Abstract
Gene transfer of dopamine-synthesizing enzymes into the striatal neurons has led to behavioral recovery in animal models of Parkinson's disease (PD). We evaluated the safety, tolerability, and potential efficacy of adeno-associated virus (AAV) vector–mediated gene delivery of aromatic -amino acid decarboxylase (AADC) into the putamen of PD patients. Six PD patients were evaluated at baseline and at 6 months, using multiple measures, including the Unified Parkinson's Disease Rating Scale (UPDRS), motor state diaries, and positron emission tomography (PET) with 6-[18F]fluoro--m-tyrosine (FMT), a tracer for AADC. The short-duration response to levodopa was measured in three patients. The procedure was well tolerated. Six months after surgery, motor functions in the OFF-medication state improved an average of 46% based on the UPDRS scores, without apparent changes in the short-duration response to levodopa. PET revealed a 56% increase in FMT activity, which persisted up to 96 weeks. Our findings provide class IV evidence regarding the safety and efficacy of AADC gene therapy and warrant further evaluation in a randomized, controlled, phase 2 setting.
Introduction
Dopamine replacement has been the standard pharmacotherapy for motor impairment in Parkinson's disease (PD). Although virtually all patients benefit from levodopa at an early stage of the disease, severe loss of nigrostriatal nerve terminals in advanced PD leads to profoundly decreased activities of dopamine-synthesizing enzymes, including aromatic -amino acid decarboxylase (AADC), an essential enzyme that converts levodopa to dopamine. Failure to respond to levodopa therapy may result from a reduction in AADC activity, decreased dopamine storage capacity in synaptic vesicles, postsynaptic changes in striatal output neurons, and abnormalities of nondopaminergic neurotransmitter systems.1,2 Systemic administration of high-dose levodopa enhances oscillations in motor performance and complications, including hallucinations, due to dopaminergic stimulation of the mesolimbic system.
One potential treatment for advanced PD is gene therapy to restore striatum-selective dopamine production. In addition to AADC, tyrosine hydroxylase, which converts -tyrosine to levodopa, and guanosine triphosphate cyclohydrolase I, which catalyzes biosynthesis of the essential tyrosine hydroxylase cofactor, tetrahydrobiopterine, are necessary for efficient synthesis of dopamine.2 Viral vector–mediated gene transfer of these dopamine-synthesizing enzymes has been shown to achieve behavioral recovery in animal PD models, with efficient transduction of striatal neurons that escape degeneration.3,4,5,6 When tyrosine hydroxylase and guanosine triphosphate cyclohydrolase I are expressed in the striatum, levodopa can be synthesized continuously. This strategy would be useful for reducing motor fluctuations associated with intermittent levodopa intake. Gene transfer of AADC alone in combination with oral levodopa administration would be a safer strategy for initial clinical trials. In the latter approach, the patients still need to take levodopa to control motor symptoms, but excess production of dopamine could be avoided by reducing the dose of levodopa. We assessed the safety, tolerability, and the potential efficacy of intraputaminal infusion of recombinant adeno-associated virus (AAV) serotype 2 vector encoding human AADC (AAV-hAADC-2) in patients with mid- to late-stage PD. We also examined whether the short-duration response to levodopa, the antiparkinsonian response that parallels the plasma levodopa levels, would change after gene therapy.7
Results
Patient disposition and baseline characteristics
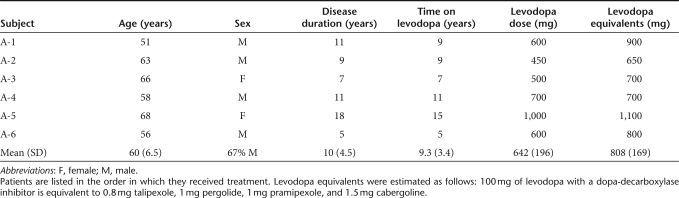
Six patients (4 men, 2 women), mean age 60 (range, 51–68) years, were enrolled (Table 1). The mean disease duration was 10 (range, 5–18) years, and time on levodopa was 9.3 (range, 5–15) years. The average baseline daily levodopa and levodopa equivalent doses were 642 and 808 mg, respectively.
