Abstract
Presently, in vivo methods to efficiently and broadly transduce all major cell types throughout both the central (CNS) and peripheral adult nervous system (PNS) are lacking. In this study, we hypothesized that during early fetal development neural cell populations, including neural stem cells (NSCs), may be accessible for gene transfer via the open neural groove. To test this hypothesis, we injected lentiviral vectors encoding a green fluorescent protein (GFP) marker gene into the murine amniotic cavity at embryonic day 8. This method (i) efficiently and stably transduced the entire nervous system for at least 80% of the lifespan of the mice, (ii) transduced all major neural cell types, and (iii) transduced adult NSCs of the subventricular zone (SVZ) and subgranular zones (SGZs). This simple approach has broad applications for the study of gene function in nervous system development and adult NSCs and may have future clinical applications for treatment of genetic disorders of the nervous system.
Introduction
The neural groove of a mouse fetus at gestational day 8 (E8; Theiler stage 12–13) is open to the amniotic cavity. This embryonic ectoderm gives rise to the brain, spinal cord, peripheral nervous system (PNS), autonomic nervous system, and neural crest. Despite the importance of these tissues in normal physiology as well as neurological diseases, a method to efficiently and stably transduce these tissues in a globally distributed manner has been lacking. We hypothesized that in utero injection of lentiviral vector into the amniotic cavity at early gestational ages would enable delivery of gene products to cells within the neural groove and lead to transgene expression in neural-tube-derived tissues in adult mice.
New neurons are continuously generated from neural stem cells (NSCs) and progenitor cells in adult rodent and primate brains.1 NSCs are defined as self-renewing, multipotent cells that generate neurons, astrocytes, and oligodendrocytes in the adult brain. In the adult animal, production of new neurons occurs primarily in two brain regions. NSCs residing in the subventricular zone (SVZ) of the lateral ventricles migrate to give rise to neurons of the olfactory bulb.2 And NSCs in the subgranular zone (SGZ) of the dentate gyrus of the hippocampus migrate locally to give rise to granular hippocampal neurons.3
We observed strong and stable transduction throughout the CNS and PNS in mice ranging in age from E12 up to 2 years. Furthermore, because increased numbers of green fluorescent protein (GFP)–expressing neurons were observed in areas of known neuronal proliferation, such as the dentate gyrus of the hippocampus and the olfactory bulb, we hypothesized and subsequently demonstrated the in vivo transduction of NSCs.
This approach has substantial advantages over existing techniques. For example, stereotactic injection of lentivirus into adult mice results in a spatially limited area of transduction and is relatively inefficient in transducing NSCs.4 In contrast, our results provide a straightforward and efficient means to genetically modify neural precursors and their progeny in vivo. The high frequency of transduction and the global distribution within the CNS and PNS will enable a number of new experimental and clinical approaches including: (i) functional studies of specific genes during nervous system development and adult neurogenesis without the creation of a transgenic animal; (ii) evaluation of the potential impact of gene therapeutics independent of the delivery challenges that currently exist in adult recipients; (iii) studies of the influence of mixed genetic phenotypes in neural development and neurological disease using varying dosages of vector to transduce predetermined proportions of neural cells; (iv) rapidly create models of neurological disease; and (v) delivery of therapeutic vector to treat clinical disorders. Regarding, this last use, as the techniques to diagnose genetic diseases in utero continue to progress, this approach may serve as a basis for future clinical treatments of genetically based disorders of the nervous system. Thus, this simple approach has multiple applications for the study of gene functions in nervous system development, adult NSCs, and neurological disease.
Results
Viability of fetuses following early intra-amniotic gene transfer
Intra-amniotic gene transfer at 8 days of gestation was performed on a total of 93 BALB/c fetuses of which 42 recipients survived postnatally: a survival rate of 45%. In comparison, in uninjected BALB/c mice the postnatal survival of E8 fetuses is ~80%. There were no maternal deaths or morbidities. All fetal recipients that were born alive survived to late adulthood unless they were harvested for analysis. Care was taken to avoid injuring the fetus with the needle and, in contrast to previous reports,5 no cases of exencephaly were observed. Thus, after the immediate postinjection period, there does not seem to be any morbidity or mortality associated with the injection procedure and all mice born were phenotypically normal.
Broad, strong, and stable transduction of the nervous system
A human immunodeficiency virus–based lentiviral construct in which GFP expression was driven by the constitutively active, human cytomegalovirus (CMV) immediate-early promoter (CMV-GFP) was packaged in a vesicular stomatitis virus envelope.6 The brains of all recipients, which were harvested at ages ranging from E12 to 2 years of age, were grossly GFP+ in all regions.
Specifically, GFP expression in large number of CNS and PNS cells was clearly present by E12 (Figure 1a–h). At E12, the frequency of cells expressing GFP, which morphologically appeared to be predominantly neurons, was consistent throughout all areas of the CNS and in the areas of the PNS that were visualized including the spinal ganglia. GFP expression steadily increased during the fetal period and was visible without antibody by E14. In postnatal brain sections, the use of GFP antibody resulted in a 5–10% increased frequency of transduced cells (data not shown). However, as we were interested in cells with higher level transgene expression, no GFP antibody was used to image or quantify histology in recipients killed after birth. All postnatal images and data presented here relied on the optical detection of the GFP protein.
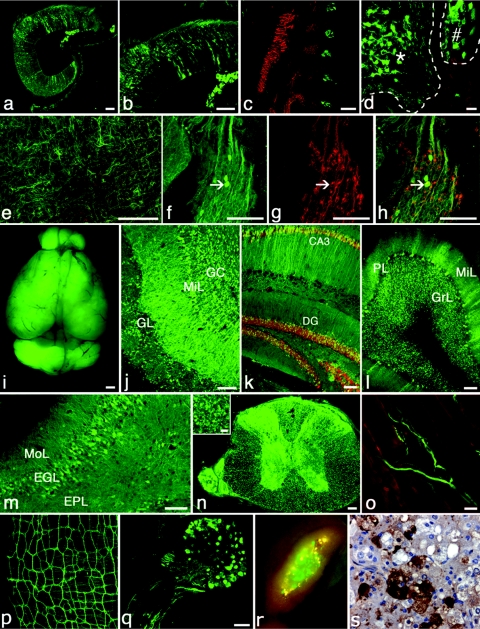
Figure 1.
