Abstract
Inherited skin blistering conditions collectively named epidermolysis bullosa (EB) cause significant morbidity and mortality due to the compromise of the skin's barrier function, the pain of blisters, inflammation, and in some cases scaring and cancer. The simplex form of EB is usually caused by dominantly inherited mutations in KRT5 or KRT14. These mutations result in the production of proteins with dominant-negative activity that disrupt polymerization of intermediate filaments in the basal keratinocyte layer and result in a weak epidermal–dermal junction. The genome of adeno-associated virus (AAV) vectors can recombine with chromosomal sequence so that mutations can be corrected, or production of proteins with dominant-negative activity can be disrupted. We demonstrate a clinically feasible strategy for efficient targeting of the KRT14 gene in normal and EB-affected human keratinocytes. Using a gene-targeting vector with promoter trap design, targeted alteration of one allele of KRT14 occurred in 100% of transduced cells and transduction frequencies ranged from 0.1 to 0.6% of total cells. EBS patient keratinocytes with precise modifications of the mutant allele are preferentially recovered from targeted cell populations. Single epidermal stem cell clones produced histologically normal skin grafts after transplantation to athymic mice and could generate a sufficient number of cells to transplant the entire skin surface of an individual.
Introduction
Epidermolysis bullosa (EB) is the term used to describe a group of inherited skin diseases that exhibit frequent blistering as the primary phenotype.1,2 The group is further divided into dystrophic, junctional, hemidesmosomal, and simplex subtypes based on the cleavage plane of the blister and the affected gene. With the exception of the simplex form, most EB is inherited in an autosomal recessive pattern. EB simplex (EBS) is caused by KRT5 and KRT14 mutations that usually result in proteins with dominant-negative activity3,4 and cause abnormal polymerization of intermediate filaments within the basal keratinocyte layer.5 Mutational hotspots exist in both KRT5 and KRT14 such that 70% of affected individuals have mutations in one of five locations.6,7 EBS symptoms usually manifest at birth with erythema, widespread blistering, and areas of denuded skin.8 Secondary complications arise as a result of recurrent blistering and include skin infections, sepsis, nail dystrophy, and pigmentary changes. Current treatment strategies are limited to the use of shoes and clothing that minimize blister formation, lancing of blisters, and prompt treatment of cellulitis with antibiotics.8
The EBs are a promising category of disease targets for gene therapy strategies because epidermal stem cells reside abundantly in the skin, can be cultured ex vivo, and autologous transplants of modified cells can be performed. Skin tissue contains two types of stem cells: follicular stem cells that reside in the lateral bulge of the hair follicle and contribute to the hair follicle cycle and wound repair,9 and interfollicular stem cells that are located in the basal layer of the epidermis. Interfollicular stem cells contribute mainly to homeostasis, renewing the epidermis as layers are continually sloughed.10 In the mid 1970s, conditions were established to grow interfollicular stem cells in culture allowing them to be studied directly, and used therapeutically for the treatment of full thickness burns.11,12 In an elegant set of experiments, the same group defined three colony phenotypes that arise from single cells in culture, and described them by colony morphology and size.13 Cells that give rise to colonies with a “holoclone” phenotype come from the interfollicular stem cell niche and contain small undifferentiated and highly proliferative cells that can be grown almost indefinitely.14 In a recent successful gene therapy clinical trial for the treatment of recessively inherited junctional EB, cells with a holoclone phenotype were found in the palms and a few unaffected areas of the 36-year-old patient and used to generate skin equivalents for disease treatment.15
Gene therapy for recessively inherited conditions can be accomplished by adding another copy of the mutated gene; however, genetic disorders such as EBS are resistant to this approach because of the dominant-negative activity of proteins produced by the mutated gene. Therefore, a gene-targeting approach was used to model treatment strategies for EBS. This approach has not been applied therapeutically due to limitations of efficiency, and the inability to generate large numbers of modified cells for transplantation. Adeno-associated virus (AAV)–mediated gene targeting is an efficient method of gene alteration used in primary human cell culture, and offers a safe, effective, and permanent treatment for individuals with EBS. The observation of mutation hot spots in KRT5 and KRT14 suggests that the construction of a few gene-targeting vectors could treat the cells of multiple individuals from different families, simplifying the therapeutic approach in this patient group. Techniques for keratinocyte culture, stratification on artificial matrices, and successful transplantation of skin equivalents to human recipients have been established.16 Modification of cells by AAV-mediated gene targeting before transplantation represents the final challenge for affecting a gene therapy strategy to treat this dominantly inherited condition and would allow modified cells to be incorporated into existing autologous transplantation protocols.
We demonstrate efficient targeting of KRT14, the ability to recover KRT14-targeted stem cells with a holoclone phenotype, and transplantation of skin equivalents made from either targeted holoclones, or polyclonal populations of modified cells. Using this clinically feasible approach, human skin equivalents functioned normally after transplantation by providing a barrier to water loss and infection and had a histologically normal appearance. This is the first report that demonstrates both efficient transduction of human keratinocytes by AAV vectors, and efficient purification and functional characterization of keratinocyte populations containing targeted genetic alterations. The number of keratinocytes can be expanded in culture so that clinically significant skin grafts can be generated from modified cells. These results form the basis of future studies that should allow clinical application of this strategy for the treatment of EBS or other EB types where no effective treatments have been found.
Results
AAV vectors pseudotyped with capsids from serotype 6 isolates efficiently transduce normal human keratinocytes
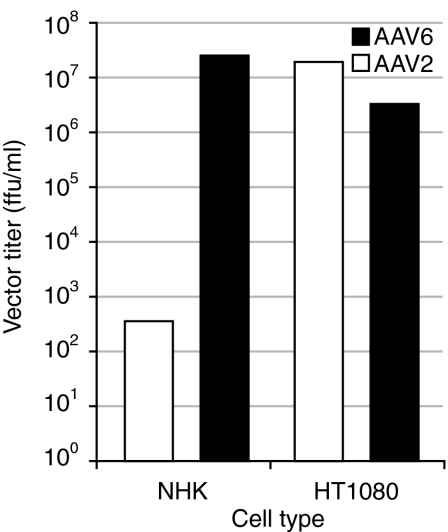
Gene-targeting approaches require efficient infection of cells so that sufficient numbers of independent transduction events due to homologous recombination can be collected for analysis and ultimately for therapeutic use. Human keratinocytes are resistant to transduction by AAV vectors pseudotyped with type 2 capsid serotypes.17,18 We generated AAV vector preparations using a plasmid containing the human placental alkaline phosphatase reporter and packaging plasmids containing capsid genes from serotypes 2 and 6. Transduction efficiencies were compared in human keratinocytes, or human HT1080 cells to determine whether a type 6 capsid serotype allows efficient transduction of normal human keratinocytes. Vectors pseudotyped with capsids from serotype 2 isolates transduced human keratinocytes poorly when transduction efficiencies in keratinocytes were compared with efficiencies in HT1080 cells. However, transduction frequencies increased by 5 logs when the same vector was packaged with capsids from a serotype 6 isolate(Figure 1).
Figure 1.
