Abstract
Myogenic cell transplantation is an experimental approach for the treatment of myopathies. In this approach, transplanted cells need to fuse with pre-existing myofibers, form new myofibers, and generate new muscle precursor cells (MPCs). The last property was fully reported following myoblast transplantation in mice but remains poorly studied with human myoblasts. In this study, we provide evidence that the intramuscular transplantation of postnatal human myoblasts in immunodeficient mice generates donor-derived MPCs and specifically donor-derived satellite cells. In a first experiment, cells isolated from mouse muscles 1 month after the transplantation of human myoblasts proliferated in vitro as human myoblasts. These cells were retransplanted in mice and formed myofibers expressing human dystrophin. In a second experiment, we observed that inducing muscle regeneration 2 months following transplantation of human myoblasts led to myofiber regeneration by human-derived MPCs. In a third experiment, we detected by immunohistochemistry abundant human-derived satellite cells in mouse muscles 1 month after transplantation of postnatal human myoblasts. These human-derived satellite cells may correspond totally or partially to the human-derived MPCs evidenced in the first two experiments. Finally, we present evidence that donor-derived satellite cells may be produced in patients that received myoblast transplantation.
Introduction
Transplantation of cells with the ability to differentiate into skeletal muscle is an approach under study for the treatment of some myopathies, mainly those of recessive genetic origin. Cells potentially useful for this purpose need to have one of the following properties (ideally the three): (i) ability to fuse with pre-existing myofibers, (ii) ability to form new myofibers, and (iii) ability to produce myogenically committed stem cells. The first property allows integrating exogenous nuclei in the myofibers of the recipient. Exogenous nuclei can thus express therapeutic genes in myofibers that previously suffered a genetic disorder. The second property would be important to treat skeletal muscles in which there were severe loss of myofibers. The third property ensures a permanent source of normal myogenically committed stem cells in the recipient.
Myoblasts were the first myogenic cells to be proposed for this therapeutic approach.1 In postnatal life, myoblasts derive from satellite cells, the committed stem cells of skeletal muscles. Satellite cells can be isolated from muscle biopsies by standard cell-culture techniques and can be easily expanded in vitro to produce large amounts of myoblasts, maintaining their capacity to fuse and to differentiate into myofibers.2 Myoblasts were the first myogenic cells transplanted in mice,3 dogs,4 monkeys,5 rabbits,6 and pigs.7 They were also the first cells to be tested in clinical trials (see ref. 8 for a summary of these clinical trials).
From the three properties mentioned above, the first one was profusely demonstrated with myoblasts.9 In humans, occasional observations of improved expression of dystrophin following normal myoblast transplantation in Duchenne muscular dystrophy (DMD) patients were reported in the clinical trials conducted in the 1990s.8 However, these results were limited and erratic due to lack of data about the appropriate transplantation parameters. More recent clinical trials, based on data obtained with nonhuman primate experiments, showed that donor-derived dystrophin can be expressed in myofibers of DMD patients implanted with normal myoblasts.10,11,12 The second property (formation of new myofibers) was observed in different mouse experiments of myoblast transplantation.13,14,15,16,17,18 More important, a clinical observation was encouraging for the use of this second property in the clinic: putative neo-formed small dystrophin+ myofibers were observed in DMD patients transplanted with normal myoblasts.12
The third property (to give rise to myogenically committed stem cells) was observed in several studies of mouse myoblast transplantation into mouse muscles. On one hand, experiments of muscle regeneration showed that some of the transplanted myoblasts remained as muscle precursor cells (MPCs) that can later participate in muscle regeneration.19,20 On the other hand, histological analyses showed that some of the transplanted myoblasts formed satellite cells.16,17,21 So far, only few studies have addressed this issue with human myoblasts.22,23,24 From these, only one study that used fetal human myoblasts presented evidence that human myoblasts transplanted in immunodeficient mice form functional donor-derived MPCs.23 However, myoblasts transplanted in clinical trials are from postnatals and have not fetal origin, which may imply different biological properties as discussed below.
We conducted this study to verify whether the intramuscular transplantation of postnatal human myoblasts produces functional donor-derived MPCs and whether it produces specifically donor-derived satellite cells. We performed experiments of human myoblast transplantation in immunodeficient mice, and completed them by analyzing muscle biopsies of DMD patients transplanted with normal myoblasts.