Broad and efficient transduction of the nervous system with a lentiviral construct expressing CMV-GFP (green in all). Expression after E8 intra-amniotic injection is demonstrated in sagittal sections of E12 (a, b) mouse hindbrain, (e) cortex, and (c, d, f–h) dorsal root ganglia, which are located adjacent to myosin (MF20)-positive somites (red, c). Both the (*, d) developing neural tube and (#, d) adjacent dorsal root ganglion are well transduced. CMV-GFP-expressing neurons within the dorsal root ganglion are identified by co-expression of TrkB (red, g, h). Two years after E8 intra-amniotic delivery, strong and stable CMV-GFP expression is present throughout the CNS: macroscopic view of (i) whole brain for CMV-GFP fluorescence, (j) olfactory bulb, (k) hippocampus (NeuN in red), (l) cerebellum, (m) cerebral cortex with a small portion of internal granular layer in lower right corner, (n) spinal cord with strong GFP expression in both anterior and posterior horns as well as frequent GFP+ axons (inset, corticospinal tract) traveling in both motor and sensory tracts, (o) trigeminal nerve innervating the massetter muscle (red), (p) Auerbach's (myenteric) plexus on the small intestine, (q) dorsal root ganglia with spinal nerve, and (r) gross and (s) histological image of transduced adrenal medulla. Red in o and r is background autofluorescence imaged with rhodamine filter set. Within the adrenal, GFP+ cells are found primarily in the medulla and are surrounded by highly autofluorescent adrenal cells (yellow, r) in the zona reticularis.35 Anti-GFP staining is brown in s. Bar = 20 µm: d, inset of n, o; 100 µm: a–c, e–n, q. CA3, CA3 region of hippocampus; CMV, cytomegalovirus; DG, dentate gyrus; EGL, external granular layer, EPL, external pyramidal layer; GC, granule cells; GFP, green fluorescent protein; GL, glomerular layer; GrL, granular layer; MiL, mitral layer; MoL, molecular layer, PL, Purkinje layer.
Remarkably, GFP expression was stably maintained for at least 2 years, the latest time point evaluated. Examination of brain sections from postnatal mice of various ages (10 days to 2 years) did not reveal any reduction in GFP expression nor in the frequency of GFP-expressing cells over time. Specifically, GFP expression was sufficiently strong at 21 and 24 months that GFP fluorescence was visibly obvious in grossly dissected brains (Figure 1i). Upon sectioning and examination by confocal microscopy, large numbers of cells throughout the CNS and PNS strongly expressed GFP. Within the CNS, representative sections are shown for the olfactory bulb, hippocampus, cerebellum, cortex, and spinal cord (Figure 1j–n, Supplementary Figure S1 a–e,h). The PNS appeared to be broadly transduced as well with GFP expression observed in the cranial and spinal nerves including brachial plexus (extending at least to ulnar, radial, and median nerves), lumbosacral plexus (extending at least to femoral and sciatic nerves), intercostal nerves, and various unidentified nerves in distal limbs (Figure 1o). The intestinal myenteric (Auerbach's) plexus (Figure 1p, Supplementary Figure S1f,g) was strongly transduced. In addition, neural crest derivatives such as dorsal root ganglia (Figure 1d,q) and adrenal medulla (Figure 1r,o) were also highly transduced. Although not every nerve was visualized, it was apparent that the majority of the PNS expressed GFP in all anatomic regions and no areas of the PNS were observed that lacked GFP expression.
Broad transduction of all three main neural cell types
After intra-amniotic delivery of CMV-GFP lentivirus, the transduction efficiency for the major cell types in the CNS (neurons, astrocytes, oligodendrocytes, ependymal cells, and microglia) was evaluated histologically. Of these cell types, neurons were the most efficiently and broadly transduced: ~35–45% of neurons throughout the entire CNS strongly expressed GFP (Table 1, Figures 1j–n and 2a–d,l,m,q,r). In the cerebellum, 46% of Purkinje neurons were transduced and were clearly identifiable when GFP+ by the distinct morphology of their large dendritic extensions (Figure 2h,v,w).
Table 1. Frequency of transduced cells by cell type, vector, and CNS region.
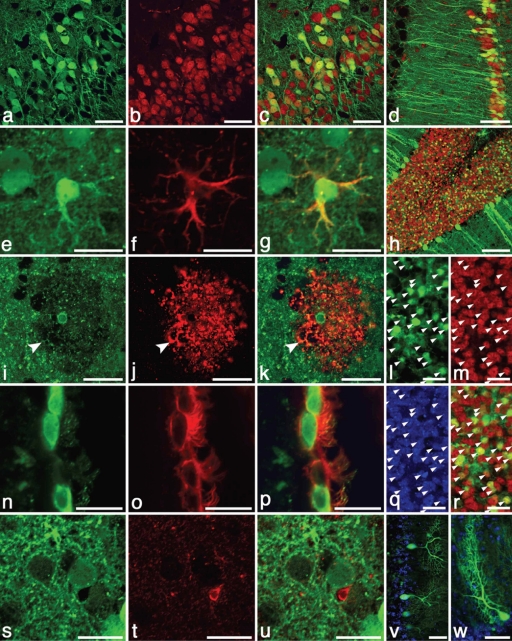
Figure 2.
Strong and persistent expression of CMV-GFP (green in all panels) in neurons and astrocytes but not microglial cells occurred after E8 intra-amniotic lentiviral delivery. Neurons in the cerebral cortex (red, a–c) and hippocampus (d) that co-express NeuN (red, b–d) are strongly transduced with GFP. Even 2 years after injection, astrocytes (e–g) throughout the CNS stably expressed CMV-GFP as well as the astrocyte-specific protein GFAP (red, f–g). Although rare, an oligodendrocyte (i–k) expressing O4 (red, j,k) in the caudate nucleus appears to express CMV-GFP (i,k) as indicated by the GFP-containing cell body that was centrally located within the oligodendrocyte by three-dimensional confocal analysis. The arrow indicates colocalization of green cytoplasm and O4 staining (arrow) but it is not clear whether the GFP is from a myelinated axon or the oligodendrocyte extension. In intact tissue sections, GFP distributes poorly to oligodendrocyte extensions and O4 often does not stain the cell bodies of oligodendrocytes.7 Ependymal cells (n–p) expressing CD24 (red, o,p) of the lateral ventricle were also transduced with GFP. However, no GFP-expressing microglial cells (Iba1 staining, red, t,u) were observed. Even at 2 years of age, GFP expression remains strong throughout the CNS as demonstrated by GFP-expressing cerebellar neurons (h; red is NeuN), granular neurons in the cerebellum (l,m,q,r; NeuN is red, m,r; Hoescht-stained nuclei in blue, q), and morphologically distinct Purkinje neurons (v,w; Hoescht-stained nuclei in blue). Bar = 20 µm: e–g, i–w, 50 µm: a–c,v,w; 100 µm: d,h). CMV, cytomegalovirus; CNS, central nervous system; GFP, green fluorescent protein.
