Abstract
Due to the lack of acid α-glucosidase (GAA) activity, Pompe mice develop glycogen storage pathology and progressive skeletal muscle dysfunction with age. Applying either gene or enzyme therapy to reconstitute GAA levels in older, symptomatic Pompe mice effectively reduces glycogen storage in skeletal muscle but provides only modest improvements in motor function. As strategies to stimulate muscle hypertrophy, such as by myostatin inhibition, have been shown to improve muscle pathology and strength in mouse models of muscular dystrophy, we sought to determine whether these benefits might be similarly realized in Pompe mice. Administration of a recombinant adeno-associated virus serotype 8 vector encoding follistatin, an inhibitor of myostatin, increased muscle mass and strength but only in Pompe mice that were treated before 10 months of age. Younger Pompe mice showed significant muscle fiber hypertrophy in response to treatment with follistatin, but maximal gains in muscle strength were achieved only when concomitant GAA administration reduced glycogen storage in the affected muscles. Despite increased grip strength, follistatin treatment failed to improve rotarod performance. These findings highlight the importance of treating Pompe skeletal muscle before pathology becomes irreversible, and suggest that adjunctive therapies may not be effective without first clearing skeletal muscle glycogen storage with GAA.
Introduction
Pompe disease, a lysosomal storage disorder, is characterized by progressive degenerative myopathy. Deficiency of the lysosomal enzyme acid α-glucosidase (GAA) results in massive accumulation of glycogen in lysosomes and autophagosomes of striated and smooth muscle, leading to skeletal muscle weakness and cardiorespiratory failure.1,2 Patients present clinically with a spectrum of disease severity that inversely correlates with residual enzyme activity.
A Pompe mouse model (6neo/6neo) has been described that shows negligible enzyme activity and a pattern of glycogen storage similar to human patients.3 Mice are healthy at birth and breed normally, but demonstrate muscle pathology and weakness with age. Detailed ultrastructural studies have drawn parallels between the muscle pathology in the Pompe mouse and affected humans.4,5
We have shown previously that systemic delivery of an adeno-associated virus serotype 8 (AAV8) vector encoding GAA in young, presymptomatic Pompe mice reduced glycogen to basal levels and prevented motor function loss.6 However, when this treatment was applied to older, disease impaired Pompe mice, only partial motor function improvement resulted. In an effort to improve functional outcome in these older mice, we evaluated the potential of an adjuvant, namely follistatin, to improve motor function.
Inhibiting myostatin, a negative regulator of muscle growth, has been shown to increase muscle mass and improve function in some mouse models of neuromuscular disorders.7,8,9,10,11,12 These effects may be attributed, at least in part, to enhanced muscle regeneration.13,14 Although various myostatin inhibitors have been described,7,8,9,10,15,16,17 follistatin can modulate other regulators of muscle mass in addition to myostatin. For example, follistatin administration to Mstn−/− mice caused muscle mass increases beyond that stimulated by myostatin depletion18,19 suggesting that this may be a more potent approach than targeting myostatin alone. Intramuscular administration of gene therapy vectors expressing follistatin have increased muscle mass and strength in both young and aged mdx mice,11 as well as in nonhuman primates.20
To determine whether systemic follistatin could affect global musculature and improve functional efficacy in aged Pompe mice, we used liver-directed AAV8 vectors to provide the secreted form of human follistatin.21,22 We administered AAV8-GAA to symptomatic Pompe mice to clear glycogen from the muscles, and co-administered AAV8-follistatin to stimulate muscle regeneration and/or hypertrophy. We chose liver-directed AAV8 as a delivery vehicle to generate prolonged circulating levels of the therapeutic proteins, and to prevent complications from neutralizing antibodies to the expressed transgenes,6,23,24,25,26,27 thereby enabling us to perform long-term functional analyses of the treated Pompe mice.
Results
Pompe mice demonstrate progressive motor function deficits with age
In previous studies, we had observed that as Pompe mice aged, their muscle strength and coordination degenerated, as measured by rotarod and wire hang tests.6 Administration of AAV8-GAA to asymptomatic 3-month-old Pompe mice prevented this decline in motor function. However, treating symptomatic 10-month-old Pompe mice did not normalize their performance in these tests.6 To evaluate the potential of follistatin to improve muscle strength in 10-month-old Pompe mice, we applied less demanding functional tests in an attempt to discern more incremental changes in muscle strength, e.g., by deploying a decreased rate of acceleration on the rotarod test. Despite a slower rotarod acceleration rate, the pattern of functional decline in the Pompe mice over time remained unchanged, with latency declining to ~10 seconds by 17 months of age (Figure 1a). In contrast, all three parental wild-type strains maintained their initial latency of ~70 seconds under the same conditions.
Figure 1.
Pompe mice demonstrate motor function deficits. Pompe mice and age-matched wild-type mice were subjected to a series of assays to evaluate motor function and muscle strength. Testing was performed once a month. (a) Accelerating rotarod measures muscle strength, coordination, and endurance. Pompe mice demonstrated significant deterioration in performance as they aged compared to the relatively stable performance of the wild-type strains. (b) Pompe mice showed reduced front limb grip strength compared to 129S2 or B6.129 mice, but performance was similar to C57B6 wild type. (c) Pompe mice showed significantly reduced force compared to all wild-type strains when grip strength testing included all limbs. Data are expressed as average ± SEM (n = 10/group); asterisks denote *P < 0.05, **P < 0.01, ***P < 0.001, Pompe compared to all wild-type strains; carets denote ∧P < 0.05, ∧∧P < 0.01, Pompe compared to 129S2 or B6.129 only.
Performance on the rotarod requires endurance and coordination as well as muscle strength. For a more specific measure of muscle strength, grip strength tests were performed. Grip strength measurements showed that compared to wild-type mice, Pompe mice were significantly impaired at an early age, but in contrast to the rotarod results, did not decline drastically with increasing age (Figure 1b,c). When the front limbs alone were tested, there was a modest but significant difference in strength between Pompe and 129S2 or B6.129 wild-type mice, but no difference was observed between Pompe and C57B6 mice (Figure 1b). Testing both front and rear limbs showed a greater differential between Pompe and all the wild-type strains, with the wild-type (but not Pompe) mice demonstrating increasing strength over time (Figure 1c). These data support our observations that Pompe mice exhibit more functional deterioration in the hind limbs.