Results
Human MPCs were isolated from mouse muscles transplanted with postnatal human myoblasts
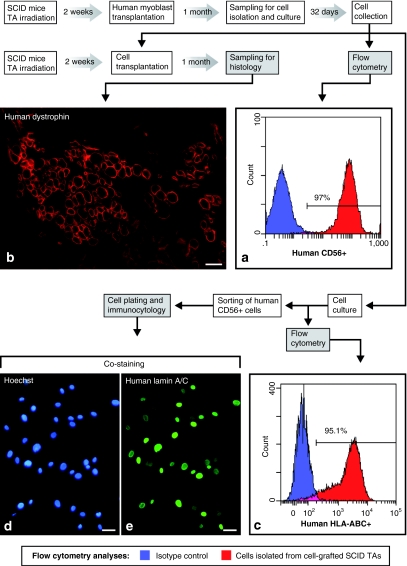
Postnatal human myoblasts were proliferated in vitro and were grafted in both previously irradiated Tibialis anterior (TA) muscles of three severe combined immunodeficiency (SCID) mice. These TA muscles were collected 4 weeks later and pooled to enzymatically isolate cells for in vitro culture. The cells isolated from these TA muscles were proliferated for 32 days. These cells will be designated hereafter as post-transplantation cells (cellspt). When confluent, a sample of these cultured cellspt was tested by flow cytometry with a monoclonal antibody (mAb) specific for human CD56. This cell population was consistently positive for the human CD56 antigen, i.e., ~97% were clearly discriminated from the isotype control (Figure 1a). The final amount of cellspt collected after the 32-day culture was of 44 × 106. From these, we transplanted 3 × 106 cells per muscle in previously irradiated TA muscles of SCID mice. These TA muscles were sampled 1 month later, and the histological analysis showed several myofibers expressing human dystrophin, identified with the NCL-Dys3 mAb (Figure 1b).
Figure 1.
Human muscle precursor cells were isolated from mouse muscles 1 month after transplantation of human myoblasts. (a) The cells isolated from the transplanted muscles are designated as post-transplantation cells (cellspt). A flow cytometry analysis shows that 97% of the cellspt proliferated from these mouse muscles were clearly human CD56+ after 32 days in culture. (b) A sample of these cellspt was retransplanted in severe combined immunodeficient mouse muscles and produced human-dystrophin+ myofibers that were observed 1 month later by immunofluorescence in a muscle cross-section. A sample of the cellspt was also proliferated again to perform further analyses. (c) A flow cytometry analysis shows that 95.1% of these cellspt were human HLA-ABC+. These cellspt were also sorted by flow cytometry using the antihuman CD56 antibody and were plated and briefly cultured for immunocytology: most nuclei in these (d, Hoechst blue fluorescence) cultures expressed (e, green fluorescence) human lamin A/C. Bar = 50 µm.
The remnant nongrafted cellspt were later cultured for further confirmation of their human origin. We performed a flow cytometry analysis of the whole cell population using a mouse antihuman HLA-ABC mAb: 95.1% of the cells clearly expressed human HLA-ABC (Figure 1c). We also performed immunocytology detection of human lamin A/C both with the whole cellpt population and with a subpopulation of the cellspt separated by flow cytometry for their expression of human CD56. Almost all nuclei in both cases showed different intensities of lamin A/C labeling (Figure 1d,e). Human lamin A/C-negative nuclei corresponded to mitosis, as previously reported.25
We performed a similar enzymatic dissociation of both TA muscles of three SCID mice in which a sham experiment was done, i.e., the muscles were treated as for myoblast transplantation (i.e., they were irradiated) but not injected with human cells. No cell plated in vitro from these muscles. The human specificity of the anti-CD56 antibody was confirmed with myoblasts obtained from nonirradiated neonatal mice, which showed no labeling with this antibody.
