Abstract
A major limitation to the use of immunotherapy in the treatment of cancer has been the localized immune suppressive environment within the tumor. Although there is evidence that tumor-selective (oncolytic) viruses may help to overcome this immune suppression, a primary limitation to their use has been limited systemic delivery potential, especially in the face of antiviral immunity. We recently demonstrated that tumor-trafficking immune cells can efficiently deliver oncolytic viral therapies to their tumor targets. These cells act as both a therapeutic agent and also a carrier vehicle for the oncolytic virus. Here, we demonstrate that such delivery is also possible in the face of pre-existing antiviral immunity, so overcoming the limited systemic delivery of naked, cell-free virus. It was also found that treatment of previously immunized mice or repeat treatments leading to immunization resulted in a switch from a primarily oncolytic to an immunotherapeutic mechanism of action. Furthermore, repeat cycles of treatment with combination immune cell-viral therapy resulted in increased tumor infiltration of effector T-cells and a general reduction in the levels of known immune suppressive lymphocyte populations. This therefore represents a novel and effective means to overcome localized immune suppression within the tumor micoenvironment.
Introduction
Oncolytic viruses are therapeutic agents that display natural or engineered selective replication in cells with a malignant phenotype. They comprise a therapeutic platform that has recently seen significant advances with the development of new agents and demonstrated efficacy against a number of tumor types.1,2,3,4 Systemic delivery and potent antitumor effects have been demonstrated in preclinical models with a variety of oncolytic viral vectors and an accumulation of clinical data have consistently demonstrated the safety and, in many cases, therapeutic potential of oncolytic viruses.3,5,6,7,8 In addition, because these viral agents, despite replicating exclusively within the tumor, are eventually cleared by the host immune response, leading to antiviral immunity, they must be capable of overcoming tumor-mediated localized immune suppression. However, one significant limitation to these therapeutic approaches that has not been addressed is the severely reduced ability of these vectors to be delivered systemically once such an immune response develops. This is of particular importance as the induction of an immune response in an otherwise naive patient will severely reduce the treatment window within which multiple cycles of the same therapeutic can be applied. Although, the use of immune suppressive drugs has been proposed,9,10,11 this may raise safety concerns by increasing the potential toxicity of the viruses. In addition, the use of immune suppression may reduce the overall antitumor benefits, as it is apparent that an immune response targeting infected cancer cells can help to clear these cells12 and can even lead to an adaptive immune response targeting tumor-associated antigens as a form of in situ vaccination.12,13 Novel approaches are therefore needed to enhance viral delivery to the tumor in immunized hosts, to enhance the therapeutic effects of the viruses under these conditions, and so to allow repeat cycles of treatment. Without addressing these issues it is unlikely that the potential of oncolytic viruses will be realized in the clinic.
Although oncolytic viral therapies have been limited in their application due to the effective induction of adaptive immunity, several therapeutic platforms that rely on immune targeting of the tumor (such as vaccine therapy or immune cell therapies) are instead frequently limited by the immune suppressive nature of the tumor. It appears that even when a cellular immune response targeting the tumor or a tumor antigen is successfully produced, the cells are unable to infiltrate the tumor or the response is subverted once within the tumor.14,15,16 Therefore, unlike the case with oncolytic viruses, the failure to induce a productive immune response in the tumor is often the limiting factor with this therapeutic approach. These opposing interactions with the host immune response may therefore become an advantage when immune cell and oncolytic viral therapies are combined.
We have recently described an approach that enhances delivery and therapeutic potential of oncolytic strains of vaccinia virus by preinfecting tumor-trafficking immune cells as carrier vehicles that also serve to amplify the therapy in the target tissue.17,18,19 Here, we initially extended this work to examine the delivery potential of this approach in the face of pre-existing antiviral immunity. It was found that immune cell carriers could indeed deliver virus in the face of an antiviral immune response. However, successful delivery of virotherapy in immunized mice (either through cell-based delivery, or through direct injection into the tumor) was associated with limited viral replication in the tumor. This presumably indicates that the oncolytic potential of the virus has been curtailed by rapid immune-mediated clearance of virus or infected cells. Yet, despite this loss of viral oncolysis, antitumor effects were still seen that were equivalent or even greater than those seen in nonimmunized mice. It is therefore apparent that if evasion of circulating antibody and delivery to the tumor is achieved, oncolytic viruses can produce therapeutic effects in previously immunized hosts and that these effects are no longer mediated by the direct oncolytic potency of the virus. Instead these antitumor effects are primarily mediated through the cellular immune response induced within the tumor. Types and levels of tumor infiltrating lymphocytes were determined under different conditions, demonstrating that multiple rounds of therapeutic treatment with a combined immune cell-oncolytic viral therapy results in the induction of an immunotherapeutic mechanism of action, leading to a general increase in the level of CD4+ and CD8+ T-cells within the tumor and a reduction in the levels of a variety of lymphocyte populations associated with the creation of a localized immune suppressive environment. This therefore represents a potential novel and effective means to overcome tumor-mediated immunosuppression.
Results
CIK cells can conceal vvDD from neutralizing antibody
We have previously described a cancer therapy involving a combination of two biological agents, cytokine-induced killer (CIK) cells (an natural killer (NK)-T cell-like population) and oncolytic vaccinia virus strain (vvDD).17 The oncolytic vvDD contains deletions in the viral thymidine kinase (TK) and viral growth factor (VGF) genes, and so selectively replicates in tumors where cell cycle control has been disrupted and/or regulation of the EGF-R/Ras signaling pathway has been lost.20 In this approach, the CIK cells are infected with virus before their systemic delivery to a tumor-bearing mouse, so that the virus is able to utilize the ability of CIK cells to traffic to tumor targets and achieve more effective delivery to the tumor than cell-free virus. However, we have not previously examined the effectiveness of this approach in immunized mice, or after repeat cycles of the therapy. Because the main barrier to systemic delivery in preimmunized hosts is the presence of circulating antibody in the plasma, we began by examining the effects of neutralizing antibody in vitro.