Administration of AAV8-follistatin increased lean and quadriceps mass in adult wild-type mice
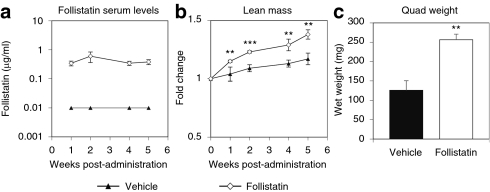
The objective of these studies was to determine whether the concomitant delivery of follistatin and GAA could increase muscle mass and correct the functional deficits beyond that which had been noted earlier with GAA alone in aged Pompe mice. Before initiating long-term, labor-intensive functional studies in Pompe mice, 1 × 1011 DNase resistant particles of AAV8-follistatin or vehicle were initially administered into 3-month-old wild-type (C57B6) mice to measure expression levels of follistatin and its effects on muscle mass. Virus-treated mice exhibited a sustained circulating level of ~0.4 µg/ml follistatin (Figure 2a) that correlated with a significant increase (~15%) in lean mass compared to vehicle-treated animals (Figure 2b). After 9 weeks, mice treated with AAV8-follistatin showed a doubling of the quadriceps mass over vehicle-treated mice (265 ± 20 mg versus 126 ± 24 mg; Figure 2c). These data indicated that sustained serum levels of ~0.4 µg/ml follistatin were sufficient to stimulate significant skeletal muscle hypertrophy in adult wild-type mice.
Figure 2.
AAV8-Follistatin increased lean mass and quadriceps mass in adult wild-type mice. Male C57B6 mice (3 months of age) were injected systemically with AAV8-follistatin or vehicle and evaluated over 5 weeks. (a) Serum was collected at the indicated time points and human follistatin levels measured using an enzyme-linked immunosorbent assay. (b) Follistatin-treated mice showed a significant increase in whole-body lean mass compared to vehicle-treated as determined by EchoMRI. (c) At killing, the right quadriceps muscle was excised and weighed. The quadriceps mass of the follistatin-treated mice was twice that of the vehicle-treated controls. Data are expressed as average ± SEM (n = 4–5/group); asterisks denote **P < 0.01, ***P < 0.001, follistatin compared to vehicle.
Systemically delivered follistatin improved motor function in younger but not older GAA-treated Pompe mice
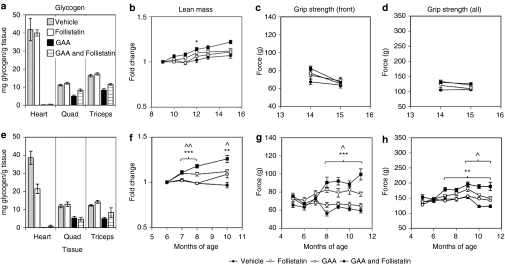
Previously, we had reported that although enzyme augmentation therapy is able to reduce the lysosomal glycogen stores in the muscles of Pompe mice, this therapeutic strategy alone could not completely reverse the accompanying myopathy, particularly in aged Pompe mice.6,28 To address this challenge, follistatin was co-administered as an adjuvant with GAA to determine whether stimulating muscle regeneration and/or hypertrophy could improve the functional response beyond that observed with GAA treatment alone. Pompe mice were treated beginning at 10 months, an age at which we have characterized extensive muscle pathology and dysfunction.6 Cohorts of Pompe mice (n = 10) were administered AAV8-GAA, AAV8-follistatin, a mixture of both AAV8-GAA and AAV8-follistatin, or vehicle. GAA and follistatin expression levels were similar across the different treatment groups, whether viral vectors were administered alone or in combination (~10 µg/ml GAA and ~1 µg/ml follistatin; data not shown). As expected, the skeletal and heart muscles of mice treated with AAV8-GAA showed significant decreases in glycogen levels (Figure 3a), similar to our previous results in Pompe mice of this age.6 There was a trend toward an increase in lean mass in animals administered AAV8-follistatin in combination with AAV8-GAA (Figure 3b). However, the provision of follistatin with or without GAA to these relatively old Pompe mice did not improve their grip strength (Figure 3c,d).
Figure 3.
Systemic follistatin improved motor function in younger but not older GAA-treated Pompe mice. Ten- (top panels) or seven- (bottom panels) month-old male Pompe mice were treated with AAV8-GAA, AVV8-Follistatin, a mixture of the GAA and follistatin vectors, or vehicle and were killed ~5 months after virus administration. (a) AVV8-GAA treatment significantly reduced glycogen levels in muscles of mice treated at 10 months of age. (b) Whole body lean mass, measured by Echo MRI, and (c,d) muscle strength, evaluated using grip strength assays, showed little change regardless of treatment when vectors were administered at 10 months of age. (e) Muscles from animals treated at 7 months showed a similar extent of glycogen clearance as in the older mice (40–60%). Mice treated at 7 months of age with GAA and follistatin showed significant increases in (f) lean mass and (g,h) grip strength over those treated with GAA alone. Data are expressed as average ± SEM (n = 4–10/group); Asterisks denote *P < 0.05, **P < 0.01, ***P < 0.001 GAA and follistatin compared to GAA alone; carets donote ∧P < 0.05, ∧∧P < 0.01, follistatin compared to vehicle. (P < 0.05 GAA & follistatin compared to follistatin alone for the last two time points in f and h).
An analogous study was conducted in 7-month-old Pompe mice, an age at which they showed only a moderate decline in rotarod function (Figure 1a). Treatment with the viral vectors generated similar serum levels of GAA and follistatin to those described above (data not shown). At 4–5 months post-treatment an analysis of Pompe mice administered AAV8-GAA showed a 40–60% reduction in their skeletal muscle glycogen levels (Figure 3e). This extent of glycogen clearance was comparable to that seen in the older mice (above). However, in contrast to the results with the older Pompe mice, those that had been treated earlier (starting at 7 months of age) with AAV8-follistatin demonstrated a significant increase in lean mass compared to those treated with AAV8-GAA alone (Figure 3f). A significant increase in the mass of the quadriceps muscles was also noted in the AAV8-follistatin-treated mice (Supplementary Figure S1). Associated with these gains in muscle mass were significant improvements in grip strength (Figure 3g,h). The force generated when all limbs were tested in mice treated with follistatin and GAA (180–200 g) was significantly greater than that produced in the vehicle- or GAA-treated mice (120–150 g). However, treatment with GAA and follistatin did not increase grip strength near to the levels seen in the wild-type strains (225–305 g; Figure 1). Importantly, the response to follistatin (as measured by both muscle mass and grip strength) was greater in Pompe mice that had also been treated with AAV8-GAA to decrease muscle lysosomal glycogen stores (Figure 3f–h; Supplementary Figure S1). In summary, Pompe mice that had been treated earlier responded better to follistatin, suggesting that the muscle pathology became more difficult to reverse with age. Follistatin and GAA worked synergistically to increase muscle mass and improve muscle function in the younger Pompe mice.