MPCs derived from postnatal human myoblast transplantation participate in later muscle regeneration
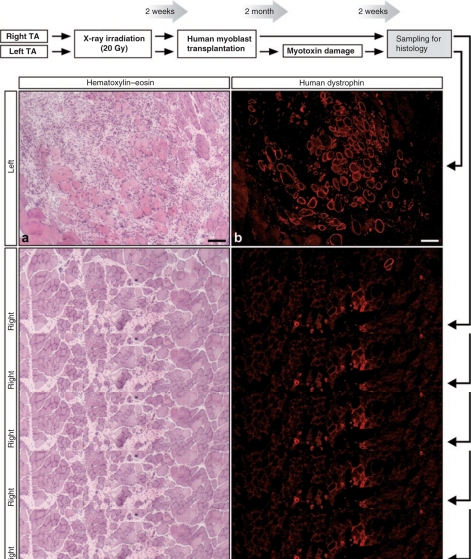
Human myoblasts were transplanted in the previous experiment in both irradiated TA muscles of six SCID mice. Two months later, the left TA muscles were damaged by the intramuscular injection of a myotoxin (cardiotoxin). Two weeks later, both TA muscles were harvested for histological analysis. Different amounts of myofibers expressed human dystrophin in all the TA muscles (Figure 2b,d,e). In the left TA, there were large regions with ongoing myofiber regeneration and mononuclear cell infiltration due to the recent cardiotoxin damage (Figure 2a). Regenerating regions were not observed in the right muscles, not damaged with cardiotoxin 2 weeks before (Figure 2c). Many myofibers into the regenerating regions of the left TA expressed human dystrophin (Figure 2b). No labeling was observed with the mAb used to detect human dystrophin in the TAs of a sham experiment done in four SCID mice, i.e., in muscles irradiated and cardiotoxin-treated at the same time points, but without cell transplantation. The quantification of the maximum amount of human-dystrophin+ myofibers per muscle cross-section in each TA is illustrated in Figure 2e. A mean of 245 ± 161 human-dystrophin+ myofibers was present in the left TA muscles (recently damaged with cardiotoxin) and a mean of 100 ± 59 human-dystrophin+ myofibers was observed in the contralateral TA muscles that did not receive such damage. The level of significance for the difference between both groups was of P = 0.06 in a unilateral paired Student t-test (post-transplantation damage versus non post-transplantation damage).
Figure 2.
Human MPCs remaining in SCID mouse muscles transplanted with human myoblasts regenerated myofibers following a second damage 2 months post-transplantation. (a–d) The images show serial cross-sections of both TA muscles of a SCID mouse transplanted with human myoblasts and submitted to a second damage in the left TA 2 months post-transplantation. The sections were stained with hematoxylin–eosin to observe the (a,c) structure of the muscle and (b,d: the images show the sections with the greatest amount of human-dystrophin+ myofibers in this mouse) for fluorescent immunodetection of human dystrophin. (a) Recent muscle destruction and ongoing regeneration is evident in the left TA. (c) In the right TA, there is some fibrosis probably due to the cell transplantation but regeneration is completed. (b) Most myofibers around and into the area of recent muscle destruction in the left TA express human dystrophin strongly suggesting that regeneration was carried out essentially by human MPCs. Bar = 100 µm. (e) The graphic shows the quantification of human-dystrophin+ myofiber profiles in each mouse (the section with the greatest amount of human-dystrophin+ myofibers in each TA was used for quantification). MPC, muscle precursor cell; SCID, severe combined immunodeficient; TA, Tibialis anterior.
Human satellite cells in mouse muscles transplanted with postnatal human myoblasts
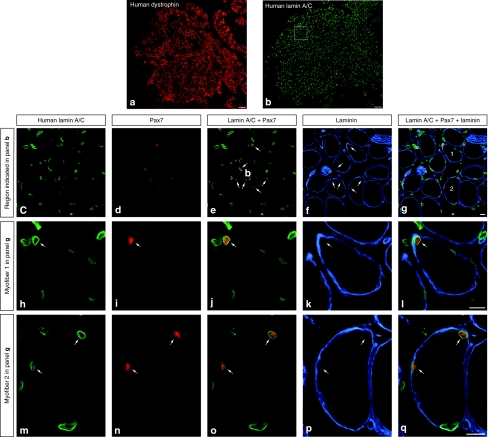
Myoblast transplantation was done as in the previous experiments in six SCID mice. The cell-grafted TAs were collected 1 month later for histological analysis. The cross-sections of these muscles exhibited many myofibers expressing human dystrophin (Figure 3a). In serial sections, abundant human nuclei (detected with an antihuman lamin A/C mAb) were present in the regions with human-derived myofibers, i.e., expressing human dystrophin (Figure 3b). Human nuclei were observed into or in the periphery of myofibers, and in the interstitium. Codetection of Pax7 and human lamin A/C revealed that several human nuclei expressed Pax7 (Figure 3c–e). Codetection of laminin with Pax7 and human lamin A/C revealed that several Pax7+ human nuclei were situated in the periphery of the myofibers and inside the basal lamina (Figure 3c–q). Pax7 was never observed in myofiber internal nuclei or in nuclei under the sarcolemma when codetected with human dystrophin. Approximately 15% of the human laminin A/C+ nuclei were Pax7+, and ~75% of the human laminin A/C+/Pax7+ nuclei were situated in the periphery of myofibers under the basal lamina. Therefore, ~11.25% of the human-derived nuclei present in the SCID muscles may be considered as satellite cells.