After delivery of CMV-GFP vector, GFP-expressing astrocytes were present but at a much lower frequency than neurons (Table 1, Figure 2e–g). Although the proportion of GFP-expressing neurons was roughly constant throughout all brain and spinal cord regions, the frequency of GFP-expressing astrocytes had clear regional differences within the CNS; GFP-expressing astrocytes (GFAP+) were rare (<1%) in midbrain and cortex but within the medulla ~10% of astrocytes expressed GFP.
Oligodendrocytes that appeared to be transduced were very rare in intact tissue sections (Figure 2i–k). Antibody against myelin basic protein colocalized with many GFP+ extensions but high-resolution confocal imaging revealed that this appeared to be GFP+ axons traveling through GFP−, myelin sheaths (Supplementary Figure S1i–k). Because GFP is typically excluded from myelin sheaths in mature, GFP-expressing oligodendrocytes, this was not surprising. Staining with O4 antibody, which detects late oligodendrocyte progenitors, identified a small population of oligodendrocytes, a few of which appeared to express GFP within the oligodendrocyte cell body by three-dimensional confocal analysis (Figure 2i–k). In intact tissue sections, GFP distributes poorly to oligodendrocyte extensions and O4 often does not stain the cell bodies of oligodendrocytes.7 Because of the nonoverlapping patterns of O4 and myelin basic protein with cytoplasmic GFP in oligodendrocytes in intact tissue sections, it was difficult to conclusively demonstrate transduction of oligodendrocytes in vivo.
Approximately 50% of ependymal cells lining the lateral ventricles were transduced. Determination of the exact percentage was complicated because GFP+ ependymal cells were usually found in large patches of GFP+ cells; in many sections, all ependymal cells expressed GFP and in other sections all ependymal cells lacked GFP expression. The large patches of GFP+ ependymal cells suggests the transduction of ependymal progenitor cells that are present during development (Figure 2n–p).
A total of 300 Iba1+ microglial cells were visualized and none were found to express GFP (Figure 2s–u). Given the hematopoietic origin of microglial cells and the observation that <0.3% of blood cells in these mice express GFP (data not shown), this result is not surprising.
To determine whether the low frequency of GFP+ astrocytes was due to a failure to express GFP as opposed to a failure to transduce astrocyte progenitors, the CMV-promoter elements in the original GFP reporter construct were replaced with the full-length 2.2 kb human GFAP promoter. The recipients of the GFAP-driven vector (GFAP-GFP) were killed at 10 and 30 days of age, at which time numerous GFP+ astrocytes were observed. Approximately 10% of GFAP-expressing astrocytes expressed GFP. And in all cells expressing both GFAP and GFAP-GFP, the pattern of GFP expression confirmed that all these cells exhibited the classic morphology of astrocytes (Figure 3a–d, Supplementary Figure S2e-g,k–m). In contrast to CMV-GFP, in recipients receiving the GFAP-GFP vector the ratio of GFP+ astrocytes to GFP− astrocytes was roughly consistent throughout the hind-, mid-, and forebrain with the exception that in the hippocampus almost one-quarter of astrocytes expressed GFP (Table 1, Supplementary Figure S2a,k–m). Taken together, it appears that the vesicular stomatitis virus–packed lentiviral vector was able to readily transduce astrocytes throughout the CNS but the CMV immediate-early promoter was suboptimal for transgene expression in this cell population.
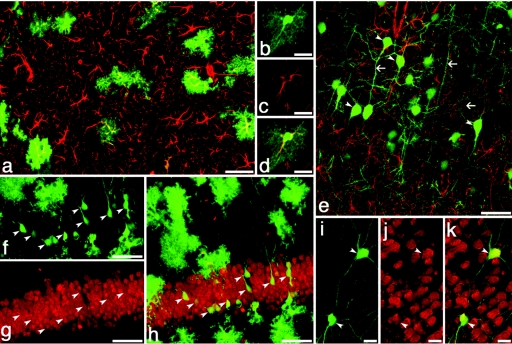
Figure 3.
GFAP-promoter region driven GFP reveals efficient transduction of astrocytes and neurons. (a–k) GFAP-GFP green in all. GFP-expressing astrocytes colocalize with antibody staining for GFAP (red, a–e) in the cortex (a) and hippocampus (b–d). A subset of GFAP-GFP transduced neurons (arrowheads, e–k) expressing GFP in the adult (7 months old) hippocampus (f–h) and caudate (i–k) are identifiable by morphology including long axonal extensions (arrows) and antibody staining for NeuN (red; g,h,j,k). Despite the expression of GFP from GFAP regulatory elements, these neurons lack expression of native GFAP (red, e). Bar = 20 µm: b–d, i–k; 50 µm: a,e–h. GFAP, GFP-expressing astrocyte; GFP, green fluorescent protein.
One clear advantage of the GFAP promoter was a substantial loss of GFP expression in tissues outside the nervous system. Although the CMV-GFP vector was also efficient at transducing most ectodermal tissues (skin, mammary gland, cochlea, ectodermal tissues of the eye),8,9 and other organs (kidney, skeletal muscle, thyroid, thymus)10 no GFP expression was observed outside the nervous system when the GFAP promoter was used.
