Abstract
Adenoviral (AdV) transfer of sodium iodide symporter (NIS) gene has translational potential, but relatively low levels of transduction and subsequent radioisotope uptake limit the efficacy of the approach. In previous studies, we showed that combining NIS gene delivery with external beam radiotherapy (EBRT) and DNA damage repair inhibitors increased viral gene expression and radioiodide uptake. Here, we report the therapeutic efficacy of this strategy. An adenovirus expressing NIS from a telomerase promoter (Ad-hTR-NIS) was cytotoxic combined with relatively high-dose (50 µCi) 131I therapy and enhanced the efficacy of EBRT combined with low-dose (10 and 25 µCi) 131I therapy in colorectal and head and neck cancer cells. Combining this approach with ataxia-telangiectasia mutated (ATM) or DNA-dependent protein kinase (DNA-PK) inhibition caused maintenance of double-stranded DNA breaks (DSBs) at 24 hours and increased cytotoxicity on clonogenic assay. When the triplet of NIS-mediated 131I therapy, EBRT, and DNA-PKi was used in vivo, 90% of mice were tumor-free at 5 weeks. Acute radiation toxicity in the EBRT field was not exacerbated. In contrast, DNA-PKi did not enhance the therapeutic efficacy of EBRT plus adenovirus-mediated HSVtk/ganciclovir (GCV). Therefore, combining NIS gene therapy and EBRT represents an ideal strategy to exploit the therapeutic benefits of novel radiosensitizers.
Introduction
The role of the sodium iodide symporter (NIS) in mediating radioiodine uptake underpins the unique clinical status of thyroid cancer as a tumor that can be cured by systemic administration of unsealed radioisotope sources, even in the setting of disseminated disease.1,2 In recent years, there has been a growing appreciation of the potential value of using NIS as a means of achieving therapeutic or imaging goals in nonthyroidal tumors. This work has largely focused on viral vector–mediated delivery of NIS and 131I to nonthyroid tumor cells in in vitro and in vivo therapeutic models,3,4,5,6,7,8,9,10,11 but in the past 2 years NIS-expressing vectors have also been administered to patients in early phase clinical trials.12,13 Although, as yet, the levels of radioisotope uptake that have been achieved have been modest and unlikely to deliver therapeutic benefit in single-agent strategies, the potential for exploiting NIS-mediated radioisotopic therapy is considerable, especially if combined with standard therapies such as external beam radiotherapy (EBRT) and radiosensitizing drugs.14,15 In certain tumor types, such as head and neck cancer, even relatively small increases in the radiation dose absorbed by the tumor through radioisotope delivery may yield substantial improvements in tumor control.
We have previously shown that gene expression from replication-defective adenoviral (AdV) vectors is upregulated after EBRT16 and that this process is further enhanced by inhibition of repair of DNA damage.17 We hypothesized that combining AdV-mediated NIS gene therapy with EBRT and DNA repair inhibition should be associated with an increase in cytotoxicity and improvement of the therapeutic effect. Clinically, the above strategy has enormous translational potential for achieving tumor-specific escalation of radiation dose at various tumor sites. In this article, we describe the combination therapeutic effects of NIS gene delivery, EBRT and novel radiosensitizing drugs and demonstrate that this approach has significant potential for future clinical development.
Results
Ad-hTR-NIS mediates 131I-induced cytotoxicity in vitro
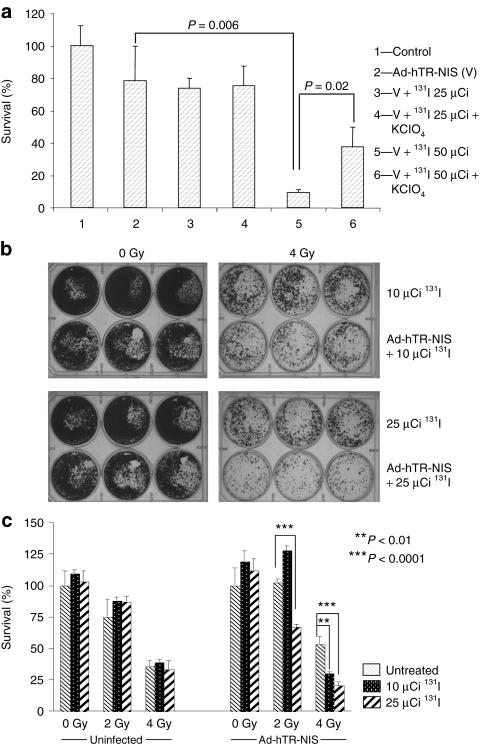
Infection with Ad.hTR-NIS followed by radioiodide therapy was associated with a dose-dependent increase in cytotoxicity. No apparent therapeutic effect—compared to the infected untreated control—was observed after treatment with 25 µCi of 131I. However, when the dose of radioiodide was increased to 50 µCi, a marked increase in cytotoxicity was observed with <10% of cells surviving (P = 0.006) (Figure 1a). Furthermore, the radioiodide-induced cytotoxic effect was significantly inhibited by the competitive inhibitor perchlorate (P = 0.02). These observations clearly show that exogenous NIS transfer followed by 131I therapy is associated with in vitro cytotoxicity.
Figure 1.