Figure 3.
Human satellite cells detected in mouse muscles transplanted with myoblasts from an adult human donor. The panels correspond to cross-sections of a severe combined immunodeficient mouse muscle sampled 4 weeks after transplantation of human myoblasts from a 43-year-old donor. (a,b) Two topographic serial sections show that a large region of this muscle is composed of human-dystrophin+ myofibers (a, red fluorescence) and is filled by human nuclei (b, green fluorescence using an antihuman lamin A/C monoclonal antibody). (c–g) The region in the white rectangle in b, illustrating the codetection of human nuclei (human lamin A/C+), Pax7 (red fluorescence), and basal lamina (laminin, blue fluorescence) observed by confocal microscopy. The two myofibers numbered 1 and 2 are shown under higher magnification in the rows below to better exhibit the labeling of each antibody. Arrows indicate several human nuclei expressing Pax7+ in the periphery of myofibers and inside the basal lamina. Bar = 100 µm (a,b); 10 µm (c–q).
Putative donor-derived satellite cells detected in humans after myoblast transplantation
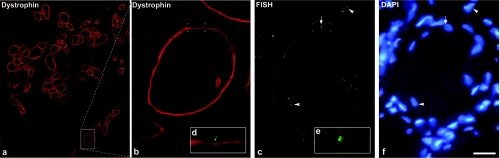
As a corollary, we wanted to determine whether myoblast transplantation could produce donor-derived satellite cells in humans. We analyzed muscle sections of DMD patients that received normal myoblast allo-transplantations in a clinical trial. Some patients received myoblasts from their mother, and this made possible to detect donor-derived nuclei by the presence of two X chromosomes using fluorescent in situ hybridization (FISH). Nuclei containing two close FISH+ dots were observed into myofibers and in mononucleated cells. Some nuclei with two close FISH+ dots were in juxtaposition to the external side of the sarcolemma of dystrophin+ myofibers, in such a manner that the nucleus indented slightly the sarcolemma, a characteristic of satellite cells (Figure 4).
Figure 4.
Putative donor-derived satellite cells in humans after myoblast transplantation. The images correspond to a cross-section of the cell-grafted muscle of a Duchenne muscular dystrophy patient, 1 month after normal myoblast transplantation. We codetected dystrophin (red fluorescence), X chromosomes by FISH (green-fluorescent dots), and nuclei (DAPI: blue fluorescence). (a) A region with several dystrophin+ myofibers is shown. This patient had 18.6% dystrophin+ myofibers in the myoblast-transplanted muscle and <0.5% dystrophin+ myofibers in the contralateral nontransplanted muscle. The area in the rectangle is enlarged in b. (c) The most evident pairs of FISH dots indicating female (donor-derived) nuclei are pointed: arrowheads indicate pairs of FISH dots inside dystrophin+ myofibers, and the arrow indicates a pair of FISH dots adjacent to the exterior side of the dystrophin+ sarcolemma. The Cy3 fluorescence of dystrophin appears with the filter used here for FITC, and the picture was taken in this form to allow confirming the position of the FISH dots in reference to the sarcolemma. The region containing the pair of FISH dots, circumscribed with white corners in b and c, is enlarged in the insets (d,e). d is a merge of the FISH dots with the dystrophin fluorescence, to better evidence the position of this donor-derived nucleus in a satellite cell position. There is a concavity in the sarcolemma under the FISH dots, which may correspond to the indentation of the myofiber profile produced by satellite cells. (f) In spite of some diffusion, DAPI is shown to indicate the nuclei corresponding to the pairs of FISH dots pointed in c. Bar = 25 µm. DAPI, 4′-6-Diamidino-2-phenylindole; FISH, fluorescent in situ hybridization; FITC, fluorescein isothiocyanate.