Interestingly, within the CNS, 4–8% of neurons (clearly identifiable by NeuN staining and morphology) expressed GFAP-driven GFP suggesting that the GFAP promoter elements failed to limit GFP expression to the astrocyte population. Although in some cases these were located in known areas of neurogenesis such as the hippocampus and olfactory bulb (Figure 3f–h, Supplementary Figure S2a,k–m) and could represent recently created neurons, these cells were also observed in areas such as the cerebral cortex where adult neurogenesis is limited (Supplementary Figure S2h–j). Interestingly, in most cases where GFAP-GFP-expression occurred in neurons, the expression of GFP was strong and it was uncommon to observe low or intermediate levels of GFP expression within neurons.
Transduction of NSCs
To determine whether NSCs had been transduced, dividing cells were labeled with BrdU. Clear BrdU staining was observed in subset of cells expressing the neuronal marker NeuN in both the olfactory bulb as well as the hippocampus, confirming the neurogenesis continued into adulthood in recipient mice (Figure 4a–j, Supplementary Figure S3a–y). Age-matched, non-lentivirus-injected mice that received BrdU had a similar frequency of BrdU+ neurons (positive controls) and age-matched mice that received E8 lentivirus but not BrdU injections did not have any cells that appeared to be BrdU+ (negative controls). All images were obtained with high-power objectives and small pinhole size (Airy unit <1.5) so that optical section thicknesses remained under 2 µm to allow unequivocal confirmation that the BrdU and GFP were within the same cell.
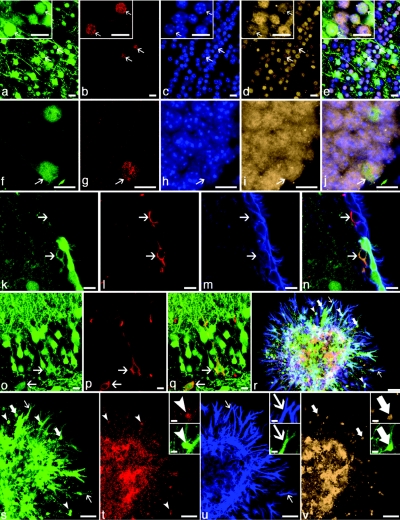
Figure 4.
Evidence for transduction of NSCs after CMV-GFP intra-amniotic injection. GFP expression (green in all) was observed in (a–j, arrows) newly formed neurons that had (red; b,g) divided in adulthood as demonstrated by incorporation of BrdU. Also shown in a–j is the neuronal marker NeuN (tan; d,i,), Hoescht-stained nuclei (blue; c,h) and four-color composite images (e, j). GFP-expressing, new neurons were observed throughout the CNS but were most frequent in the (a–e) olfactory bulbs and (f–j) hippocampi of adult mice. (k–q) GFP-transduced (green), putative NSCs were observed. A single cell expressing GFAP (red; l) that was located immediately underneath ependymal cells expressing CD24 (blue, m) in the subventricular zone expressed a low-level of GFP (lower arrow, k–n). Despite the cytoplasmic localization of GFP (which is occasionally observed in cells weakly expressing GFP), both a hue-based analysis (not shown) and the GFAP+ but GFP− cell (upper arrow) provide reassurance of the authenticity of the GFP-signal. Two cells with neuronal morphology (o–q, arrows) co-expressing GFP (o,q) and Nestin (red; p,q), a marker of neural stem and progenitor cells, were observed in the CA3 region of adult hippocampus. Low-density neurosphere cultures (r–v) demonstrate that a single, GFP-expressing cell gives rise to cells of the three major neural lineages: myelin basic protein–expressing oligodendrocytes (red, arrowheads, r,t), GFAP-expressing astrocytes (blue, thin arrows, r,u), and NeuN-expressing neurons (tan, thick arrows, r,v). Representative cells from each image are shown at higher magnification (insets). Although GFP expression appears heterogeneous, all cells within the neurosphere expressed GFP. The limited dynamic range of the detectors in the confocal microscope amplifies the contrast differences between cells, which was likely due to both varying densities of cells in the expanding neurosphere as well as the sensitivity of the CMV promoter to the surrounding chromatin state which varies with cell type. Bar = 10 µm: a–q and insets in t–v; 50 µm: r–v. CA3, CA3 region of hippocampus; CMV, cytomegalovirus; CNS, central nervous system; GFAP, GFP-expressing astrocyte; GFP, green fluorescent protein; NSC, neuronal stem cell.
Within the olfactory bulb 23% of BrdU+ neurons (NeuN+) expressed GFP (Figure 4a–e, Supplementary Figure S3a–j), indicating successful transduction of approximately one-quarter of the NSCs within the SVZ. Within the hippocampus, 35% of BrdU+ neurons (NeuN+) in the dentate gyrus expressed GFP (Figure 4f–j, Supplementary Figure S3k–y), indicating that approximately one-third of NSCs in the SGZ of the hippocampus were transduced.
Neural progenitors of the SVZ are GFAP+, have a distinct morphology compared to typical astrocytes, and reside immediately below the ependymal layer. Several cells were observed that appeared to be transduced neural progenitors of the SVZ (Figure 4k–n). Similarly, neural progenitors of the SGZ are nestin+, sit at the basal layer of the SGZ of the hippocampus, and have a branched morphology. We observed transduced, nestin+ cells with the anatomical location and morphology of NSCs in the SGZ (Figure 4o–q).
Another key criterion to document NSCs is the ability of single cells to generate cell colonies in culture containing all three major brain lineages. Thus, the brains from mice that had received CVM-GFP at E8 were dissociated and cultured at low density to assay for single-cell derived neurospheres. When cultured at low density, as was done here, it has been demonstrated that the resulting colonies are derived from single cells.11 This was confirmed in our experiments where approximately one-quarter of NSC expressed GFP. All colonies evaluated all were homogenously GFP+ or lacked GFP expression in all cells. Several GFP+ neurospheres were observed and when placed in differentiation conditions, these neurospheres generated substantial numbers of cells of all three major neural lineages: neurons, astrocytes, and oligodendrocytes (Figure 4r–v).
Taken together, the generation of new GFP+ neurons in adulthood, the histological presence of cells with markers and morphologies of NSCs, and generation of GFP+ neurospheres that differentiated into all three major neural lineages provides evidence for the in utero transduction of NSCs.
Discussion
Early intra-amniotic gene transfer with high-titer lentiviral vector before closure of the neural tube, results in efficient, stable, and broad transduction throughout the entire CNS and PNS. The technique is straightforward, rapid, and can be used with any pre-existing wild-type or transgenic murine models.