Provision of follistatin did not improve rotarod performance in GAA-treated Pompe mice
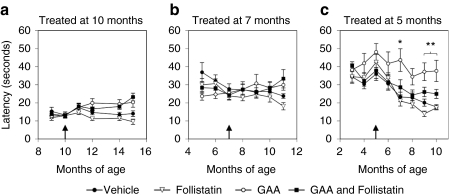
We had previously shown that treating young Pompe mice (3 months of age) with AAV8-GAA preserved muscle endurance and coordination as measured by rotarod, but treating older Pompe mice (10 months of age) did not restore their ability to function in this assay. The mice described in this study were similarly tested for muscle endurance and coordination using the rotarod to determine whether follistatin treatment could improve functional outcome. The mice were treated with AAV8 GAA and AAV8-follistatin starting at 10, 7, or 5 months of age, i.e., where they demonstrate different degrees of rotarod dysfunction (Figure 1a). We reasoned that such a comparison might reveal the impact of pre-existing myopathy on treatment efficacy. Surprisingly, follistatin failed to improve rotarod performance, either alone or in combination with GAA, regardless of the age at treatment (Figure 4a–c). Indeed, in mice treated at 5 months of age, which had up to 90% clearance of skeletal muscle glycogen and a significant follistatin-mediated increase in lean mass (Supplementary Figure S2), those treated with GAA alone tended to perform best (Figure 4c). Thus, despite measurable follistatin-mediated increases in muscle mass and grip strength, systemic follistatin failed to rescue their performance on the rotarod.
Figure 4.
Provision of follistatin did not improve rotarod performance in GAA-treated Pompe mice. Male Pompe mice were treated with GAA and follistatin vectors (as previously described) at (a) 10 months, (b) 7 months, or (c) 5 months of age (as indicated by a black arrow), and tested for endurance and coordination on a rotarod. The follistatin-treated mice showed no significant improvement in rotarod performance at any age. In mice treated at 5 months of age, which had up to 90% clearance of glycogen in skeletal muscles, there was a trend for the mice treated with GAA alone to perform best. Data are expressed as average ± SEM (n = 4–10/group); Asterisks denote *P < 0.05, **P < 0.01, GAA compared to follistatin or vehicle.
Pompe mice treated with follistatin demonstrate muscle fiber hypertrophy
Follistatin and other myostatin inhibitors reportedly can stimulate muscle fiber hypertrophy.7,9,10 To determine whether the observed increase in muscle mass in the follistatin-treated Pompe mice was similarly due to muscle fiber hypertrophy, cross-sections of quadriceps muscle were analyzed for fiber size. The images showed that the muscle fibers of Pompe mice treated at 7 months of age with both GAA and follistatin were larger than those treated with GAA alone or vehicle (Figure 5a,b); these differences were not as apparent in Pompe mice treated with GAA and follistatin starting at 10 months of age (Figure 5c,d).
Figure 5.
Histological evaluation of muscle fiber hypertrophy. Representative sections of formalin-fixed, hematoxylin and eosin stained quadriceps from the mice described in Figure 3. Photomicrographs depict how muscle fibers were outlined (in black) and the cross-sectional area measured using Aperio software. Qualitatively, in mice that had been treated at 7 months of age, (a) vehicle-treated animals had fibers with a smaller cross-sectional area than (b) mice treated with AAV8-GAA and AAV8-follistatin. In mice treated at 10 months of age, this difference between (c) vehicle and (d) AAV8-GAA plus AAV8-follistatin was less apparent. There were some unusually large fibers found in the follistatin-treated mice (white arrowheads). White areas within the cytoplasm likely represent vacuolar glycogen storage, or central cores of autophagic debris. Bar = 100 µm.
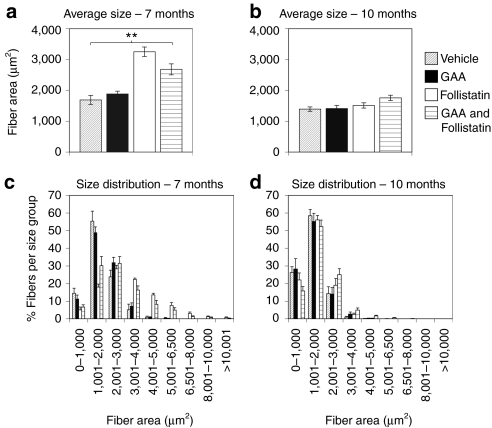
To quantify the differences in fiber size, 250–500 fibers/mouse were outlined and the cross-sectional area of each fiber determined. Results for each treatment group were displayed in terms of their average fiber area (Figure 6a,b) and fiber size distribution (Figure 6c,d). When Pompe mice were treated at 7 months of age, follistatin generated significantly larger muscle fibers compared to those administered either GAA alone or vehicle (Figure 6a). In the 10-month-old Pompe mice, however, the more modest increase in fiber size in the group treated with GAA and follistatin only attained significance when compared to the vehicle-treated mice (Figure 6b). A proportion of the fibers in Pompe mice treated with follistatin at 7 months of age were large, with some being unusually large (>4,000 µm2); vehicle- and GAA alone-treated animals showed smaller sized fibers and almost none in the very large size categories (Figure 6c). In contrast, Pompe mice that had been treated with follistatin at 10 months of age showed little difference in their fiber size distribution regardless of their treatment (Figure 6d). These histological data correlate well with the lean mass and grip strength data noted earlier, i.e., follistatin treatment stimulated significant muscle fiber hypertrophy in Pompe mice only when they were treated before 10 months of age.
Figure 6.