Discussion
This study shows that human postnatal myoblasts proliferated in vitro and intramuscularly transplanted, not only fuse to form human-derived myofibers but, in addition, remain as functional MPCs into the recipient mouse muscles. The study shows also that intramuscularly transplanted human postnatal myoblasts gave rise to donor-derived satellite cells in mice and probably (although not conclusive) in humans.
This phenomenon was previously demonstrated in experiments of mouse myoblast transplantation in mice. First evidence was given by transplanting myoblasts into nonirradiated muscles and collecting them up to 5 months later for cell culture: 1–2% of the myoblast colonies obtained from these muscles were from donor origin.19 These donor-derived myoblasts fused into myotubes in vivo and formed myofibers after transplantation.19 Irintchev et al. were the first to detect satellite cells produced by transplanted myoblasts.16,17 In a first study, they destroyed mouse muscles and, thereafter, they partially reconstituted them by myoblast transplantation.16 In these reconstituted muscles, they identified some donor-derived mononuclear cells expressing M-cadherin in the border with myofibers, a characteristic of satellite cells.26,27 In a second study, they created ectopic muscles following subcutaneous myoblast implantation.17 These neo-formed muscles also showed M-cadherin+ cells adjacent to myofibers, indenting the sarcolemma under the basal lamina in a typical satellite cell position.17 Gross and Morgan20 transplanted mouse myoblasts into irradiated muscles and performed four muscle destructions at 3-week intervals using notexin, a myotoxin that destroys myofibers but not satellite cells. Regenerating donor-derived myofibers were observed after each muscle injury, confirming that some transplanted myoblasts remained as MPCs able to participate in muscle regeneration even after repeated muscle damages. Heslop et al.21 identified donor-derived cells remaining as satellite cells in myofibers isolated from muscles grafted with mouse myoblasts.
From a clinical perspective, these results with mouse myoblasts needed to be confirmed with human myoblasts. Indeed, differences between rodents and humans are striking in muscle regeneration,28 and this could be reflected in the capacity of exogenous myoblasts to produce satellite cells. So far, three studies addressed this issue with human myoblasts by partial analyses. The first of them suggested that some human nuclei in isolated human/mouse hybrid myofibers could be satellite cells.22 A subsequent study grafted fetal human myoblasts into muscles of immunodeficient mice.23 Cell-grafted muscles were sampled 4 weeks later, human cells were isolated from them, and they were retransplanted in other immunodeficient mice. Myofibers expressing human proteins were observed 4 weeks later in these mice. However, these results were obtained with myoblasts from a 14-week gestation fetus, whereas myoblasts grafted in clinical myoblast transplantation were of postnatal origin.8 Indeed, myoblasts responsible of muscle histogenesis in the early fetus and myoblasts derived from satellite cells in postnatal life may be different in terms of post-transplantation myogenesis. Fetal and postnatal myoblasts have different behavior and morphology in culture.29 Moreover, postnatal myoblasts survive better transplantation than fetal myoblasts.30 A recent study quantified the human myoblast–derived satellite cells following transplantation in immunodeficient mice, although only as a control of the transplantation of CD133+ cells and without showing histological pictures of the human myoblast–derived satellite cells.24
In this study, we cultured postnatal human myoblasts for transplantation in muscles of SCID mice, following a protocol that we previously used in human myoblast xenotransplantation.12,31,32 One month post-transplantation, we were able to proliferate cells from these myoblast-grafted muscles, and most of these cells expressed human CD56. Expression of human lamin A/C and human HLA-ABC further confirmed the human-derived status of these cells. Since 1 month post-transplantation muscle regeneration is fully completed, this experiment revealed that not all transplanted myoblasts fused into myofibers but that some of them remained as MPCs able to proliferate in vitro as CD56+ myoblasts. The prevalence of human cells in these cultures can be explained, first, by the pretransplantation treatment of the cell-grafted muscles. The cell-grafted muscles were treated with high doses of ionizing radiation, which inhibited most cell proliferation and muscle regeneration in mouse models.33,34,35,36 Indeed, no cells plated from SCID muscles irradiated similarly and injected with cardiotoxin (without cells) in our sham experiment. Although the presence of some radiation-resistant MPCs was suggested by some experiments of regeneration in mice,20 differences in the irradiation protocol can explain this discrepancy. We used X-rays delivered by a 6 MV linear accelerator, and we placed a bolus to deliver the maximum radiation energy in the TA. This irradiation conditions could cause more deleterious effects to the muscle MPCs than γ rays delivered by a cell irradiator with a 137cesium source, as used by others.20 Our cultures of human cells derived from the human myoblast–transplanted mouse muscles were transplanted in other mice and they fused again into myofibers. It is important to note that this was obtained with cells that proliferated for 21 days (~12 cell doublings) before the first transplantation, and that they produced MPCs able to proliferate for at least 32 days in culture before the second transplantation.