AAV vector transduction frequencies in normal human keratinocytes and HT1080 cells. The titer of an AAV vector containing the human placental alkaline phosphatase gene and packaged in capsids from either serotype 2 or serotype 6 AAV isolates was determined by quantifying probe hybridization signals on alkaline Southern blots. An equal number of genome containing particles was added to cultures of normal human keratinocytes (NHK) or HT1080 cells in parallel. Cells were stained for alkaline phosphatase activity 3 days after infection and foci counted to determine vector titers on each cell type. AAV, adeno-associated virus; ffu, focus-forming unit.
Design of vectors for targeted disruption of human KRT14 genes
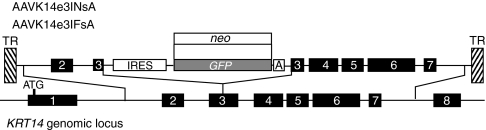
Permanent transduction of replicating cells by AAV vectors occurs by integration of vector genomes at sites of double-strand break repair,19 or by homologous recombination of vector and chromosomal sequences.20 Because vector integration at random genomic locations occurs in ~3–10% of cells at high infection multiplicities,21 homologous recombination usually represents a fraction of total transduction events. A number of strategies have been developed to enhance detection of transduction events that occur by homologous recombination while ignoring transduction that occurs as a result of integration at random genomic locations. Vector designs that require promoter trapping for expression of marker genes can shift the balance of detection toward recombinants because most integration at random locations does not trap the activity of an active promoter.22 A promoterless gene expression cassette containing an internal ribosomal entry site was designed to result in the disruption of KRT14 transcription by insertion into exon 3 of KRT14. GFP expression results from the activity of the KRT14 promoter allowing detection of cells containing targeted insertions. GFP expression resulting from integration at random locations requires the relatively rare event of insertion of the cassette into an exon of an actively transcribed gene (Figure 2).
Figure 2.
Human KRT14 gene-targeting vector. The AAV vector used for targeting the human KRT14 gene is shown above a graphic of the genomic locus. Exons are depicted as black boxes and numbered, with introns indicated by adjoining lines. The IRES-GFP or IRES-neo expression cassettes are shown and areas of sequence homology are indicated by thin adjoining lines. The insertion site for the expression cassettes within exon 3 is also shown. A, synthetic polyadenylation signal; AAV, adeno-associated virus; GFP, green fluorescent protein; IRES, internal ribosomal entry site from encephalomyocarditis virus; TR, terminal repeats.
Transduction frequencies of keratin gene-targeting vectors in normal human keratinocytes
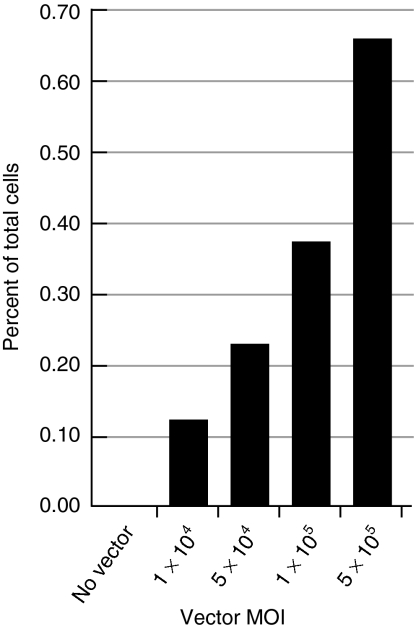
The KRT14 gene-targeting vector (Figure 2) was packaged with capsid proteins from a serotype 6 isolate23 and the percentage of GFP expressing normal human keratinocytes was determined by flow cytometry 7 days after infection of human keratinocytes. Transduction frequencies were typical of other targeting vectors with promoter trap design,21 ranged from 0.1 to 0.6% of total cells, and increased with increasing multiplicity of infection (Figure 3).
Figure 3.
KRT14-targeting vector transduction frequencies in normal human keratinocytes. Normal human keratinocytes were infected with the AAVK14e3IFsA gene-targeting vector and transduction measured by determining the percent of GFP positive cells using fluorescence-activated cell sorting. Because 100% of GFP positive clones contained targeted insertions at the KRT14 locus (see Figure 4a), these percentages also reflect the targeting frequency in normal human keratinocytes. MOI, multiplicity of infection.
Analysis of transduced keratinocyte clones
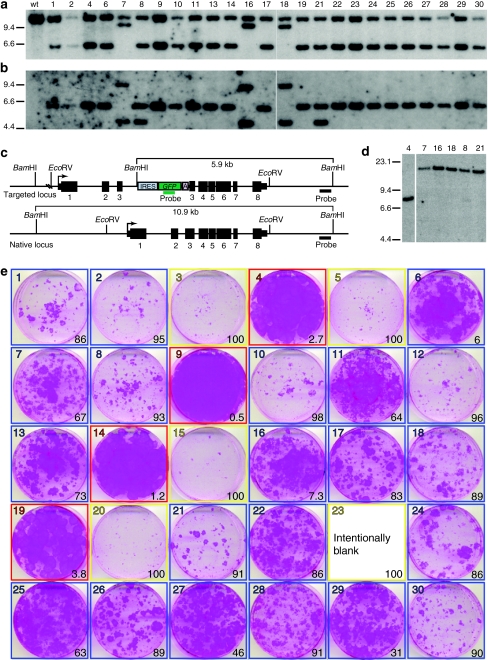
GFP positive cells transduced with the AAVK14e3IFsA vector were collected using fluorescence-activated cell sorting and placed in cell culture dishes at limiting dilution. Keratinocyte colonies generated from single cells were ring cloned and expanded for analysis by genomic Southern, a colony forming efficiency assay, and transplantation of skin equivalents to athymic mice. Out of 30 GFP positive clones, 25 grew sufficiently for DNA analysis and all 25 clones contained a targeted insertion of the vector within exon 3 of the KRT14 gene as evidenced by a shift in size of the BamHI fragment from 10.9 to 5.9 kb on Southern blots hybridized with the KRT14 probe (Figure 4a,c). Analysis of the KRT14 target site in three clones revealed a 9.1-kb vector–chromosome junction fragment (Figure 4a, clones 7, 16, and 18). The same blot was stripped of the KRT14 probe and hybridized with a DNA fragment from the GFP gene. A common size fragment was detected in genomic DNA from each of the targeted clones with the exception of clones 7, 16, and 18 where two GFP-hybridizing fragments of 4.6 and 9.1 kb molecular weight were observed (Figure 4b). DNA from two additional clones showed hybridization to an additional GFP-containing fragment (Figure 4b, clones 8 and 21). To determine whether the additional signals result from vector integration at random locations within the genome, genomic DNA from clones 7, 8, 16, 18, and 21 was digested with the EcoRV enzyme that does not cut within the targeting vector sequence. Vectors present at random genomic locations should have unique fragment lengths depending on their proximity to EcoRV sites in the genome. Hybridization of the GFP probe to these samples revealed a single DNA fragment demonstrating that multiple vector copies integrated at the same locus (Figure 4d). These data demonstrate the efficiency of the promoter trap strategy, and the accuracy of gene targeting in keratinocytes using these vectors.
Figure 4.