Table 1. Patients' baseline characteristics.
Primary end point
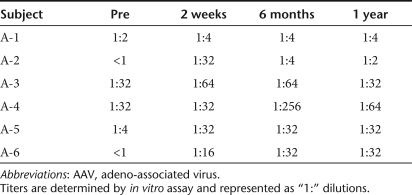
The procedure was well tolerated. All patients completed all protocol-defined visits. One patient (patient A-2) had a venous hemorrhage in the right frontal lobe just below a burr hole that was found on CT scan 3 days after infusion. The patient used his left arm less frequently than his right arm for 3 weeks; this was assumed to reflect mild frontal lobe dysfunction and resolved completely. Mild, transient headache around the burr holes was present for 2 days after surgery in all patients. There were no significant laboratory test abnormalities. All patients had mildly increased titers of anti-AAV2-neutralizing antibodies 6 months after treatment, which tended toward baseline concentrations thereafter (Table 2).
Table 2. Changes in neutralizing AAV2 antibody titers in sera following gene therapy.
Clinical evaluations
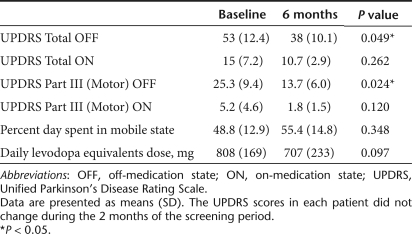
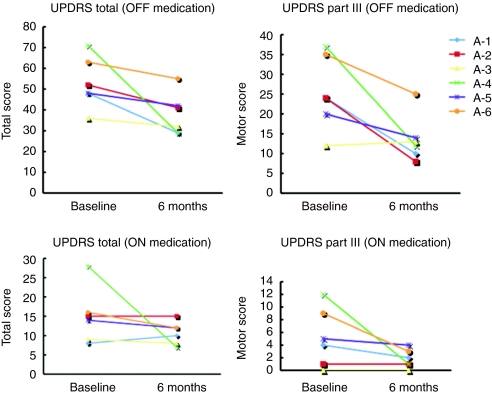
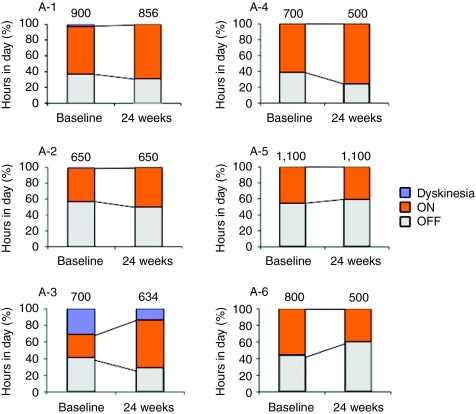
The clinical results are summarized in Table 3. Intraputaminal AAV-hAADC-2 infusion significantly improved both total and motor scores of the unified Parkinson's disease rating scale (UPDRS) in the OFF state. Five of six patients showed substantial improvement in UPDRS motor ratings in the OFF state (Figure 1). Changes in the UPDRS ON state and the percent of ON state hours in a day were not significant. One patient with relatively mild motor symptoms at baseline did not improve on UPDRS (A-3 in Figure 1). However, this patient showed a remarkable increase in mobile time as measured by the diaries (28% at baseline to 58% at 6 months after gene transfer; Figure 2). The daily dose of levodopa was unchanged in two patients (A-2 and A-5) and reduced in three patients (A-1, A-3, and A-5) at 6 months. Patient A-6, who had daytime sleepiness, preferred to reduce pramipexole instead of levodopa after gene therapy.
Table 3. Clinical outcomes of six patients.
Figure 1.