Pompe mice demonstrated significant muscle fiber hypertrophy only when treated at 7 months of age. Muscle fiber hypertrophy was quantified from the slides described above. The area (µm2) of each outlined muscle fiber was calculated for 250–500 fibers of 7–8 mice/treatment group. (a) The average fiber size increased significantly in the Pompe mice treated with AAV8-follistatin at 7 months of age, but (b) not if treated at 10 months. (c,d) Fiber size distribution was also evaluated. Pompe mice treated at 7 months of age with follistatin showed a shift toward larger sized fibers (and fewer smaller sized fibers), with some that were extremely large. Mice treated at 10 months of age showed only a slight trend toward larger muscle fibers. Data are expressed as average ± SEM (n = 7–8 per group); Asterisks denote **P < 0.01, follistatin treatment groups compared to GAA alone or vehicle (there was no statistical difference between mice treated with GAA and follistatin compared to mice treated with follistatin alone).
As the muscle fibers in Pompe mice age, they show an increased incidence of centralized nuclei.6,28 Central nucleation is also a feature of other mouse models of muscular dystrophy and reflects rounds of muscle degeneration and regeneration. In muscular dystrophy mice, treatment with myostatin inhibitors increased the percentage of fibers with centralized nuclei, which was attributed to treatment-stimulated increases in muscle regeneration.7,29 In wild-type mice, very few muscle fibers exhibited a phenotype characterized by centrally localized nuclei (data not shown). In contrast, in vehicle-treated Pompe mice at 7 or 10 months of age, ~35% of fibers had centralized nuclei (Supplementary Figure S3). Pompe mice treated at either 7 or 10 months of age with follistatin had an increased percentage of central nucleated fibers compared to vehicle-treated controls, and this response was somewhat greater in the mice treated at the younger age. These results suggest that follistatin may have stimulated some regeneration of Pompe muscles, even in the older mice.
Discussion
Enzyme replacement therapy was approved by the US Food and Drug Administration and the European Medicines Agency in 2006 for patients with Pompe disease. Clinical trials in infants demonstrate improved survival and improvement in hypertrophic cardiomyopathy.30,31,32,33 A randomized, placebo-controlled, multicenter trial in adults demonstrated stabilization of musculoskeletal outcomes and respiratory parameters.34 The presentation, natural history, and progression of Pompe is extremely heterogeneous, as is the response to treatment, and some patients continue to show muscle weakness despite enzyme replacement therapy.35,36 Data generated in aged Pompe mice demonstrated incomplete functional recovery following gene or protein therapy,6,28 which suggested that the aged Pompe mouse might serve as a model for patients with poor motor function response. We have used aged Pompe mice to evaluate the potential of follistatin as an adjuvant to stimulate muscle cell regeneration and/or hypertrophy. We hypothesized that generating new muscle tissue with follistatin in the presence of GAA to treat the defect in glycogenosis would improve motor function in the aged Pompe mouse.
We constructed an AAV8 vector encoding the secreted isoform of human follistatin (FS344),17 which has been shown to bind to myostatin and inhibit its activity. Intramuscular delivery of an AAV1 vector expressing FS344 effected an increase in the size and strength of the injected muscles in mdx mice and in primates.11,20 Pilot experiments in wild-type mice following liver-directed delivery of our AAV8-follistatin vector demonstrated bioactivity of the follistatin secreted into the circulation, as illustrated by increases in total body weight, gross lean mass, and the size of the quadriceps muscle similar to that reported for intramuscular delivery.11 In contrast to these effects in wild-type mice, studies in aged Pompe mice (10 months of age) showed little effect of follistatin on skeletal muscle, despite the somewhat higher circulating levels (0.4 µg/ml versus 1 µg/ml, respectively). No measurable increase in gross muscle mass or improved grip strength could be discerned in mice treated with both AAV8-GAA and AAV8-follistatin compared to those treated with AAV8-GAA alone. Histological analysis of the mice in this study did show some evidence of a response to follistatin, viz., an increase in the percentage of central nuclei in the follistatin-treated groups and a trend toward muscle fiber hypertrophy, but these effects did not translate into measurable functional efficacy.
In younger (7-month-old) Pompe mice, however, treatment with the combination of vectors did increase muscle mass and strength over GAA treatment alone. We also documented significant muscle fiber hypertrophy in response to follistatin in the 7-month-old mice that was not evident when the mice were treated at 10 months of age. The positive response to follistatin in the younger mice was likely due to the fact that there was less established myopathy at the time of vector administration. Because similar levels of transgene expression were documented in both age groups, a disparity in GAA or follistatin exposure cannot explain the observed differences in efficacy. Glycogen clearance following AAV8-GAA treatment of the aged mice was incomplete, as had been seen previously,6 but substrate levels were not appreciably different between the two age groups being compared.
Previous studies suggested that 10-month-old mice should be capable of responding to myostatin inhibition. For example, in a transgenic model, the muscles of 24-month-old Mstn−/− mice were twice the size, and retained an elevated capacity for regeneration compared to age-matched normal mice.13 In addition, administration of a myostatin antagonist increased muscle regeneration and grip strength in a 24-month-old mouse model of sarcopenia.14 These results suggest that it is more likely that the disease-associated muscle pathology in the Pompe mice rather than age interfered with the response to follistatin.
We also observed that 7-month-old Pompe mice treated with follistatin alone showed a small increase in lean mass and grip strength, but the muscles responded more robustly to follistatin when substrate had been depleted by concomitant GAA administration. This result suggests that lysosomal glycogen stores in the skeletal muscle somehow interfere with a response to follistatin. These results may not be surprising as the enlarged, glycogen-filled lysosomes and autophagosomes characteristic of this disease have been shown to interrupt the contractile apparatus in the muscle of the 6neo/6neo mouse,3 and interfere in mechanical force generation in a similar GAA knockout mouse model.37
Despite the significant increases in grip strength measured in the follistatin-treated Pompe mice described above, we did not see improvement in rotarod performance, even when treatment was initiated at 5 months of age. In fact, 5-month-old mice treated with GAA alone tended to perform the best in this functional assay. This observation indicates that muscle function was not completely normalized by follistatin treatment. It should also be noted that there was less improvement in grip strength when hindlimbs were included in the assessment. Although the front limb grip strength values approached those of normal mice, when mice were allowed to grip the test apparatus with all of their limbs only a partial correction of force was achieved. There is typically more obvious muscle dysfunction in hindlimbs of this mouse model, as they become splayed and weak as the mice age. Our data imply that the hindlimb dysfunction was not fully corrected, which likely affected rotarod performance. Disparity between muscle hypertrophy and motor function outcomes following myostatin inhibition has been reported previously. For example, Qiao et al.10 demonstrated increased grip strength but reduced treadmill endurance in mdx mice treated with myostatin propeptide. Amthor et al.38 described an increase in muscle mass that did not translate to a corresponding increase in specific force in myostatin knockout mice, and postulated that there are qualitative differences in the muscle generated during myostatin deficiency, e.g., changes in fiber type distribution and a reduced number of mitochondria.