Clonal analysis of KRT14-targeted normal human keratinocytes. Normal human keratinocytes were transduced with AAVK14e3IFsA and GFP positive cells sorted for clonal analysis. Thirty clones were examined for evidence of targeted insertions at the KRT14 locus, and for the growth phenotype of colonies on irradiated NIH-3T3-J2 feeders. Four of these clones were subsequently used to construct skin equivalents that were transplanted to nude mice for further analysis. (a) Southern blot analysis of BamHI digested genomic DNA from 25 clones that grew sufficiently for DNA to be collected. The blot is probed with a DNA fragment from the KRT14 locus (outside of vector homology). (b) The same blot as in a, with KRT14 probe removed and probed with a GFP gene fragment. (c) Diagram of the KRT14-targeted and native locus. Restriction enzyme sites and relevant fragment sizes are indicated as well as the position of both the KRT14 probe and the GFP probe used for Southern in a and b. Transcription start sites are indicated by bent arrows. Exons are depicted as black boxes and numbered below with introns shown as a connecting line. 5′ and 3′ untranslated regions are shown as narrow boxes. (d) Genomic DNA from clones 7, 16, 18, 8, and 21 was digested with EcoRV and probed with a GFP gene fragment to determine if multiple vector copies present in these clones were composed as tandem integrants at the KRT14 locus, or at multiple different integration sites. Genomic DNA from clone 4 is used as a control. (e) Colony forming efficiency (CFE) assay used to define which targeted clones are paraclones (100% abortive colonies, outlined in yellow), meroclones (6–99% abortive colonies, outlined in blue), or holoclones (<5% abortive colonies, outlined in red). The photographs are of 6-cm diameter culture dishes containing formaldehyde-fixed colonies stained with rhodamine B. Numbers in the lower right of each box indicate the percentage of abortive colonies on the dish as determined by colony diameter size of <4 mm after 12 days of growth. Colonies from clone 23 were pooled with cells from the other dishes to generate enough DNA for Southern analysis so no picture was obtained from this CFE assay for this clone.
Clones 8 and 21 contain a tandem head to tail insertion of two vectors such that a target site vector:chromosome junction fragment and a unit length vector fragment are generated when DNA is digested with an enzyme that cuts once within vector sequence (BamHI) and probed with a DNA fragment from GFP. Clones 7, 16, and 18 also contain two tandem vector insertions at the KRT14 locus with an additional 3.2 kb of vector sequence added to the right junction producing a 9.1 kb vector:chromosome junction fragment. Concatomerization of AAV vectors has been previously described24,25 and vector genomes in nondividing cells are present as large predominantly head to tail concatomers where gene expression occurs predominantly from episomal forms.26
The number of clones resulting from independent KRT14-targeting events in the sorted cell populations is calculated to be >1,000 based on the number of cells used to initiate targeting experiments and a transduction frequency of 0.5%. However, as multiple keratinocyte colonies are being pooled before sorting, the possibility exists that the same clone could be isolated more than once. To determine whether similar gene-targeting frequencies, and similar frequencies of integration at random genomic locations are observed if cells are cloned immediately after application of the gene-targeting vector, the GFP expression cassette was replaced with a neomycin phosphotransferase gene and G418 selection applied 1 day after infection. Southern blot analysis of genomic DNA from 14 G418 resistant colonies isolated by ring cloning demonstrated that all clones contained an insertion of the gene expression cassette in exon 3 of the KRT14 locus. None of the 14 clones contained vectors integrated at random genomic locations as evidenced by probing the same blot with a DNA fragment homologous to the neo gene supporting the results obtained by sorting and cloning GFP positive cells (Supplementary Figure S1).
Growth characteristics of KRT14-targeted keratinocyte clones
Colonies generated from sorted GFP positive cells were similar in size, and showed only subtle morphologic differences when chosen for expansion and characterization. A colony forming efficiency assay based on colony size and morphology has been previously described13 and colonies having a holoclone phenotype have been shown to have an almost unlimited capacity for expansion, capability for transplantation and differentiation,14,27,28 and contain cell surface antigens also present on stem cells defined by other criteria.29 The colony forming efficiency assay was performed on cells from all 30 GFP positive clones. One quarter of the cells from a single colony were seeded to dishes containing irradiated mouse feeders, allowed to proliferate for 12 days, and fixed and stained with rhodamine B. Colonies were counted and categorized as greater than or less than 4 mm in diameter. Colonies of keratinocyte clones that contained cells that generate large (>4 mm diameter) daughter colonies 95% of the time are classified as holoclones (Figure 4e, clones 4, 9, 14, and 19 outlined in red). Colonies of cells that give rise to a mixture of large daughter colonies and small abortive colonies are classified as meroclones (Figure 4e, clones outlined in blue), and those with cells that only form abortive daughter colonies (<4 mm in diameter) are classified as paraclones (Figure 4e, clones 3, 5, 15, 20, and 23 outlined in yellow).13 Of the 30 clones analyzed, 13% were classified as holoclones, 70% as meroclones, and 16% as paraclones (Figure 4e). These frequencies are similar to those observed in unmodified cells from skin biopsies13 and demonstrate the feasibility of a gene-targeting approach to skin blistering conditions.
Efficient KRT14 targeting in keratinocytes from EBS affected patients
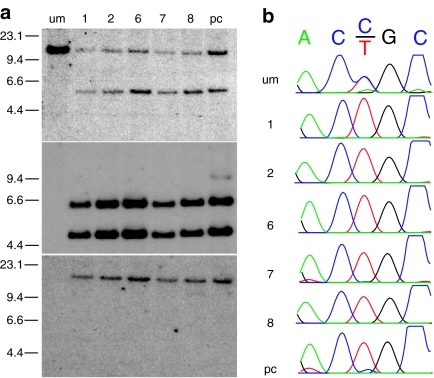
Keratinocytes isolated from the skin of a patient with EBS (Dowling-Meara subtype), and containing a common mutation that results in a change of amino acids at position 125 of the protein sequence (R125C) were transduced with the AAVK14e3IFsA vector. Ten GFP positive clones and a polyclonal GFP positive cell population were collected for Southern blot analysis and sequencing. Five clones grew sufficiently for DNA analysis performed by probing genomic DNA with a KRT14 gene fragment (Figure 5a, top) and a GFP gene fragment (Figure 5a, middle). Each clone and the polyclonal population showed targeted insertion of the vector within exon 3 of the KRT14 gene again demonstrated by a shift in the size from 10.9 to 5.9 kb of the KRT14 hybridizing BamHI fragment. However, in contrast to clones obtained by targeting normal cells, every clone, and the majority of the polyclonal cell population, contained a minimum of two vector copies at the KRT14 locus demonstrated by a KRT14–GFP junction fragment (5.9 kb) and an additional GFP-hybridizing fragment of 4.6 kb similar to clones 8 and 21 isolated from transduction of normal cells (Figures 4b and 5a, middle). The location of both GFP-hybridizing fragments at the KRT14 locus was confirmed by digestion of DNA from the same cells with EcoRV and hybridization with a DNA fragment from the GFP gene (Figure 5a, bottom). Again hybridization to a single DNA fragment with the same size as a concatomer of two vector copies within exon 3 of the KRT14 gene was observed. A faint GFP-hybridizing fragment of ~13.8 kb size is seen in the polyclonal population indicating that a small percentage of cells have as many as three vector copies at the KRT14 locus (Figure 5a, lanes middle labeled pc).