Changes in UPDRS scores. Absolute changes in scores from baseline to 6 months for individual patients. OFF, off-medication state; ON, on-medication state; UPDRS, Unified Parkinson's Disease Rating Scale.
Figure 2.
Evaluation of patients' diaries and daily doses of levodopa equivalents. For each 30-minute interval throughout the day, the patients recorded whether they were mobile (ON), immobile (OFF), or asleep. They also recorded the time with troublesome dyskinesias (Dyskinesia). The graph shows the percentage of hours in a day spent in each condition at baseline and at 6 months. The numbers on the bars indicate the mean daily doses of levodopa equivalents (mg). OFF, off-medication state; ON, on-medication state.
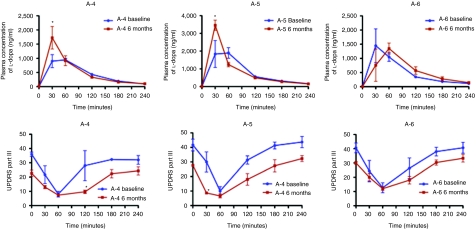
The last three patients underwent the levodopa test after our institutional review board confirmed the safety of AADC gene transfer in the first three patients. The short-duration response to levodopa did not change significantly after gene therapy in these three patients, though UPDRS motor scores at 6 months showed slight improvement at 30 minutes in patient 5 and at 120 minutes in patient 4 after levodopa intake (Figure 3). Significantly higher peak plasma levodopa concentrations were observed in these two patients after gene therapy.
Figure 3.
Short-duration response to levodopa. Comparison of short-duration response to levodopa before (blue) and after gene therapy (brown) in three patients (A-4, A-5, and A-6). Patients took 100 mg of levodopa with 25 mg benserazide orally after 20 hours without dopaminergic medication. Values represent means and SE of three trials. Upper panels: plasma levodopa levels; lower panels: Unified Parkinson's Disease Rating Scale motor scores. *P < 0.05.
The mini-mental state examination (MMSE) and geriatric depression scale (GDS) scores did not change significantly.
PET analysis
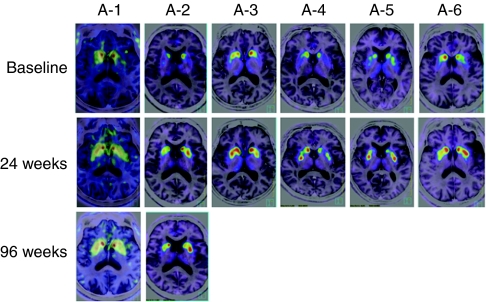
PET imaging revealed increased 6-[18F]fluoro--m-tyrosine (FMT), a tracer for AADC, activity 4 weeks postoperatively, which persisted at 6-month evaluation (Figure 4). The mean increase in FMT uptake from baseline in the combined (right and left) putamen at 24 weeks was 56%. Two patients (A-1 and A-2) who had PET scans 96 weeks after surgery showed persistently increased FMT uptake. In these two patients, motor performance in the OFF state also maintained its improvement at 96 weeks.
Figure 4.
FMT-PET images. Axial images at the level of the putamen are shown before and 24 weeks after gene therapy for all six patients. Increased FMT uptake persisted until 96 weeks in two patients. The 4-week images are not shown because they are similar to the 24-week images. FMT, 6-[18F]fluoro--m-tyrosine; PET, positron emission tomography.
Discussion
Extensive preclinical studies on both rodent and nonhuman primate models of PD have shown that AAV vectors can express exogenous genes for a long time in the brain target areas without significant toxicity.3,4,6,8,9 Recently, three phase I clinical trials of gene therapy for advanced PD demonstrated that AAV vector–mediated gene delivery into the subthalamic nucleus or putamen was safe and tolerable.10,11,12,13 In this study, the safety of the AAV vectors for clinical use in the human brain was confirmed. Although one patient developed a venous hemorrhage in the subcortical white matter along the trajectory, it is well known that cerebral bleeding occasionally occurs in association with surgical procedures for deep brain stimulation in which electrodes are inserted into the basal ganglia through the frontal lobe white matter.14,15 PET imaging in this patient showed that putaminal AADC expression was not affected by the subcortical venous hemorrhage and persisted up to 96 weeks. Thus, the venous hemorrhage was probably due to the surgical procedure and not gene transduction.