Others have demonstrated a dramatic expansion of autophagosomes leading to centralized cores of cellular debris in type II muscles of Pompe mice.4 Similar findings have been described in muscle fibers from Pompe patients.5 The associated disorganized microtubular network and reduced cycling of the cation-independent mannose-6 phosphate receptor (required for GAA uptake) interfered with trafficking of GAA to the lysosomes, and led to the subsequent resistance of these damaged fibers to glycogen clearance.4,39 Based on our observations, we contend that as this muscle pathology progresses in the Pompe mouse, the affected fibers become unresponsive to follistatin due to the retention of glycogen and the accumulation of autophagic debris. We had previously observed thickening of the endomysium and mild fibrosis in the muscles of older Pompe mice.6 Although we did not specifically evaluate levels of fibrosis in the current studies, this pathology could also have contributed to a lack of functional response to follistatin in the older mice. Thus, these other pathologic features, in addition to glycogen storage per se, could contribute to the incomplete functional correction observed in our studies, particularly in the 10-month-old Pompe mice.
In summary, we did not achieve our original goal of improving function in 10-month-old Pompe mice by using systemic follistatin as an adjuvant to GAA treatment. We were able to show some positive effects of follistatin in younger Pompe mice, and this response was more significant when glycogen stores in the skeletal muscles were reduced. However, even in the younger mice motor dysfunction was not completely alleviated. It is difficult to predict whether patients might respond positively to treatment with an adjuvant like follistatin, as histopathology of muscle from Pompe patients before and after enzyme replacement therapy showed a variable response in terms of glycogen clearance.40,41 Importantly, our data imply that patients will be more likely to benefit from either GAA or muscle-enhancing molecules like follistatin if treated early, before irreversible muscle pathology has developed.
Materials and Methods
Construction of plasmids and vectors. A synthetic human follistatin-344 complementary DNA (FST344 GenBank accession NM_013409) was generated with 5′ EcoRI and 3′ SfiI sites and subcloned into pUC (Genescript, Piscataway, NJ). Plasmid pDC190-follistatin was generated by ligating the 1,140-bp follistatin complementary DNA into the EcoRI/SfiI sites of pDC190.25 This put follistatin expression under the control of a hepatocyte-restricted human serum albumin promoter (DC190). The follistatin expression cassette was then subcloned into the MfeI–KpnI sites of the AAV2 previral vector pAAV/SP70. Due to viral packaging issues, pAAV/SP70-follistatin was modified. Plasmid pAAV/SP70-follistatin was cut with KpnI, blunt ended with Klenow and cut with SfiI. A 1,367-bp fragment of the human α1-antitrypsin 3′ intron stuffer sequence was generated from PmlI and SfiI digest of pAAV/SP70-IGF142 and ligated into pAAV/SP70-follistatin. The DC190-follistatin vector DNA was packaged into AAV8 capsid using standard triple plasmid transfection into HEK 293 cells. The AAV8/DC190-follistatin (AAV8-follistatin) virus was purified by iodixanol-gradient centrifugation followed by ion exchange chromatography over Hitrap Q HP column. AAV8/DC190-GAA (AAV8-GAA) has been previously described.6 Virus titers were determined by real time PCR with primers to the bovine growth hormone polyadenylation sequence, and quantified as DNase-resistant particles.25
Animal studies. The Pompe6neo/6neo (Pompe) mice used in these studies have a disruption of exon 6 in the GAA gene resulting in a lack of enzyme activity.3 Pompe, 129S2/SvPasCrl (129S2), C57BL/6NCrl (C57B6) (Charles River Laboratories, Waltham, MA), and B6.129SF2/J (B6.129) (Jackson Laboratory, Bar Harbor, ME) were housed in small groups with free access to food and water in an AAALAC accredited facility. All studies were reviewed and approved by the institutional animal care and use committee. Test articles were administrated via tail-vein injection in 0.2–0.3 ml. Blood samples were collected from the orbital venous plexus under anesthesia (3% isoflurane) using microhematocrit capillary tubes (VWR, Bridgeport, NJ), and collected into BD gold microtainer tubes (VWR). Serum was separated by centrifugation at 10,000g for 10 minutes and stored at −80 °C. Once a month animals were transferred from the housing area into separate rooms for functional testing or measurement of body mass index (see below). At the end of each study, mice were killed by CO2 asphyxiation or intraperitoneal injection of Euthasol (pentobarbital sodium and phenytoin sodium; Virbac, Fort Worth, TX), after which tissues were removed and either fixed in 10% neutral-buffered formalin or snap frozen on dry ice and stored at −80 °C for later use. In some cases, individual dissected tissues were weighed before processing.
It should be noted that a large percentage of the mice treated with AAV8-follistatin developed prolapsed rectums between 3 and 6 months postadministration. This was seen in Pompe mice treated with follistatin alone, or a combination of follistatin and GAA, but was not observed in any GAA or vehicle-treated mice. Prolapsed rectums were also observed in wild-type mice treated with follistatin, indicating that this was not a consequence of the Pompe disease phenotype. This effect seemed to be somewhat dose-dependent as we did not observe prolapses in mice that had lower follistatin expression levels (0.1 compared to 0.5–2 µg/ml serum). It is possible that this side effect is follistatin specific, and not a general response to myostatin inhibition, as follistatin has been shown to bind several members of the transforming growth factor-β family of regulatory proteins in addition to myostatin,17,18,19 which can inhibit activin signaling in a variety of tissues (for a review see refs. 43,44).