Therapeutic activity of NIS-mediated radioisotope therapy. (a) Sodium iodide symporter (NIS)–mediated 131I cytotoxicity in colorectal (HCT116) cancer cells. Cells were infected with Ad-hTR-NIS [multiplicity of infection (MOI) = 1] and treated with 131I (25 µCi or 50 µCi) 72 hours later. Cell survival was assessed by clonogenic assay. No increase in cytotoxicity was observed at a dose of 25µCi 131I compared to the virus alone control. A marked and significant reduction in survival (P = 0.006) was observed when the dose of 131I was increased to 50 µCi. Furthermore, this effect was partially abrogated in the presence of perchlorate (KClO4) (P = 0.02). Data are representative of at least three repeat experiments. (b) Effect of external beam radiotherapy (EBRT; 0 or 4 Gy) combined with Ad-hTR-NIS (MOI = 1) and radioiodide (10 or 25 µCi). Representative photographs of 6-well plates showing reduction in clonogenic survival with the triple therapy (EBRT, virus, radioisotope). Data are representative of at least three repeat experiments. (c) Formal quantitation of the data based on counting tumor clonogens. EBRT (2 Gy) did not induce an independent cytotoxic effect but was associated with significant enhancement of the therapeutic efficacy of 25 µCi of 131I in NIS-infected cells (P < 0.0001). Similarly, combining EBRT (4 Gy) with 10 µCi (P < 0.01) or 25 µCi (P < 0.0001) 131I therapy was associated with a significant increase in cytotoxicity in infected cells. Data are representative of at least three repeat experiments.
Enhanced cytotoxic effect of NIS gene delivery combined with EBRT
We have previously shown that EBRT enhances NIS expression from AdV vectors16 and that this translates into increased iodide uptake in NIS-transduced tumor cells.17 Therefore, we hypothesized that combining EBRT with AdV-mediated NIS transfer followed by administration of 131I should be associated with increased therapeutic efficacy. Cells (HCT116, SIHN-5B) were grown to 70–80% confluency in a 6-well plate, irradiated (0, 2, 4 Gy), then infected with Ad-hTR-NIS (multiplicity of infection = 1) 24 hours later and treated with 131I (10 or 25 µCi) at 72 hours after infection. Because the aim of the experiment was to examine the interaction between therapeutic NIS gene delivery and EBRT, relatively low doses of 131I were used so that they were unlikely to have an independent cytotoxic effect. Cell survival was assessed by clonogenic assay as described above.
Treatment of uninfected HCT116 cells with EBRT and 131I yielded no additional cytotoxicity over and above that seen with EBRT alone. In contrast, enhancement of the therapeutic efficacy of adenovirus-mediated NIS gene therapy in combination with EBRT was seen in HCT116 cells at relatively low doses of 131I (10 and 25 µCi). This effect was observed when 2 Gy was combined with 25 µCi 131I (P < 0.0001) and when 4 Gy was combined with 10 µCi (P < 0.01) and 25 µCi (P < 0.0001) 131I (Figure 1b,c). In SIHN-5B cells, Ad-hTR-NIS infection followed by 10 µCi or 25 µCi 131I was not associated with a cytotoxic effect. However, combination of NIS gene delivery and 4 Gy EBRT was associated with significant enhancement of cytotoxicity after 10 µCi (P < 0.05) or 25 µCi (P < 0.001) of 131I. In contrast to the HCT116 cells, Ad-hTR-NIS infection followed by 131I treatment did not enhance the effect of 2 Gy of EBRT in SIHN-5B cells (Supplementary Figure S1).
Inhibition of DNA repair increases phosphorylated γH2AX focus formation after NIS-mediated 131I uptake
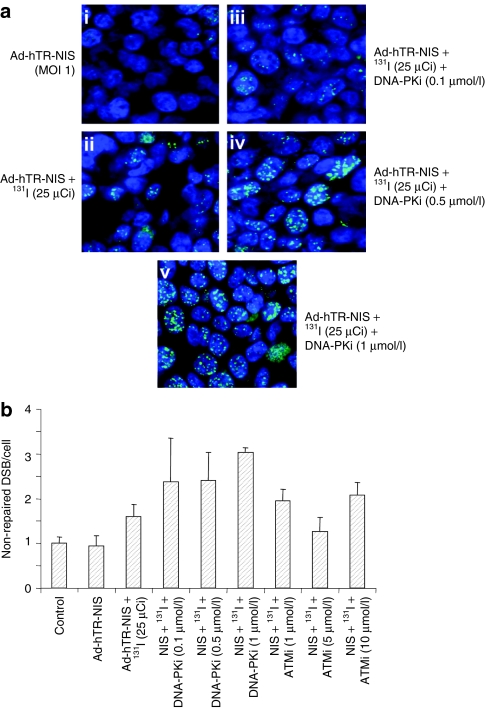
We have previously shown that targeted agents that inhibit DNA repair increase EBRT-induced gene expression from replication-defective AdV vectors through a mechanism that involves maintenance of DNA double-strand breaks (DSBs) (measured as phospho-γH2AX foci). Therefore, we assessed the effect of DNA repair inhibitors in maintaining phospho-γH2AX foci after NIS-mediated 131I radionuclide therapy. HCT116 cells were infected with the Ad-hTR-NIS vector (multiplicity of infection = 1) and treated with 25 µCi of 131I 72 hours later at a time when NIS expression is maximal (data not shown). Cells were exposed to varying concentrations of the DNA-dependent protein kinase (DNA-PK) inhibitor (KU0057788; 0.1, 0.5, 1 µmol/l) or the ataxia-telangiectasia mutated (ATM) inhibitor (KU0055993; 1, 5, 10 µmol/l) from 1 hour before to an hour after treatment with 131I. The concentrations of the DNA repair inhibitors were selected as being nontoxic but capable of maintaining EBRT-induced DSB (data not shown). Twenty-four hours after treatment with 131I, cells were fixed with 4% paraformaldehyde for 1 hour at room temperature. Cells were subsequently stored at 4 °C for a period of approximately three 131I half-lives until the radioactivity had declined to safe levels. The presence of γH2AX foci formation was assessed using confocal microscopy after appropriate primary and secondary antibody staining.