Although the present trial was a small, open-label study, and the nonblinded, uncontrolled analysis limits the interpretation, the initial efficacy outcomes are encouraging. Our patients showed improved motor performance in the OFF state. Levodopa has a relatively short plasma half-life (60–90 minutes), and antiparkinsonian effects observed after levodopa administration have generally been recognized as short- and long-duration responses. The short-duration response roughly parallels the plasma levodopa concentrations and is thought to be closely linked to dyskinesia, whereas the long-duration response builds up over weeks and improves trough (worst) motor performance in the OFF state.7 Because the pattern of the short-duration response to levodopa did not change after gene therapy in our patients, the beneficial effect on the OFF state appears to be attributed to augmentation of the long-term response to levodopa.16 In the preclinical studies with animal models of PD, AAV vectors mainly transduced medium spiny neurons that have dopamine receptors, and extracellular dopamine was increased in the striatum after administration of levodopa.5,17 The mechanism underlying the long-duration response is not sufficiently understood, and future study is necessary to determine how nonphysiologic production of dopamine in the striatal neurons could enhance the response. It has been reported that the sustained long-duration response to levodopa is greater in patients treated with higher single doses of levodopa.18 Thus, it is likely that increased dopamine in the putamen after gene transfer may enhance the stable long-duration response. Motor fluctuations in PD are associated with increased response to levodopa with a deeper trough in motor performance, rather than shortening of the response. Improving trough or OFF state motor function by augmenting the long-term response would likely reduce motor fluctuation.16 Two of three patients in whom the short-duration response to levodopa was studied showed increased peak plasma levodopa concentrations after gene therapy. This finding may simply reflect variable absorbance of levodopa, and it remains to be elucidated whether changes in gastrointestinal absorption could be related to better motor performance in the OFF state.19
Activities and levels of AADC mRNA and protein are profoundly reduced in advanced PD,2 but there are still several types of AADC-containing cells in the striatum, such as serotonin neurons, intrinsic dopamine neurons, AADC-containing “D” neurons, and glial cells.20 These cells may act as a local source of dopamine. However, dopamine produced in nondopamine cells may not be taken up into dopamine cells and stored in synaptic vesicles, as dopamine transporter and vesicular monoamine transporter 2 are also reduced in advanced PD. The functional efficacy of dopamine produced from exogenous levodopa in these cells may be limited, at least in primates.2,3 Striatal output neurons, main targets in AADC gene therapy, play a principal role in dopamine modulation of motor function in the basal ganglia. Dopamine synthesized in the striatal neurons themselves may more easily stimulate both synaptic and extrasynaptic receptors.
Results of a similar phase I protocol were reported recently for the 10 patients treated with AAV-hAADC-2 (ref. 10). That study used the same vector preparations as this study. The subjects were divided into two groups that received the same or one-third dose of the vector used in this study, respectively. Although the present patients had slightly milder initial symptoms, the patients treated with the same dose of vector in the two studies showed similar improvement in the OFF state and putaminal FMT uptake on PET. These findings provide independent confirmation of the safety, tolerability, and potential efficacy of AADC gene therapy. Future studies focusing on optimal vector dosing and defining the relationship between vector dose and clinical effects are necessary.21
In conclusion, these data indicate that AAV vector–mediated gene transfer of AADC is safe and may benefit advanced PD patients.
Materials and Methods
Study design. The protocol and consent forms were approved by the institutional review board. The protocol was also reviewed by the committee of the Ministry of Health, Labour and Welfare of Japan. A data safety monitoring board reviewed the ongoing study. All subjects reviewed the consent form and provided their written, informed consent.
