt of isoniazid preventive therapy (p<0.001), higher time updated CD4 count (p<0.001), virologic suppression during follow up (p<0.001), and lower baseline WHO clinical stage (p<0.001).
Table 2.
Associations with mortality and multivariable model with co-trimoxazole
| 12 month survival probability | Unadjusted hazard ratio (HR)* | p | Adjusted hazard ratio (HR)* | p | |
|---|---|---|---|---|---|
| Co-trimoxazole | |||||
| No | 0.87 | Referent | <0.001 | Referent | <0.001 |
| Yes | 0.92 | 0.60 (0.53–0.68) | 0.64 (0.57–0.72) | ||
| Age (years) | |||||
| ≤30 | 0.92 | Referent | 0.03 | Referent | 0.2 |
| 31–35 | 0.91 | 1.1 (0.90–1.3) | 1.1 (0.88–1.3) | ||
| 36–40 | 0.89 | 1.2 (0.99–1.4) | 1.2 (0.97–1.4) | ||
| 41–45 | 0.88 | 1.3 (1.1–1.5) | 1.3 (1.0–1.5) | ||
| >45 | 0.88 | 1.3 (1.1–1.5) | 1.2 (0.98–1.4) | ||
| Sex | |||||
| Men | 0.88 | Referent | <0.001 | Referent | 0.1 |
| Women | 0.92 | 0.75 (0.66–0.86) | 0.89 (0.77–1.0) | ||
| CD4, cART initiation (cells/mm3) | |||||
| <200 | 0.87 | Referent | <0.001 | Referent | <0.001 |
| 200–350 | 0.95 | 0.32 (0.27–0.39) | 0.38 (0.32–0.47) | ||
| >350 | 0.96 | 0.31 (0.23–0.44) | 0.35 (0.25–0.49) | ||
| Log10 HIV RNA at cART initiation | 2.1 (1.9–2.4) | <0.001 | |||
| WHO stage at cART initiation | |||||
| Stage 1 or 2 | 0.94 | Referent | <0.001 | Referent | <0.001 |
| Stage 3 | 0.90 | 1.6 (1.4–1.9) | 1.4 (1.2–1.6) | ||
| Stage 4 | 0.85 | 3.1 (2.7–3.5) | 2.2 (1.9–2.6) | ||
| Prior tuberculosis | |||||
| No | 0.91 | Referent | <0.001 | Referent | 0.03 |
| Yes | 0.86 | 1.6 (1.4–1.8) | 1.2 (1.0–1.3) | ||
| Time updated CD4 (cells/mm3) | |||||
| <50 | 0.56 | Referent | <0.001 | ||
| 50–100 | 0.79 | 0.36 (0.31–0.42) | |||
| 100–200 | 0.90 | 0.15 (0.13–0.17) | |||
| 200–350 | 0.96 | 0.060 (0.051–0.072) | |||
| >350 | 0.97 | 0.040 (0.033–0.053) | |||
| Time updated virologic suppression | |||||
| ≥400 | 0.68 | Referent | <0.001 | Referent | <0.001 |
| <400 | 0.95 | 0.11 (0.10–0.13) | 0.12 (0.10–0.13) | ||
| Isoniazid preventive therapy | |||||
| None | 0.89 | Referent | <0.001 | ||
| Received | 0.94 | 0.56 (0.44–0.70) | |||
controlling for clinic level effects using frailty random effects
We assessed the proportional hazards assumptions for each covariate. CD4 at cART initiation failed to meet proportional hazards assumptions (interaction with cART duration) (p=0.04). However, as our goal was to model the effectiveness of CPT by different CD4 strata at cART initiation, we included CD4 at cART initiation in the final model. All the other covariates met the proportional hazards assumption (interaction with duration on cART, p>0.1). In addition, there were no interactions between the effect of CPT on mortality and any of the other covariates (P>0.1).