Previous attempts at in utero delivery for CNS transduction observed a high frequency of exencephaly and characterized the technique only in the embryonic nervous system.5 In contrast, we demonstrate here that exencephaly can be entirely avoided with careful technique. Furthermore, we extend the previous studies by demonstrating that transduction is stable in adult mice for at least 2 years, that adult stem cells are transduced, and that we demonstrate that distinct promoters (are useful for targeting specific cell populations within the CNS.
Importantly, transgene expression appeared to be stable from mid-gestation (E12) until at least 2 years (80% of a mouse's natural life). Another key advantage of this system is that the expression of a transgene product occurs before immune system maturation. This allows transgene presentation in the thymus, which recapitulates normal mechanisms of tolerance for self-antigen by mechanisms involving both clonal deletion of reactive T cells and development of peripheral mechanisms of tolerance such as T-regulatory cells.12 Tolerance induction for even strongly immunogenic antigens has been experimentally validated.13,14,15 For example, when fully major histocompatibility mismatched cells are transplanted during this period, recipients develop both central and peripheral tolerance to the cells, accepting them without immunosuppression for the life of the recipient.13,16,17 Thus there is strong experimental evidence that the immune response can be avoided, even for highly immunogenic antigens by this approach.
It is clear from this study and others that transgene expression is not an accurate indicator of transduction efficiency for different cell types as the substitution of the CMV promoter with a GFAP promoter increased the frequency of GFP-expressing astrocytes 10- to 20-fold. Even in young transgenic mice expressing GFP from the same GFAP promoter used here (i.e., every cell has a GFAP-GFP transgene), only 50 and 80% of astrocytes in the cortex and striatum, respectively, expressed GFP.18,19 And by 1 year of age, only 40% of striatal astrocytes expressed GFP.18 We also tested another constitutive promoter, the murine leukemia virus–derived MND promoter, but it had more limited CNS expression (data not shown) when delivered by the same lentiviral vector. The use of cell type-specific promoters, which are well described in the literature, is anticipated for future studies.20,21
The human GFAP promoter used was highly successful in that it strongly expressed GFP in astrocytes, which is consistent with previous reports indicating that transgene expression levels with this promoter can reach 0.1% of total brain protein.22 The strong expression of GFP in nonastrocytic cells, which appeared to be exclusively neurons, has been reported previously.23,24 Whether GFAP+ neurons represent a distinct neuronal population, possibly with progenitor properties, is controversial.24 In our vector, the Woodchuck hepatitis virus post-transcriptional regulatory element was incorporated to increase overall expression of GFP. However, Woodchuck hepatitis virus post-transcriptional regulatory element is reported to increase gene expression in neurons more than other cell types and thus could have conveyed some neuron-specific properties to our CMV-GFP vector.25,26 It is also likely that the CMV promoter used, which is prone to silencing in some cell types, played a role in the neuron-dominated pattern of GFP expression.27
The strongest evidence of adult neurogenesis is typically thymidine analogue uptake into proliferating neuronal progenitors that is detected by immunohistochemistry.28 Other criteria include expression of nestin in cells with neuronal morphology, expression of GFAP combined with a distinct morphology and anatomical location in the SVZ, and differentiation of clonally derived neurospheres into multiple CNS lineages.29 Each of these criteria was evaluated in this study. Transduction of NSCs was indicated by all criteria but the BrdU labeling study was particularly clear, with a number of obviously BrdU+ neurons demonstrating strong expression of GFP. The generation of GFP+ neurons in a mouse of at least 3 months of age provides strong evidence that NSCs were transduced in utero. Nestin is an intermediate filament protein that is expressed in neural stem and progenitor cells as well as rare differentiated astrocytes in the adult nervous system.30,31 However, when combined with neuronal location and morphology, nestin expression is a reliable indicator of a neural stem or progenitor cell.
Although considerable debate exists regarding the identity of NSCs and progenitor cells, consensus is emerging that GFAP expression occurs in some or most NSCs.29,32 Within the SVZ, GFAP expression must be combined with morphology to identify putative NSCs.29 Other histological markers of NSCs exist including Musashi-1 and -2, SOX2, prominin-1, and SSEA1, but they are also expressed by CNS cell types other than NSC.29 Given the controversy regarding NSC markers, it is not clear that any current set of markers would provide definitive evidence for NSC transduction. GFAP expression combined with cellular morphology as it relates to the ependymal cells appears to currently be the most reasonable approach to identify cells that are histologically consistent with NSCs. Taken together, all four analyses (BrdU labeling, nestin staining plus morphology in the hippocampus, GFAP staining plus morphology in the SVZ, and clonal neurosphere assays) indicate the stable transduction of NSC and their progeny.
Other studies have previously demonstrated the effectiveness of lentivirus to transduce NSC populations4 but such studies (where vector was delivered locally into adult brains) transduced spatially limited areas of the CNS in contrast to the widespread transduction that was observed in our study. Thus, selection of the route and developmental stage of delivery can be used to achieve distinct patterns of transduction.
Intra-amniotic delivery of lentiviral vector promises to be an important tool in studies of CNS and PNS biology and disease. The broad and efficient transduction observed, including the dorsal root ganglia, create opportunities for a number of novel studies, particularly those focused on NSCs, neuronal development, and mature neurons. A key feature of this approach is the speed at which genes can be broadly introduced into the nervous system of experimental animals and the ability to create mixed genetic backgrounds within neuronal populations in vivo. Ultimately, as the ability to diagnose and treat genetically based neurological disorders during fetal development advances, this approach may serve as a platform for novel strategies to treat a wide variety of neurological disorders.
Materials and Methods
Mice. BALB/c mice were mated in our breeding colony (breeding stock from Jackson Laboratories, Bar Harbor, ME). Animals were housed in the Laboratory Animal Facility of the Abramson Pediatric Research Center at the Children's Hospital of Philadelphia and were maintained in sterilized plastic microisolator cages and given sterilized standard laboratory chow and tap water ad libitum. Litters were housed with the dam until weaning. All experimental protocols were reviewed and approved by the Institutional Animal Care and Use Committee at the Children's Hospital of Philadelphia and followed guidelines set forth in the National Institutes of Health “Guide for Care and Use of Laboratory Animals.” To achieve accurate time-dated pregnancies, females were paired with males in the evening and separated the following morning (E0); mice were palpated for pregnancy at E8.