This 24-week, phase I, open-label study was primarily designed to evaluate the safety and tolerability of intraputaminal AAV-hAADC-2 infusion in idiopathic PD. Patients were evaluated preoperatively and monthly postoperatively for 6 months, using multiple measures, including the UPDRS, motor state diaries, the MMSE, the short form of the GDS, and laboratory tests. The UPDRS was done in the practically defined OFF state 12 hours after withdrawal of all antiparkinsonian medications, and in the ON state 1 hour after administration of the usual morning dose of medication. Motor scores for the UPDRS can range from 0 to 56, with higher scores indicating poorer function. Using diaries that separated the day into half-hour segments, the patients recorded their mobility during the 4 days before admission and for another 4 days at 6 months after admission. They were trained to rate their condition as sleeping, immobile, mobile without troublesome dyskinesias, or mobile with troublesome dyskinesias. The total number of hours spent in each of these categories was calculated, and the differences between the baseline and the 6-month scores were compared between the groups.
The short-duration response to levodopa was evaluated in three patients (patients 4–6) at baseline and 6 months after gene transfer; they took 100 mg of levodopa orally with 25 mg benserazide after 20 hours without dopaminergic medication. Motor symptoms based on UPDRS motor (part III) and plasma levodopa concentrations were assessed at baseline and 30 minutes, 1, 2, 3, and 4 hours after levodopa intake.
Patients. The main entry criteria were: age 45–75 years; diagnosis of moderate to advanced PD, defined as Hoehn and Yahr Stage IV and UPDRS in the practically defined OFF condition of at least 20; at least 5 years of levodopa therapy; a minimum 8-point improvement in the UPDRS motor score after levodopa intake; and motor complications not satisfactorily controlled with medical therapy. The main exclusion criteria were atypical parkinsonism, dementia (MMSE score <20), and previous neurosurgical treatment for PD.
Vector and stereotaxic infusion. The vector used in this trial was a recombinant AAV2 with an expression cassette consisting of a human cytomegalovirus immediate-early promoter, followed by the human growth hormone first intron, complementary DNA of human AADC, and simian virus 40 polyadenylation signal sequence.3,4,5,6 Clinical grade AAV-hAADC-2 was manufactured by Avigen (Alameda, CA) and provided by Genzyme (Boston, MA). The patients received AAV-hAADC-2 via bilateral intraputaminal infusions. Two target points were determined in the putamen that were sufficiently separated from each other in dorsolateral directions and identified on a magnetic resonance image. One burr hole was trepanned in each side of the cranial bone, through which the vector was injected into the two target points via the two-track insertion route. The vector-containing solution was prepared to a concentration of 1.5 × 1012 vector genome/ml, and 50 µl per point of the solution were injected at 1 µl/min; each patient received 3 × 1011 vector genome of AAV-hAADC-2.
Neutralizing antibody titers against AAV2 were determined by measuring β-galactosidase activities in HEK293 cells transduced with 5 × 103 vector genome/cell of AAV2 vectors expressing β-galactosidase in various dilutions of sera.22
PET. The AADC expression level in the putamen was assessed on PET imaging with FMT 6 days before surgery and 1 and 6 months after gene transfer. All patients stopped dopaminergic medications 18 hours before PET and took 2.5 mg/kg of carbidopa orally 1 hour before FMT injection. Subsequently, 0.12 mCi/kg of FMT in saline were infused into an antecubital vein, and a 90-minute dynamic acquisition sequence was obtained. The PET and magnetic resonance imaging data were co-registered with a fusion processing program (Syntegra; Philips, Amsterdam, The Netherlands) to produce the fusion images. Radioactivities within volumes of interest drawn in the putamen and occipital lobe were calculated between 80 and 90 minutes after tracer injection. A change in putaminal FMT uptake from baseline to 24 weeks was assessed using the putaminal–occipital ratio of radioactivities.