Mortality and Co-trimoxazole
The adjusted model included baseline CD4 group, time updated HIV RNA suppression, history of prior TB, WHO stage at cART initiation, sex, age, and receipt of CPT (Table 2). These factors were selected, as described in the Methods, to include covariates that either were indications for CPT use or affected the association between CPT and mortality. Neither isoniazid preventive therapy nor time updated CD4 count affected the hazard ratio for CPT in an adjusted model. In the final adjusted model, CPT use remained strongly associated with decreased mortality (adjusted HR: 0.64, 95% CI 0.57–0.72, p<0.001).
Co-trimoxazole use, CD4 count, and WHO clinical stage at cART initiation
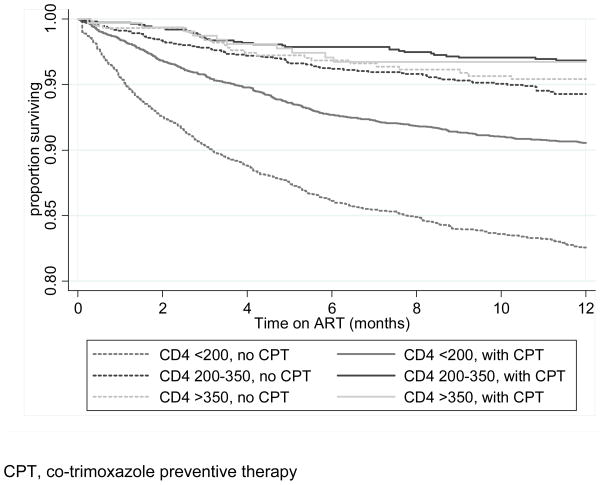
We graphically evaluated the impact of CPT by baseline CD4 group via Kaplan-Meier survival curves (Figure 1). To assess for differences in association between CPT and mortality by baseline CD4 count we assessed for an interaction (effect modification). When including patients in all WHO stages, data were consistent with no effect modification of CPT by CD4 strata (p=0.8), indicating a similar association between CPT and mortality across the three baseline CD4 groups. The adjusted association of CPT on mortality stratified by CD4 group at cART initiation was as follows: CD4 <200 cells/mm3, HR 0.64 (95% CI: 0.56–0.72); CD4 200–350 cells/mm3, HR 0.62 (95% CI: 0.41–0.94), and CD4 >350 cells/mm3, HR 0.80 (95% CI: 0.38–1.7) (Table 3). We also restricted analysis to patients with WHO stage 1 and 2 at baseline to address the question of whether CPT has a measurable impact among individuals with higher CD4 and no history of WHO clinical stage 3 or 4 conditions. Limiting the analysis to patients with WHO clinical stage 1 or 2 disease resulted in the hazard ratios for CPT and mortality becoming close to, or above, unity amongst those with CD4 >200 cells/mm3. The test for effect modification for those with WHO clinical stage 1 or 2 was consistent with the changing association by CD4 count (effect modification, p=0.08).
Figure 1.
Kaplan-Meier survival curve by co-trimoxazole use and baseline CD4 group
Table 3.
Association between co-trimoxazole and mortality stratified by baseline CD4
| Stratified by baseline CD4 (cells/mm3) | Deaths (n)/Person time (yrs) | Unadjusted* | Adjusted*† | Deaths (n)/Person time (yrs) | Adjusted*† (WHO stage 1 or 2 only) | |
|---|---|---|---|---|---|---|
| <200 | No CPT | 626/2818 | Referent | Referent | 130/928 | Referent |
| CPT | 515/4857 | 0.51 (0.45–0.58) | 0.64 (0.56–0.72) | 133/1979 | 0.52 (0.40–0.68) | |
| 200–350 | No CPT | 83/1336 | Referent | Referent | 14/534 | Referent |
| CPT | 33/992 | 0.58 (0.38–0.87) | 0.62 (0.41–0.94) | 12/462 | 0.92 (0.42–2.0) | |
| >350 | No CPT | 23/463 | Referent | Referent | 6/241 | Referent |
| CPT | 10/284 | 0.77 (0.36–1.6) | 0.80 (0.38–1.7) | 6/129 | 1.7 (0.53–5.2) | |
| p-value for effect modification | 0.5 | 0.8 | 0.08 | |||
Controlling for clinic level effects using frailty random effects
Adjusted for age, sex, WHO HIV clinical stage, history of tuberculosis, and time updated HIV RNA suppression
CPT, co-trimoxazole preventive therapy
Finally, we performed a sensitivity analysis, limiting events to known deaths and not counting medical separations as mortality events, and had similar findings to the combined endpoint of association between CPT and mortality by CD4 at cART initiation (not shown).