In our second experiment, we produced extensive damage in cell-grafted muscles by injecting cardiotoxin 2 months after myoblast transplantation. This damage was done in one side, and the contralateral cell-grafted muscle was left as a control. Both muscles were sampled 2 weeks postdamage, i.e., before completing muscle regeneration, to identify recently damaged and regenerating regions. Several myofibers in the regenerating areas expressed human dystrophin. This observation, together with the fact that the muscles were irradiated previous to transplantation (inhibiting the regenerative capacity of the recipient cells) indicates that muscle regeneration was predominantly done by human MPCs.
We used immunohistochemistry to obtain morphological evidence of human MPCs in the cell-grafted muscles, and to observe whether they have characteristics of satellite cells. Immunohistochemically, satellite cells are identified by CD34, Pax7, and M-cadherin expression.37,38 In our hands, immunodetection of human M-cadherin and human CD34 was hampered by a weak specific reaction versus a significant background. The most efficient combination was the codetection of human lamin A/C, Pax7, and laminin. Labeling with these antibodies was clear and their combination insured detection of human nuclei (human lamin A/C+) with a marker of satellite cells (Pax7) in the position of satellite cells, i.e., in the periphery of the myofibers under the basal lamina (laminin immunodetection). The best anti-Pax7 antibody in our hands was a mouse mAb and we tried codetection even if the antihuman lamin A/C was also a mouse IgG. We expected that epitopes recognized by the first anti-mouse-IgG antibody will be saturated enough to prevent substantial attachment of the second anti-mouse-IgG antibody. In fact, controls omitting the primary mAb in the second reaction showed no attachment of the second anti-mouse-IgG antibody. In addition, the pattern of each staining was different and characteristic of each antigen: lamin A/C labeled the nuclear periphery and Pax7 showed its characteristic intranuclear pattern. Several human nuclei located in the periphery of myofibers, under the basal lamina, expressed Pax7, and this was consistent evidence that they corresponded to satellite cells. Although quadruple labeling with dystrophin (to evidence the sarcolemma) was not possible, three elements confirmed that Pax7+ nuclei inside the basal lamina corresponded to mononuclear cells outside the sarcolemma: (i) no internal myonuclei were Pax7+; (ii) in sections co-stained for dystrophin (instead of laminin) all Pax7+ nuclei were outside the sarcolemma; and (iii) Pax7 was never expressed after fusion in our myoblast cultures, in agreement with the well-known dynamics of Pax7 expression.39 The presence of Pax7+ human nuclei in the interstitium indicates that not all donor-derived MPCs remained in a niche of satellite cells. An interesting question is thus whether MPCs with biological characteristics of satellite cells can remain out of the niche of satellite cells. The observation that cells with molecular markers of satellite cells are present in myoblast cultures40,41 could indicate that MPCs with characteristics of satellite cells can remain out of their niche at least in vitro.
Our FISH observations in muscle biopsies of DMD patients transplanted with normal myoblasts showed that donor-derived mononuclear cells remained in the recipient's muscle. The location of some of them, in apposition to the dystrophin-labeled sarcolemma, suggests that they could correspond to putative satellite cells.
In conclusion:
-The intramuscular transplantation of postnatal human myoblasts in mice gave rise to donor-derived MPCs into the recipient muscles, that could: (i) be isolated to be further proliferated as human myoblasts in vitro, able to be successfully retransplanted; and (ii) participate in subsequent muscle regeneration.
-The intramuscular transplantation of proliferated postnatal human myoblasts in mice formed donor-derived satellite cells, which may correspond totally or partially to the donor-derived MPCs evidenced experimentally.