Figure 5.
Southern blot and sequence analysis of KRT14-targeted keratinocytes from a patient with epidermolysis bullosa simplex (EBS). Ten GFP positive colonies were selected after transduction of patient keratinocytes with the AAVK14e3IFsA gene-targeting vector and sorting for GFP fluorescence. A polyclonal population of sorted GFP positive cells was also analyzed in parallel. Five of the 10 clones grew sufficiently to allow isolation of genomic DNA and analysis by Southern blot. (a) Upper panel: genomic DNA samples from the unmodified patient cells, five GFP positive clones, and the polyclonal KRT14-targeted cells were digested with BamHI and probed with a gene fragment from the KRT14 gene (top) or the GFP gene fragment (middle) as in Figure 4a,b. The same samples were digested with EcoRV and probed with a GFP gene fragment (bottom) as in Figure 4d. The migration positions and size of HindIII digested ΛDNA fragments are shown in kb at the left of each panel. (b) Sequence tracings of the region immediately surrounding the patient's mutation from vector–chromosome junction fragments of each clone and the polyclonal population amplified by PCR using primers located within GFP and 5′ of the location of the EBS-causing mutation in exon 1. Wild type sequence is ACCGC and the EBS-causing mutation is ACTGC producing a R125C substitution of amino acids in the KRT14 protein. pc, polyclonal; um, unmodified.
KRT14-targeted keratinocytes from EBS-affected patients are enriched for targeting events at the mutant allele
Vector:chromosome junction fragments were amplified from the targeted allele of each clone, and from the polyclonal cell population to determine whether cells targeted at the mutant allele are present more frequently after growth in culture. All five clones and the vast majority of cells in the polyclonal population contained targeted insertions of the GFP expression cassette at the mutant allele (Figure 5b) suggesting that cells without the mutant protein are at a growth advantage in culture, and that a tandem insertion of at least two vector copies more effectively eliminated transcription of the mutant allele.
KRT14-targeted keratinocyte holoclones and polyclonal cell populations stratify and function normally when transplanted to athymic mice
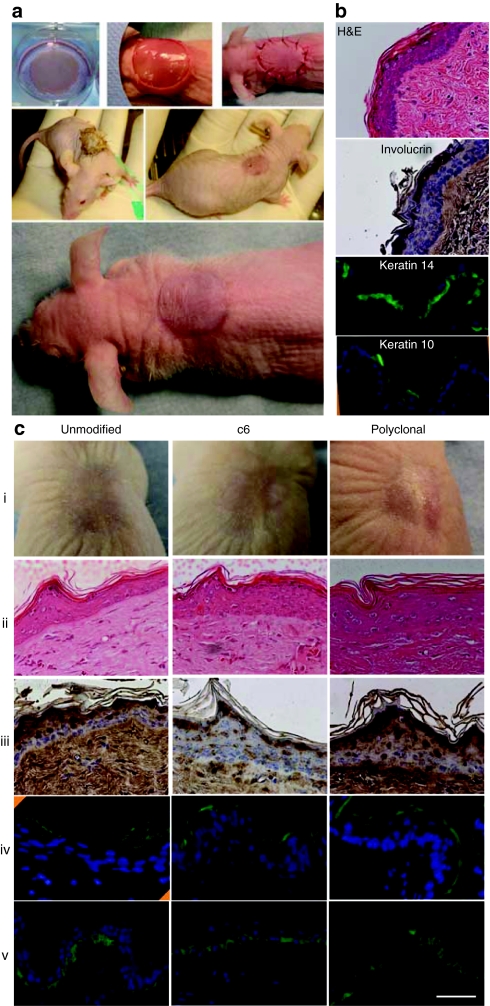
To determine whether KRT14-targeted keratinocytes retain the ability to form a normally functioning stratified epidermal cell layer in vivo, skin equivalents were constructed from each KRT14-targeted normal keratinocyte clone having a holoclone colony phenotype (Figure 4e, clones 4, 9, 14, 19) and from an EBS-affected individual (Figure 5, unmodified, clone 6, and polyclonal). Full thickness skin grafts were transplanted to the dorsal surface of nude mice and bandaged by suturing devitalized full thickness skin from the graft site over the human graft. The devitalized mouse skin was sloughed by day 8 in each case, and a mature skin appearance was present 8–10 days after surgery (Figure 6a). Mice were killed 13–20 weeks later for histological analysis of the engrafted tissues.
Figure 6.
Gross appearance and histological analysis of human–mouse xenografts containing KRT14-targeted keratinocytes. Unmodified cells, polyclonal cell populations, or characterized KRT14-targeted clones described in Figures 4 and 5 were cultured on dermal equivalents composed of polymerized human fibrin containing normal human dermal fibroblasts. Skin equivalents were transplanted to the backs of nude mice and grafts were biopsied 10–20 weeks later and characterized for human cell content using an antibody specific for human involucrin. Grafts were also characterized for appropriate stratification by hematoxylin and eosin (H&E) staining, and the distribution of cells containing terminal differentiation markers involucrin, keratin 10, and basal marker keratin 14. (a) Upper panels show the skin equivalent of a representative holoclone (Figure 4e, clone 19) after growth on a fibrin matrix containing normal fibroblasts from the same biopsy, and detached from the well of a 6-well dish (left), transfer of the skin equivalent to a similar sized wound created on the back of nu/nu mouse (center), and replacement of the mouse skin after devitalization by freeze thawing three times (right). Middle two panels show the gross appearance of the devitalized patch 3 days (left) and the underlying graft 8 days (right) after surgery. Bottom panel shows the appearance of the mature graft 4 weeks after surgery. (b) Histology of the graft shown in a with H&E staining and the distribution of involucrin, keratin 10 and keratin 14 labeled cells within the epidermis of the graft. The involucrin antibody is specific for the human protein and did not stain mouse epidermis. (c) The same characterization as above using grafts from unmodified (um), clonal (c6) or polyclonal (pc) populations of KRT14 gene-targeted keratinocytes from a patient with EBS (see Figure 5 for molecular characterization of these cells). (i) gross appearance; (ii) H&E stained sections, and immunostained sections using (iii) antihuman-specific involucrin antibody, (iv) antibody to keratin 10, and (v) antibody to keratin 14. Bar = 20 µm.
The gross appearance of the grafts was normal and the engrafted human skin functioned effectively by physically protecting underlying tissues, preventing infection, and limiting water loss (Figure 6a,c). Hematoxylin and eosin staining of paraffin-embedded tissue sections revealed an organized, stratified epidermal layer with cornification and keratinization in the upper epidermal layers (Figure 6b). The engrafted skin generally contained more cell layers than the adjacent mouse skin and could be distinguished microscopically by the lack of dermal structures in the engrafted tissue, as well as specific staining for human involucrin in the apical layer of keratinocytes with a antibody specific for the human protein (Figure 6b,c). Normal skin architecture and organization is demonstrated by KRT14 immunostaining in the basal layer (Figure 6b,c), and KRT10 immunostaining in the apical layers of the graft (Figure 6b,c). The KRT14-targeted patient clone 6 maintained a normal karyotype after expansion to over 1013 cells demonstrating the proliferative capacity of single clones, and the ability to generate grafts that could cover large skin surfaces (Supplementary Figure S2).