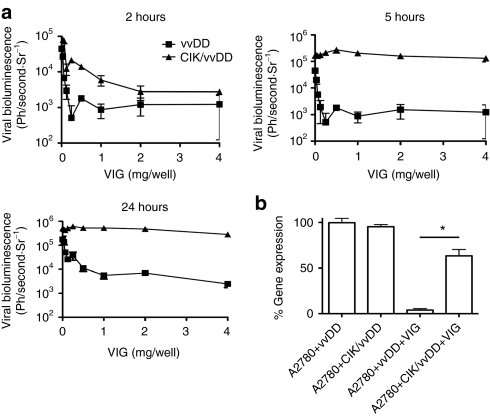
We incorporated vaccinia immunoglobulin (VIG) as a natural source of polyclonal antivaccinia antibodies, including neutralizing antibody. Because VIG is raised following immunization with the dryvax vaccine strain of vaccinia, and we tested neutralization of the vvDD strain (which is in a western reserve backbone), we initially compared the ability of the VIG to neutralize different viral strains (Supplementary Figure S1). It was found that all viruses were equally susceptible to neutralization. Therefore, we examined the ability of VIG to prevent delivery of virus to tumors within CIK cells. CIK cells were expanded from human peripheral blood and preinfected with vvDD expressing luciferase for different periods of time before being exposed to different doses of VIG, centrifuged and washed (to remove unbound VIG) and added to a monolayer of the A2780 tumor cell line. Subsequent infection of tumor cells was assayed by bioluminescence imaging (BLI). We observed a window of time during which the virus was effectively concealed within the CIK cell and was unaffected by the presence of neutralizing antibody (between 5 and up to at least 24 hours after infection) (Figure 1a). CIK cells were therefore preinfected for 5 hours before use in future experiments.
Figure 1.
CIK infection shields vvDD from neutralizing antibody. (a) 100,000 PFU of vvDD-expressing luciferase, alone or premixed with CIK cells for 2, 5, or 24-hours were exposed to increasing doses of vaccinia immunoglobulin (VIG) for 2 hours. CIK cells were then pelleted and layered over a monolayer of A2780 cells. Bioluminescence was measured 72 hours later, results are triplicates. (b) Experiment was repeated, only CIK cells preinfected with vvDD-luc for 5 hours or vvDD were mixed with 10.0 mg/ml VIG and layered directly onto A2780 cells. Bioluminescence was read 72 hours later and expressed as a percentage of the light produced with no VIG present. Results are triplicates (*P < 0.05). CIK, cytokine-induced killer; PFU, plaque-forming unit; vvDD, vaccinia virus.
In further experiments, CIK cells that had been infected with vaccinia for 5 hours were mixed continuously with neutralizing antibody and target, A2780 cancer cells. This study was used to determine whether the cell-to-cell transmission from CIK cells to the tumor target could occur in the presence of neutralizing antibody. This cell-to-cell spread was only marginally affected by the presence of neutralizing antibody (Figure 1b), whereas virus alone was almost completely neutralized.
CIK cells can deliver vvDD systemically to tumors in the face of antiviral immunity
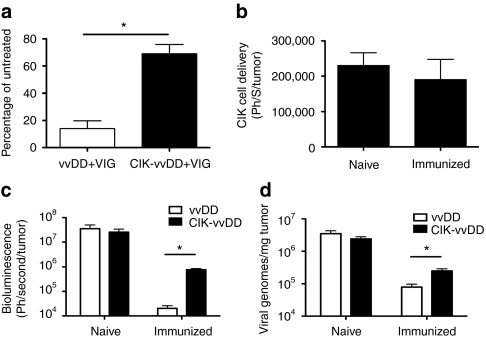
We next examined the ability of infected CIK cells to systemically deliver vvDD virus to tumors in the face of different components of the adaptive immune response in animal models. In the initial model tested, we pretreated tumor-bearing mice (subcutaneous CMT64 tumors, a mouse colorectal cancer) with high doses of VIG, such that levels of circulating neutralizing antibody equivalent to those seen in fully immunized hosts were present for at least 72-hours after treatment to assess the effects of both neutralizing and non-neutralizing antibody. The levels of neutralizing antibody in the serum of mice 72 hours after VIG treatment was equivalent to levels in fully immunized mice, and also in serum from humans previously immunized with vaccinia virus (Supplementary Figure S2). BLI of luciferase expressed from the virus was used to determine the levels of viral gene expression within the tumor (as well as infection elsewhere in the animal). We and others have shown that bioluminescence from virally encoded luciferase is tightly correlated with viral load in different tissues.21,22 In addition, although virus within CIK cells does produce some bioluminescence it is more than three logs less than the signal produced in tumor cells (Supplementary Figure S3). Therefore BLI signal (following delivery of labeled virus within CIK cells) will be primarily that produced from infected tumor cells. It was found that when vvDD-luc+ alone was used, the addition of high levels of circulating antibody reduced the amount of luciferase signal from the tumor site at 72-hours after intravenous injection of the virus to 15% of the value obtained when no VIG was used (Figure 2a). In contrast, when virus was delivered within CIK cells, the signal was only reduced by 30% in the presence of VIG (i.e., VIG reduced bioluminescent signals to 70% of that seen with no VIG) (Figure 2a). This mirrors the in vitro data, and highlights the value of cell-based delivery in avoiding circulating antibody.
Figure 2.
CIK cells can deliver vvDD to the tumor in the face of neutralizing antibody. (a) Mice-(C57BL/6) bearing subcutaneous CMT64 tumors were pretreated with intraperitoneal (i.p.) injections of VIG (200 mg/mouse) or PBS and 12 hours later intravenous (i.v.) with vvDD-luc (1 × 107 PFU) or 1 × 107 CIK cells premixed with 1 × 107 PFU vvDD-luc for 5 hours. Viral gene expression from within the tumor was determined after 72 hours by bioluminescence imaging. Percentage of BLI signal for VIG treated relative to PBS-treated mice are shown. Mice were bled at the time of imaging to verify continued presence of neutralizing antibody in serum at this point (n = 4/group). (b) vvDD-infected CIK cells can traffic to the tumor in immunized mice. Mice either immunized with i.p. injection of WR, or naive, were implanted subcutaneously with CMT64 cells 14 days later. Once tumors had formed, they were treated i.v. with CIK-vvDD as before. CIK cells were expanded from a C57BL/6-luciferase transgenic mouse, such that they expressed luciferase. BLI (indicative of numbers of CIK cells) within the tumor were determined after 72-hours (n = 5/group). (c) Viral replication in the tumor is reduced in immunized mice. Experiment as in b repeated, only vvDD-luc and unlabeled CIK cells used, and compared to vvDD-luc delivered alone. Viral gene expression (BLI signal) within the tumor for immunized and naive mice is shown (n = 4/group) at 72 hours after treatment. (d) Experiment as in c repeated, only mice sacrificed at 72 hours after treatment and relative viral genomes in the tumor determined by Q-PCR (n = 3/group) (*P < 0.05). BLI, bioluminescence imaging; CIK, cytokine-induced killer; PBS, phosphate-buffered saline; PFU, plaque-forming units; Q-PCR, quantitative PCR; VIG, vaccinia immunoglobulin; vvDD, vaccinia virus; WR, western reserve.