Both ATM and DNA-PK inhibitors were associated with maintenance of phospho-γH2AX foci at 24 hours after 131I therapy compared to the untreated control (Figure 2a,b). Quantitation of this effect by counting foci at confocal microscopy showed that DNA-PKi was associated with a dose-dependent increase in the number of residual phospho-γH2AX foci at 24 hours (Figure 2a,b). A similar effect was seen with the ATMi (Figure 2b).
Figure 2.
DNA repair inhibitors maintain NIS-mediated radioisotope-induced DNA double-strand breaks. Effect of DNA-dependent protein kinase (DNA-PK) inhibitor on maintenance of 131I-mediated phosphorylated γH2AX foci (green dots against blue staining of nuclei) in Ad-hTR-NIS-infected HCT116 cells. (a) Representative confocal microscopic images show (i) very scanty foci in virus-infected cells, (ii) with an increase after treatment with 131I. (iii–v) A dose-dependent increase in γH2AX foci formation was seen in Ad-hTR-NIS-infected cells at 24 hours after application of 25 µCi 131I. (b) Relative number of phospho-γH2AX foci/cell (normalized to the untreated control and displayed as nonrepaired DSBs/cell). Data show a two- to threefold increase in the number of unrepaired DSBs relative to control after treatment with Ad-hTR-NIS, 131I, and DNA repair inhibitors (ATMi and DNA-PKi). Data are representative of at least three repeat experiments. ATMi, ataxia-telangiectasia mutated inhibitor; DSB, double-stranded breaks; NIS, sodium iodide symporter.
The above data show that DNA repair inhibitors are effective in maintaining DNA damage for up to 24 hours after radionuclide therapy. Because radiation-induced cytotoxicity is closely related to cell death mechanisms triggered by DNA damage, increased quantity, and duration of DNA damage observed after use of DNA repair inhibitors should translate into a superior therapeutic response.
Evaluation of the effect of DNA repair inhibition on NIS-mediated 131I therapy
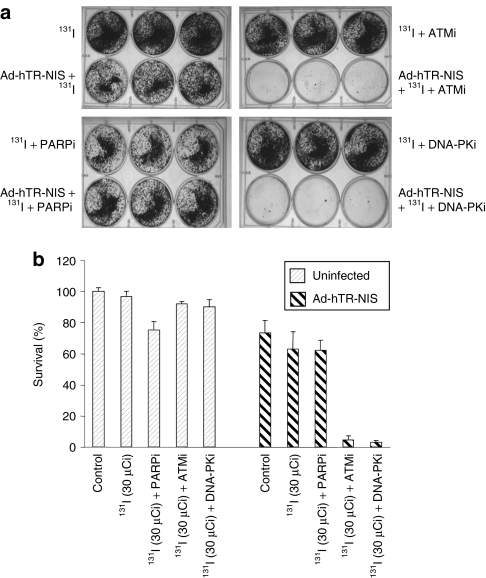
Subsequent experiments were aimed at evaluating the ability of DNA repair inhibitors to sensitize to NIS-mediated 131I therapy. HCT116 cells were infected with Ad-hTR-NIS (multiplicity of infection = 1) and 72 hours later, DNA repair inhibitors [poly-(ADP-ribose) polymerase inhibitor (PARPi): 2 µmol/l, ATMi: 5 µmol/l, DNA-PKi: 1 µmol/l] were added 60 minutes before treatment with 30 µCi 131I. Cell survival was assessed 10–14 days later using clonogenic assay. The concentrations of ATM and DNA-PK inhibitor selected for the study were previously shown to be effective in maintaining DNA damage after 131I therapy. Although the effect of PARPi on 131I-induced DNA damage was not assessed, the concentration used was derived from previous 50% inhibitory concentration curves and other combined studies with EBRT where it was found to be associated with a mild-to-moderate increase in radiosensitivity (data not shown).
Ad-hTR-NIS infection followed by 30 µCi of 131I was associated with no increase in cytotoxicity compared to the untreated control (virus alone). However, when the same treatment was combined with ATMi (5 µmol/l) or DNA-PKi (1 µmol/l), a massive increase in cytotoxicity was observed at clonogenic assay (Figure 3a). In contrast, no increase in cytotoxicity was observed in the presence of the PARPi. Reassuringly, none of the drugs caused radiosensitization in the uninfected cells following administration of 131I (Figure 3a,b).
Figure 3.
NIS-mediated 131I cytotoxicity is enhanced by DNA repair inhibitors. HCT116 cells were infected with Ad-hTR-NIS (multiplicity of infection = 1) and treated with 131I (30 µCi) 72 hours later in the presence or absence of DNA repair inhibitors. (a) Photographs of representative 6-well plates stained with crystal violet to show viable colonies. (b) Cell survival expressed as a % of control based on counting of viable colonies. NIS delivery followed by 30 µCi of 131I was associated with a modest therapeutic effect in the absence of DNA repair inhibitors. However, combined treatment with ATM (5 µmol/l) and DNA-PK inhibitors (1 µmol/l) caused a massive increase in cytotoxicity such that clonogenic survival was reduced to <5%. In contrast, PARP inhibition (2 µmol/l) was not associated with an increase in NIS-mediated 131I cytotoxicity. None of the DNA repair inhibitors enhanced 131I cytotoxicity in cells that were not infected with Ad-hTR-NIS. Data are representative of at least three repeat experiments. ATMi, ataxia-telangiectasia mutated inhibitor; DNA-PKi, DNA-dependent protein kinase inhibitor; NIS, sodium iodide symporter; PARPi, poly-(ADP-ribose) polymerase inhibitor.
Attempts to combine NIS gene delivery, EBRT, and DNA repair inhibition were associated with profound cytotoxic effects such that it was not possible to assess the triple combination in vitro (data not shown).