Discussion
We have demonstrated a 36% reduction in mortality when co-trimoxazole was included at cART initiation. This is an important finding as the majority of studies from resource limited settings evaluating CPT involved individuals not yet on cART. Importantly, the reduction in mortality was seen at all CD4 counts at cART initiation among individuals unrestricted by WHO clinical stage. This demonstrates a wide range of benefit from co-trimoxazole in South Africa despite low levels of malaria, Pneumocystis jirovecii infection, and high levels of bacterial resistance to co-trimoxazole [14–18]. In addition, it suggests that the WHO co-trimoxazole recommendations have broad applicability among cART initiators in differing African settings.
Our findings build on several studies of adults not receiving cART [2–4, 19–21] and one study of adult cART recipients [22]. A study conducted in the pre-cART era in the Ivory Coast randomized patients with both HIV and TB (WHO stage 3 or 4 disease) to receive either CPT or placebo [4]. In that study, the use of CPT reduced mortality by 46%. In subgroup analysis, the benefit was statistically significant up to a CD4 count of 350 cells/mm3. Two other studies also focusing on CPT among TB patients, one from Zambia and the other from South Africa, also identified mortality reduction with CPT use, with hazard ratios of 0.79 and 0.68, respectively [18, 20]. Neither of these studies presented results stratified by CD4 count.
Two cohort studies from pre-ART adult populations, with or without a history of TB, also identified a benefit at lower CD4 counts and more advanced disease, but little mortality reduction among individuals with higher CD4 counts. A study from Uganda, identified an association between CPT and mortality with a hazard ratio of 0.54 [3]. However, the association was statistically significant only for individuals with CD4 <200 cells/mm3 or WHO clinical stage 3 or 4 disease. Very similar results regarding benefit only at lower CD4 counts were reported from a pre-ART cohort in South Africa in which CPT reduced mortality with a hazard ratio of 0.56 [16].
The one on-cART study was a clinic level comparison performed in Malawi [19]. In that study, the hazard ratio for 6 month mortality comparing clinics dispensing CPT and those not dispensing CPT was 0.68. As it was a clinic level comparison, results could not be stratified by CD4 count.
A study that did not demonstrate reduced mortality from CPT was a clinical trial from the Ivory Coast that was stopped early based on predetermined differences in the combined end-point of mortality and any hospitalization [2]. The researchers did identify higher rates of pneumonia, diarrhea associated with isosporiasis, malaria, fevers, and bacteremia in the placebo group with an overall hazard ratio for events in the CPT arm of 0.57. The reduction in events was seen for all CD4 strata; no stratification by WHO clinical stage was reported.
Our results are perhaps remarkable in their consistency with prior findings from regions with higher prevalence of malaria and diarrheal disease and some with reportedly lower prevalence of co-trimoxazole resistant bacteria. Thus they both help to generalize evidence for CPT use across African settings to individuals with CD4 counts >200 cells/mm3 and to extend the findings to cART initiators. In addition, we have identified a group of individuals who may not benefit from CPT in our setting, individuals with both CD4 count >200 cells/mm3 and WHO clinical stage 1 or 2 disease. As those individuals had a low likelihood of benefit, the risk for adverse reactions related to co-trimoxazole may be greater than clinical benefit.
