Abstract
Glycogen storage disease type Ia (GSD-Ia), also known as von Gierke disease, is caused by a deficiency of glucose-6-phosphatase-α (G6Pase), a key enzyme in glucose homeostasis. From birth, affected individuals cannot maintain normal blood glucose levels and suffer from a variety of metabolic disorders, leading to life-threatening complications. Gene therapy has been proposed as a possible option for treatment of this illness. Vectors have been constructed from feline immunodeficiency virus (FIV), a nonprimate lentivirus, because the wild-type virus does not cause disease in humans. Previously, we have shown that these vectors are capable of integrating stably into hepatocyte cell lines and adult murine livers and lead to long-term transgene expression. In the current work, we have assessed the ability to attenuate disease symptoms in a murine model of GSD-Ia. Single administration of FIV vectors containing the human G6Pase gene to G6Pase-α−/− mice did not change the biochemical and pathological phenotype. However, a double neonatal administration protocol led to normalized blood glucose levels, significantly extended survival, improved body weight, and decreased accumulation of liver glycogen associated with the disease. This approach shows a promising paradigm for treating GSD-Ia patients early in life thereby avoiding long-term consequences.
Introduction
Glycogen storage disease type I (GSD-I, von Gierke disease) is a group of autosomal recessive metabolic disorders that occur in 1 out of 100,000 births. GSD-Ia represents over 80% of cases and is caused by a mutation in the glucose-6-phosphatase-α (G6Pase) enzyme.1 G6Pase is a key enzyme in glucose homeostasis that catalyzes the terminal step in the gluconeogenesis and glycogenolysis pathways. This enzyme is located in the endoplasmic reticulum and is expressed mainly in the liver and kidneys. Patients afflicted with GSD-Ia develop hypoglycemia due to their inability to utilize stored glycogen or generate adequate amounts of glucose between meals. Furthermore, their liver and kidneys are enlarged as a result of glycogen and fat accumulation. Additional complications of the disease include growth retardation, hyperlipidemia, gout, hepatic adenomas with a risk of malignancy, osteoporosis, and renal failure.1 Current treatment of GSD-Ia involves continuous infusion of glucose or frequent oral administration of uncooked cornstarch in order to control the symptomatic hypoglycemia; however, these regimens do not cure the disease and long-term complications commonly develop.2 Moreover, efficacy of the dietary therapy is frequently limited due to poor compliance.
Genetically engineered G6Pase knockout (KO) mice and canine colonies carrying a naturally occurring G6Pase mutation both exhibit disease symptoms similar to human patients3,4 and enable the testing of diverse therapeutic approaches to the disease. G6Pase-deficient mice survive, at most, a few weeks even upon daily administration of glucose.5,6 A recent report describes a specific husbandry protocol that allowed ~60% of KO mice to withstand weaning and live to adulthood; however, they required extensive palliative care, and the liver histology and glycogen accumulation did not improve with age.7 In an attempt to provide life-long correction of G6Pase, gene therapy approaches have been employed using adeno- and adeno-associated viral vectors.5,6,8,9,10,11 In the mouse model, efficacy proved highest with the use of adeno-associated viral–based vectors that partially restored G6Pase activity and prolonged survival; however, the mice succumbed to the disease, and the therapy did not completely correct blood glucose levels to those observed in wild-type littermates.5,10,11 Furthermore, in some cases, renal disease was still documented because the transgene was mainly expressed in the liver.5
Adeno-associated viral vectors integrate into the host cell genome at very low frequencies and remain primarily in an episomal form,12,13 thus providing prolonged transgene expression only in the absence of cell division. In human trials, these vectors were shown to induce an immune response resulting in the production of neutralizing antibodies that led to elimination of the transduced cells.14 In contrast to adeno-associated virus, lentiviral vectors efficiently integrate into the cellular genome, providing long-term expression even in cells that are actively dividing.15 Furthermore, an immune response is not elicited to vector components, even upon repeated viral administration.16 Ongoing clinical trials using lentiviral vectors to treat X-linked adrenoleukodystrophy, Parkinson's disease, and β-thalassemia17,18,19 have shown promising results. Due to safety concerns and public fear of human immunodeficiency virus–based delivery systems, vectors were developed from feline immunodeficiency virus (FIV), a nonprimate lentivirus that infects cats and is phylogenetically only distantly related to the primate lentiviruses. Despite repeated exposure to FIV, neither seroconversion nor other evidence of infection in humans has been observed, mainly due to inefficient replication of the virus in human cells owing to the negligible transcriptional activity of the FIV long-terminal repeat (LTR).20,21
The FIV vector used in the present study has been deleted of the enhancer located in the U3 region of the 3′LTR, thus ensuring self-inactivation following transduction (self-inactivating vector).22 In addition, it contains a mutated woodchuck hepatitis post-transcriptional regulatory element (WPRE) located downstream of the transgene, which serves to enhance transgene expression and prevent transcription read-through into cellular genes.23,24,25 The mutation in WPRE ablates the promoter and the translation initiation site of the downstream woodchuck X-protein and thus prevents its potential oncogenic activity.26
Previously, we utilized FIV-based vectors to stably express reporter genes in liver cells in vitro and in vivo.27 Because neonatal intervention in a clinical setting may drastically improve the phenotype in the most critical years of an infant's development, the goal of our present study was to evaluate a novel approach for GSD-Ia therapy based on transduction of neonates with FIV-based vectors harboring the G6Pase gene. Herein, we report that this treatment alleviated disease symptoms and prolonged survival. Thus, we believe that our study can be used as a paradigm for treatment of other metabolic diseases requiring correction at an early stage of life.