-There is some evidence of putative donor-derived satellite cell formation after the intramuscular transplantation of proliferated postnatal human myoblasts in humans.
Material and Methods
Animals. Twenty-five SCID Balb/c mice were used as recipients for human myoblast transplantations and for sham tests. The Animal Care Committee of the CHUL hospital approved all the procedures used in this study.
Cell culture. We proliferated human myoblasts from skeletal muscle samples obtained postmortem in a normal 13-month-old baby and from deltoid muscle biopsies of two healthy adults (39 and 43 years old). In the three cases, the muscle fragments were digested with 0.2% collagenase (Sigma-Aldrich, St Louis, MO) in Hank's balanced salt solution for 1 hour, followed by 0.125% trypsin (Gibco, Grand Island, NY) in Hank's balanced salt solution for 45 minutes. The isolated cells were cultured for 4 weeks in MB-1 medium (Hyclone, Logan, UT) supplemented with 15% fetal bovine serum (Hyclone), 10 ng/ml basic fibroblast growth factor (Feldan, St Laurent, Quebec, Canada), 0.5 mg/ml bovine serum albumin (Sigma-Aldrich), 0.39 µg/ml dexamethasone (Sigma-Aldrich), and 5 µg/ml human insulin (Eli Lilly Canada, Toronto, Ontario, Canada).
Cells were also isolated from the TA muscles of three SCID mice grafted 1 month before with human myoblasts (see below for transplantation details) and of three SCID mice used as sham controls (see below for details). These TA muscles were aseptically dissected, and then digested and cultured as described above for human muscle samples. When cells were obtained from these cultures, they were used for flow cytometry analysis, immunocytology, and intramuscular transplantation in other SCID mice.
Cell transplantation. In all the cases, both TAs of SCID mice were X-ray irradiated (20 Gy) in one session, at least 1 week before transplantation or sham experiments. Irradiation was done using a 6 MV linear accelerator (Siemens, Malvern, PA), placing only the distal part of the mice posterior limbs into the irradiation field, and placing a 1-cm thick bolus over them to deliver the maximum radiation energy in the TA muscles. For transplantation, 2–3 × 106 human myoblasts, resuspended in 20 µl of Hank's balanced salt solution containing 10 µg/ml cardiotoxin (Sigma-Aldrich), were implanted in each TA through ~20 percutaneous microinjections done with glass micropipettes (Drummond Scientific, Broomall, PA).
The cell-grafted TA muscles of six SCID mice were sampled 4 weeks post-transplantation for histological detection of satellite cells. The other cell-grafted mice were used for two experiments.
In the first experiment, both TAs of three SCID mice were grafted with human myoblasts as described above, and these cell-grafted TA muscles were harvested 4 weeks after transplantation to isolate their cells for culture (described above). After 32 days in culture, a sample of these cells was used for flow cytometry analysis of human CD56 (described below). From the rest, 3 × 106 cells were transplanted in each TA muscles of three other SCID mice. The cell-grafted TA muscles in this second set of SCID mice were harvested 4 weeks later for histological analysis. The cells that were not transplanted were briefly cultured and used for a flow cytometry analysis of human HLA-ABC expression and immunocytology detection of human lamin A/C (described below).
In the second experiment, the left TA muscles of the myoblast-transplanted SCID mice were damaged 2 months post-transplantation by the intramuscular injection of 20 µl cardiotoxin (100 µg/ml) per muscle. In these mice, we collected the left and right TA muscles 2 weeks after the cardiotoxin-induced damage. Sham experiments were done as controls for the two last experiments. In both, the same procedure was done in the same number of animals with the only difference that no myoblasts were grafted and only 20 µl of 10 µg/ml cardiotoxin in Hank's balanced salt solution were injected.