Our study is further important because the large size of the study population with 14,097 cART cART initiators and 1,289 events, provided power to identify even small differences in mortality. However, several limitations are worth noting. First, it is an observational cohort analysis and individuals were not randomly assigned to receive CPT. As a result, despite adjusting for multiple factors, residual confounding could remain and affect the results. As CPT is generally reserved for individuals with more advanced HIV, residual confounding may be more likely to lead to a finding of non-benefit or increased mortality with CPT rather than the benefit we observed. We believe that clinic level effects regarding CPT use and mortality, such as both better care and CPT use, are unlikely to be driving the association because we adjusted for clinic site in all analyses. A strength of our cohort is the extensive ascertainment of deaths via human resources records in the workplace cohort and follow-up of individuals and deregistration of individuals who died. However, we likely have under-ascertained mortality with greater absolute numbers under-ascertained in the non-CPT group, the group with higher mortality. This is suggested by the higher proportion censored in the non-CPT group. We believe that under ascertainment of death could reduce our power, but if it affected the strength of association it would be to bias towards the null hypothesis. Another limitation is that we did not identify cause of death, thus we are unable to describe the specific causes of death that CPT reduced. However, as our goal was to assess the overall effectiveness of CPT, cause of death was not a focus. Finally, we did not assess adherence to CPT, however, our goal was to evaluate CPT in a routine clinical setting with expected variation in adherence.
Although we do not have data on the cause of death for the patients in our study, we believe that the effect size of CPT is plausible based on available data on cause of death among persons living with HIV in Africa. Bacterial pathogens, especially Salmonella species and Streptococcus pneumoniae, Pneumocystis, and diarrhea have been reported to be present at death among 18–50% of persons with HIV in southern Africa [17, 18, 23, 24]. Of note, the studies reporting the highest fraction of bacterial infections also had the most complete microbiological culture ascertainment. Thus we speculate that bacterial infection, either in isolation or in co-infection with other diseases, was a major contributor to mortality in our population, and that use of CPT reduced this cause of death.
Our findings will be important to help guide clinicians in deciding when to use CPT in conjunction with cART. Our results indicate that, in resource-limited settings, CPT should be started among all adults with a CD4 count <200 cells/mm3 or WHO clinical stage 3 or 4. This message is critically important as early mortality during cART is, at times, viewed as a consequence of pre-existing illness and not amenable to prevention with prophylactic therapies. As the majority of cART initiators, in our and other cohorts in Africa, start with a CD4 count considerably below 200 cells/mm3, this is a simple and immediately implementable intervention to reduce high early mortality after cART initiation. A second important question regards when CPT can be safely discontinued. In our cohort, most patients received approximately 9 months of CPT. However, we have not assessed to optimal time to discontinuation of CPT and can only speculate that 9 to 12 months, or more, may be appropriate. Given the low cost and profound impact on mortality, efforts should be redoubled to achieve high levels of CPT uptake within cART programmes at, or before, cART initiation.
Acknowledgments
Support: This work was supported by the Aurum Institute. CJH was supported by NIH DK074348, REC by NIH AI5535901 and AI016137, and ADG by a UK Department of Health Public Health Career Scientist Award.
Footnotes
Conflicts of interest: CJH: none, KLF: none, SC: none, CI: none, REC: none, GAD: none, GJC: none
This work was presented in part at the 17th Conference on Retroviruses and Opportunistic Infections, San Francisco, California, February 17–21, 2010.