Results
Expression of human G6Pase in liver and kidney cell lines following transduction with an FIV-based vector
To test exogenous expression of human G6Pase (hG6Pase), Huh7 (a human hepatocellular carcinoma), HO-15 (hepatocytes derived from G6Pase KO mice), and COS-1 (monkey kidney epithelial cells) were transduced with FIV lentiviral vector particles carrying the hG6Pase gene at a multiplicity of infection of 15 or 1,500. The transgene was driven either by the ubiquitous elongation factor 1-α (EF-1α) promoter or the liver-specific human α-1 antitrypsin (hAAT) promoter (FIV-hAAT-G6Pase). Genomic DNA and total RNA were extracted between 10 and 28 days after transduction, and PCR or RT-PCR was performed using primers specific for the exogenously inserted G6Pase gene. Proviral DNA and RNA transcripts of the hG6Pase gene were detected in all three cells lines (data not shown). RT-PCR results were further verified by northern blot analysis (data not shown). To evaluate the enzymatic activity of the expressed transgene, lysates extracted from cells 10 days after transduction were tested for their ability to hydrolyze glucose-6-phosphate. Extracts from FIV-treated cells yielded a 2- to 11-fold increase in G6Pase activity over untransduced cells (Table 1) indicating that exogenously expressed G6Pase is functional.
Table 1. Biochemical analysis reveals G6Pase activity in FIV-transduced cell lines.
Expression and biodistribution of reporter genes in FIV-transduced neonates
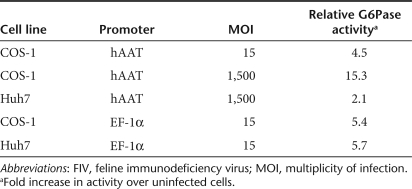
In order to monitor efficiency and biodistribution of transgene expression following neonatal transduction, 1-day-old BALB/c mice were injected via the temporal vein with FIV-based vectors (3 × 107 particles) carrying the luciferase (luc) reporter gene driven either by the hAAT or the cytomegalovirus (CMV) promoter. Bioluminescence evaluation at 2- or 4-week intervals revealed stable transgene expression exclusively in the liver of all treated mice (n = 11) regardless of promoter type, with the hAAT promoter driving significantly stronger hepatic luciferase expression than the CMV promoter (Figure 1a,b). Importantly, luc expression driven by the ubiquitous CMV promoter was detected solely in the liver, indicating that temporal vein injection of neonates leads to restricted delivery to this organ (Figure 1b). To quantitate the number of transduced hepatocytes and to determine vector copy number as a function of time following neonatal transduction, an additional cohort of mice was injected with a viral vector carrying the EGFP reporter gene driven by the hAAT promoter. The mice were killed at 2 weeks of age and at 1-month intervals thereafter (n = 16). The number of EGFP+ hepatocytes was quantified by immunohistochemistry and ranged from 3 ± 1.3% at 2 weeks to a peak of 17 ± 2.3% at 4 months after transduction (Figure 1c,d). In correlation with the kinetics of EGFP expression, the number of proviral copies in the transduced livers (as determined by qPCR) also increased with time, peaking at the age of 3–4 months and remaining stable thereafter (data not shown).
Figure 1.
FIV vectors produce stable and prolonged transgene expression following neonatal administration. One-day-old BALB/c mice were administered 3 × 107 FIV viral particles carrying the gene encoding luciferase or EGFP driven by the liver-specific hAAT promoter or the CMV promoter. (a) At various times after treatment, luciferase expression was measure by CCCD camera imaging; mean ± SD; P < 0.0001. (b) Representative bioluminescence photographs of mice taken 6 months after transduction with the viral vectors, FIV-hAAT-Luc (upper panel) or FIV-CMV-Luc (lower panel). (c) Immunohistochemical detection of EGFP+ cells in liver sections analyzed at the indicated time points following transduction. Bar = 100 µm. (d) Computerized quantification of EGFP+ cells as represented in c using the Ariol automated image analysis system. Data are shown as mean ± SD. EGFP, enhanced green fluorescent protein; FIV, feline immunodeficiency virus.