Functional assays and measurement of body mass index. The 6neo/6neo Pompe mice have both C57B6 and 129S2 genetic background. Both of these strains, and a B6.129 cross, were used as wild-type control strains in the evaluation of functional assays. Muscle coordination and endurance were assessed on a rotarod using the Smartrod program (AccuScan Instruments, Columbus, OH). Mice were placed on a 30-mm diameter spindle that was elevated 18 inches. The spindle was programmed to accelerate from 0–30 r.p.m. over 90 seconds. The time to fall (latency) was recorded automatically with a light beam sensor at the bottom of the apparatus. Each animal was subjected to three trials with ~5-minutes rest between trials and the average latency was determined. Muscle strength was tested using a Chatillon grip strength meter (Columbus Instruments, Columbus, OH). Mice were encouraged to grip a wire grid attached to the force meter with their front limbs alone, or with both the front and rear limbs. Once a firm grasp had been established the animals were pulled away until they lost their grip. The maximal force generated was recorded electronically, and the average from two trials per time point was reported. It should be noted that these functional assays show some study-to-study variability and are best evaluated against internal control groups, i.e., quantitative comparisons between studies are not always valid. During the course of the studies animals were weighed (weekly or monthly) and their whole-body mass composition measured using an EchoMRI 3-in-1 Whole Body Composition Analyzer (Echo Medical Systems, Houston, TX), which uses nuclear magnetic resonance to determine lean and fat mass.
Measurement of glycogen, GAA, and follistatin levels. Frozen tissue samples were weighed and suspended in sterile water at a concentration of 100 mg/ml in a 2-ml microcentrifuge tube. A 5-mm stainless steel bead (Qiagen, Valencia, CA) was added to the tube and the tissue was disrupted using a TissueLyser (Qiagen) at 30 Hz for 10 minutes in a cold room (4 °C). Samples were stored frozen at −80 °C, thawed, centrifuged at 14,000 g for 15 minutes at 4 °C, and aliquots stored at −80 °C. To measure glycogen, lysates were defrosted and digested with α-amylglucosidase (0.54 mg/ml; Sigma-Aldrich, St Louis, MO) in 25 mmol/l potassium acetate buffer, pH 5.5. Glucose released from tissues, and the levels of endogenous glucose from parallel undigested samples, were quantitated with the Amplex Red glucose/glucose oxidase assay kit (Molecular Probes, Eugene, OR) according to manufacturer's instructions. Glycogen levels were determined by subtracting the undigested glucose levels from the digested samples. Bovine liver glycogen (Sigma-Aldrich) was used as a reference standard. Serum GAA levels were determined using an enzyme-linked immunosorbent assay as previously described.6 Serum follistatin levels were determined using an hFollistatin enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN).
Histopathology. Mouse quadriceps were fixed in 10% neutral-buffered formalin (VWR) and embedded in paraffin. Five-micron sections were cut and stained with hematoxylin–eosin. All slides were scanned at an absolute magnification of ×200 using the Aperio ScanScope XTsystems (Aperio Technologies, Vista, CA). The background illumination levels were calibrated using a prescan procedure. All acquired images were subsequently labeled, placed in dedicated project folders, and stored on a local server. Slides were viewed and analyzed remotely using desktop personal computers employing the web-based ImageScope viewer (Aperio Technologies). To evaluate the mean and distribution of muscle fiber sizes, outlines were manually drawn around the periphery of individual fibers and the cross-sectional area calculated automatically by the software, then the data were exported into Excel (Microsoft, Redmond, WA) for further analysis. Between 250 and 500 fibers/mouse were measured. To evaluate fiber size distribution, fibers were grouped into nine size categories and the percentage of fibers that fell within each category was determined for the different treatment groups.
Statistical analysis. All data are reported as means with corresponding SEM. Studies comparing multiple groups were evaluated using analysis of variance, followed by Bonferroni's multiple comparison post-test. When only two groups were compared, Student's t-test was used for analysis. Statistics were performed using GraphPad Prism software (GraphPad Software, La Jolla, CA). A P value of <0.05 was considered statistically significant.
SUPPLEMENTARY MATERIAL Figure S1. Pompe mice treated with follistatin at 7 months of age showed an increase in quadriceps mass. Figure S2. Glycogen levels and lean mass measurements from Pompe mice treated with follistatin at 5 months. Figure S3. Histological evaluation of muscle fiber regeneration. Materials and Methods.
Acknowledgments
We thank members of Comparative Medicine, Virus Production, and Histology; as well as Nelson Yew, Jennifer Nietupski, Alida D'Angona, Bill Weber, and Nilesh Pande for technical assistance. We also acknowledge Brad Hodges, Allison McVie-Wylie, Rod Moreland, Jonathan Fidler, Lisa Stanek, and Brian Kasper (Ohio University) for help with experimental design. For critical review of the manuscript, we thank Ed Kaye, Joan Keutzer, and Jennifer Tousignant. All of the authors are employees of and shareholders in Genzyme Corporation.
Supplementary Material
Pompe mice treated with follistatin at 7 months of age showed an increase in quadriceps mass.
Glycogen levels and lean mass measurements from Pompe mice treated with follistatin at 5 months.
Histological evaluation of muscle fiber regeneration.
References
- Hirschhorn R., and, Reuser AJJ.2001. Glycogen storage disease type ii: acid α-glucosidase (acid maltase) deficiency. In: Scriver, CR, Beaudet, AL, Sly, WS, Valle, D (eds). The Metabolic and Molecular Bases of Inherited Disease, Vol. III. McGraw-Hill: New York, pp 3389–3420.