We next looked to examine the delivery of virus to tumors within CIK cells in fully immunized hosts. In these studies, mice were first immunized with a single intraperitoneal injection of 1 × 106 plaque-forming unit of western reserve strain of vaccinia 14 days before the implantation of tumors, and ~24 days before application of therapy. Successful immunization was confirmed by assay of neutralizing antibody in the plasma of these mice. In this model, CIK cells were expanded from a transgenic C57BL/6 mouse strain expressing luciferase from all hematopoietic cell lineages, such that CIK cells could be obtained labeled with luciferase without the need for any ex vivo manipulation. These CIK-luc cells were preinfected with vvDD (without luciferase) such that we could use BLI to assay CIK trafficking, proliferation, and persistence (rather than viral biodistribution). It was seen that at 72-hours after intravenous injection of CIK-luc cells infected with vvDD into vaccinia immunized or naive mice, there was no significant difference in CIK cell levels in the tumors (as measured by BLI) (Figure 2b and Supplementary Figure S4). This demonstrated that infected CIK cells are capable of trafficking to the tumor even in the face of full antiviral immunity.
Having determined that vvDD-infected CIK cells could still traffic to the tumor in immunized mice, we examined whether CIK cells could transfer the virus to the cancer cells. We therefore repeated the previous experiment, but utilizing vvDD-luc+ to preinfect nonlabeled CIK cells (Figure 2c). This demonstrated that, even though viral gene expression was again increased when CIK delivery was utilized to overcome the host immune response (relative to naked vvDD), only about 3% of the viral gene expression relative to nonimmunized hosts was seen. The level of delivery of virus to the tumor was confirmed by quantitative-PCR assay of viral genomes within the tumor (Figure 2d), in this case ~10% of the genomes measured in tumors of nonimmunized mice were seen in the case of immunized mice (when CIK-mediated delivery was used). It is presumed that this will include some viral particles that reach the tumor, but do not produce significant gene expression due to rapid immune-mediated clearance. Similar effects were seen when vvDD was injected directly into the tumor in immunized and nonimmunized animals (Supplementary Figure S5). This loss of viral gene expression and viral replication would clearly be expected to block the oncolytic effect of the therapy.
vvDD-CIK dual biotherapy produces significant antitumor effects in immunized hosts despite limited viral replication
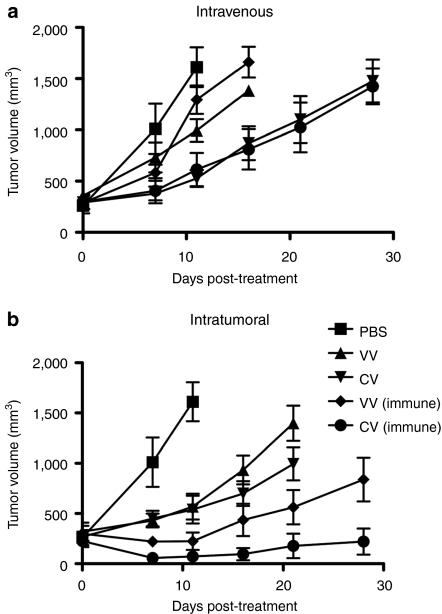
To examine whether any therapeutic benefit remained in this preimmunized model, we repeated these experiments, but followed tumor burden over time. Surprisingly, antitumor effects were still seen (Figure 3a). When CIK-mediated delivery of the virus was used, equivalent levels of antitumor effects were seen in both naive and immunized animals, despite the fact that only 3% of the level of viral gene expression was seen in the tumor in the immunized relative to nonimmunized mice. This implies that different mechanisms of tumor destruction must be mediating the therapeutic effects in the immunized mice.
Figure 3.
Immunized mice treated with vvDD therapies still display antitumor effects. Mice were immunized or treated with PBS 14 days before subcutaneous implantation of CMT64 cells. Once palpable tumors had formed (50–100 mm3; day 0), mice were treated with (a) i.v. or (b) intratumoral (IT) (bottom) injections of PBS; vvDD (V); or CIK-vvDD (CV) as before. Tumor volume was determined by caliper measurement. N = 8/group. For i.v. therapy, at day 11, vvDD (P = 0.025), CIK-vvDD (P = 0.0009), and CIK-vvDD in immunized mice (P = 0.0043) all showed significant improvement over PBS control. vvDD in naive was significantly enhanced over vvDD in immunized mice (P = 0.02 at day 16), but CIK-vvDD effects were not significantly different between immunized and naive mice. In IT-treated groups, all treatments resulted in enhanced efficacy over PBS by day 7 (P values of 0.002 [vvDD] 0.002 [vvDD in immunized mice] 0.003 [vvDD-CIK] and 0.001 [vvDD-CIK in immunized mice]); both treatments were more effective in immunized mice (P values of 0.022 for vvDD at day 21 and 0.039 for vvDD-CIK as soon as day 7). CIK, cytokine-induced killer; PBS, phosphate-buffered saline; vvDD, vaccinia virus.
To determine whether the presence of virus within the tumor was sufficient to induce these antitumor effects in immunized mice these experiments were repeated with intratumoral (IT) injection of the therapies. In this case, the antitumor effects were actually increased in immunized animals (despite dramatic reductions in viral gene expression), and this was true both for naked virus, or virus preinfected into CIK cells. It therefore appears that antitumor effects can be produced even in fully immunized mice (despite greatly reduced viral gene expression), as long as virus can be delivered to the tumor. CIK cells alone produced no antitumor effects in this tumor model (data not shown).
vvDD-CIK therapeutic effects in immunized mice correspond to enhanced effector T-cell infiltration
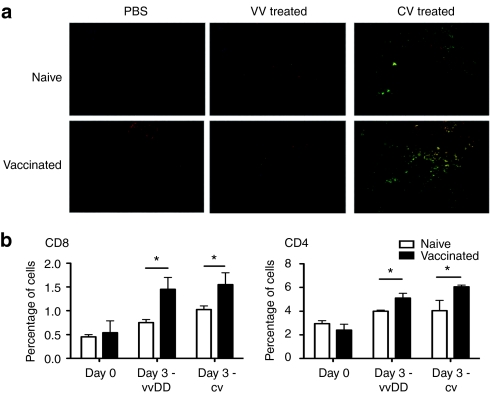
It is likely that the antitumor effect seen in the immunized mice is immune mediated. We therefore determined whether an increase in the level of tumor infiltrating lymphocytes was seen in immunized mice following systemic (IV) viral delivery to the tumor (Figure 4). Immunofluorescence microscopy was used to examine the levels of viral gene expression [green fluorescent protein (GFP)] and of CD3+ T-cells (red) within the tumor 72-hours after different treatments in naive or immunized mice (Figure 4a). It was seen that a large influx of CD3+ cells within the tumor correlated with viral delivery in immunized mice, and so with antitumor effects in the absence of viral replication. Further experiments were performed to define the types of T-cell and to quantify their levels (through flow cytometry on dissociated tumor samples) (Figure 4b). In this experiment, virus (or viral-infected CIK cells) was delivered directly into the tumor to better control and to synchronize viral delivery. It was seen that for either CIK-mediated or direct IT injection of vvDD, before immunization led to an early additional influx of both CD4+ and CD8+ T-cells.