Alleviation of disease symptoms in G6Pase-α KO mice following neonatal transduction
In our experiments, G6Pase-α−/− newborns died within the first 3 days of life (n = 10), though their life span was extended up to 22 ± 6.5 days (n = 7) upon subcutaneous administration of glucose every 12 hours. In order to prolong survival and alleviate disease symptoms, we administered 3 × 107 FIV viral particles carrying the hG6Pase gene into neonatal G6Pase-α−/− mice via the temporal vein. In addition, glucose was administered every 12 hours. Blood glucose levels of homozygous KO mice receiving only glucose injections never rose above 10 mg/dl (n = 7). In contrast, blood glucose levels in 6 of 15 transduced mice increased to levels ranging from 16 to 128 mg/dl; however, survival of transduced mice was not prolonged over those that received glucose injections alone. Furthermore, the mice continued to suffer from hypoglycemia, their lipid profile exhibited the typical pathological values, and they exhibited severe hepatomegaly and nephromegaly (data not shown).
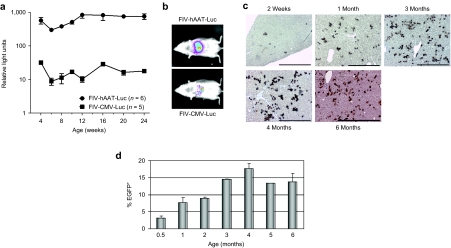
Previous studies showed that repeated viral administrations improved gene therapy efficacy.5,11 Therefore, we administered an initial dose of 3 × 107 FIV-hAAT-G6Pase viral particles on day 1 to a new cohort of mice, as described above, and a second dose of 9 × 107 viral particles at 7 days of age via the retro-orbital sinus. These animals were supplemented with subcutaneous glucose injections, as in the previous cohort, until blood glucose levels rose above 30 mg/dl and were then killed at 4 (n = 5) and 6 months (n = 5) of age. All animals that survived weaning (10/12 mice) lived until the termination of the experiment, whereas untreated mice died within the first 3 weeks (Figure 2a). Fatalities in the treated group that occurred during the first month of life were most likely due to suboptimal vector administration. In accordance with survival, the transduced mice exhibited continuously rising blood glucose levels that reached age-matched control values at 8 weeks after birth (Figure 2b). In addition, body weight of treated mice was only slightly below that of healthy controls (Figure 2c). At the termination of the experiment, viral vector copy numbers were determined in the livers of treated animals by qPCR, and were found to range from 0.03 to 120 copies per 100 cells. Notably, no direct correlation was observed between vector copy number and blood glucose levels (Figure 2d).
Figure 2.
Double administration of FIV-hAAT-G6Pase to knockout neonatal mice leads to prolonged survival and improvement in disease symptoms. G6Pase-α−/− mice were administered a double injection of viral particles or plasmid DNA at 1 and 6 days after birth. (a) Survival curves of mice receiving FIV viral particles carrying the G6Pase-α gene (n = 18), the EGFP reporter gene (n = 2), or naked plasmid DNA containing the G6Pase-α gene (n = 3) (log-rank test; P < 0.0001). (b) Blood glucose levels were measured biweekly, after transduction with the FIV-hAAT-G6Pase vector. Between 4 and 16 mice were sampled at each time point in the treated KO mice group, and between 1 and 8 mice in the healthy age-matched control group (mean ± SD). No significant difference (P < 0.05) was observed between the two groups after the 8th week of surveillance. (c) Mice were weighed at birth, and at 4, 8, 16, and 24 weeks of age; virally transduced G6Pase-α−/− mice (treated KO) n = 31, 6, 3, 5, 5; untreated healthy age-matched siblings (control) n = 45, 3, 2, 4, 2, respectively. Mean ± SD is shown. (d) Proviral vector copy number (VCN) (columns) was measured in livers of FIV-transduced G6Pase-α−/− mice at 4 and 6 months after treatment and compared to serum glucose levels; C, untransduced control. FIV, feline immunodeficiency virus; GFP, green fluorescent protein; KO, knockout.
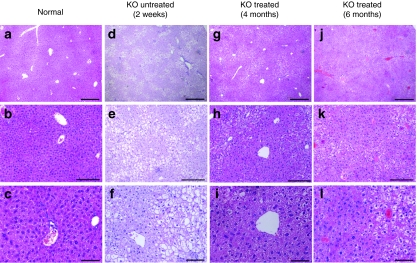
Next, we determined G6Pase enzymatic activity in the microsomal fraction of the livers of treated and control mice. As seen in Figure 3a, at 3 months of age, enzyme activity of treated homozygotes was significantly higher than 2-week-old untreated animals. At 6 months of age, G6Pase activity reached 56% of that detected in age-matched healthy controls. Following double FIV administration, hepatic glycogen accumulation was diminished (Figure 3b), and blood cholesterol and triglycerides levels were dramatically reduced (3.7- and 20-fold, respectively) compared to untreated mice (Table 2). Levels of serum creatinine were within normal limits (data not shown). Evaluation of the relative weight of livers and kidneys of G6Pase-α−/− treated mice revealed persistent organomegaly, which was not reversed by the double viral infusion (Table 3). Nevertheless, histological examination revealed less hepatic fat deposition (steatosis) and fewer areas containing hepatocytes with abnormal morphology in the treated livers as compared to untreated controls (Figure 4). In contrast to the liver, kidney tissue morphology was not improved by the liver-directed treatment and showed typical histopathological findings (data not shown).