Statistical analysis. Values at baseline and 6 months after gene transfer were compared using Student's t-test (paired analyses). A two-sided P value <0.05 was taken to indicate significant differences. Two-way analysis of variance with Bonferroni correction of P values was used for the short-duration response to levodopa.
Acknowledgments
This study was supported by grants from the Japanese Government: a grant-in-aid from the Research Committee of CNS Degenerative Diseases via the MHLW and grants from the Ministry of Education, Culture, Sports, Science and Technology. We thank Hiroshi Ichinose and Toshiharu Nagatsu for their helpful comments, and Naomi Takino, Hitomi Miyauchi, Keiko Ayabe, and Tetsuo Ito for their technical assistance. We also thank Avigen and Genzyme for providing clinical grade AAV vector.
REFERENCES
- Fahn S. The history of dopamine and levodopa in the treatment of Parkinson's disease. Mov Disord. 2008;23 Suppl 3:S497–S508. doi: 10.1002/mds.22028. [DOI] [PubMed] [Google Scholar]
- Nagatsu T., and, Sawada M. Biochemistry of postmortem brains in Parkinson's disease: historical overview and future prospects. J Neural Transm Suppl. 2007. pp. 113–120. [DOI] [PubMed]
- Bankiewicz KS, Eberling JL, Kohutnicka M, Jagust W, Pivirotto P, Bringas J, et al. Convection-enhanced delivery of AAV vector in parkinsonian monkeys; in vivo detection of gene expression and restoration of dopaminergic function using pro-drug approach. Exp Neurol. 2000;164:2–14. doi: 10.1006/exnr.2000.7408. [DOI] [PubMed] [Google Scholar]
- Bankiewicz KS, Forsayeth J, Eberling JL, Sanchez-Pernaute R, Pivirotto P, Bringas J, et al. Long-term clinical improvement in MPTP-lesioned primates after gene therapy with AAV-hAADC. Mol Ther. 2006;14:564–570. doi: 10.1016/j.ymthe.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Muramatsu S, Fujimoto K, Ikeguchi K, Shizuma N, Kawasaki K, Ono F, et al. Behavioral recovery in a primate model of Parkinson's disease by triple transduction of striatal cells with adeno-associated viral vectors expressing dopamine-synthesizing enzymes. Hum Gene Ther. 2002;13:345–354. doi: 10.1089/10430340252792486. [DOI] [PubMed] [Google Scholar]
- Shen Y, Muramatsu SI, Ikeguchi K, Fujimoto KI, Fan DS, Ogawa M, et al. Triple transduction with adeno-associated virus vectors expressing tyrosine hydroxylase, aromatic--amino-acid decarboxylase, and GTP cyclohydrolase I for gene therapy of Parkinson's disease. Hum Gene Ther. 2000;11:1509–1519. doi: 10.1089/10430340050083243. [DOI] [PubMed] [Google Scholar]
- Nutt JG. Pharmacokinetics and pharmacodynamics of levodopa. Mov Disord. 2008;23 Suppl 3:S580–S584. doi: 10.1002/mds.22037. [DOI] [PubMed] [Google Scholar]
- Leriche L, Björklund T, Breysse N, Besret L, Grégoire MC, Carlsson T, et al. Positron emission tomography imaging demonstrates correlation between behavioral recovery and correction of dopamine neurotransmission after gene therapy. J Neurosci. 2009;29:1544–1553. doi: 10.1523/JNEUROSCI.4491-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramatsu S, Tsukada H, Nakano I., and, Ozawa K. Gene therapy for Parkinson's disease using recombinant adeno-associated viral vectors. Expert Opin Biol Ther. 2005;5:663–671. doi: 10.1517/14712598.5.5.663. [DOI] [PubMed] [Google Scholar]