Lentiviral vectors. The starting materials for generating a self-inactivating human immunodeficiency virus-1-based vector were kindly provided by I. Verma (Salk Institute, La Jolla, CA).33 Modifications of the CS-CG vector included deletion of the remaining right U3 region except for 23 nucleotides downstream of the 3′ppt, deletion of the residual envelope and ancillary gag/pol sequences, insertion of the central DNA FLAP, insertion of the Rev response element, and insertion of the Woodchuck hepatitis virus post-transcriptional regulatory element, which is modified eliminating the initiation codon for the Woodchuck hepatitis X protein. For the CMV-GFAP vector, the EGFP (Clontech Laboratories, Palo Alto, CA) was located downstream of the human CMV immediate-early promoter and modified so that all stop codons from the transcription start site to the translation initiation site were removed. For the GFAP promoter–driven vectors, the CMV-promoter elements were replaced with the full-length 2,210 bp human GFAP promoter, gfa2 (a kind gift from M. Brenner, University of Alabama), to create the GFAP-GFP vector. Viral vectors pseudotyped with vesicular stomatitis virus envelope were generated and titered as previously described.6 Briefly, the titer (transducing units) was determined by quantifying GFP+ 293T cells after serial dilution of vector.
Intra-amniotic vector injection at E8. Gestational day 8 (E8) fetuses in time-dated mice were injected with vector using an ultrasound-guided injection system (Vevo 660; VisualSonics, Toronto, Canada; Supplementary Figure S4). Pregnant mice were anesthetized with isoflurane and placed supine on the ultrasound table. The abdominal hair was removed by a chemical hair remover (Nair; Church & Dwight, Princeton, NJ) and the surgical area was disinfected with alcohol. A 1-cm ventral midline incision was made through the skin, abdominal wall, and peritoneum and a segment of the uterus containing fetuses was exteriorized and covered in prewarmed sterile ultrasound gel (Acquasonic; Parker Laboratories, Fairfield NJ). The fetuses were scanned using a 40 MHz probe to confirm appropriate developmental stage. With ultrasound the preturning Theiler stages 12a/12b could be distinguished from the turning (Theiler stage 13) and post-turning (Theiler stage 14) stages when the rostral neural tube begins to close and the anterior neuropore forms and closes. Only preturning mice were injected. Pulled and beveled glass microcapillary pipettes (outer diameter 1.14 mm, inner diameter, 0.53 mm; Humagen, Charlottesville, VA) were prefilled with mineral oil (Sigma, St Louis, MO), connected to the micropipette holder, and loaded with vector. Under two-dimensional visualization the micropipette tip was inserted through the uterine wall into the amniotic cavity. A 350 nl of vector (2 × 1010 transducing units/ml) was injected using an automated syringe and the micropipette was retracted. This procedure was repeated until all fetuses were injected. Upon completion of the injections, the uterus was returned to the abdominal cavity and the incision was closed in two layers using running 4-0 nonabsorbable sutures.
BrdU administration. BrdU (5′-bromo-2′-deoxuridine) (Sigma) 50 mg/kg/day was injected intraperitoneally daily for 4 days into 3-month-old animals that had received an E8 intra-amniotic lentiviral injection and their tissues were harvested 3 weeks later. Age-matched BrdU-injected mice and BrdU-uninjected mice were examined as positive and negative controls.
Immunohistochemistry. The tissue specimens for histology and immunohistochemistry were fixed by intracardiac perfusion with 4% formalin in phosphate-buffered saline/azide. The 50-µm thick vibratome sections were stained with antibodies as floating sections as follows: (i) blocked with 20% normal goat serum with 0.25% Triton-X for 1 hour; (ii) primary antibody in 3% normal goat serum with 0.1% Triton-X for 12 hours; (iii) washed 3× (iv) secondary antibody in 3% normal goat serum with 0.1% Triton-X for 6 hours; and (v) washed and mounted with Hoechst dye. Sections stained for BrdU were pretreated in 2 N HCl for 10 minutes followed by 0.1 N sodium borate pH 8.5 for 10 minutes. The antibodies and dilutions were as follows NeuN (1:500; Millipore, Billerica, MA; MAB 377), GFAP (1:500; Dako, Glostrup, Denmark; Z0334), CD 24 (1:1,000; eBioscience, San Diego, CA; 14-0242), myelin basic protein (1:100; Abcam, Cambridge, MA; A7349-2), O4 (1:500; R&D Systems, Minneapolis, MN; HWW0307111), BrdU (1:500; Accurate Chemicals, Westbury, NY; H9505), GFP (1:2,000, Abcam; Ab13970), Nestin (1:2,000, Developmental Studies Hybridoma Bank, Iowa City, IA; R401), and secondary antibodies (all at 1:1,000; Molecular Probes/Invitrogen, Carlsbad, CA).
Neurospheres. Brains were removed from E8-injected fetuses at E16. Single cells were suspended in Dulbecco's modified Eagle's medium/F12 1:1 with N2, penicillin/streptomycin, and fibroblast growth factor at a density of 10 cells/µl. After 1 week floating neurospheres reach appropriate size and are spun at 50g for 3 minutes. Neurospheres are resuspended in differentiation media Dulbecco's modified Eagle's medium/F12 1:1 with N2, penicillin/streptomycin and fetal bovine serum 1% and applied to ornithine-coated chamber slides. Neurospheres adhere to the slides and differentiate. They are then fixed in 4% formalin in phosphate-buffered saline/azide and stained for NeuN, GFAP, and MBP.