Reference List
- 1.Mofenson LM, Brady MT, Danner SP, Dominguez KL, Hazra R, Handelsman E, et al. Guidelines for the Prevention and Treatment of Opportunistic Infections among HIV-exposed and HIV-infected children: recommendations from CDC, the National Institutes of Health, the HIV Medicine Association of the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the American Academy of Pediatrics. MMWR Recomm Rep. 2009;58(RR-11):1–166. [PMC free article] [PubMed] [Google Scholar]
- 2.Anglaret X, Chene G, Attia A, Toure S, Lafont S, Combe P, et al. Early chemoprophylaxis with trimethoprim-sulphamethoxazole for HIV-1-infected adults in Abidjan, Cote d’Ivoire: a randomised trial. Cotrimo-CI Study Group. Lancet. 1999;353(9163):1463–1468. doi: 10.1016/s0140-6736(98)07399-1. [DOI] [PubMed] [Google Scholar]
- 3.Mermin J, Lule J, Ekwaru JP, Malamba S, Downing R, Ransom R, et al. Effect of co-trimoxazole prophylaxis on morbidity, mortality, CD4-cell count, and viral load in HIV infection in rural Uganda. Lancet. 2004;364(9443):1428–1434. doi: 10.1016/S0140-6736(04)17225-5. [DOI] [PubMed] [Google Scholar]
- 4.Wiktor SZ, Sassan-Morokro M, Grant AD, Abouya L, Karon JM, Maurice C, et al. Efficacy of trimethoprim-sulphamethoxazole prophylaxis to decrease morbidity and mortality in HIV-1-infected patients with tuberculosis in Abidjan, Cote d’Ivoire: a randomised controlled trial. Lancet. 1999;353(9163):1469–1475. doi: 10.1016/s0140-6736(99)03465-0. [DOI] [PubMed] [Google Scholar]
- 5.Sesay M, Chimzizi R, Chotpitayasunondh T, Crowley S, Duncombe C, El Sadr W, et al. Guidelines on co-trimoxazole prophylaxis for HIV-related infections among children, adolescents, and adults in resource-limited settings. Geneva, Switzerland: World Health Organization; 2006. [Google Scholar]
- 6.Lynen L, Jacobs J, Colebunders R. Co-trimoxazole prophylaxis in tropical countries in the era of highly active antiretroviral therapy: do we know enough? Trans R Soc Trop Med Hyg. 2007;101(11):1059–1060. doi: 10.1016/j.trstmh.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Anglaret X, Sylla-Koko F, Bonard D, Combe P, Coulibaly M, Aoussi E, et al. Susceptibilities to co-trimoxazole of pathogens isolated from blood and stool specimens in Abidjan, Ivory Coast, 1994 to 1996. J Clin Microbiol. 1997;35(7):1915. doi: 10.1128/jcm.35.7.1915-1915.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nchabeleng M. Cotrimoxazole resistance amongst commensal organisms in AIDS patients commencing cotrimoxazole prophylaxis. [Abstract]. First South African AIDS Conference; Durban. 2003. [Google Scholar]
- 9.Govender N, Cohen C, editors. GERMS-SA Annual Report 2008. South Africa: NICD; 2008. Group for Enteric Respiratory and Meningeal Disease Surveillance in South Africa. [Google Scholar]
- 10.Lawn SD, Little F, Bekker LG, Kaplan R, Campbel E, Orrell C, et al. Changing mortality risk associated with CD4 cell response to antiretroviral therapy in South Africa. AIDS. 2009;23(3):335–342. doi: 10.1097/QAD.0b013e328321823f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lawn SD, Harries AD, Anglaret X, Myer L, Wood R. Early mortality among adults accessing antiretroviral treatment programmes in sub-Saharan Africa. AIDS. 2008;22(15):1897–1908. doi: 10.1097/QAD.0b013e32830007cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lawn SD, Myer L, Orrell C, Bekker LG, Wood R. Early mortality among adults accessing a community-based antiretroviral service in South Africa: implications for programme design. AIDS. 2005;19(18):2141–2148. doi: 10.1097/01.aids.0000194802.89540.e1. [DOI] [PubMed] [Google Scholar]