Combined treatment with Ad-hTR-NIS, EBRT, and DNA repair inhibition has significant in vivo activity
The primary aim of these studies was to evaluate the combined in vivo therapeutic effect of AdV-mediated NIS gene therapy, EBRT, and DNA repair inhibition. Previous in vitro studies have demonstrated that there may be several steps of mutual interaction where these therapeutic modalities may positively influence each other. Therefore, the in vivo studies were designed not to assess the efficacy of any individual treatment, but to assess potential synergistic interactions between the different modalities as a means of improving the overall treatment outcome.
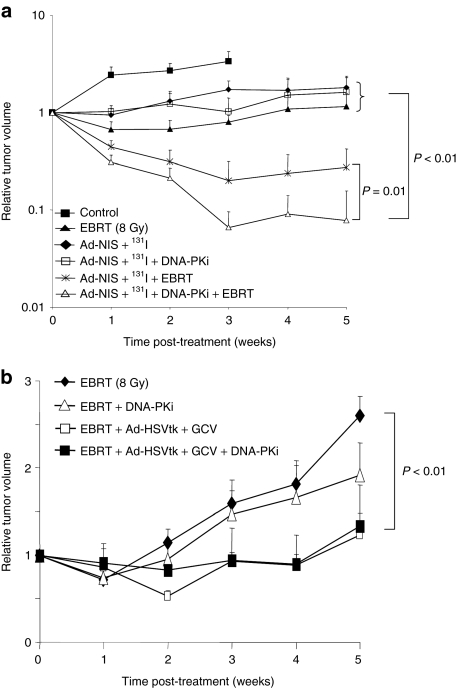
Female MF1 nude mice bearing bilateral tumor xenografts (5–8 mm in diameter) were divided into the following study groups: (i) untreated control (no EBRT, no virus infection, no 131I, no DNA-PKi); (ii) EBRT (8 Gy) alone; (iii) EBRT plus 131I; (iv) EBRT plus DNA-PKi (25 mg/kg); (v) Ad-hTR-NIS (1 × 108 plaque-forming units in 100 µl) plus 131I; (vi) Ad-hTR-NIS plus 131I plus DNA-PKi; (vii) EBRT plus Ad-hTR-NIS plus 131I; and (viii) EBRT plus Ad-hTR-NIS plus 131I plus DNA-PKi. EBRT was delivered to anesthetized mice in relevant groups (ii–iv, vii, viii) as described. For animals randomized to receive the DNA-PKi (groups iv, vi, viii), an intraperitoneal injection of KU0060648 (25 mg/kg) in 100 µl was administered just before radiation and again 24 hours later. In selected animals (groups v–viii), 1 × 108 plaque-forming units of Ad-hTR-NIS was injected intratumorally 24 hours after radiation. 131I (1 mCi) was injected intraperitoneally 48 hours after infection in appropriate animals (groups iii, v–viii). Tumors were measured at baseline and then at weekly intervals. Mice were culled when their tumors doubled in size or reached a maximum size of 1.5 cm in any dimension. The experiment was terminated at 5 weeks (Figure 4a).
Figure 4.
In vivo therapeutic efficacy of NIS gene therapy with EBRT and radiosensitisers. (a) Combined in vivo effects of adenovirus-mediated NIS gene therapy, EBRT, and DNA repair inhibitors in nude mice bearing HCT116 xenografts. Relative tumor volume over time showing that combining Ad-NIS/131I and EBRT (with or without DNA-PKi) was significantly more active than control, EBRT alone, Ad-NIS/131I, and Ad-NIS/131I plus DNA-PKi groups (P < 0.01). The addition of DNA-PKi to Ad-NIS/131I and EBRT was associated with a statistically significant improvement relative to Ad-NIS/131I and EBRT alone (P = 0.01). Controls for Ad-NIS alone, Ad-NIS and EBRT and Ad-NIS plus DNA-PKi were not performed. (b) Combined in vivo effects of adenovirus-mediated suicide gene therapy (Ad-CMV-HSVtk plus GCV), EBRT, and DNA repair inhibition. Combined treatment schedules [Ad-CMV-HSVtk plus GCV plus EBRT, with (P < 0.01) or without DNA-PKi (P < 0.05)] were statistically significantly better than EBRT alone or EBRT plus DNA-PKi. However, the addition of DNA-PKi to Ad-CMV-HSVtk plus GCV and EBRT was not associated with a significant benefit compared to the combined effects of EBRT and gene therapy (P > 0.1). ATMi, ataxia-telangiectasia mutated inhibitor; DNA-PKi, DNA-dependent protein kinase inhibitor; EBRT, external beam radiotherapy; GCV, ganciclovir; NIS, sodium iodide symporter.
Tumor growth delay curves are shown in Figure 4a and Supplementary Figure S2. Mice treated with EBRT showed a reduction in the rate of tumor growth compared to unirradiated controls, but this was not increased in uninfected tumors after the administration of 131I or the DNA-PK inhibitor (Supplementary Figure S2). Mice bearing NIS-infected tumors showed a reduction in the rate of tumor growth following administration of 131I, without objective tumor shrinkage. Furthermore, the tumor response was not altered after addition of DNA-PKi. Therefore, both EBRT and NIS-mediated 131I therapy were associated with similar effects that involved reduction in the rate of tumor growth but no evidence of major response. However, when the two modalities were combined a marked increase in tumor response was observed with objective shrinkage of nearly all tumors (Figure 4a), with 50% (4 of 8) of the tumors demonstrating a complete response at 5 weeks. The therapeutic effect of using the combined protocol was statistically significant (independent samples t-test) at most time points compared to either modality alone. When NIS-mediated 131I therapy was combined with EBRT and DNA-PKi, a dramatic increase in tumor response was observed with 90% (9 of 10) mice achieving a complete response at 5 weeks. The other mouse showed a nearly complete response followed by slow regrowth. The superior effect of combining EBRT with NIS-mediated 131I therapy and DNA repair inhibition was statistically significant at all time points (independent samples t-test) compared to either modality alone.