Imaging and quantification. Images of gross tissue were taken with a Leica (Wetzlar, Germany) fluorescent stereomicroscope (MZ16FA) equipped with an HQ GFP bandpass filterset (Chroma Technology, Bellows Falls, VT). Images of tissue sections were taken on Zeiss 510 Laser Scanning Confocal microscopes (three or four lasers, Carl Zeiss International, Oberkochen, Germany) with optical sections corresponding to <1.5 Airy units (usually 1 Airy unit) to allow thin optical sections to be discriminated and colocalization of GFP and identifying proteins to be unequivocally determined. Most images were captured with light paths utilizing series of sputter filters (ET filters; Chroma Technology, Bellows Falls, VT) with improved wavelength selection and transmission.34 Even with multitracking, when four colors were imaged simultaneously, a slight amount of Alexa 633 emission was observed when imaging Alexa 594. In this case, the ratio of the absolute signal from Alexa 633 alone was used to proportionally mask the Alexa 594 signal on a pixel-by pixel-basis to eliminate the detection of Alexa 633 in the channel for Alexa 594. Frequency of GFP-expressing cell types was determined in representative sections from at least three recipients. Briefly, CNS regions were identified, confocal images were obtained and all cells labeled with GFP or an identifying antibody were quantified by computer-aided analysis using a custom Photoshop CS3 script that translates a morphometric mask into a series of detection events that are then individually analyzed for intensity values in the relevant excitation and emission filter paths (GFP, fluorophore-labeled antibody, etc.) relative to intensity in a filter path without an added fluorophore (i.e., autofluorescence). The individual cell events were displayed for each light path, confirmed manually, and rare manual corrections were made for each individual event with reference to florescent visual inspection of the original tissue section. Because GFP was very bright, even without antibody staining, cells with unclear GFP intensity were very rare and categorized as negative for GFP expression when encountered.
SUPPLEMENTARY MATERIAL Figure S1. GFP expression (green in all) after intra-amniotic injection of CMV-GFP lentivirus at E8. Figure S2. Expression of GFP (green in all) in astrocytes and neurons after E8 intra-amniotic delivery of GFAP-GFP lentiviral vector. Figure S3. Further evidence for transduction of NSCs. Figure S4. High-resolution-ultrasound image of a glass needle about to enter the amniotic cavity of an E8 fetus and deliver lentiviral vector.
Acknowledgments
This study was supported in part by the Ruth and Tristram C. Colket Jr. Chair in Pediatric Surgery (A.W.F.) and by Children's Hospital of Philadelphia Institutional Development Funds (T.R.B.). We thank Carol Schneider and Antonetta Radu for their support and assistance. The authors declare no conflicts of interest related to this article or its content or publication.
Supplementary Material
GFP expression (green in all) after intra-amniotic injection of CMV-GFP lentivirus at E8.
Expression of GFP (green in all) in astrocytes and neurons after E8 intra-amniotic delivery of GFAP-GFP lentiviral vector.
Further evidence for transduction of NSCs.
High-resolution-ultrasound image of a glass needle about to enter the amniotic cavity of an E8 fetus and deliver lentiviral vector.
REFERENCES
- Abrous DN, Koehl M., and, Le Moal M. Adult neurogenesis: from precursors to network and physiology. Physiol Rev. 2005;85:523–569. doi: 10.1152/physrev.00055.2003. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, García-Verdugo JM., and, Tramontin AD. A unified hypothesis on the lineage of neural stem cells. Nat Rev Neurosci. 2001;2:287–293. doi: 10.1038/35067582. [DOI] [PubMed] [Google Scholar]
- Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- Consiglio A, Gritti A, Dolcetta D, Follenzi A, Bordignon C, Gage FH, et al. Robust in vivo gene transfer into adult mammalian neural stem cells by lentiviral vectors. Proc Natl Acad Sci USA. 2004;101:14835–14840. doi: 10.1073/pnas.0404180101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaiano N, Kohtz JD, Turnbull DH., and, Fishell G. A method for rapid gain-of-function studies in the mouse embryonic nervous system. Nat Neurosci. 1999;2:812–819. doi: 10.1038/12186. [DOI] [PubMed] [Google Scholar]
- Sena-Esteves M, Tebbets JC, Steffens S, Crombleholme T., and, Flake AW. Optimized large-scale production of high titer lentivirus vector pseudotypes. J Virol Methods. 2004;122:131–139. doi: 10.1016/j.jviromet.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Naka H, Nakamura S, Shimazaki T., and, Okano H. Requirement for COUP-TFI and II in the temporal specification of neural stem cells in CNS development. Nat Neurosci. 2008;11:1014–1023. doi: 10.1038/nn.2168. [DOI] [PubMed] [Google Scholar]
- Endo M, Zoltick PW, Chung DC, Bennett J, Radu A, Muvarak N, et al. Gene transfer to ocular stem cells by early gestational intraamniotic injection of lentiviral vector. Mol Ther. 2007;15:579–587. doi: 10.1038/sj.mt.6300092. [DOI] [PubMed] [Google Scholar]
- Endo M, Zoltick PW, Peranteau WH, Radu A, Muvarak N, Ito M, et al. Efficient in vivo targeting of epidermal stem cells by early gestational intraamniotic injection of lentiviral vector driven by the keratin 5 promoter. Mol Ther. 2008;16:131–137. doi: 10.1038/sj.mt.6300332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo M, Henriques-Coelho T, Zoltick PW, Stitelman DH, Peranteau WH, Radu A, et al. The developmental stage determines the distribution and duration of gene expression after early intra-amniotic gene transfer using lentiviral vectors. Gene Ther. 2010;17:61–71. doi: 10.1038/gt.2009.115. [DOI] [PubMed] [Google Scholar]
- Coles-Takabe BL, Brain I, Purpura KA, Karpowicz P, Zandstra PW, Morshead CM, et al. Don't look: growing clonal versus nonclonal neural stem cell colonies. Stem Cells. 2008;26:2938–2944. doi: 10.1634/stemcells.2008-0558. [DOI] [PubMed] [Google Scholar]