- Kishnani PS., and, Howell RR. Pompe disease in infants and children. J Pediatr. 2004;144 5 Suppl:S35–S43. doi: 10.1016/j.jpeds.2004.01.053. [DOI] [PubMed] [Google Scholar]
- Raben N, Nagaraju K, Lee E, Kessler P, Byrne B, Lee L, et al. Targeted disruption of the acid alpha-glucosidase gene in mice causes an illness with critical features of both infantile and adult human glycogen storage disease type II. J Biol Chem. 1998;273:19086–19092. doi: 10.1074/jbc.273.30.19086. [DOI] [PubMed] [Google Scholar]
- Fukuda T, Ewan L, Bauer M, Mattaliano RJ, Zaal K, Ralston E, et al. Dysfunction of endocytic and autophagic pathways in a lysosomal storage disease. Ann Neurol. 2006;59:700–708. doi: 10.1002/ana.20807. [DOI] [PubMed] [Google Scholar]
- Raben N, Takikita S, Pittis MG, Bembi B, Marie SK, Roberts A, et al. Deconstructing Pompe disease by analyzing single muscle fibers: to see a world in a grain of sand. Autophagy. 2007;3:546–552. doi: 10.4161/auto.4591. [DOI] [PubMed] [Google Scholar]
- Ziegler RJ, Bercury SD, Fidler J, Zhao MA, Foley J, Taksir TV, et al. Ability of adeno-associated virus serotype 8-mediated hepatic expression of acid alpha-glucosidase to correct the biochemical and motor function deficits of presymptomatic and symptomatic Pompe mice. Hum Gene Ther. 2008;19:609–621. doi: 10.1089/hum.2008.010. [DOI] [PubMed] [Google Scholar]
- Bogdanovich S, Krag TO, Barton ER, Morris LD, Whittemore LA, Ahima RS, et al. Functional improvement of dystrophic muscle by myostatin blockade. Nature. 2002;420:418–421. doi: 10.1038/nature01154. [DOI] [PubMed] [Google Scholar]
- Ohsawa Y, Hagiwara H, Nakatani M, Yasue A, Moriyama K, Murakami T, et al. Muscular atrophy of caveolin-3-deficient mice is rescued by myostatin inhibition. J Clin Invest. 2006;116:2924–2934. doi: 10.1172/JCI28520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani M, Takehara Y, Sugino H, Matsumoto M, Hashimoto O, Hasegawa Y, et al. Transgenic expression of a myostatin inhibitor derived from follistatin increases skeletal muscle mass and ameliorates dystrophic pathology in mdx mice. FASEB J. 2008;22:477–487. doi: 10.1096/fj.07-8673com. [DOI] [PubMed] [Google Scholar]
- Qiao C, Li J, Jiang J, Zhu X, Wang B, Li J, et al. Myostatin propeptide gene delivery by adeno-associated virus serotype 8 vectors enhances muscle growth and ameliorates dystrophic phenotypes in mdx mice. Hum Gene Ther. 2008;19:241–254. doi: 10.1089/hum.2007.159. [DOI] [PubMed] [Google Scholar]
- Haidet AM, Rizo L, Handy C, Umapathi P, Eagle A, Shilling C, et al. Long-term enhancement of skeletal muscle mass and strength by single gene administration of myostatin inhibitors. Proc Natl Acad Sci USA. 2008;105:4318–4322. doi: 10.1073/pnas.0709144105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchida K. Myostatin inhibition by a follistatin-derived peptide ameliorates the pathophysiology of muscular dystrophy model mice. Acta Myol. 2008;27:14–18. [PMC free article] [PubMed] [Google Scholar]
- Wagner KR, Liu X, Chang X., and, Allen RE. Muscle regeneration in the prolonged absence of myostatin. Proc Natl Acad Sci USA. 2005;102:2519–2524. doi: 10.1073/pnas.0408729102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siriett V, Salerno MS, Berry C, Nicholas G, Bower R, Kambadur R, et al. Antagonism of myostatin enhances muscle regeneration during sarcopenia. Mol Ther. 2007;15:1463–1470. doi: 10.1038/sj.mt.6300182. [DOI] [PubMed] [Google Scholar]
- Whittemore LA, Song K, Li X, Aghajanian J, Davies M, Girgenrath S, et al. Inhibition of myostatin in adult mice increases skeletal muscle mass and strength. Biochem Biophys Res Commun. 2003;300:965–971. doi: 10.1016/s0006-291x(02)02953-4. [DOI] [PubMed] [Google Scholar]
- Amthor H, Nicholas G, McKinnell I, Kemp CF, Sharma M, Kambadur R, et al. Follistatin complexes Myostatin and antagonises Myostatin-mediated inhibition of myogenesis. Dev Biol. 2004;270:19–30. doi: 10.1016/j.ydbio.2004.01.046. [DOI] [PubMed] [Google Scholar]
- Sidis Y, Mukherjee A, Keutmann H, Delbaere A, Sadatsuki M., and, Schneyer A. Biological activity of follistatin isoforms and follistatin-like-3 is dependent on differential cell surface binding and specificity for activin, myostatin, and bone morphogenetic proteins. Endocrinology. 2006;147:3586–3597. doi: 10.1210/en.2006-0089. [DOI] [PubMed] [Google Scholar]
- Lee SJ. Quadrupling muscle mass in mice by targeting TGF-beta signaling pathways. PLoS ONE. 2007;2:e789. doi: 10.1371/journal.pone.0000789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilson H, Schakman O, Kalista S, Lause P, Tsuchida K., and, Thissen JP. Follistatin induces muscle hypertrophy through satellite cell proliferation and inhibition of both myostatin and activin. Am J Physiol Endocrinol Metab. 2009;297:E157–E164. doi: 10.1152/ajpendo.00193.2009. [DOI] [PubMed] [Google Scholar]
- Kota J, Handy CR, Haidet AM, Montgomery CL, Eagle A, Rodino-Klapac LR, et al. Follistatin gene delivery enhances muscle growth and strength in nonhuman primates. Sci Transl Med. 2009;1:6ra15. doi: 10.1126/scitranslmed.3000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimasaki S, Koga M, Esch F, Cooksey K, Mercado M, Koba A, et al. Primary structure of the human follistatin precursor and its genomic organization. Proc Natl Acad Sci USA. 1988;85:4218–4222. doi: 10.1073/pnas.85.12.4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito S, Sidis Y, Mukherjee A, Xia Y., and, Schneyer A. Differential biosynthesis and intracellular transport of follistatin isoforms and follistatin-like-3. Endocrinology. 2005;146:5052–5062. doi: 10.1210/en.2005-0833. [DOI] [PubMed] [Google Scholar]
- Pastore L, Morral N, Zhou H, Garcia R, Parks RJ, Kochanek S, et al. Use of a liver-specific promoter reduces immune response to the transgene in adenoviral vectors. Hum Gene Ther. 1999;10:1773–1781. doi: 10.1089/10430349950017455. [DOI] [PubMed] [Google Scholar]