Figure 4.
Prior immunization leads to increased levels of T-cell infiltration into the tumor. (a) Mice treated as in (Figure 3) were sacrificed 72-hours after treatment and tumors either frozen and sectioned for staining with anti-CD3 antibody (red) and viral GFP expression (green) (top) or (b) tumors were dissociated and cells stained with fluorophore labeled antibodies to CD3 and CD4 or CD8 and percentage positively stained cells determined by flow cytometry (bottom) (n = 4/group) (*P < 0.05). GFP, green fluorescent protein; vvDD, vaccinia virus.
Repeat cycles of vvDD-CIK therapy leads to further enhanced effector lymphocyte infiltration into the tumor
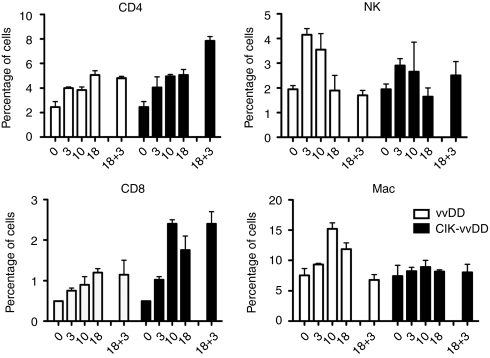
In order to model the scenario of repeat cycles of therapy in a cancer patient (rather than the treatment of a patient presenting with pre-existing acquired immunity to the virus), tumor-bearing mice were treated intravenously with vvDD alone or CIK cells preinfected with vvDD. We followed changes in the level and type of tumor infiltrating lymphocyte both following the initial treatment and then after a subsequent second cycle (we incorporated an IT injection of vvDD for the second cycle of therapy so as to better control the timing and level of virus arriving in the tumor, but our previous results indicate similar results would be achieved with intravenous delivery of CIK cells carrying vvDD). Changes in levels of different lymphocytes within the tumor were followed postmortem by flow cytometry of dissociated tumor cells (Figure 5). It was found that both CD4 and CD8+ T-cells were recruited into the tumor over time following vvDD or CIK-vvDD systemic therapy in naive mice, with levels peaking after 10–18 days. The addition of a second round of IT vvDD therapy maintained these T-cells at high levels (when vvDD was used as the initial therapy), or further boosted T-cell infiltration (for CIK-vvDD initial therapy).
Figure 5.
Second round of viral therapy leads to enhanced T-cell infiltration into the tumor. Mice- (C57BL/6) bearing subcutaneous CMT64 tumors were treated with an intravenous injection of vvDD or CIK-vvDD as before (day 0). Mice were sacrificed at the indicated days after treatment and tumors dissociated postmortem for antibody staining and flow cytometry analysis (n = 3/group at each time). A second treatment of 1 × 107 PFU vvDD was delivered via IT injection at day 18 after the initial treatments. Dissociated tumor cells were stained for CD3+CD4+ (CD4+ T-cells); CD3+CD8+ (CD8+ T-cells); CD3-NK1.1+ (NK cells); F4/80+ (macrophages). CIK, cytokine-induced killer; IT, intratumoral; PFU, plaque-forming unit; vvDD, vaccinia virus.
We further looked at NK cell and macrophage infiltration into the tumor. It was seen that both therapies (vvDD or CIK-vvDD) induced NK cell infiltration into the tumor, with levels peaking 3–10 days after treatment, but no significant further increase of NK cell levels in the tumor were seen following a “boost” with a second round of IT vvDD therapy. Macrophage infiltration was only significantly induced by vvDD systemic therapy, and not CIK-vvDD therapy. When CIK cells were used alone there was no significant change in any of these cell populations at 3 days post-treatment (Supplementary Figure S6), however, the levels of CD4+ and CD8+ cells did trend toward an increase (the CIK therapy itself would not be expected to produce a significant increase in CD3+ cells in the tumor).
Repeat cycles of vvDD-CIK therapy leads to reduced levels of immune suppressive lymphocyte populations in the tumor
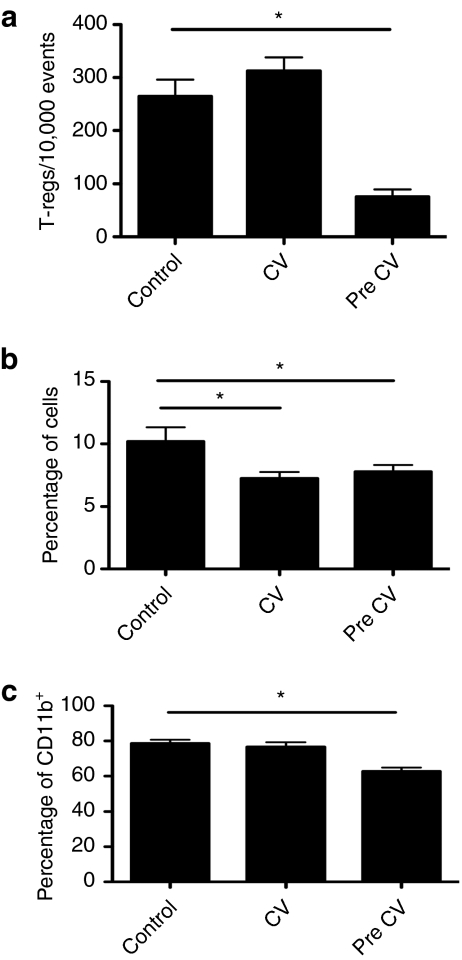
We next looked at the effects of CIK-vvDD single and repeat treatments on the levels of known immune suppressive cell types (Figure 6). We examined levels of regulatory T-cells (CD4+CD25+ FoxP3+), monocyte-derived suppressor cells (CD11b+IL4Ra+ Gr-1+CD11c–) and the percentage of tumor-associated macrophages (CD11b+) with a Th2 phenotype (CD206+). It was found that levels of monocyte-derived suppressor cells were suppressed by a single treatment of CIK-vvDD, and remained low after a second round of treatment. Regulatory T-cells and the percentage of CD206+ macrophages were unchanged after the first round of therapy, but were significantly reduced only after the second round of treatment. It therefore appears that CIK-vvDD therapy is capable of boosting T-cell infiltration after both a primary and secondary round of therapy, whereas reducing the levels of known immune suppressive cell types, especially after repeat treatments.