- Christine CW, Starr PA, Larson PS, Eberling JL, Jagust WJ, Hawkins RA, et al. Safety and tolerability of putaminal AADC gene therapy for Parkinson disease. Neurology. 2009;73:1662–1669. doi: 10.1212/WNL.0b013e3181c29356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberling JL, Jagust WJ, Christine CW, Starr P, Larson P, Bankiewicz KS, et al. Results from a phase I safety trial of hAADC gene therapy for Parkinson disease. Neurology. 2008;70:1980–1983. doi: 10.1212/01.wnl.0000312381.29287.ff. [DOI] [PubMed] [Google Scholar]
- Kaplitt MG, Feigin A, Tang C, Fitzsimons HL, Mattis P, Lawlor PA, et al. Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson's disease: an open label, phase I trial. Lancet. 2007;369:2097–2105. doi: 10.1016/S0140-6736(07)60982-9. [DOI] [PubMed] [Google Scholar]
- Marks WJ, Jr, Ostrem JL, Verhagen L, Starr PA, Larson PS, Bakay RA, et al. Safety and tolerability of intraputaminal delivery of CERE-120 (adeno-associated virus serotype 2-neurturin) to patients with idiopathic Parkinson's disease: an open-label, phase I trial. Lancet Neurol. 2008;7:400–408. doi: 10.1016/S1474-4422(08)70065-6. [DOI] [PubMed] [Google Scholar]
- Ben-Haim S, Asaad WF, Gale JT., and, Eskandar EN. Risk factors for hemorrhage during microelectrode-guided deep brain stimulation and the introduction of an improved microelectrode design. Neurosurgery. 2009;64:754–62; discussion 762. doi: 10.1227/01.NEU.0000339173.77240.34. [DOI] [PubMed] [Google Scholar]
- Weaver FM, Follett K, Stern M, Hur K, Harris C, Marks WJ, Jr, et al. CSP 468 Study Group. (2009Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: a randomized controlled trial JAMA 30163–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt JG, Carter JH, Lea ES., and, Sexton GJ. Evolution of the response to levodopa during the first 4 years of therapy. Ann Neurol. 2002;51:686–693. doi: 10.1002/ana.10189. [DOI] [PubMed] [Google Scholar]
- Sánchez-Pernaute R, Harvey-White J, Cunningham J., and, Bankiewicz KS. Functional effect of adeno-associated virus mediated gene transfer of aromatic -amino acid decarboxylase into the striatum of 6-OHDA-lesioned rats. Mol Ther. 2001;4:324–330. doi: 10.1006/mthe.2001.0466. [DOI] [PubMed] [Google Scholar]
- Zappia M, Oliveri RL, Bosco D, Nicoletti G, Branca D, Caracciolo M, et al. The long-duration response to -dopa in the treatment of early PD. Neurology. 2000;54:1910–1915. doi: 10.1212/wnl.54.10.1910. [DOI] [PubMed] [Google Scholar]
- Cersosimo MG., and, Benarroch EE. Neural control of the gastrointestinal tract: implications for Parkinson disease. Mov Disord. 2008;23:1065–1075. doi: 10.1002/mds.22051. [DOI] [PubMed] [Google Scholar]
- Kitahama K, Geffard M, Araneda S, Arai R, Ogawa K, Nagatsu I, et al. Localization of -DOPA uptake and decarboxylating neuronal structures in the cat brain using dopamine immunohistochemistry. Brain Res. 2007;1167:56–70. doi: 10.1016/j.brainres.2007.05.081. [DOI] [PubMed] [Google Scholar]
- Forsayeth JR, Eberling JL, Sanftner LM, Zhen Z, Pivirotto P, Bringas J, et al. A dose-ranging study of AAV-hAADC therapy in Parkinsonian monkeys. Mol Ther. 2006;14:571–577. doi: 10.1016/j.ymthe.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Yamamoto S, Hayashi T, Kodera M, Mizukami H, Ozawa K, et al. A convenient enzyme-linked immunosorbent assay for rapid screening of anti-adeno-associated virus neutralizing antibodies. Ann Clin Biochem. 2009;46:508–510. doi: 10.1258/acb.2009.009077. [DOI] [PubMed] [Google Scholar]