There was no evidence of excessive local or systemic toxicity in the mice receiving combined-modality treatment schedules. The skin in the treated area did not show any excessive erythema or inflammation following EBRT or 131I therapy. However, after 3–4 weeks the skin around the primary tumor area was paler than the surrounding skin. This phenomenon was more pronounced in mice who demonstrated a good response at the site of their primary tumors.
The above data conclusively demonstrate that EBRT enhances the effectiveness of NIS gene therapy with or without the presence of DNA repair inhibitors. In both circumstances, the effect of adding EBRT was associated with a significant increase in tumor response compared to either modality alone. With respect to DNA repair inhibitors their incorporation in the treatment algorithm was associated with a significantly [P = 0.01 (Mann–Whitney U test) higher response rate at 5 weeks (complete response = 90% versus 50%)] compared to the dual-modality strategy of EBRT and NIS gene therapy.
DNA repair inhibition does not enhance the in vivo therapeutic effect of EBRT combined with Ad-CMV-HSVtk/ganciclovir gene therapy
In the NIS model described above, the enhanced therapeutic efficacy of DNA-PKi combined with EBRT and Ad-hTR-NIS may be explained by increased NIS gene expression due to maintenance of DNA DSB and radiosensitization to EBRT and/or radioisotope therapy. In order to test whether DNA repair inhibition was able to enhance the therapeutic efficacy of a transgene that did not mediate increased radiation dose delivery to the tumor, we repeated the in vivo therapeutic studies with Ad-CMV-HSVtk. Again, HCT116 xenograft tumors were grown in nude mice and treated in the following groups: (i) untreated control (no EBRT, no virus infection, no ganciclovir (GCV), no DNA-PKi); (ii) GCV alone; (iii) EBRT (8 Gy) alone; (iv) EBRT plus DNA-PKi (25 mg/kg); (v) Ad-CMV-HSVtk (1 × 108 plaque-forming units in 100 µl); (vi) Ad-CMV-HSVtk plus GCV; (vii) EBRT plus Ad-CMV-HSVtk plus GCV; and (viii) EBRT plus Ad-CMV-HSVtk plus GCV plus DNA-PKi. EBRT, intratumoral injections of adenovirus and DNA-PKi were administered as per the experiments involving Ad-hTR-NIS. Mice in the appropriate groups (ii, vi–viii) received 100 mg/kg of GCV intraperitoneally twice a day for a period of 7 days after injection of the virus. Tumor growth was monitored as described above.
EBRT caused a significant reduction in tumor growth compared to the unirradiated controls, but without evidence of major response (Supplementary Figure S3). The radiation response was not altered with the addition of DNA-PK inhibitor (Figure 4b). GCV alone or following intratumoral injection of Ad-CMV-HSVtk was not associated with an effect on tumor growth. However, when Ad-CMV-HSVtk plus GCV was combined with EBRT, an improvement in tumor response was observed that was statistically significantly better than EBRT alone (independent samples t-test). However, the addition of DNA-PKi to the combined treatment protocol was not associated with a further increase in tumor response, in contrast to results from the previous experiments with AdV-mediated NIS gene therapy (Figure 4b). None of the mice in the combined treatment groups experienced obvious local or systemic toxicity. Some mice developed skin discoloration at the site of the primary tumor 3–4 weeks post-treatment, which was more marked in mice with a good tumor response.
Discussion
In these studies, we have shown that NIS-mediated radioisotopic therapy is capable of enhancing the therapeutic efficacy of EBRT at clinically relevant doses (2 or 4 Gy) (Figure 1b,c). In fact, by studying radioisotope doses (10 and 25 µCi) that were unable to mediate a direct therapeutic effect, we were able to demonstrate that even relatively low doses of radioisotopic therapy caused significant enhancement of the effect of EBRT. Quantitation of the number of residual DNA breaks at 24 hours after treatment with 131I revealed that NIS-mediated radioisotopic therapy caused DNA DSB and that concomitant treatment with DNA repair inhibitors (DNA-PKi and ATMi) was associated with increased maintenance of these breaks (Figure 2a,b). This finding complements our previous observation that EBRT is able to maintain DNA DSB and that this phenomenon is associated with increased AdV-mediated gene expression.17 It was not possible to test the level of AdV-mediated gene expression after radioisotope-induced DNA damage because of issues relating to radiation safety. However, subsequent therapeutic experiments in which DNA repair inhibitors were combined with radioisotope delivery with or without the addition of EBRT, lent support to the notion that maintenance of radionuclide-induced DNA damage may enhance adenovirus-mediated gene expression.
The combination of NIS gene delivery, 131I radioisotopic therapy and DNA repair inhibition (DNA-PKi and ATMi) was shown to be extremely effective (Figure 3a,b), even with a radioisotope dose that was incapable of mediating an independent therapeutic effect. This is an important consideration for future clinical translation of this approach, given the fact that it is likely that relatively low levels of tumor transduction will be achieved (even with a replication-competent vector) and will result in modest levels of radioisotope uptake and dose delivery in tumor deposits.
The specific nature of the radiosensitization was demonstrated by the fact that DNA repair inhibition did not enhance the effect of 131I treatment of cells that were not infected with a NIS-expressing virus. The profound nature of the radiosensitizing effect of DNA-PKi and ATMi precluded subsequent in vitro analysis of the triple combination of NIS gene therapy, EBRT, and DNA repair inhibition. Instead, these studies were conducted in in vivo models, which showed a statistically significant enhancement of activity for the combination. Indeed, remarkable responses were seen for both groups that received NIS gene therapy and EBRT, but the greatest antitumor efficacy occurred when DNA-PKi was added to the combination. In this situation, 90% of the tumors showed complete regression out to 5 weeks (Figure 4a).