- Merianos D, Heaton T., and, Flake AW. In utero hematopoietic stem cell transplantation: progress toward clinical application. Biol Blood Marrow Transplant. 2008;14:729–740. doi: 10.1016/j.bbmt.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Hayashi S, Peranteau WH, Shaaban AF., and, Flake AW. Complete allogeneic hematopoietic chimerism achieved by a combined strategy of in utero hematopoietic stem cell transplantation and postnatal donor lymphocyte infusion. Blood. 2002;100:804–812. doi: 10.1182/blood-2002-01-0016. [DOI] [PubMed] [Google Scholar]
- Waddington SN, Nivsarkar MS, Mistry AR, Buckley SM, Kemball-Cook G, Mosley KL, et al. Permanent phenotypic correction of hemophilia B in immunocompetent mice by prenatal gene therapy. Blood. 2004;104:2714–2721. doi: 10.1182/blood-2004-02-0627. [DOI] [PubMed] [Google Scholar]
- Zhang CC., and, Lodish HF. Insulin-like growth factor 2 expressed in a novel fetal liver cell population is a growth factor for hematopoietic stem cells. Blood. 2004;103:2513–2521. doi: 10.1182/blood-2003-08-2955. [DOI] [PubMed] [Google Scholar]
- Peranteau WH, Endo M, Adibe OO., and, Flake AW. Evidence for an immune barrier after in utero hematopoietic-cell transplantation. Blood. 2007;109:1331–1333. doi: 10.1182/blood-2006-04-018606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merianos DJ, Tiblad E, Santore MT, Todorow CA, Laje P, Endo M, et al. Maternal alloantibodies induce a postnatal immune response that limits engraftment following in utero hematopoietic cell transplantation in mice. J Clin Invest. 2009;119:2590–2600. doi: 10.1172/JCI38979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehner T, Böntert M, Eyüpoglu I, Prass K, Prinz M, Klett FF, et al. Bone marrow-derived cells expressing green fluorescent protein under the control of the glial fibrillary acidic protein promoter do not differentiate into astrocytes in vitro and in vivo. J Neurosci. 2003;23:5004–5011. doi: 10.1523/JNEUROSCI.23-12-05004.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolte C, Matyash M, Pivneva T, Schipke CG, Ohlemeyer C, Hanisch UK, et al. GFAP promoter-controlled EGFP-expressing transgenic mice: a tool to visualize astrocytes and astrogliosis in living brain tissue. Glia. 2001;33:72–86. [PubMed] [Google Scholar]
- Jakobsson J, Ericson C, Jansson M, Björk E., and, Lundberg C. Targeted transgene expression in rat brain using lentiviral vectors. J Neurosci Res. 2003;73:876–885. doi: 10.1002/jnr.10719. [DOI] [PubMed] [Google Scholar]
- Sims K, Ahmed Z, Gonzalez AM, Read ML, Cooper-Charles L, Berry M, et al. Targeting adenoviral transgene expression to neurons. Mol Cell Neurosci. 2008;39:411–417. doi: 10.1016/j.mcn.2008.07.020. [DOI] [PubMed] [Google Scholar]
- Smith JD, Sikes J., and, Levin JA. Human apolipoprotein E allele-specific brain expressing transgenic mice. Neurobiol Aging. 1998;19:407–413. doi: 10.1016/s0197-4580(98)00076-1. [DOI] [PubMed] [Google Scholar]
- Su M, Hu H, Lee Y, d'Azzo A, Messing A., and, Brenner M. Expression specificity of GFAP transgenes. Neurochem Res. 2004;29:2075–2093. doi: 10.1007/s11064-004-6881-1. [DOI] [PubMed] [Google Scholar]
- Casper KB., and, McCarthy KD. GFAP-positive progenitor cells produce neurons and oligodendrocytes throughout the CNS. Mol Cell Neurosci. 2006;31:676–684. doi: 10.1016/j.mcn.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Glover CP, Bienemann AS, Hopton M, Harding TC, Kew JN., and, Uney JB. Long-term transgene expression can be mediated in the brain by adenoviral vectors when powerful neuron-specific promoters are used. J Gene Med. 2003;5:554–559. doi: 10.1002/jgm.381. [DOI] [PubMed] [Google Scholar]
- Glover CP, Bienemann AS, Heywood DJ, Cosgrave AS., and, Uney JB. Adenoviral-mediated, high-level, cell-specific transgene expression: a SYN1-WPRE cassette mediates increased transgene expression with no loss of neuron specificity. Mol Ther. 2002;5 5 Pt 1:509–516. doi: 10.1006/mthe.2002.0588. [DOI] [PubMed] [Google Scholar]
- Baskar JF, Smith PP, Nilaver G, Jupp RA, Hoffmann S, Peffer NJ, et al. The enhancer domain of the human cytomegalovirus major immediate-early promoter determines cell type-specific expression in transgenic mice. J Virol. 1996;70:3207–3214. doi: 10.1128/jvi.70.5.3207-3214.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Encinas JM., and, Enikolopov G. Identifying and quantitating neural stem and progenitor cells in the adult brain. Methods Cell Biol. 2008;85:243–272. doi: 10.1016/S0091-679X(08)85011-X. [DOI] [PubMed] [Google Scholar]
- Chojnacki AK, Mak GK., and, Weiss S. Identity crisis for adult periventricular neural stem cells: subventricular zone astrocytes, ependymal cells or both. Nat Rev Neurosci. 2009;10:153–163. doi: 10.1038/nrn2571. [DOI] [PubMed] [Google Scholar]
- Lendahl U, Zimmerman LB., and, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60:585–595. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- Gilyarov AV. Nestin in central nervous system cells. Neurosci Behav Physiol. 2008;38:165–169. doi: 10.1007/s11055-008-0025-z. [DOI] [PubMed] [Google Scholar]
- Morshead CM., and, van der Kooy D. Disguising adult neural stem cells. Curr Opin Neurobiol. 2004;14:125–131. doi: 10.1016/j.conb.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Miyoshi H, Blömer U, Takahashi M, Gage FH., and, Verma IM. Development of a self-inactivating lentivirus vector. J Virol. 1998;72:8150–8157. doi: 10.1128/jvi.72.10.8150-8157.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley MW, Tani EM., and, Skoog L. Metaplastic carcinoma of the breast: fine-needle aspiration cytology of seven cases. Diagn Cytopathol. 1989;5:22–28. doi: 10.1002/dc.2840050106. [DOI] [PubMed] [Google Scholar]
- Shimokawa I, Higami Y, Horiuchi S, Iwasaki M., and, Ikeda T. Advanced glycosylation end products in adrenal lipofuscin. J Gerontol A Biol Sci Med Sci. 1998;53:B49–B51. doi: 10.1093/gerona/53a.1.b49. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
GFP expression (green in all) after intra-amniotic injection of CMV-GFP lentivirus at E8.
Expression of GFP (green in all) in astrocytes and neurons after E8 intra-amniotic delivery of GFAP-GFP lentiviral vector.
Further evidence for transduction of NSCs.
High-resolution-ultrasound image of a glass needle about to enter the amniotic cavity of an E8 fetus and deliver lentiviral vector.