Figure 6.
Repeat treatments with CIK-vvDD therapy leads to reduction of tumor immunosuppression. Mice- (C57BL/6) bearing subcutaneous CMT64 tumors were treated intravenously with PBS; CIK-vvDD; or two rounds of CIK-vvDD therapy 7 days apart. Mice were sacrificed 5-days later and tumors dissociated and cells stained with labeled antibodies for determination of levels of different cell types within the tumor. These included (a) regulatory T-cells (CD4+CD25+FoxP3+); (b) monocyte-derived suppressor cells (CD11b+IL4Ra+Gr-1+CD11c–); and (c) Th2 tumor-associated macrophages (CD11b+CD206+) (n = 5/group) (*P < 0.05). CIK, cytokine-induced killer; PBS, phosphate-buffered saline; vvDD, vaccinia virus.
Discussion
We have previously demonstrated that immune cells, such as CIK cells, can act as efficient delivery vehicles to carry oncolytic viruses, such as the vaccinia strain vvDD, systemically to their tumor targets. In addition, the cytolytic immune cells are able to take advantage of the additional tumor killing capabilities of the viral agent with increased recognition of infected tumor targets than uninfected malignant cells.17 This led to synergistic therapeutic benefits between the two agents. Having determined the effectiveness of this approach in several preclinical models, we sought to determine whether cell-based delivery of therapeutic viruses could still be achieved in the face of pre-existing antiviral immunity.
Initially, we found that the preinfected CIK cells could protect the viral agent from neutralizing antibody, and that the oncolytic virus could be passed from CIK cell to tumor cell despite constant exposure to high levels of neutralizing antibody. The virus must therefore either be capable of passing from the immune cell to the tumor target without any exposure to the surrounding extracellular environment (e.g., through the immune synapse), or stoichiometric effects allow rapid transfer of the virus, meaning the effects of neutralizing antibodies can be avoided. This in line with several other recent reports that have also shown that cell-based delivery vehicles can conceal oncolytic viral strains from an antiviral immune response, but none have incorporated a tumor-targeting cell type as we describe here.23,24
These observations were extended in vivo, into mouse tumor models with high levels of circulating neutralizing antibody. Although CIK-mediated delivery of the virus was most efficient (with 70% delivery relative to naive mice), high doses of oncolytic virus delivered intravenously also had a limited capability of overcoming circulating neutralizing antibody. This in itself is an important observation, as many ongoing clinical trials with oncolytic therapies as single agents involve multiple cycles of treatment, often weeks apart, and so later cycles will undoubtedly have to contend with an induced immune response.
However, although neutralizing antibody is the major determinant of viral removal from the blood stream in immunized hosts it is not the only factor, and infected cells in particular may additionally be targeted by other components of the adaptive immune response, including effector T-cells. As such, we determined that infected CIK cells were able to reach the tumor as effectively as uninfected CIK cells, even in fully immunized hosts. However, it was also observed, that despite being delivered to the tumor, very little viral gene expression within the tumor was observed in immunized mice. This may be surprising as (i) we know the virus can be delivered to the tumor (with 70% efficiency) within CIK cells in the face of neutralizing antibody, and (ii) we know that infected CIK cells are equally efficient at getting to the tumor in nonimmunized and immunized mice. This implies therefore that the host cellular immune response is rapidly removing the virus from within the tumor once it reaches its target, and that this is reducing the ability of the virus to express genes (and so replicate).
Interestingly, despite the reduction in viral replication, antitumor effects were still seen, and these were equivalent to, or even better than were seen in naive animals. It was hypothesized that this may be due to a switch from a primarily oncolytic to a primarily immunotherapeutic means of tumor destruction. Previous reports have shown that vesicular stomatitis virus delivered to tumors within T-cell carriers can induce a potent immune response,25 and that oncolytic reovirus can produce an immunotherapeutic antitumor effect under certain conditions of reduce replication,26 but both of these reports relied on ovalbumin-expressing tumor models, and importantly, did not describe the ability of a single therapy to destroy the same tumor by different mechanisms of action depending on external conditions. An ability to switch from oncolytic to immunotherapeutic tumor killing, if harnessed effectively, could significantly enhance the therapeutic potential of this approach. Similar results were seen when viral therapy was injected directly into the tumors of immunized mice (i.e., reduced viral gene expression with increased antitumor effects), but this would have limited clinical application, where many tumors are poorly accessible for IT injection, or where disseminated disease is present.
Further investigation determined that the therapeutic effects of vvDD-CIK dual biotherapy in immunized hosts correlated with an influx of CD4+ and CD8+ T-cells into the tumor. Although this correlates with the antitumor effects seen in the preimmunized mice, it is difficult to assess the relative roles of these and other lymphocyte populations, as use of transgenic knockouts, or antibody-mediated depletion of different immune cell types would be expected to lead to increased viral replication, and so there would be a switch back from an immune to an oncolytic mechanism of tumor destruction.
Finally, we wished to model the scenario of repeat cycles of therapy in a single cancer patient, as opposed to the examination of the effects of pre-existing antiviral immunity in a tumor-bearing host. One of the most efficient means to induce a robust immune response against a tumor antigen during vaccination strategies has been to incorporate a prime-boost approach, with several rounds of exposure to the same antigen.27 We have also previously shown that oncolytic viral therapy, resulting in viral-mediated destruction of tumor cells and release of tumor antigens and other costimulatory molecules, is capable of inducing an adaptive immune response against the tumor itself.22 It is therefore possible that multiple rounds of oncolytic viral therapy (as long as delivery to the tumor is achieved) will prime and boost an antitumor immune response. We therefore looked in more detail at the levels, types and activities of the immune cells found within the tumor under different conditions to determine which cells are attracted to, or proliferate in, the tumor as a result of initial vaccinia infection (in naive mice), or subsequent repeat cycles of treatment. The more pertinent question therefore, was whether CIK-mediated delivery raised the potential to repeat treat with the same virus, and whether this would lead to enhanced antitumor immune effects, and so we sought to model this situation in a preclinical setting. Comparing the levels of T-cells in the tumor 72 hours after IT vvDD therapy in mice previously treated with the oncolytic virus (Figure 5) to mice where immunity was raised by immunization with vaccinia before implantation of the tumor (Figure 4b), it was seen that the “prime-boost” strategy of multiple rounds of therapy in a tumor-bearing mouse results in the greatest infiltration of T-cells into the tumor.