Figure 3.
Restoration of hepatic G6Pase-α activity and reduction in hepatic glycogen accumulation in knockout mice following FIV-hAAT-G6Pase transduction. (a) G6Pase-α enzyme activity in microsomes extracted from liver samples was measured at various times after FIV treatment. Healthy siblings (control), n = 2; untreated KO mice at 12 days, n = 3; treated KO mice at 3, 8, and 24 weeks of age, n = 6, 4, 4, respectively. Data are shown as % activity relative to healthy controls ± SD. Values of all treated mice were significantly higher than untreated KO mice (P < 0.05). (b) Liver glycogen content of FIV-transduced mice (n = 5 at 4 and 6 months) relative to healthy controls (n = 3 at 4 months and n = 2 at 6 months); mean fold increase ± SD is shown (P < 0.05 between all groups). (c) The level of ALT and AST liver enzymes was measured in the serum of treated G6Pase-α−/− mice (treated KO) (n ≥ 3) and healthy age-matched controls (n ≥ 2) at 4 (4m) and 6 (6m) months after transduction. *P < 0.05. ALT, alanine transaminase; AST, aspartate transaminase.
Table 2. Cholesterol and triglyceride levels of FIV-treated G6Pase knockout mice.
Table 3. Liver and kidney weights of FIV-transduced G6Pase−/− mice and healthy siblings.
Figure 4.
Reduced lipid accumulation in the livers of G6Pase-α−/− mice following feline immunodeficiency virus vector administration. Representative photomicrographs of liver hematoxylin and eosin–stained sections from (a–c) normal, (d–f) untreated 2-week-old G6Pase-α KO pups, and treated G6Pase-α KO mice (g–i) 4 months and (j–l) 6 months after transduction. Original magnification ×40 in a,d, and g (bar = 100 µm); ×100 in b,e, and h (bar = 50 µm); ×200 in c, f, and i (bar = 20 µm). KO, knockout.
Previous studies demonstrated the presence of chronic low-grade inflammatory hepatitis in GSD-Ia patients and G6Pase-α−/− mice.28,29 In our study, serum levels of alanine transaminase and aspartate transaminase liver enzymes at 4 and 6 months were slightly elevated in treated G6Pase-α−/− mice as compared to healthy controls (Figure 3c); however, upon histological examination, no apparent necrotic foci were seen in untreated controls, treated mice, or healthy siblings (Figure 4).
Discussion
GSD-Ia is a hereditary metabolic disorder that causes significant morbidity and mortality. Modern medicine offers only supportive treatment to alleviate suffering but fails to cure the disease. Advances in gene therapy approaches hold great promise in curing inherited disorders,30 such as GSD-Ia. Herein, we describe the first attempt to target this disease in a murine model by using a nonprimate FIV-based lentiviral vector to introduce the wild-type G6Pase transgene into the liver. We report that viral transduction of neonates resulted in progressively normalized blood glucose levels that significantly prolonged their life span. Furthermore, serum lipid profiles, liver glycogen storage, and body weight were markedly improved, although kidneys and livers remained enlarged. Although our studies were limited to 6 months, no tumor development was observed following transduction with FIV, a possible risk factor associated with integrating viral vectors.31
We found that a single administration of viral particles carrying the transgene to 1-day-old neonates resulted in mild glucose elevation in some mice but did not prolong survival. When a second viral load was added at day 7, which contained three times the number of viral particles that were given to the 1-day-old neonates, we saw a marked improvement in blood glucose levels and survival. We believe that viral load is a major factor in determining the outcome of the treatment, and the ineffectiveness of a single administration to 1-day-old neonates is related to the low viral dose administered to these pups, whose size limits administration to small volumes. In accordance, administration of FIV-EGFP to 1-day-old pups showed that only 3% of hepatocytes expressed the transgene and this may indicate that, in our disease model, this viral dose did not provide adequate serum glucose levels to reverse the phenotypic hypoglycemia. The limitation in the volume of viral particles administered should be negligible in large animals or human clinical trials.
Following a double FIV administration, liver histology revealed areas of normally appearing hepatocytes, as well as regions of cells bearing a high content of glycogen, possibly providing an explanation for the unresolved hepatomegaly. Our biodistribution studies with the luciferase reporter gene driven by the CMV promoter failed to detect transgene expression in organs other than the liver; therefore, as expected, kidneys of the FIV-G6Pase-treated mice displayed typical pathological findings in the form of glycogen accumulation. Regardless of the excess glycogen that was observed in the kidneys even after two viral administrations, this study and others5,9,11 clearly show that hepatic G6Pase-α expression in the liver is sufficient to promote long-term survival in parallel with a substantial improvement in many clinical parameters.