- 13.Lawn SD, Myer L, Bekker LG, Wood R. CD4 cell count recovery among HIV-infected patients with very advanced immunodeficiency commencing antiretroviral treatment in sub–Saharan Africa. BMC Infect Dis. 2006;6:59. doi: 10.1186/1471-2334-6-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pemba L, Charalambous S, von GA, Magadla B, Moloi V, Seabi O, et al. Impact of cotrimoxazole on non-susceptibility to antibiotics in Streptococcus pneumoniae carriage isolates among HIV-infected mineworkers in South Africa. J Infect. 2008;56(3):171–178. doi: 10.1016/j.jinf.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 15.Crewe-Brown HH, Reyneke MP, Khoosal M, Becker PJ, Karstaedt AS. Increase in trimethoprim-sulphamethoxazole (co-trimoxazole) resistance at Chris Hani Baragwanath Hospital, Soweto, in the AIDS era. S Afr Med J. 2004;94(6):440–442. [PubMed] [Google Scholar]
- 16.Liebowitz LD, Slabbert M, Huisamen A. National surveillance programme on susceptibility patterns of respiratory pathogens in South Africa: moxifloxacin compared with eight other antimicrobial agents. J Clin Pathol. 2003;56(5):344–347. doi: 10.1136/jcp.56.5.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martinson NA, Karstaedt A, Venter WD, Omar T, King P, Mbengo T, et al. Causes of death in hospitalized adults with a premortem diagnosis of tuberculosis: an autopsy study. AIDS. 2007;21(15):2043–2050. doi: 10.1097/QAD.0b013e3282eea47f. [DOI] [PubMed] [Google Scholar]
- 18.Murray J, Sonnenberg P, Nelson G, Bester A, Shearer S, Glynn JR. Cause of death and presence of respiratory disease at autopsy in an HIV-1 seroconversion cohort of southern African gold miners. AIDS. 2007;21 (Suppl 6):S97–S104. doi: 10.1097/01.aids.0000299416.61808.24. [DOI] [PubMed] [Google Scholar]
- 19.Badri M, Ehrlich R, Wood R, Maartens G. Initiating co-trimoxazole prophylaxis in HIV-infected patients in Africa: an evaluation of the provisional WHO/UNAIDS recommendations. AIDS. 2001;15(9):1143–1148. doi: 10.1097/00002030-200106150-00009. [DOI] [PubMed] [Google Scholar]
- 20.Zachariah R, Spielmann MP, Chinji C, Gomani P, Arendt V, Hargreaves NJ, et al. Voluntary counselling, HIV testing and adjunctive cotrimoxazole reduces mortality in tuberculosis patients in Thyolo, Malawi. AIDS. 2003;17(7):1053–1061. doi: 10.1097/00002030-200305020-00015. [DOI] [PubMed] [Google Scholar]
- 21.Nunn AJ, Mwaba P, Chintu C, Mwinga A, Darbyshire JH, Zumla A. Role of co-trimoxazole prophylaxis in reducing mortality in HIV infected adults being treated for tuberculosis: randomised clinical trial. BMJ. 2008;337:a257. doi: 10.1136/bmj.a257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lowrance D, Makombe S, Harries A, Yu J, Aberle-Grasse J, Eiger O, et al. Lower early mortality rates among patients receiving antiretroviral treatment at clinics offering cotrimoxazole prophylaxis in Malawi. J Acquir Immune Defic Syndr. 2007;46(1):56–61. [PubMed] [Google Scholar]
- 23.Mzileni MO, Longo-Mbenza B, Chephe TJ. Mortality and causes of death in HIV-positive patients receiving antiretroviral therapy at Tshepang Clinic in Doctor George Mukhari Hospital. Pol Arch Med Wewn. 2008;118(10):548–554. [PubMed] [Google Scholar]
- 24.Garcia-Jardon M, Bhat VG, Blanco-Blanco E, Stepian A. Postmortem findings in HIV/AIDS patients in a tertiary care hospital in rural South Africa. Trop Doct. 2010;40(2):81–84. doi: 10.1258/td.2010.090465. [DOI] [PubMed] [Google Scholar]