- Mingozzi F, Liu YL, Dobrzynski E, Kaufhold A, Liu JH, Wang Y, et al. Induction of immune tolerance to coagulation factor IX antigen by in vivo hepatic gene transfer. J Clin Invest. 2003;111:1347–1356. doi: 10.1172/JCI16887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler RJ, Lonning SM, Armentano D, Li C, Souza DW, Cherry M, et al. AAV2 vector harboring a liver-restricted promoter facilitates sustained expression of therapeutic levels of alpha-galactosidase A and the induction of immune tolerance in Fabry mice. Mol Ther. 2004;9:231–240. doi: 10.1016/j.ymthe.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Franco LM, Sun B, Yang X, Bird A, Zhang H, Schneider A, et al. Evasion of immune responses to introduced human acid alpha-glucosidase by liver-restricted expression in glycogen storage disease type II. Mol Ther. 2005;12:876–884. doi: 10.1016/j.ymthe.2005.04.024. [DOI] [PubMed] [Google Scholar]
- Ziegler RJ, Cherry M, Barbon CM, Li C, Bercury SD, Armentano D, et al. Correction of the biochemical and functional deficits in fabry mice following AAV8-mediated hepatic expression of alpha-galactosidase A. Mol Ther. 2007;15:492–500. doi: 10.1038/sj.mt.6300066. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Jiang JL, Gumlaw NK, Zhang J, Bercury SD, Ziegler RJ, et al. Glycoengineered acid alpha-glucosidase with improved efficacy at correcting the metabolic aberrations and motor function deficits in a mouse model of Pompe disease. Mol Ther. 2009;17:954–963. doi: 10.1038/mt.2009.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons SA, Millay DP, Sargent MA, McNally EM., and, Molkentin JD. Age dependent effect of myostatin blockade on disease severity in a murine model of limb-girdle muscular dystrophy. Am J Pathol. 2006;168:1975–1985. doi: 10.2353/ajpath.2006.051316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishnani PS, Nicolino M, Voit T, Rogers RC, Tsai AC, Waterson J, et al. Chinese hamster ovary cell-derived recombinant human acid alpha-glucosidase in infantile-onset Pompe disease. J Pediatr. 2006;149:89–97. doi: 10.1016/j.jpeds.2006.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishnani PS, Corzo D, Nicolino M, Byrne B, Mandel H, Hwu WL, et al. Recombinant human acid [alpha]-glucosidase: major clinical benefits in infantile-onset Pompe disease. Neurology. 2007;68:99–109. doi: 10.1212/01.wnl.0000251268.41188.04. [DOI] [PubMed] [Google Scholar]
- Levine JC, Kishnani PS, Chen YT, Herlong JR., and, Li JS. Cardiac remodeling after enzyme replacement therapy with acid alpha-glucosidase for infants with Pompe disease. Pediatr Cardiol. 2008;29:1033–1042. doi: 10.1007/s00246-008-9267-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolino M, Byrne B, Wraith JE, Leslie N, Mandel H, Freyer DR, et al. Clinical outcomes after long-term treatment with alglucosidase alfa in infants and children with advanced Pompe disease. Genet Med. 2009;11:210–219. doi: 10.1097/GIM.0b013e31819d0996. [DOI] [PubMed] [Google Scholar]
- van der Ploeg AT, Clemens P, Corzo D, Lake S, Skrinar A, Escolar D, et al. Results from a randomized, double-blind, multicenter, multinational, placebo-controlled study of the safety and efficacy of Myozyme, recombinant human acid alpha-glucosidase (rhGAA), for the treatment of Pompe disease in juveniles and adults. Neurology. 2008;71:155. [Google Scholar]
- Winkel LP, Van den Hout JM, Kamphoven JH, Disseldorp JA, Remmerswaal M, Arts WF, et al. Enzyme replacement therapy in late-onset Pompe's disease: a three-year follow-up. Ann Neurol. 2004;55:495–502. doi: 10.1002/ana.20019. [DOI] [PubMed] [Google Scholar]
- van Capelle CI, Winkel LP, Hagemans ML, Shapira SK, Arts WF, van Doorn PA, et al. Eight years experience with enzyme replacement therapy in two children and one adult with Pompe disease. Neuromuscul Disord. 2008;18:447–452. doi: 10.1016/j.nmd.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Drost MR, Hesselink RP, Oomens CW., and, van der Vusse GJ. Effects of non-contractile inclusions on mechanical performance of skeletal muscle. J Biomech. 2005;38:1035–1043. doi: 10.1016/j.jbiomech.2004.05.040. [DOI] [PubMed] [Google Scholar]
- Amthor H, Macharia R, Navarrete R, Schuelke M, Brown SC, Otto A, et al. Lack of myostatin results in excessive muscle growth but impaired force generation. Proc Natl Acad Sci USA. 2007;104:1835–1840. doi: 10.1073/pnas.0604893104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardone M, Porto C, Tarallo A, Vicinanza M, Rossi B, Polishchuk E, et al. Abnormal mannose-6-phosphate receptor trafficking impairs recombinant alpha-glucosidase uptake in Pompe disease fibroblasts. Pathogenetics. 2008;1:6. doi: 10.1186/1755-8417-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkel LP, Kamphoven JH, van den Hout HJ, Severijnen LA, van Doorn PA, Reuser AJ, et al. Morphological changes in muscle tissue of patients with infantile Pompe's disease receiving enzyme replacement therapy. Muscle Nerve. 2003;27:743–751. doi: 10.1002/mus.10381. [DOI] [PubMed] [Google Scholar]
- Thurberg BL, Lynch Maloney C, Vaccaro C, Afonso K, Tsai AC, Bossen E, et al. Characterization of pre- and post-treatment pathology after enzyme replacement therapy for Pompe disease. Lab Invest. 2006;86:1208–1220. doi: 10.1038/labinvest.3700484. [DOI] [PubMed] [Google Scholar]
- Chu Q, Moreland R, Yew NS, Foley J, Ziegler R., and, Scheule RK. Systemic Insulin-like growth factor-1 reverses hypoalgesia and improves mobility in a mouse model of diabetic peripheral neuropathy. Mol Ther. 2008;16:1400–1408. doi: 10.1038/mt.2008.115. [DOI] [PubMed] [Google Scholar]
- Aoki F., and, Kojima I. Therapeutic potential of follistatin to promote tissue regeneration and prevent tissue fibrosis. Endocr J. 2007;54:849–854. doi: 10.1507/endocrj.kr07e-001. [DOI] [PubMed] [Google Scholar]
- Sonoyama K, Rutatip S., and, Kasai T. Gene expression of activin, activin receptors, and follistatin in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2000;278:G89–G97. doi: 10.1152/ajpgi.2000.278.1.G89. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Pompe mice treated with follistatin at 7 months of age showed an increase in quadriceps mass.
Glycogen levels and lean mass measurements from Pompe mice treated with follistatin at 5 months.
Histological evaluation of muscle fiber regeneration.