In an attempt to test the nature of the therapeutic benefit from combining DNA repair inhibitors with AdV gene delivery and EBRT, we next used the Ad-CMV-HSVtk/GCV system. Once again, AdV gene delivery was shown to enhance the effect of EBRT when the appropriate prodrug was administered, but the additional use of DNA-PKi did not further increase the therapeutic effect (Figure 4b). Therefore, it seems that the use of a DNA repair inhibitor to enhance gene expression and sensitize to radiation is most likely to be beneficial in circumstances in which the therapeutic transgene delivers a further radiation boost.
The data reported here strongly support the development of viral delivery of NIS in combination with EBRT and novel radiosensitizing compounds. This approach is ideally suited to clinical application in a range of tumor types in which radiation offers an option for durable tumor control. These proof-of-principle studies have been performed with a replication-defective adenovirus, which is unlikely to be the best platform for this approach. Instead, future studies should focus on oncolytic viruses that express NIS as a means of optimizing the therapeutic efficacy of the combination. Measles virus expressing NIS is already in the clinic and would seem to be an ideal initial model. The assessment of alternative radioisotopes, such as 186Re, 188Re, or 211At,18,19,20 would offer the prospect of integrating this approach within a standard outpatient regimen of radiation delivery.
Materials and Methods
Cell lines. HCT116 (colorectal cancer) and SIHN-5B (head and neck cancer) cells were cultured in Dulbecco's modified Eagle's medium containing 5% (vol/vol) fetal calf serum (FCS), 1% (vol/vol) glutamine, and 0.5% (vol/vol) penicillin/streptomycin. Cell lines were maintained at 37 °C and 5% CO2 in a humidified incubator.
AdV vectors. Replication-defective adenovirus vector expressing NIS under the control of the hTR promoter (Ad-hTR-NIS) has been described previously.21 Replication-defective adenovirus encoding HSVtk under the control of the constitutive CMV viral promoter (Ad-CMV-HSVtk) was generated using the AdEasy protocol (AdEasy vector system; Stratagene, La Jolla, CA) according to the manufacturer's instructions.
AdV infection of tumor cells. Infections were performed when cells were 70–75% confluent (~1 × 105 cells in 24-well plates/5 × 105 cells in 6-well plates). Viral infections were performed in Dulbecco's modified Eagle's medium supplemented with 2% FCS, at appropriate multiplicities of infection. Cells were subsequently incubated at 37 °C for at least 4 hours, followed by addition of fresh medium containing 5% FCS.
DNA repair inhibitors. PARPi (KU0058948, C21H21N4O2F),22 ATMi (KU0055993, C21H17NO3S2),23 and the DNA-PKi (KU0057788/NU7441, C25H19NO3S and KU0060648, a water soluble version of KU005788/NU7441)24 were supplied by KuDOS Pharmaceuticals (Cambridge, UK). PARPi stocks were stored at 10 mmol/l in phosphate-buffered saline (PBS) and working concentrations of 1–10 µmol/l were used. ATMi and DNA-PKi stocks were stored at 1 mmol/l in 100% dimethyl sulfoxide (VWR International, Poole, UK) and working concentrations of 1–5 µmol/l were used for ATMi and 1 µmol/l for DNA-PKi. All stock solutions were stored at ‐20 °C and protected from light.
Cell irradiation. Irradiations were performed using a Pantak H.F. 320kV X-ray machine (AGO X-RAY, Reading, UK). Before irradiation of cells, the dose rate was determined using a Farmer Sub-Standard X-ray dosimeter Mk.2/S3 according to the manufacturer's instructions. The dose rate for irradiations was 6.6–6.8 Gy/minute at 240 kVp and 10 mA. Cells were irradiated in 6- or 24-well plates (BD Labware, Franklin Lakes, NJ) or in 25 cm2 tissue culture flasks (BD Biosciences, Bedford, MA) depending on the experimental design.
γH2AX focus assay. Phosphorylated γH2AX foci were used to quantify radiation-induced DSB as described previously.17 Briefly, cells were grown on coverslips in 6-well plates, treated with DNA repair inhibitors, and irradiated 1 hour later. They were subsequently returned to the incubator at 37 °C for 24 hours before being fixed in 4% paraformaldehyde in PBS for 1 hour at room temperature. Following fixation, cells were kept in immunofluorescence fixative (1% bovine serum albumin and 2% FCS in PBS) at 4 °C until stained. At the time of staining, cells at room temperature were permeabilized with a covering volume of 0.2% (vol/vol) Triton X-100 in PBS for 10 minutes, washed, and incubated with immunofluorescence fixative in PBS for 10 minutes. Coverslips were removed and inverted onto 50 µl of mouse anti-phosphohistone γH2AX antibody diluted 1:2,000 in immunofluorescence fixative for 1 hour at room temperature. Coverslips were washed three times and then inverted onto 50 µl of fluorescein isothiocyanate–conjugated donkey anti-mouse secondary antibody diluted 1:250 in immunofluorescence fixative for 40 minutes. Cells were washed thrice with TO-PRO-3 iodide (Molecular Probes, Eugene, OR) diluted 1:10,000 in PBS in a 6-well plate on a rocking platform. Coverslips were inverted onto a drop of DakoCytomation Fluorescent mounting medium on glass slides and viewed with a Nikon Eclipse E500 confocal microscope.
Clonogenic survival assays. For studies involving EBRT alone, tumor cells were seeded in 25 cm2 flasks and irradiated 12–16 hours later (0, 2, 4 Gy). Twenty-four hours later, cells were trypsinized and plated at reducing cell densities in 24-well plates (5,000, 2,500, 1,000, and 500 cells per well). Cells were stained with 0.2% crystal violet in 7% ethanol 10–14 days later. The total number of colonies was counted and cell survival was estimated by normalization to the unirradiated control.