One limitation of the gene therapy approach is the possible induction of an immune response to the therapeutic protein or vector components, which would limit the efficacy and long-term duration of the treatment. It has previously been shown that gene therapy administered at the neonatal stage minimizes immune response to the transgene32,33 as well as to the FIV vector.34 There are also studies showing that the use of a hepatocyte-restricted promoter, as was used in our study, reduces the risk of an immune response to the transgene product in liver-directed gene transfer.35,36 Although we did not directly evaluate immune response, stable and normal serum glucose levels were observed in the treated G6Pase-α−/− mice for the duration of the experiment. Furthermore, our reporter gene studies showed that the number of transduced hepatocytes increased with time reaching a plateau at 3 months of age and remaining stable for up to 6 months. Therefore, we conclude that neonatal transduction with FIV vectors did not elicit an immune response. In addition, although a single vector administration of FIV-G6Pase to 1-day-old neonates did not yield therapeutic results, it is tempting to speculate that immune tolerance was induced by this initial exposure.
In summary, our data indicate that neonatal gene therapy using FIV-based vectors is an attractive treatment approach for early-onset inherited hepatic diseases in general and GSD-Ia in particular. The reported success of recent clinical trials using lentiviral vectors allows hope of bringing this technology into medical reality.
Materials and Methods
Virus preparation. The hG6Pase-α, the luciferase, or the GFP coding sequences were cloned into a third generation FIV-based viral self-inactivating vector driven by the EF-1α, CMV, or hAAT promoters. The vector harbored a WPRE downstream of the transgene that contained a mutated promoter and start codon of the X gene.26,37 Viral particles were produced in the 293T packaging cell line by a three-plasmid co-transfection system, pseudotyped with the VSV-G envelope, and concentrated 100-fold in a Sorvall Discovery 100 ultracentrifuge (Thermoscientific, Asheville, NC) at 25K rpm for 2 hours at 4 °C using an SW30 rotor.38 Titers of unconcentrated and concentrated viral stock preparations were determined to be approximately 107 and 109 transducing units/ml, respectively, as measured by enzyme-linked immunosorbent assay of the p24 viral core protein.
In vitro transduction and molecular analysis. Cell lines, Huh7, HO-15, and COS-1, were plated at 1–3 × 105 in a 6-well tissue culture plate. After 24 hours, the cells were infected at a multiplicity of infection of 15 or 1,500 in the presence of polybrene (5 µg/ml). Cells were passaged for 10–28 days after infection, and genomic DNA, total RNA, and protein lysates were extracted. Proviral DNA and RNA transcripts were detected by PCR and RT-PCR using primers to the hG6Pase gene (sense 5′-GACTACTACAGCAACA-3′ antisense 5′-TGAGCAGCAAGGTAGAT-3′).
Animal procedures. All animal studies were approved by the Hebrew University-Hadassah Medical School ethics review board. One-day-old BALB/c mice (Harlan Laboratories, Rehovot, Israel) were hypothermically anesthetized and were injected with 30 µl viral particles containing 3 × 107 transducing units harboring the gene encoding luciferase or EGFP. The virus was administered via the temporal vein using a 33-gauge needle (TSK STERiJECT; Air-Tite Products, Virginia Beach, VA). One-day-old G6Pase-α KO neonates3 whose blood glucose level was lower than 10 mg/dl (Accu-Chek Aviva\Performa; Roche, Indianapolis, IN) were designated as affected pups. Homozygocity was validated by PCR on genomic DNA (G6Pase: sense 5′-AAGTCCCTCTGGCCATGCCATGGG-3′ and antisense 5′-CCAAGCATCCTGTGATGCTAACTC-3′ neomycin: sense 5′-ATACGCTTGATCCGGCTACCTGCC-3′ and antisense 5′-CATTTGCACTGCCGGTAGAACTCC-3′). G6Pase-α+/+ and −/+ siblings were considered healthy controls. Affected mice received subcutaneous injections of 50 µl of a 15% glucose solution at birth and every 12 hours, with doses adjusted to body weight. Treated G6Pase-α−/− mice were transduced as described above. For double-administration studies, each treated animal received a second administration of viral particles (90 µl) at 1 week of age containing 9 × 107 transducing units via the retro-orbital sinus. Blood glucose levels were measured twice a week, and glucose was continuously administered until serum glucose levels were higher than 30 mg/dl. At 4 and 6 months after injection, the mice were deprived of food for 8 hours, weighed, bled ad mortem, and their liver and kidneys were harvested, frozen, and subjected to molecular and biochemical assays.
In vivo bioluminescent imaging. In vivo imaging was performed on BALB/c mice transduced with FIV-luc after an intraperitoneal injection of 126 mg/kg beetle luciferin with a Roper Chemiluminescence Imaging system cooled CCD camera (Roper Scientific, Trenton, NJ). The images were acquired and quantified by using the MetaVue software (Princeton Instruments, Trenton, NJ).