These data demonstrate that normal and diseased human keratinocytes can be modified by gene targeting at a specific genomic locus, expanded in culture, and engrafted to form normal functioning skin surfaces. We also show that clonal (Figure 6a–c) and polyclonal populations of KRT14-targeted keratinocytes (Figure 6c, polyclonal) form skin grafts that are histologically indistinguishable from those formed by unmodified cells (Figure 6c, unmodified). Finally, we demonstrate for the first time a growth advantage of phenotypically corrected human keratinocytes from EBS patients.
Discussion
Epidermal stem cells are attractive cell targets for gene modification given their growth characteristics, their ability to be studied in xenotransplant models,30 and their history of successful use for autologous transplantation when life threatening full thickness burns have occurred.11,31 These cells qualify as adult stem cells because of their ability to divide almost indefinitely, and the ability to terminally differentiate to specialized cell types when environmental or spaciotemporal cues are present.32 More recently epidermal stem cells have been shown to reprogram frequently to pluripotent embryonic cell states with the overexpression of only two transcription factors further demonstrating their unique characteristics among adult cell types.33
Clinically feasible gene-targeting strategies in keratinocytes have been hindered by the lack of techniques that allow efficient delivery of targeting templates to the nucleus of cells without altering their growth phenotype. Until now this has also included delivery of templates using AAV vectors because keratinocytes have proven to be resistant to transduction.18 Here, we demonstrate, the ability to efficiently transduce primary human keratinocytes in culture using AAV vectors that allow specific modification of genes and demonstrate the utility of this approach in cells where ex vivo culture, expansion, and transplantation has been widely studied and used therapeutically.
Using the gene knockout strategy demonstrated here, each KRT14 allele has a 50% chance of being disrupted resulting in a targeted cell population containing mixture of cells modified at the mutant or normal KRT14 alleles. Thus, we anticipated that it would be necessary to isolate and expand targeted clones containing insertions on the mutant allele for transplantation. Although clonal isolation and transplantation of modified cells was demonstrated to be a feasible approach, we observed that cells with disruption of transcription from the mutant allele rapidly dominate targeted cell populations after a short growth period in culture. This is an important observation for several reasons: first, it suggests that after KRT14-targeted cells are collected, they could be transplanted without clonal isolation allowing ex vivo expansion and associated risks to be minimized. Second, in vivo gene-targeting approaches may also be feasible as similar selective pressures may be active as keratinocytes naturally proliferate. In vivo application of AAV vectors would also have the benefit of allowing proliferation of targeted cells in vivo and minimize risks associated with preparative conditioning and skin transplantation.
We observed similar gene-targeting frequencies, and a low frequency of integration at off target sites whether transduction events were isolated by sorting for GFP positive cells, or selected using G418 resistance (Supplementary Figure S1). Interestingly, G418 resistant clones 3 and 10 were homozygous for targeted disruption of KRT14 raising the possibility that both alleles were targeted independently (an unlikely event that would be predicted to occur in <1 in 500 targeted clones) or more likely a strand crossover occurred centromeric to the KRT14 locus causing loss of heterozygosity from the targeted region to the telomere of chromosome 17. The latter possibility has been previously observed and selection for these events used as a technique to generate homozygous knockouts in mouse ES cells.34,35,36 The application of growth selection may have favored the isolation of these clones because of a growth advantage due to the presence of two neo genes and increased resistance to G418 explaining why such events were not observed when GFP fluorescence was used to identify targeted cells.
An AAV-mediated gene-targeting approach has several advantages that lend itself to rapid translation to the clinic. First, the high frequency of specific modifications at the KRT14 locus allow for a clinically relevant number of keratinocytes to be phenotypically corrected in a simple ex vivo transduction. Second, the selective advantage conferred by knockout of the dominant-negative form of KRT14 allows for amplification of corrected cells ex vivo before transplantation, and allows a competitive advantage for transplanted cells during engraftment. Third, keratinocyte culture has been demonstrated in synthetic media, free of animal products. Finally, the proliferative potential of keratinocyte holoclones allows for significant expansion of corrected cells to produce large, clinically relevant skin grafts. This approach can be translated to the clinic by simply removing the GFP cassette from targeted cells before transplantation, or through the use of a nonimmunogenic detection cassette such as CD24 (ref. 37) that would allow purification of modified cells. Importantly, the molecular analysis of KRT14-targeted keratinocytes shows that modifications are highly specific, that epidermal stem cells with a holoclone phenotype are not depleted from targeted cell populations, and the frequency of random integrants will be low (<4% in this study) suggesting the approach should also be safe.
Materials and Methods
All experiments performed on mice were approved by the Institutional Animal Care and Use Committee, University of Washington, Seattle, Washington.
Cell culture. Cells were grown at 37 °C in 5% CO2. NIH-3T3 J2 feeder cells were cultured in Dulbecco's modified Eagle's medium containing 4 g of glucose per liter (Gibco/Invitrogen, Carlsbad, CA), 10% heat-inactivated calf serum, penicillin, and streptomycin. Keratinocytes were cocultured with irradiated (6,000 rad) feeders of NIH-3T3 J2 variant cells (kindly provided by Dr Green), and “FAD” media formulations previously described.38
Human keratinocyte isolation from skin biopsies. Normal human keratinocytes and keratinocytes from patients with EBS were cultured from skin biopsies by 15-hour digestion of tissue fragments in a solution of Dulbecco's modified Eagle's medium and dispase (10 mg/ml) at 4 °C. The epidermis was separated from the dermal layer using forceps and floated on a drop of trypsin–EDTA–phosphate-buffered saline solution (0.5 mg/ml trypsin) for 15–20 minutes. Loose cells were collected, washed, and seeded to dishes containing irradiated NIH-3T3-J2 feeders (2.5 × 104 cells/cm2).
Human keratinocyte transduction by AAV vectors. Keratinocytes were transduced by seeding 5 × 104 cells each to multiple collagen coated wells of a 12-well dish in keratinocyte serum-free medium (Gibco/Invitrogen) on day −1. AAV vector preparations were added at various multiplicities of infection on day 0, and on day 1; cells were detached with trypsin and seeded to larger dishes containing irradiated feeders (FAD medium). These cells were expanded for 7–10 days and colonies isolated by ring cloning when G418 selection (0.15 mg of active compound/ml) was applied, or cells from multiple dishes were combined, sorted for GFP fluorescence, and isolated by ring cloning 7 days after colonies formed from sorted cells.
Plasmids and DNA analysis. The KRT14 gene-targeting vector was constructed by PCR amplification of a fragment of the gene containing bps 1,354–4,217 of the gene. An internal ribosomal entry site-GFP expression cassette was cloned into the FspI site in exon 3, and the entire sequence inserted between the inverted terminal repeat sequences cloned from a serotype 2 AAV isolate.39 Packaging plasmids pDG (ref. 40) and pDGM6 (ref. 23) used for AAV vector production have been previously described. Plasmids were purified using a plasmid maxi kit (Qiagen, Valencia, CA) Genomic DNAs were isolated and Southern blots were performed by standard techniques.