In experiments involving radioiodide treatment, cells were plated at 5 × 105 cells in 6-well plates and infected with Ad-hTR-NIS 16 hours later. Seventy-two hours after infection, cells were treated with a range of doses (10–50 µCi) of Na131I [Amersham Biosciences (GE Healthcare), Uppsala, Sweden)] in 2 ml Hank's buffered salt solution with or without potassium perchlorate (100 µmol/l). Cells were incubated at 37 °C for 90 minutes and then washed twice with ice-cold Hank's buffered salt solution and fresh medium (Dulbecco's modified Eagle's medium with 5% FCS) was added. Cells were trypsinized 24 hours later and plated at clonogenic densities (2,500 cells/well). Plates were stained 10–14 days later with 0.2% crystal violet in 7% ethanol. Colony counting was performed using the Labworks image acquisition and analysis software (UVP, Cambridge, UK). Percent cell survival was estimated by normalizing data to the uninfected untreated control.
For 131I experiments in combination with EBRT, cells were irradiated 24 hours before infection and the rest of the protocol was conducted as above. In experiments involving the use of DNA repair inhibitors, cells were treated with appropriate concentrations of drugs from 1 hour before to an hour after treatment with 131I. Cells were then washed twice with PBS and incubated for a further 24 hours at 37 °C. Subsequently, the cells were trypsinized and plated at clonogenic densities and read-out was obtained as described above.
In vivo irradiation of tumor-bearing mice. Tumor xenografts were established by injecting 1 × 106 HCT116 cells subcutaneously into bilateral flanks of 6-week-old female MF1 nude mice. At 2 weeks, animals with well-formed (5–8 mm diameter) tumors were randomized in to treatment groups (see below). Animal irradiation was performed as described previously.16,17 Briefly, animals were anesthetized by intraperitoneal injection of 100 µl of a 1:1:4 mixture of Hypnorm (fentanyl citrate 0.315 mg/ml, fluanisone 10 mg/ml; Janssen-Cilag, High Wycombe, UK), Hypnovel (midazolam 5 mg/ml; Roche Products, Welwyn Garden City, UK) and water for injection BP (Fresenius Health Care Group, Basingstoke, UK). They were positioned in an irradiation jig with the subcutaneous tumors exposed under an aperture in a 3-mm lead sheet and a localized radiation dose (0 or 8 Gy) was delivered to the tumor using the Pantak H.F. 320kV X-ray machine. Relevant groups also received treatment with intratumoral injections (100 µl) of AdV vectors or PBS, intraperitoneal injections of 131I or PBS, and intraperitoneal injections of DNA-PKi (KU0060648) or PBS. Potassium iodide was added to the drinking water during the pretreatment period in an attempt to reduce normal tissue uptake of radioiodide after administration of 131I.
Statistical analysis. Statistical analysis was performed using SPSS software (SPSS, Chicago, USA) package.
SUPPLEMENTARY MATERIAL Figure S1. Effect of EBRT on NIS mediated 131I cytotoxicity in SIHN-5B cells. Figure S2. In vivo effect of combining adenovirus-mediated NIS gene therapy with EBRT and DNA repair inhibitors in nude mice bearing HCT116 xenografts. Figure S3. Effects of EBRT and DNA repair inhibition in nude mice bearing HCT116 xenografts.
Supplementary Material
Effect of EBRT on NIS mediated 131I cytotoxicity in SIHN-5B cells.
In vivo effect of combining adenovirus-mediated NIS gene therapy with EBRT and DNA repair inhibitors in nude mice bearing HCT116 xenografts.
Effects of EBRT and DNA repair inhibition in nude mice bearing HCT116 xenografts.
REFERENCES
- Dohán O, De la Vieja A, Paroder V, Riedel C, Artani M, Reed M, et al. The sodium/iodide Symporter (NIS): characterization, regulation, and medical significance. Endocr Rev. 2003;24:48–77. doi: 10.1210/er.2001-0029. [DOI] [PubMed] [Google Scholar]
- Haq M., and, Harmer C. Thyroid cancer: an overview. Nucl Med Commun. 2004;25:861–867. doi: 10.1097/00006231-200409000-00001. [DOI] [PubMed] [Google Scholar]
- Mandell RB, Mandell LZ., and, Link CJ., Jr Radioisotope concentrator gene therapy using the sodium/iodide symporter gene. Cancer Res. 1999;59:661–668. [PubMed] [Google Scholar]
- Boland A, Ricard M, Opolon P, Bidart JM, Yeh P, Filetti S, et al. Adenovirus-mediated transfer of the thyroid sodium/iodide symporter gene into tumors for a targeted radiotherapy. Cancer Res. 2000;60:3484–3492. [PubMed] [Google Scholar]
- Nakamoto Y, Saga T, Misaki T, Kobayashi H, Sato N, Ishimori T, et al. Establishment and characterization of a breast cancer cell line expressing Na+/I- symporters for radioiodide concentrator gene therapy. J Nucl Med. 2000;41:1898–1904. [PubMed] [Google Scholar]
- Faivre J, Clerc J, Gérolami R, Hervé J, Longuet M, Liu B, et al. Long-term radioiodine retention and regression of liver cancer after sodium iodide symporter gene transfer in wistar rats. Cancer Res. 2004;64:8045–8051. doi: 10.1158/0008-5472.CAN-04-0893. [DOI] [PubMed] [Google Scholar]
- Gaut AW, Niu G, Krager KJ, Graham MM, Trask DK., and, Domann FE. Genetically targeted radiotherapy of head and neck squamous cell carcinoma using the sodium-iodide symporter (NIS) Head Neck. 2004;26:265–271. doi: 10.1002/hed.10369. [DOI] [PubMed] [Google Scholar]