When NK cell infiltration was examined, it was found that an increase in NK cell infiltration was seen after the initial round of therapy, but that this was not boosted further after repeat cycles. This is perhaps not surprising as these cells are primarily a part of the innate immune response and so their levels would not be expected to correlate with immune status. Alternatively, when levels of macrophages in the tumor were examined, it was seen that levels increased after vvDD therapy, but nor vvDD-CIK dual biotherapy. It is not clear why this occurs, but our previous results demonstrated a Th1 skewing of the immune response through an altered cytokine profile when vvDD was delivered along with CIK cells, which may help to explain this.
Because macrophages are more closely associated with tumor-mediated immune suppression than an antitumor effect, we looked at the levels of other known immune suppressive cell types, including regulatory T-cell, monocyte-derived suppressor cells, and Th2 skewed macrophages. All three of these cell populations were reduced after multiple rounds of vvDD-CIK therapy (with MSDC levels reduced after a single round of therapy). It therefore appears that CIK-vvDD therapy is capable of boosting T-cell infiltration after both a primary and secondary round of therapy, while reducing the levels of known immune suppressive cell types, especially after repeat treatments. It is likely therefore that the strongly immunogenic nature of the viral infection within the tumor coupled with the Tc1 CIK cells results in an alteration in the type and level of cytokines and chemokines being produced within the tumor environment. This switch from an immunosuppressive to more immunostimulatory profile may be able to influence the lymphocyte populations being attracted to or proliferating within the tumor environment.
The use of preinfected CIK cells as a means to deliver vvDD oncolytic virus to the tumor is therefore possible even in the face of an antiviral immune response. However, it was seen that the antitumor effects resulting from this delivery are less associated with viral oncolysis of the tumor cell, but appear to be primarily mediated by the host immune response. This is seen with an increase in the overall level of T-cells in the tumor and a reduction in the levels of a variety of immune suppressive cell types (including regulatory T-cells, monocyte-derived suppressor cells, and tumor-associated macrophages) that is most apparent after repeat rounds of therapy in tumor-bearing hosts. This therefore represents a novel and effective way to overcome localized tumor immune suppression that may ultimately be incorporated into a variety of future generation therapies.
Materials and Methods
Viral and cell-based therapies. The oncolytic virus vvDD, a western reserve strain of vaccinia containing deletions in the VGF genes and an insertional mutation in the viral TK gene has been constructed to express either GFP or firefly luciferase, as described previously.20,28
CIK cells are an autologus, ex vivo expanded cell population with phenotypic markers of NK and T-cells. Their expansion from mouse splenocytes or human peripheral blood (buffy coats provided by blood bank through institutional review board ethics committee approved protocols) involves activation and differentiation through exposure to interferon-γ and anti-CD3 antibody and expansion in interleukin-2, and has been described previously.29 In some experiments, CIK cells were expanded from luciferase and GFP-expressing transgenic mice, to obtain CIK cells expressing these reporter genes. The production of the dual biotherapy (CIK-vvDD) requires mixing of CIK cells and vvDD at a multiplicity of infection of 1.0 for the described periods of time.
Tumor cell lines and other reagents. JC (murine breast cancer syngeneic for BALB/c) and CMT64 (murine colorectal cancer from C57/BL6 background) were obtained from the Cancer Research UK cell bank. A2780 was obtained from ATCC (Manassas, VA). All cell lines have been constructed to stably express firefly luciferase via lentiviral transfection and selection. VIG (Cangene, Fort Garry, Manitoba, Canada), was kindly provided by Chris Allen (CDC, Atlanta, GA).
Neutralizing antibody assay. To measure the levels of neutralizing antibody, a luciferase assay of viral gene expression was performed. One thousand plaque-forming unit aliquots of vvDD-expressing luciferase were mixed with serial dilutions of neutralizing antibody samples (VIG or mouse serum samples) for 2 hours, before being added to a naive A2780 cell layer. Luciferase (viral gene expression) was determined after addition of luciferin substrate 72 hours later, using an IVIS200 (Xenogen product from Caliper LifeSciences, Alameda, CA). The dilution at which 50% of the viral gene expression was neutralized was determined relative to no virus (0%) and no antibody (100%) controls.
Mouse models. Tumors were formed in syngeneic mice by subcutaneous injection of 500,000 tumor cells (CMT64, nonsmall cell lung tumors, in C57BL/6). Once tumors had become palpable (50–100 mm3; tumor measurement determined by caliper) mice were treated with intravenous (tail vein) injections of 1 × 107 plaque-forming unit vvDD, 1 × 107 CIK cells, or 1 × 107 plaque-forming unit vvDD premixed with 1 × 107 CIK cells. In some cases, mice were pretreated with intraperitioneal injection of VIG. Serum samples were obtained by submandibular bleed to determine the levels of circulating neutralizing antibody. In some experiments, animals were imaged for bioluminescence produced by luciferase expressed from virus or CIK cells. For in vivo BLI 𝒹-luciferin was injected into the animals and they were anesthetized (2% isoflurane), and imaged using an IVIS200. All animal studies were performed under approved animal protocols with strict adherence to institutional guidelines. A C57BL/6 mouse strain-expressing luciferase and enhanced GFP was used in this work (a cross of the L2G85 luciferase-expressing mouse, which is in the FVB/N background30 and an enhanced GFP expressing mouse, which is on a C57BL/6 background (C57BL/6-Tg(CAG-EGFP)1Osb/J, Jackson).31,32 These strains were backcrossed into the C57BL/6 background for >10 generations).
Postmortem tumor analyses. Several assays were performed on tumor tissues obtained postmortem. Immunofluorescence microscopy was performed on cryosections obtained from samples frozen in oxytetracycline and stained with antibodies targeting GFP, CD3e (eBioscience, San Diego, CA) or Hoechst 33342 (Invitrogen, Carlsbad, CA). Appropriate secondary antibodies were used.