Immunohistochemistry. Paraffin-embedded liver sections were deparaffinized, rehydrated, and antigen retrieval was performed in 20 mmol/l citrate buffer, pH 6.0. Nonspecific staining was blocked in 3% bovine serum albumin in phosphate-buffered saline/0.25% Triton for 30 minutes. The sections were stained with rabbit anti-EGFP (Invitrogen, Carlsbad, CA) diluted 1:500 overnight at 4 °C followed by anti-rabbit HRP second antibody (DakoCytomation EnVision+ system labeled polymer-HRP; Dako, Glostrup, Denmark) for 1 hour at room temperature. DAB chromogen (SuperPicture HRP Polymer Conjugate Broad Spectrum; Zymed Laboratories, San Francisco, CA) was used for the color reaction, and each slide was counterstained with hematoxylin and eosin solution. The slides were then dehydrated, cleared, and mounted with Histomount (Zymed Laboratories). Quantification was performed using the Ariol automated image analysis system (Applied Imaging, San Jose, CA).
Glycogen content in livers. The glycogen content was measured as previously described.39 Briefly, a 2% liver homogenate was prepared in water. Next, 90 µl of homogenate were mixed with 360 µl 33% KOH solution (Sigma, St Louis, MO) and incubated for 20 minutes at 100 °C. After cooling, 125 µl of sample were added to 875 µl of water and mixed with 2 ml of anthrone solution (2 mg/ml in 95% H2SO4; Sigma), incubated for 20 minutes at room temperature, and OD was measured at 620 nm. Glycogen content was determined by comparing OD to a standard curve.
G6Pase-α enzymatic activity in transduced cell lines and murine livers. G6Pase hydrolytic activity was quantified in cell lysates and microsomes as described elsewhere.8 Briefly, cell lysates from cell lines and microsomes from frozen livers were extracted. Appropriate amounts of cell lysates or microsomal proteins were incubated at 30 °C for 10 minutes in a reaction mixture of 100 µl containing 50 mmol/l sodium cacodylate buffer, pH 6.5, 10 mmol/l Glu-6-P, and 2 mmol/l EDTA. Sample absorbance was determined at 820 nm and was related to the amount of phosphate released using a standard curve constructed with a stock of inorganic phosphate solution. Disrupted microsomal membranes were prepared by incubating intact membranes in 0.2% deoxycholate for 20 minutes at 0 °C.
Blood biochemistry. In order to obtain blood levels of triglycerides, cholesterol, creatinine, alanine transaminase and aspartate transaminase, whole blood was collected, centrifuged, and these parameters were analyzed in the serum using the Reflotron device (Roche).
Measurement of vector copy number by quantitative PCR. The qPCR assay was performed on an AB 7900 HT fast real-time PCR system (Applied Biosystems, Foster City, CA) using primer and probe sequences of the psi packaging signal that were previously published.40 A standard curve representing FIV genome copy numbers ranging from 101 to 107 copies were run in parallel. The amplification was carried out in a final reaction volume of 25 µl containing 12.5 µl of TaqMan Universal PCR Master Mix (Applied Biosystems), 100 mmol/l of each primer and probe, and 10 ng of genomic DNA. All liver samples were tested in duplicate. The linear regression coefficient of the standard curve was 0.99, and the variation between the Ct of each duplicate was <0.5. The number of vector copies per 100 cells was calculated by taking into account that the average diploid nucleus of an adult rat hepatocyte contains 7.7 pg of DNA.41
Acknowledgments
We thank the Children's Fund for Glycogen Storage Disease Research, and Alfy and Lilyan Nathan for financially supporting this work.