Vector preparations. Vectors expressing human placental alkaline phosphatase and the AAVK14e3IFsA and AAVK14e3INsA vectors were made by transfecting HEK293 cells with plasmids, pCMVHPLAP (ref. 41), pA2K14e3IFsA, or pA2K14e3INSA and helper plasmids pDG (ref. 40) for pseudotyping with capsids from serotype 2 isolates or pDGM6 (ref. 23) for pseudotyping with capsids from serotype 6 isolates. Cell lysates were treated with, benzonase and vector particles purified using an iodixanol step gradient and heparin affinity column chromatography (HiTrap; GE Healthcare, Piscataway, NJ),25 and HiTrap desalting column. AAV vector titers were based on the amount of full-length single-stranded vector genomes detected by alkaline Southern blot analysis. Southern blot hybridization signals were quantified by phosphorimager analysis (GE Healthcare).
Histology and immunohistochemistry. Hematoxylin and eosin stains, or antibody stains for human involucrin (mouse monoclonal SY5; Sigma, St Louis, MO) were performed on tissue sections fixed for 7 hours in 2% formaldehyde and embedded in paraffin. Five µm thick sections were dewaxed following standard protocols and the primary mouse antihuman involucrin antibody was added at a 1:200 dilution overnight at 4 °C. The next day the specifically bound primary antibody was detected using a biotin-conjugated goat anti-mouse secondary antibody (ImmunoCruz Staining Kit; Santa Cruz Biotechnology, Santa Cruz, CA). Keratin 10 and Keratin 14 immunohistochemistry was performed on skin biopsies prepared by sequential immersion for 2 hours in 10, 15, and 20% sucrose solutions of phosphate-buffered saline before embedding in Optimal Cutting Temperature compound. Five to ten µm thick sections were cut using a cryotome and placed on Superfrost Plus slides (Thermo Fisher Scientific, Waltham, MA). Sections were air dried for 20 minutes, fixed for 10 minutes in 2% formaldehyde, blocked with normal goat serum and stained with primary monoclonal mouse antihuman antibodies. The antibodies reactive against keratin 10 (VIK-10) and keratin 14 (RCK107; Santa Cruz Biotechnology) were used at 1:200 and 1:100 dilutions, respectively, and were coupled to Alexa 488–labeled Zenon conjugated (Invitrogen, Carlsbad, CA) Fab fragments following the manufacturer's protocol.
Formation of human skin equivalents. A human fibrin matrix containing normal human fibroblasts was prepared following procedures previously described.42,43 Primary human fibroblasts were resuspended in 3.6 ml Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and penicillin/streptomycin to a final concentration of 3.1 × 104 cells/ml and human cryoprecipitate (0.19 ml) and aprotinin (3,000 KIU/ml; Calbiochem, San Diego, CA) was added to the cell suspension. The fibrin–aprotinin solution was mixed with 0.275 ml of 0.025 mol/l calcium chloride containing 0.35 units of thrombin (Calbiochem) and 2 ml of the mixture transferred into a 9.2 cm2 culture well. The fibrin was allowed to polymerize at 37 °C for 1–2 hours and then submerged in FAD medium without epidermal growth factor and supplemented with aprotinin (150 KIU/ml). A volume of 1 × 105 human keratinocytes were seeded on the polymerized fibrin gel and allowed to proliferate until confluence was reached (typically 5–7 days). The skin equivalent was detached from the edge of the well using a sterile spatula and lifted to the prepped area of the mouse with forceps.
Transplantation of human dermal equivalents to nude mice. Nude mice (nu/nu, stock #2019; Jackson Labs, Sacramento, CA) were anesthetized with a ketamine (125 mg/kg) and xylazine (6 mg/kg) mixture and placed on a sterile field warmed with a heating pad. The dorsal skin surface was cleaned with betadine and isopropyl alcohol and a full thickness circle of skin the same diameter as the skin equivalent was removed and devitalized by freezing in liquid N2 and thawing three times. The human graft was manually detached from the well, transplanted orthotopically on the prepared surface, and the devitalized patch was sutured in place using 5.0 ethicon sutures. The surgical area was bandaged by spraying with NewSkin (Irvington, NY) and postoperatively mice were given 1 ml of saline subcutaneously and 0.1 mg/kg bupronorphine subcutaneously.
SUPPLEMENTARY MATERIAL Figure S1. Southern blot hybridization analysis of G418R Human keratinocyte clones. a) Genomic DNA from 14 G418R normal Human keratinocyte clones transduced with a KRT14 gene targeting vector identical to AAVKRT14e3IFsA but containing a neomycin phosphotransferase (neo) gene in place of GFP was probed with a genomic DNA fragment from the Human KRT14 locus. The presence of a 5.9 kb band indicates insertion of the IRES-neo expression cassette into exon 3 of the KRT14 gene. b) The same blot as in (a) stripped of the KRT14 probe and probed with a DNA fragment from the neo gene showing the absence of vectors integrated at random genomic locations, and a common sized neo-KRT14 junction fragment in all clones. c) Diagram of targeted and wild-type KRT14 loci with Bam HI fragments bracketed and regions of probe homology indicated. Numbered wide black boxes indicate translated exons, narrower black boxes indicate 5' and 3' untranslated regions. Transcription start sites are indicated by bent arrows. Figure S2. KRT14-targeted EBS patient derived keratinocyte clones can be expanded in culture and retain a normal karyotype. a) EBS patient derived KRT14 -targeted keratinocyte clone 6 shown in figures 5 and 6 was allowed to proliferate in cell culture. Every 3 days cells were detached with trypsin, counted, and reseeded at a density of 3.5 x 103 cells / cm2. The number of cells vs. days in culture is plotted. The red bar drawn at the value of 1010 cells indicates the number of cells necessary to transplant the entire skin surface of a single adult individual. b) On day 62, an aliquot of the cells was used for karyotype analysis and a normal karyotype (46 X,Y) was observed after expansion to over 1013 cells.
Acknowledgments
We are especially grateful to Dr Sybert for help in identifying subjects, obtaining samples, helpful discussion, and valued advice. We thank Dr Stephens for helpful discussion regarding mutational analysis of EB patient clones, and Dr Trobridge, Dr Block, Dr Carter, and Dr Wang for helpful discussions and technical suggestions. These studies were funded by grants from the US NIH, NIAMS.
Supplementary Material
Southern blot hybridization analysis of G418R Human keratinocyte clones. a) Genomic DNA from 14 G418R normal Human keratinocyte clones transduced with a KRT14 gene targeting vector identical to AAVKRT14e3IFsA but containing a neomycin phosphotransferase (neo) gene in place of GFP was probed with a genomic DNA fragment from the Human KRT14 locus. The presence of a 5.9 kb band indicates insertion of the IRES-neo expression cassette into exon 3 of the KRT14 gene. b) The same blot as in (a) stripped of the KRT14 probe and probed with a DNA fragment from the neo gene showing the absence of vectors integrated at random genomic locations, and a common sized neo-KRT14 junction fragment in all clones. c) Diagram of targeted and wild-type KRT14 loci with Bam HI fragments bracketed and regions of probe homology indicated. Numbered wide black boxes indicate translated exons, narrower black boxes indicate 5' and 3' untranslated regions. Transcription start sites are indicated by bent arrows.