In other studies, tumor tissues were immediately dissociated into single cell suspensions after collection through gentle “grinding” of the tissues through a cell strainer. Single cell suspensions were stained with conjugated antibodies, and the percentages of positively stained cells determined by flow cytometry (FACScaliber, BD Biosciences, San Jose, CA). Antibodies included those to CD4, CD8, NK1.1, F4/80, CD25, FoxP3, CD11b, IL4Ra, Gr-1, CD11c, and CD206 (all BD Bioscience or eBioscience).
Statistical analysis. Unpaired and paired Student's t-tests were run to determine statistical significance (defined as P < 0.05).
SUPPLEMENTARY MATERIAL Figure S1. Neutralization of different vaccinia strains by VIG. Figure S2. Levels of neutralizing antibody in mice pre-treated 72 hours earlier with 200 mg VIG; or immunized 21-day earlier with vvDD. Figure S3. Cell lines CMT64 (mouse colorectal cancer) and murine CIK cells were infected with vvDD expressing luciferase at an MOI of 1.0 for 24 hours. Figure S4. Representative figures of mice treated with CIK cells expressing luciferase and pre-infected with vvDD. Figure S5. Bioluminescence signal from the tumor (subcutaneous CMT64 tumors) following intratumoral injection of vvDD expressing luciferase. Imaging taken 72 hours after treatment. Figure S6. Levels of different lymphocyte populations found within the tumor at 3 days post-CIK therapy.
Acknowledgments
This work was supported by the Alliance of Cancer Gene Therapy and NIH awards (R01 CA140215) (S.H.T), an Interdisciplinary Center for Clinical Research (IZKF Würzburg) grant (A.B.) and NIH awards P50 CA114747 and R24 CA92862 (C.H.C.).
Supplementary Material
Neutralization of different vaccinia strains by VIG.
Levels of neutralizing antibody in mice pre-treated 72 hours earlier with 200 mg VIG; or immunized 21-day earlier with vvDD.
Cell lines CMT64 (mouse colorectal cancer) and murine CIK cells were infected with vvDD expressing luciferase at an MOI of 1.0 for 24 hours.
Representative figures of mice treated with CIK cells expressing luciferase and pre-infected with vvDD.
Bioluminescence signal from the tumor (subcutaneous CMT64 tumors) following intratumoral injection of vvDD expressing luciferase. Imaging taken 72 hours after treatment.
Levels of different lymphocyte populations found within the tumor at 3 days post-CIK therapy.
REFERENCES
- Guo ZS, Thorne SH., and, Bartlett DL. Oncolytic virotherapy: molecular targets in tumor-selective replication and carrier cell-mediated delivery of oncolytic viruses. Biochim Biophys Acta. 2008;1785:217–231. doi: 10.1016/j.bbcan.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirn DH., and, Thorne SH. Targeted and armed oncolytic poxviruses: a novel multi-mechanistic therapeutic class for cancer. Nat Rev Cancer. 2009;9:64–71. doi: 10.1038/nrc2545. [DOI] [PubMed] [Google Scholar]
- Park BH, Hwang T, Liu TC, Sze DY, Kim JS, Kwon HC, et al. Use of a targeted oncolytic poxvirus, JX-594, in patients with refractory primary or metastatic liver cancer: a phase I trial. Lancet Oncol. 2008;9:533–542. doi: 10.1016/S1470-2045(08)70107-4. [DOI] [PubMed] [Google Scholar]
- Liu TC, Hwang TH, Bell JC., and, Kirn DH. Translation of targeted oncolytic virotherapeutics from the lab into the clinic, and back again: a high-value iterative loop. Mol Ther. 2008;16:1006–1008. doi: 10.1038/mt.2008.70. [DOI] [PubMed] [Google Scholar]
- Liu TC, Galanis E., and, Kirn D. Clinical trial results with oncolytic virotherapy: a century of promise, a decade of progress. Nat Clin Pract Oncol. 2007;4:101–117. doi: 10.1038/ncponc0736. [DOI] [PubMed] [Google Scholar]
- Hu JC, Coffin RS, Davis CJ, Graham NJ, Groves N, Guest PJ, et al. A phase I study of OncoVEXGM-CSF, a second-generation oncolytic herpes simplex virus expressing granulocyte macrophage colony-stimulating factor. Clin Cancer Res. 2006;12:6737–6747. doi: 10.1158/1078-0432.CCR-06-0759. [DOI] [PubMed] [Google Scholar]
- Lorence RM, Roberts MS, O'Neil JD, Groene WS, Miller JA, Mueller SN, et al. Phase 1 clinical experience using intravenous administration of PV701, an oncolytic Newcastle disease virus. Curr Cancer Drug Targets. 2007;7:157–167. doi: 10.2174/156800907780058853. [DOI] [PubMed] [Google Scholar]
- Kumar S, Gao L, Yeagy B., and, Reid T. Virus combinations and chemotherapy for the treatment of human cancers. Curr Opin Mol Ther. 2008;10:371–379. [PubMed] [Google Scholar]
- Chang CL, Ma B, Pang X, Wu TC., and, Hung CF. Treatment with cyclooxygenase-2 inhibitors enables repeated administration of vaccinia virus for control of ovarian cancer. Mol Ther. 2009;17:1365–1372. doi: 10.1038/mt.2009.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulci G, Breymann L, Gianni D, Kurozomi K, Rhee SS, Yu J, et al. Cyclophosphamide enhances glioma virotherapy by inhibiting innate immune responses. Proc Natl Acad Sci USA. 2006;103:12873–12878. doi: 10.1073/pnas.0605496103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lun XQ, Jang JH, Tang N, Deng H, Head R, Bell JC, et al. Efficacy of systemically administered oncolytic vaccinia virotherapy for malignant gliomas is enhanced by combination therapy with rapamycin or cyclophosphamide. Clin Cancer Res. 2009;15:2777–2788. doi: 10.1158/1078-0432.CCR-08-2342. [DOI] [PubMed] [Google Scholar]
- Kirn DH, Wang Y, Liang W, Contag CH., and, Thorne SH. Enhancing poxvirus oncolytic effects through increased spread and immune evasion. Cancer Res. 2008;68:2071–2075. doi: 10.1158/0008-5472.CAN-07-6515. [DOI] [PubMed] [Google Scholar]
- Qiao J, Kottke T, Willmon C, Galivo F, Wongthida P, Diaz RM, et al. Purging metastases in lymphoid organs using a combination of antigen-nonspecific adoptive T cell therapy, oncolytic virotherapy and immunotherapy. Nat Med. 2008;14:37–44. doi: 10.1038/nm1681. [DOI] [PubMed] [Google Scholar]