REFERENCES
- Chou JY. The molecular basis of type 1 glycogen storage diseases. Curr Mol Med. 2001;1:25–44. doi: 10.2174/1566524013364112. [DOI] [PubMed] [Google Scholar]
- Chou JY., and, Mansfield BC. Gene therapy for type I glycogen storage diseases. Curr Gene Ther. 2007;7:79–88. doi: 10.2174/156652307780363152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei KJ, Chen H, Pan CJ, Ward JM, Mosinger B, Jr, Lee EJ, et al. Glucose-6-phosphatase dependent substrate transport in the glycogen storage disease type-1a mouse. Nat Genet. 1996;13:203–209. doi: 10.1038/ng0696-203. [DOI] [PubMed] [Google Scholar]
- Kishnani PS, Faulkner E, VanCamp S, Jackson M, Brown T, Boney A, et al. Canine model and genomic structural organization of glycogen storage disease type Ia (GSD Ia) Vet Pathol. 2001;38:83–91. doi: 10.1354/vp.38-1-83. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Allamarvdasht M, Pan CJ, Sun MS, Mansfield BC, Byrne BJ, et al. Long-term correction of murine glycogen storage disease type Ia by recombinant adeno-associated virus-1-mediated gene transfer. Gene Ther. 2006;13:321–329. doi: 10.1038/sj.gt.3302650. [DOI] [PubMed] [Google Scholar]
- Chou JY, Zingone A., and, Pan CJ.2002Adenovirus-mediated gene therapy in a mouse model of glycogen storage disease type 1a Eur J Pediatr 161suppl. 1): S56–S61. [DOI] [PubMed] [Google Scholar]
- Salganik SV, Weinstein DA, Shupe TD, Salganik M, Pintilie DG., and, Petersen BE. A detailed characterization of the adult mouse model of glycogen storage disease Ia. Lab Invest. 2009;89:1032–1042. doi: 10.1038/labinvest.2009.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zingone A, Hiraiwa H, Pan CJ, Lin B, Chen H, Ward JM, et al. Correction of glycogen storage disease type 1a in a mouse model by gene therapy. J Biol Chem. 2000;275:828–832. doi: 10.1074/jbc.275.2.828. [DOI] [PubMed] [Google Scholar]
- Koeberl DD, Sun B, Bird A, Chen YT, Oka K., and, Chan L. Efficacy of helper-dependent adenovirus vector-mediated gene therapy in murine glycogen storage disease type Ia. Mol Ther. 2007;15:1253–1258. doi: 10.1038/sj.mt.6300188. [DOI] [PubMed] [Google Scholar]
- Koeberl DD, Pinto C, Sun B, Li S, Kozink DM, Benjamin DK, Jr, et al. AAV vector-mediated reversal of hypoglycemia in canine and murine glycogen storage disease type Ia. Mol Ther. 2008;16:665–672. doi: 10.1038/mt.2008.15. [DOI] [PubMed] [Google Scholar]
- Koeberl DD, Sun BD, Damodaran TV, Brown T, Millington DS, Benjamin DK, Jr, et al. Early, sustained efficacy of adeno-associated virus vector-mediated gene therapy in glycogen storage disease type Ia. Gene Ther. 2006;13:1281–1289. doi: 10.1038/sj.gt.3302774. [DOI] [PubMed] [Google Scholar]
- Niemeyer GP, Herzog RW, Mount J, Arruda VR, Tillson DM, Hathcock J, et al. Long-term correction of inhibitor-prone hemophilia B dogs treated with liver-directed AAV2-mediated factor IX gene therapy. Blood. 2009;113:797–806. doi: 10.1182/blood-2008-10-181479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RH. Adeno-associated virus integration: virus versus vector. Gene Ther. 2008;15:817–822. doi: 10.1038/gt.2008.55. [DOI] [PubMed] [Google Scholar]
- Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ, et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- Zahler MH, Irani A, Malhi H, Reutens AT, Albanese C, Bouzahzah B, et al. The application of a lentiviral vector for gene transfer in fetal human hepatocytes. J Gene Med. 2000;2:186–193. doi: 10.1002/(SICI)1521-2254(200005/06)2:3<186::AID-JGM100>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Sinn PL, Arias AC, Brogden KA., and, McCray PB., Jr Lentivirus vector can be readministered to nasal epithelia without blocking immune responses. J Virol. 2008;82:10684–10692. doi: 10.1128/JVI.00227-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartier N, Hacein-Bey-Abina S, Bartholomae CC, Veres G, Schmidt M, Kutschera I, et al. Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science. 2009;326:818–823. doi: 10.1126/science.1171242. [DOI] [PubMed] [Google Scholar]
- Jarraya B, Lepitit H, Ralph S, Tani N, Boulet S, Jan C, et al. ProSavin a gene therapy approach for Parkinson's disease. Hum Gene Ther. 2009;20:1391–1392. [Google Scholar]
- Leboulch P. Conversion to transfusion independence with partial clonal dominance after lentiviral gene therapy for severe human beta-thalessemia. Hum Gene Ther. 2009;20:1369–1370. [Google Scholar]
- Barraza RA., and, Poeschla EM. Human gene therapy vectors derived from feline lentiviruses. Vet Immunol Immunopathol. 2008;123:23–31. doi: 10.1016/j.vetimm.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauter SL., and, Gasmi M. FIV vector systems. Somat Cell Mol Genet. 2001;26:99–129. doi: 10.1023/a:1021078714105. [DOI] [PubMed] [Google Scholar]