KRT14-targeted EBS patient derived keratinocyte clones can be expanded in culture and retain a normal karyotype. a) EBS patient derived KRT14 -targeted keratinocyte clone 6 shown in figures 5 and 6 was allowed to proliferate in cell culture. Every 3 days cells were detached with trypsin, counted, and reseeded at a density of 3.5 x 103 cells / cm2. The number of cells vs. days in culture is plotted. The red bar drawn at the value of 1010 cells indicates the number of cells necessary to transplant the entire skin surface of a single adult individual. b) On day 62, an aliquot of the cells was used for karyotype analysis and a normal karyotype (46 X,Y) was observed after expansion to over 1013 cells.
REFERENCES
- Uitto J., and, Richard G. Progress in epidermolysis bullosa: genetic classification and clinical implications. Am J Med Genet C Semin Med Genet. 2004;131C:61–74. doi: 10.1002/ajmg.c.30035. [DOI] [PubMed] [Google Scholar]
- Fine JD, Eady RA, Bauer EA, Bauer JW, Bruckner-Tuderman L, Heagerty A, et al. The classification of inherited epidermolysis bullosa (EB): Report of the Third International Consensus Meeting on Diagnosis and Classification of EB. J Am Acad Dermatol. 2008;58:931–950. doi: 10.1016/j.jaad.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Bonifas JM, Rothman AL., and, Epstein EH., Jr Epidermolysis bullosa simplex: evidence in two families for keratin gene abnormalities. Science. 1991;254:1202–1205. doi: 10.1126/science.1720261. [DOI] [PubMed] [Google Scholar]
- Coulombe PA, Hutton ME, Letai A, Hebert A, Paller AS., and, Fuchs E. Point mutations in human keratin 14 genes of epidermolysis bullosa simplex patients: genetic and functional analyses. Cell. 1991;66:1301–1311. doi: 10.1016/0092-8674(91)90051-y. [DOI] [PubMed] [Google Scholar]
- Livingston RJ, Sybert VP, Smith LT, Dale BA, Presland RB., and, Stephens K. Expression of a truncated keratin 5 may contribute to severe palmar–plantar hyperkeratosis in epidermolysis bullosa simplex patients. J Invest Dermatol. 2001;116:970–974. doi: 10.1046/j.1523-1747.2001.01324.x. [DOI] [PubMed] [Google Scholar]
- Ehrlich P, Sybert VP, Spencer A., and, Stephens K. A common keratin 5 gene mutation in epidermolysis bullosa simplex–Weber-Cockayne. J Invest Dermatol. 1995;104:877–879. doi: 10.1111/1523-1747.ep12607050. [DOI] [PubMed] [Google Scholar]
- Stephens K, Sybert VP, Wijsman EM, Ehrlich P., and, Spencer A. A keratin 14 mutational hot spot for epidermolysis bullosa simplex, Dowling-Meara: implications for diagnosis. J Invest Dermatol. 1993;101:240–243. doi: 10.1111/1523-1747.ep12365079. [DOI] [PubMed] [Google Scholar]
- Pfender EG., and, Bruckner AL.updated 2008Epidermolysis Bullosa Simplex. In: GeneReviews at Gene Tests: Medical Genetics Information Resource (database online) Copyright, University of Washington, Seattle. 1997-2010; . < http://www.genetests.org >. Accessed 19 Mar 2010. [Google Scholar]
- Ito M, Liu Y, Yang Z, Nguyen J, Liang F, Morris RJ, et al. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med. 2005;11:1351–1354. doi: 10.1038/nm1328. [DOI] [PubMed] [Google Scholar]
- Jones PH., and, Watt FM. Separation of human epidermal stem cells from transit amplifying cells on the basis of differences in integrin function and expression. Cell. 1993;73:713–724. doi: 10.1016/0092-8674(93)90251-k. [DOI] [PubMed] [Google Scholar]
- Gallico GG, 3rd, O'Connor NE, Compton CC, Kehinde O., and, Green H. Permanent coverage of large burn wounds with autologous cultured human epithelium. N Engl J Med. 1984;311:448–451. doi: 10.1056/NEJM198408163110706. [DOI] [PubMed] [Google Scholar]
- Rheinwald JG., and, Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975;6:331–343. doi: 10.1016/s0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- Barrandon Y., and, Green H. Three clonal types of keratinocyte with different capacities for multiplication. Proc Natl Acad Sci USA. 1987;84:2302–2306. doi: 10.1073/pnas.84.8.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathor MB, Ferrari G, Dellambra E, Cilli M, Mavilio F, Cancedda R, et al. Clonal analysis of stably transduced human epidermal stem cells in culture. Proc Natl Acad Sci USA. 1996;93:10371–10376. doi: 10.1073/pnas.93.19.10371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavilio F, Pellegrini G, Ferrari S, Di Nunzio F, Di Iorio E, Recchia A, et al. Correction of junctional epidermolysis bullosa by transplantation of genetically modified epidermal stem cells. Nat Med. 2006;12:1397–1402. doi: 10.1038/nm1504. [DOI] [PubMed] [Google Scholar]
- Nanchahal J, Dover R., and, Otto WR. Allogeneic skin substitutes applied to burns patients. Burns. 2002;28:254–257. doi: 10.1016/s0305-4179(01)00107-3. [DOI] [PubMed] [Google Scholar]
- Braun-Falco M, Eisenried A, Büning H., and, Ring J. Recombinant adeno-associated virus type 2-mediated gene transfer into human keratinocytes is influenced by both the ubiquitin/proteasome pathway and epidermal growth factor receptor tyrosine kinase. Arch Dermatol Res. 2005;296:528–535. doi: 10.1007/s00403-005-0547-y. [DOI] [PubMed] [Google Scholar]
- Gagnoux-Palacios L, Hervouet C, Spirito F, Roques S, Mezzina M, Danos O, et al. Assessment of optimal transduction of primary human skin keratinocytes by viral vectors. J Gene Med. 2005;7:1178–1186. doi: 10.1002/jgm.768. [DOI] [PubMed] [Google Scholar]
- Miller DG, Petek LM., and, Russell DW. Adeno-associated virus vectors integrate at chromosome breakage sites. Nat Genet. 2004;36:767–773. doi: 10.1038/ng1380. [DOI] [PubMed] [Google Scholar]
- Russell DW., and, Hirata RK. Human gene targeting by viral vectors. Nat Genet. 1998;18:325–330. doi: 10.1038/ng0498-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata R, Chamberlain J, Dong R., and, Russell DW. Targeted transgene insertion into human chromosomes by adeno-associated virus vectors. Nat Biotechnol. 2002;20:735–738. doi: 10.1038/nbt0702-735. [DOI] [PubMed] [Google Scholar]
- Gossler A, Joyner AL, Rossant J., and, Skarnes WC. Mouse embryonic stem cells and reporter constructs to detect developmentally regulated genes. Science. 1989;244:463–465. doi: 10.1126/science.2497519. [DOI] [PubMed] [Google Scholar]