- Gulley JL, Arlen PM, Tsang KY, Yokokawa J, Palena C, Poole DJ, et al. Pilot study of vaccination with recombinant CEA-MUC-1-TRICOM poxviral-based vaccines in patients with metastatic carcinoma. Clin Cancer Res. 2008;14:3060–3069. doi: 10.1158/1078-0432.CCR-08-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RS, Walker AI., and, Ward SJ. Cancer vaccines: will we ever learn. Expert Rev Anticancer Ther. 2009;9:67–74. doi: 10.1586/14737140.9.1.67. [DOI] [PubMed] [Google Scholar]
- Elkord E, Dangoor A, Burt DJ, Southgate TD, Daayana S, Harrop R, et al. Immune evasion mechanisms in colorectal cancer liver metastasis patients vaccinated with TroVax (MVA-5T4) Cancer Immunol Immunother. 2009;58:1657–1667. doi: 10.1007/s00262-009-0674-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne SH, Negrin RS., and, Contag CH. Synergistic antitumor effects of immune cell-viral biotherapy. Science. 2006;311:1780–1784. doi: 10.1126/science.1121411. [DOI] [PubMed] [Google Scholar]
- Thorne SH., and, Contag CH. Combining immune cell and viral therapy for the treatment of cancer. Cell Mol Life Sci. 2007;64:1449–1451. doi: 10.1007/s00018-007-6550-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne SH., and, Contag CH. Integrating the biological characteristics of oncolytic viruses and immune cells can optimize therapeutic benefits of cell-based delivery. Gene Ther. 2008;15:753–758. doi: 10.1038/gt.2008.42. [DOI] [PubMed] [Google Scholar]
- Thorne SH, Hwang TH, O'Gorman WE, Bartlett DL, Sei S, Kanji F, et al. Rational strain selection and engineering creates a broad-spectrum, systemically effective oncolytic poxvirus, JX-963. J Clin Invest. 2007;117:3350–3358. doi: 10.1172/JCI32727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luker KE, Hutchens M, Schultz T, Pekosz A., and, Luker GD. Bioluminescence imaging of vaccinia virus: effects of interferon on viral replication and spread. Virology. 2005;341:284–300. doi: 10.1016/j.virol.2005.06.049. [DOI] [PubMed] [Google Scholar]
- Kirn DH, Wang Y, Le Boeuf F, Bell J., and, Thorne SH. Targeting of interferon-beta to produce a specific, multi-mechanistic oncolytic vaccinia virus. PLoS Med. 2007;4:e353. doi: 10.1371/journal.pmed.0040353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong HT, Hasegawa K, Dietz AB, Russell SJ., and, Peng KW. Evaluation of T cells as carriers for systemic measles virotherapy in the presence of antiviral antibodies. Gene Ther. 2007;14:324–333. doi: 10.1038/sj.gt.3302880. [DOI] [PubMed] [Google Scholar]
- Power AT, Wang J, Falls TJ, Paterson JM, Parato KA, Lichty BD, et al. Carrier cell-based delivery of an oncolytic virus circumvents antiviral immunity. Mol Ther. 2007;15:123–130. doi: 10.1038/sj.mt.6300039. [DOI] [PubMed] [Google Scholar]
- Prestwich RJ, Ilett EJ, Errington F, Diaz RM, Steele LP, Kottke T, et al. Immune-mediated antitumor activity of reovirus is required for therapy and is independent of direct viral oncolysis and replication. Clin Cancer Res. 2009;15:4374–4381. doi: 10.1158/1078-0432.CCR-09-0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao J, Wang H, Kottke T, Diaz RM, Willmon C, Hudacek A, et al. Loading of oncolytic vesicular stomatitis virus onto antigen-specific T cells enhances the efficacy of adoptive T-cell therapy of tumors. Gene Ther. 2008;15:604–616. doi: 10.1038/sj.gt.3303098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall JL, Hoyer RJ, Toomey MA, Faraguna K, Chang P, Richmond E, et al. Phase I study in advanced cancer patients of a diversified prime-and-boost vaccination protocol using recombinant vaccinia virus and recombinant nonreplicating avipox virus to elicit anti-carcinoembryonic antigen immune responses. J Clin Oncol. 2000;18:3964–3973. doi: 10.1200/JCO.2000.18.23.3964. [DOI] [PubMed] [Google Scholar]
- McCart JA, Ward JM, Lee J, Hu Y, Alexander HR, Libutti SK, et al. Systemic cancer therapy with a tumor-selective vaccinia virus mutant lacking thymidine kinase and vaccinia growth factor genes. Cancer Res. 2001;61:8751–8757. [PubMed] [Google Scholar]
- Lu PH., and, Negrin RS. A novel population of expanded human CD3+CD56+ cells derived from T cells with potent in vivo antitumor activity in mice with severe combined immunodeficiency. J Immunol. 1994;153:1687–1696. [PubMed] [Google Scholar]
- Cao YA, Bachmann MH, Beilhack A, Yang Y, Tanaka M, Swijnenburg RJ, et al. Molecular imaging using labeled donor tissues reveals patterns of engraftment, rejection, and survival in transplantation. Transplantation. 2005;80:134–139. doi: 10.1097/01.tp.0000164347.50559.a3. [DOI] [PubMed] [Google Scholar]
- Okabe M, Ikawa M, Kominami K, Nakanishi T., and, Nishimune Y. ‘Green mice' as a source of ubiquitous green cells. FEBS Lett. 1997;407:313–319. doi: 10.1016/s0014-5793(97)00313-x. [DOI] [PubMed] [Google Scholar]
- Hadjantonakis AK, Macmaster S., and, Nagy A. Embryonic stem cells and mice expressing different GFP variants for multiple non-invasive reporter usage within a single animal. BMC Biotechnol. 2002;2:11. doi: 10.1186/1472-6750-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Neutralization of different vaccinia strains by VIG.
Levels of neutralizing antibody in mice pre-treated 72 hours earlier with 200 mg VIG; or immunized 21-day earlier with vvDD.
Cell lines CMT64 (mouse colorectal cancer) and murine CIK cells were infected with vvDD expressing luciferase at an MOI of 1.0 for 24 hours.
Representative figures of mice treated with CIK cells expressing luciferase and pre-infected with vvDD.
Bioluminescence signal from the tumor (subcutaneous CMT64 tumors) following intratumoral injection of vvDD expressing luciferase. Imaging taken 72 hours after treatment.
Levels of different lymphocyte populations found within the tumor at 3 days post-CIK therapy.