- Cengic N, Baker CH, Schütz M, Göke B, Morris JC., and, Spitzweg C. A novel therapeutic strategy for medullary thyroid cancer based on radioiodine therapy following tissue-specific sodium iodide symporter gene expression. J Clin Endocrinol Metab. 2005;90:4457–4464. doi: 10.1210/jc.2004-2140. [DOI] [PubMed] [Google Scholar]
- Scholz IV, Cengic N, Baker CH, Harrington KJ, Maletz K, Bergert ER, et al. Radioiodine therapy of colon cancer following tissue-specific sodium iodide symporter gene transfer. Gene Ther. 2005;12:272–280. doi: 10.1038/sj.gt.3302410. [DOI] [PubMed] [Google Scholar]
- Dwyer RM, Bergert ER, O'connor MK, Gendler SJ., and, Morris JC. In vivo radioiodide imaging and treatment of breast cancer xenografts after MUC1-driven expression of the sodium iodide symporter. Clin Cancer Res. 2005;11:1483–1489. doi: 10.1158/1078-0432.CCR-04-1636. [DOI] [PubMed] [Google Scholar]
- Willhauck MJ, Sharif Samani BR, Klutz K, Cengic N, Wolf I, Mohr L, et al. Alpha-fetoprotein promoter-targeted sodium iodide symporter gene therapy of hepatocellular carcinoma. Gene Ther. 2008;15:214–223. doi: 10.1038/sj.gt.3303057. [DOI] [PubMed] [Google Scholar]
- Msaouel P, Dispenzieri A., and, Galanis E. Clinical testing of engineered oncolytic measles virus strains in the treatment of cancer: an overview. Curr Opin Mol Ther. 2009;11:43–53. [PMC free article] [PubMed] [Google Scholar]
- Barton KN, Stricker H, Brown SL, Elshaikh M, Aref I, Lu M, et al. Phase I study of noninvasive imaging of adenovirus-mediated gene expression in the human prostate. Mol Ther. 2008;16:1761–1769. doi: 10.1038/mt.2008.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingorani M, White CL, Agrawal VK, Vidal L, Melcher A., and, Harrington KJ. Combining radiation and cancer gene therapy: a potential marriage of physical and biological targeting. Curr Cancer Drug Targets. 2007;7:389–409. doi: 10.2174/156800907780809787. [DOI] [PubMed] [Google Scholar]
- Harrington KJ, Melcher A, Vassaux G, Pandha HS., and, Vile RG. Exploiting synergies between radiation and oncolytic viruses. Curr Opin Mol Ther. 2008;10:362–370. [PubMed] [Google Scholar]
- Hingorani M, White CL, Zaidi S, Merron A, Peerlinck I, Gore ME, et al. Radiation-mediated up-regulation of gene expression from replication-defective adenoviral vectors: implications for sodium iodide symporter gene therapy. Clin Cancer Res. 2008;14:4915–4924. doi: 10.1158/1078-0432.CCR-07-4049. [DOI] [PubMed] [Google Scholar]
- Hingorani M, White CL, Merron A, Peerlinck I, Gore ME, Slade A, et al. Inhibition of repair of radiation-induced DNA damage enhances gene expression from replication-defective adenoviral vectors. Cancer Res. 2008;68:9771–9778. doi: 10.1158/0008-5472.CAN-08-1911. [DOI] [PubMed] [Google Scholar]
- Willhauck MJ, Sharif Samani BR, Gildehaus FJ, Wolf I, Senekowitsch-Schmidtke R, Stark HJ, et al. Application of 188rhenium as an alternative radionuclide for treatment of prostate cancer after tumor-specific sodium iodide symporter gene expression. J Clin Endocrinol Metab. 2007;92:4451–4458. doi: 10.1210/jc.2007-0402. [DOI] [PubMed] [Google Scholar]
- Carlin S, Akabani G., and, Zalutsky MR. In vitro cytotoxicity of (211)at-astatide and (131)I-iodide to glioma tumor cells expressing the sodium/iodide symporter. J Nucl Med. 2003;44:1827–1838. [PubMed] [Google Scholar]
- Petrich T, Quintanilla-Martinez L, Korkmaz Z, Samson E, Helmeke HJ, Meyer GJ, et al. Effective cancer therapy with the alpha-particle emitter [211At]astatine in a mouse model of genetically modified sodium/iodide symporter-expressing tumors. Clin Cancer Res. 2006;12:1342–1348. doi: 10.1158/1078-0432.CCR-05-1576. [DOI] [PubMed] [Google Scholar]
- Groot-Wassink T, Aboagye EO, Wang Y, Lemoine NR, Keith WN., and, Vassaux G. Noninvasive imaging of the transcriptional activities of human telomerase promoter fragments in mice. Cancer Res. 2004;64:4906–4911. doi: 10.1158/0008-5472.CAN-04-0426. [DOI] [PubMed] [Google Scholar]
- Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–921. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- Hickson I, Zhao Y, Richardson CJ, Green SJ, Martin NM, Orr AI, et al. Identification and characterization of a novel and specific inhibitor of the ataxia-telangiectasia mutated kinase ATM. Cancer Res. 2004;64:9152–9159. doi: 10.1158/0008-5472.CAN-04-2727. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Thomas HD, Batey MA, Cowell IG, Richardson CJ, Griffin RJ, et al. Preclinical evaluation of a potent novel DNA-dependent protein kinase inhibitor NU7441. Cancer Res. 2006;66:5354–5362. doi: 10.1158/0008-5472.CAN-05-4275. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Effect of EBRT on NIS mediated 131I cytotoxicity in SIHN-5B cells.
In vivo effect of combining adenovirus-mediated NIS gene therapy with EBRT and DNA repair inhibitors in nude mice bearing HCT116 xenografts.
Effects of EBRT and DNA repair inhibition in nude mice bearing HCT116 xenografts.