- Zufferey R, Dull T, Mandel RJ, Bukovsky A, Quiroz D, Naldini L, et al. Self-inactivating lentivirus vector for safe and efficient in vivo gene delivery. J Virol. 1998;72:9873–9880. doi: 10.1128/jvi.72.12.9873-9880.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashimoto T, Urbinati F, Perumbeti A, Jiang G, Zarzuela A, Chang LJ, et al. The woodchuck hepatitis virus post-transcriptional regulatory element reduces readthrough transcription from retroviral vectors. Gene Ther. 2007;14:1298–1304. doi: 10.1038/sj.gt.3302979. [DOI] [PubMed] [Google Scholar]
- Zufferey R, Donello JE, Trono D., and, Hope TJ. Woodchuck hepatitis virus posttranscriptional regulatory element enhances expression of transgenes delivered by retroviral vectors. J Virol. 1999;73:2886–2892. doi: 10.1128/jvi.73.4.2886-2892.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schambach A, Galla M, Maetzig T, Loew R., and, Baum C. Improving transcriptional termination of self-inactivating gamma-retroviral and lentiviral vectors. Mol Ther. 2007;15:1167–1173. doi: 10.1038/sj.mt.6300152. [DOI] [PubMed] [Google Scholar]
- Kingsman SM, Mitrophanous K., and, Olsen JC. Potential oncogene activity of the woodchuck hepatitis post-transcriptional regulatory element (WPRE) Gene Ther. 2005;12:3–4. doi: 10.1038/sj.gt.3302417. [DOI] [PubMed] [Google Scholar]
- Condiotti R, Curran MA, Nolan GP, Giladi H, Ketzinel-Gilad M, Gross E, et al. Prolonged liver-specific transgene expression by a non-primate lentiviral vector. Biochem Biophys Res Commun. 2004;320:998–1006. doi: 10.1016/j.bbrc.2004.06.044. [DOI] [PubMed] [Google Scholar]
- Kim SY, Weinstein DA, Starost MF, Mansfield BC., and, Chou JY. Necrotic foci, elevated chemokines and infiltrating neutrophils in the liver of glycogen storage disease type Ia. J Hepatol. 2008;48:479–485. doi: 10.1016/j.jhep.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Chen LY, Yiu WH, Weinstein DA., and, Chou JY. Neutrophilia and elevated serum cytokines are implicated in glycogen storage disease type Ia. FEBS Lett. 2007;581:3833–3838. doi: 10.1016/j.febslet.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor TP., and, Crystal RG. Genetic medicines: treatment strategies for hereditary disorders. Nat Rev Genet. 2006;7:261–276. doi: 10.1038/nrg1829. [DOI] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S, Garrigue A, Wang GP, Soulier J, Lim A, Morillon E, et al. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J Clin Invest. 2008;118:3132–3142. doi: 10.1172/JCI35700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponder KP. Immunology of neonatal gene transfer. Curr Gene Ther. 2007;7:403–410. doi: 10.2174/156652307782151434. [DOI] [PubMed] [Google Scholar]
- Madoiwa S, Yamauchi T, Hakamata Y, Kobayashi E, Arai M, Sugo T, et al. Induction of immune tolerance by neonatal intravenous injection of human factor VIII in murine hemophilia A. J Thromb Haemost. 2004;2:754–762. doi: 10.1111/j.1538-7933.2004.00671.x. [DOI] [PubMed] [Google Scholar]
- Sinn PL, Burnight ER., and, McCray PB., Jr Progress and prospects: prospects of repeated pulmonary administration of viral vectors. Gene Ther. 2009;16:1059–1065. doi: 10.1038/gt.2009.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak S., and, Herzog RW. Progress and prospects: immune responses to viral vectors. Gene Ther. 2010;17:295–304. doi: 10.1038/gt.2009.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Domenico C, Di Napoli D, Gonzalez Y Reyero E, Lombardo A, Naldini L., and, Di Natale P. Limited transgene immune response and long-term expression of human alpha-L-iduronidase in young adult mice with mucopolysaccharidosis type I by liver-directed gene therapy. Hum Gene Ther. 2006;17:1112–1121. doi: 10.1089/hum.2006.17.1112. [DOI] [PubMed] [Google Scholar]
- Themis M, Waddington SN, Schmidt M, von Kalle C, Wang Y, Al-Allaf F, et al. Oncogenesis following delivery of a nonprimate lentiviral gene therapy vector to fetal and neonatal mice. Mol Ther. 2005;12:763–771. doi: 10.1016/j.ymthe.2005.07.358. [DOI] [PubMed] [Google Scholar]
- Zhang B, Xia HQ, Cleghorn G, Gobe G, West M., and, Wei MQ. A highly efficient and consistent method for harvesting large volumes of high-titre lentiviral vectors. Gene Ther. 2001;8:1745–1751. doi: 10.1038/sj.gt.3301587. [DOI] [PubMed] [Google Scholar]
- Seifter S., and, Dayton S. The estimation of glycogen with the anthrone reagent. Arch Biochem. 1950;25:191–200. [PubMed] [Google Scholar]
- Sinn PL, Goreham-Voss JD, Arias AC, Hickey MA, Maury W, Chikkanna-Gowda CP, et al. Enhanced gene expression conferred by stepwise modification of a nonprimate lentiviral vector. Hum Gene Ther. 2007;18:1244–1252. doi: 10.1089/hum.2006.127. [DOI] [PubMed] [Google Scholar]
- Bibbiani C, Tongiani R., and, Viola-Magni MP. I. Quantitative determination of the amount of DNA per nucleus by interference microscopy. J Cell Biol. 1969;42:444–451. doi: 10.1083/jcb.42.2.444. [DOI] [PMC free article] [PubMed] [Google Scholar]