- Gregorevic P, Blankinship MJ, Allen JM, Crawford RW, Meuse L, Miller DG, et al. Systemic delivery of genes to striated muscles using adeno-associated viral vectors. Nat Med. 2004;10:828–834. doi: 10.1038/nm1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell DW., and, Hirata RK. Human gene targeting favors insertions over deletions. Hum Gene Ther. 2008;19:907–914. doi: 10.1089/hum.2008.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin SK, Collis P, Hermonat PL., and, Muzyczka N. Adeno-associated virus general transduction vectors: analysis of proviral structures. J Virol. 1988;62:1963–1973. doi: 10.1128/jvi.62.6.1963-1973.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai H, Storm TA., and, Kay MA. Recruitment of single-stranded recombinant adeno-associated virus vector genomes and intermolecular recombination are responsible for stable transduction of liver in vivo. J Virol. 2000;74:9451–9463. doi: 10.1128/jvi.74.20.9451-9463.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larcher F, Del Rio M, Serrano F, Segovia JC, Ramírez A, Meana A, et al. A cutaneous gene therapy approach to human leptin deficiencies: correction of the murine ob/ob phenotype using leptin-targeted keratinocyte grafts. FASEB J. 2001;15:1529–1538. doi: 10.1096/fj.01-0082com. [DOI] [PubMed] [Google Scholar]
- Larcher F, Dellambra E, Rico L, Bondanza S, Murillas R, Cattoglio C, et al. Long-term engraftment of single genetically modified human epidermal holoclones enables safety pre-assessment of cutaneous gene therapy. Mol Ther. 2007;15:1670–1676. doi: 10.1038/sj.mt.6300238. [DOI] [PubMed] [Google Scholar]
- Senoo M, Pinto F, Crum CP., and, McKeon F. p63 Is essential for the proliferative potential of stem cells in stratified epithelia. Cell. 2007;129:523–536. doi: 10.1016/j.cell.2007.02.045. [DOI] [PubMed] [Google Scholar]
- Barrandon Y, Li V., and, Green H. New techniques for the grafting of cultured human epidermal cells onto athymic animals. J Invest Dermatol. 1988;91:315–318. doi: 10.1111/1523-1747.ep12475646. [DOI] [PubMed] [Google Scholar]
- Ronfard V, Rives JM, Neveux Y, Carsin H., and, Barrandon Y. Long-term regeneration of human epidermis on third degree burns transplanted with autologous cultured epithelium grown on a fibrin matrix. Transplantation. 2000;70:1588–1598. doi: 10.1097/00007890-200012150-00009. [DOI] [PubMed] [Google Scholar]
- Blanpain C., and, Fuchs E. Epidermal stem cells of the skin. Annu Rev Cell Dev Biol. 2006;22:339–373. doi: 10.1146/annurev.cellbio.22.010305.104357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aasen T, Raya A, Barrero MJ, Garreta E, Consiglio A, Gonzalez F, et al. Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nat Biotechnol. 2008;26:1276–1284. doi: 10.1038/nbt.1503. [DOI] [PubMed] [Google Scholar]
- Mortensen RM. Double knockouts. Production of mutant cell lines in cardiovascular research. Hypertension. 1993;22:646–651. doi: 10.1161/01.hyp.22.4.646. [DOI] [PubMed] [Google Scholar]
- Mortensen RM, Zubiaur M, Neer EJ., and, Seidman JG. Embryonic stem cells lacking a functional inhibitory G-protein subunit (alpha i2) produced by gene targeting of both alleles. Proc Natl Acad Sci USA. 1991;88:7036–7040. doi: 10.1073/pnas.88.16.7036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- te Riele H, Maandag ER, Clarke A, Hooper M., and, Berns A. Consecutive inactivation of both alleles of the pim-1 proto-oncogene by homologous recombination in embryonic stem cells. Nature. 1990;348:649–651. doi: 10.1038/348649a0. [DOI] [PubMed] [Google Scholar]
- Bergoglio V, Larcher F, Chevallier-Lagente O, Bernheim A, Danos O, Sarasin A, et al. Safe selection of genetically manipulated human primary keratinocytes with very high growth potential using CD24. Mol Ther. 2007;15:2186–2193. doi: 10.1038/sj.mt.6300292. [DOI] [PubMed] [Google Scholar]
- Simon M., and, Green H. Enzymatic cross-linking of involucrin and other proteins by keratinocyte particulates in vitro. Cell. 1985;40:677–683. doi: 10.1016/0092-8674(85)90216-8. [DOI] [PubMed] [Google Scholar]
- Samulski RJ, Chang LS., and, Shenk T. A recombinant plasmid from which an infectious adeno-associated virus genome can be excised in vitro and its use to study viral replication. J Virol. 1987;61:3096–3101. doi: 10.1128/jvi.61.10.3096-3101.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm D, Kern A, Rittner K., and, Kleinschmidt JA. Novel tools for production and purification of recombinant adenoassociated virus vectors. Hum Gene Ther. 1998;9:2745–2760. doi: 10.1089/hum.1998.9.18-2745. [DOI] [PubMed] [Google Scholar]
- Gregorevic P, Schultz BR, Allen JM, Halldorson JB, Blankinship MJ, Meznarich NA, et al. Evaluation of vascular delivery methodologies to enhance rAAV6-mediated gene transfer to canine striated musculature. Mol Ther. 2009;17:1427–1433. doi: 10.1038/mt.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Rio M, Larcher F, Serrano F, Meana A, Muñoz M, Garcia M, et al. A preclinical model for the analysis of genetically modified human skin in vivo. Hum Gene Ther. 2002;13:959–968. doi: 10.1089/10430340252939069. [DOI] [PubMed] [Google Scholar]
- Meana A, Iglesias J, Del Rio M, Larcher F, Madrigal B, Fresno MF, et al. Large surface of cultured human epithelium obtained on a dermal matrix based on live fibroblast-containing fibrin gels. Burns. 1998;24:621–630. doi: 10.1016/s0305-4179(98)00107-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Southern blot hybridization analysis of G418R Human keratinocyte clones. a) Genomic DNA from 14 G418R normal Human keratinocyte clones transduced with a KRT14 gene targeting vector identical to AAVKRT14e3IFsA but containing a neomycin phosphotransferase (neo) gene in place of GFP was probed with a genomic DNA fragment from the Human KRT14 locus. The presence of a 5.9 kb band indicates insertion of the IRES-neo expression cassette into exon 3 of the KRT14 gene. b) The same blot as in (a) stripped of the KRT14 probe and probed with a DNA fragment from the neo gene showing the absence of vectors integrated at random genomic locations, and a common sized neo-KRT14 junction fragment in all clones. c) Diagram of targeted and wild-type KRT14 loci with Bam HI fragments bracketed and regions of probe homology indicated. Numbered wide black boxes indicate translated exons, narrower black boxes indicate 5' and 3' untranslated regions. Transcription start sites are indicated by bent arrows.
KRT14-targeted EBS patient derived keratinocyte clones can be expanded in culture and retain a normal karyotype. a) EBS patient derived KRT14 -targeted keratinocyte clone 6 shown in figures 5 and 6 was allowed to proliferate in cell culture. Every 3 days cells were detached with trypsin, counted, and reseeded at a density of 3.5 x 103 cells / cm2. The number of cells vs. days in culture is plotted. The red bar drawn at the value of 1010 cells indicates the number of cells necessary to transplant the entire skin surface of a single adult individual. b) On day 62, an aliquot of the cells was used for karyotype analysis and a normal karyotype (46 X,Y) was observed after expansion to over 1013 cells.