Sampling. Mice were killed by intracardiac perfusion with heparinized saline under deep anesthesia. The TA muscles were dissected, mounted in embedding medium, and snap-frozen in liquid nitrogen. Serial cross-sections of 10–12 µm were obtained in a cryostat at –25 °C. We also analyzed muscle biopsies of DMD patients that were transplanted with normal myoblasts in a previous clinical trial.12
Histology. In all cases, muscle sections were stained with hematoxylin–eosin to observe the muscle structure. According to the objective of each analysis, different proteins were detected by immunohistochemistry using the mAb and polyclonal antibodies (pAb) detailed below. Human dystrophin was detected in SCID mice with the NCL-Dys3 mAb (1:50; Novocastra, Newcastle upon Tyne, UK), which does not react with mouse dystrophin. To detect Pax7, we used a mouse mAb antihuman and anti-mouse Pax7 (1:50; R&D Systems, Minneapolis, MN). Human nuclei were detected with a mouse antihuman lamin A/C mAb, which does not react with mouse lamins (1:100; Vector Laboratories, Burlingame, CA). To evidence the basal lamina, a rabbit anti-mouse/human laminin pAb (1:100; Abcam, Cambridge, MA) was used. Depending on the immunodetection, sections were either unfixed or fixed in methanol –20 °C (5 minutes) and acetone –20 °C (10 minutes). The sections were incubated later 30 minutes with a blocking solution made generally of 10% fetal bovine serum in phosphate-buffered saline or 10% fetal bovine serum+10% goat serum+0.2% Triton X-100 (Sigma-Aldrich)+2% bovine serum albumin in phosphate-buffered saline (this last blocking solution was used mostly in triple immunodetection techniques). According to the antigen to be detected, sections were incubated either 1 hour at room temperature or overnight at 4 °C with the primary antibodies in phosphate-buffered saline with 1% fetal bovine serum. For techniques done with only one primary antibody, the slides were incubated 30 minutes in a biotinylated anti-mouse IgG antibody (1:200; Dako, Copenhagen, Denmark), followed by 30 minutes in streptavidin-Cy3 (1:700; Sigma-Aldrich). For double immunolabeling (codetection of two antigens with two different primary antibodies), an anti-mouse IgG antibody conjugated to Alexa Fluor 488 (1:300; Molecular Probes, Eugene, OR) was also used as second antibody. For triple immunolabeling (codetection of three antigens with three different antibodies) an anti-rabbit IgG antibody conjugated to Alexa Fluor 647 (Molecular Probes) was also added as second antibody for laminin detection. Double and triple immunolabelings were done by sequential series of complete incubations for each antibody. Triple incubations for codetection of Pax7, lamin A/C, and laminin were done according to the following sequence: mouse anti-Pax7 → anti-mouse IgG biotin → streptavidin-Cy3 → mouse anti-lamin A/C → anti-mouse IgG Alexa Fluor 488 → rabbit anti-laminin → anti-rabbit IgG Alexa Fluor 647. In this case, a 30-minute incubation with the blocking solution was also done before the second reaction. For these double and triple immunolabelings, controls omitting the first antibody in the second and third reaction were done.
Three different controls for the immunohistochemical reactions were used. (i) Sections of SCID TAs of the sham experiments (irradiation and injection of cardiotoxin without cells) as negative controls for the human antigens detected in the myoblast-grafted SCID mouse muscles. (ii) Sections of SCID TAs in which cell transplantations were performed similarly but with human nonmyogenic cells (amniotic liquid-derived cells) sampled 1 month post-transplantation. (iii) Sections of normal human muscle, as positive controls for the human antigens detected in the myoblast-grafted SCID mouse muscles. We used sections from our frozen stock for the two last controls, while sham experiments were done as detailed above.
Flow cytometry. Cell cultures from SCID mouse muscles grafted with human myoblasts were harvested and incubated 15 minutes with a mouse antihuman CD56 mAb conjugated to phycoerythrin (Beckman Coulter, Fullerton, CA) at 0.5 µg per 106 cells, to be analyzed by flow cytometry. The same test was performed on mouse myoblasts from our frozen stock (obtained from nonirradiated neonatal mice) as a control of the human specificity of this antibody. We used also the same antibody to cell-sort human CD56+ cells for immunocytological detection of human lamin A/C (see below). Flow cytometry analysis of cell cultures from SCID mouse muscles grafted with human myoblasts were also done following 1-hour incubation of the harvested cells with a mouse antihuman HLA-ABC mAb (1:200; BD Biosciences, Mississauga, Ontario, Canada), followed by a 30-min incubation with an anti-mouse IgG antibody conjugated to Alexa Fluor 488 (1:300; Molecular Probes). The specificity of this antibody for human cells was previously tested. In all cases, isotype controls were done. Incubations were at room temperature.
Immunocytology. Cell cultures from SCID mouse muscles grafted with human myoblasts were analyzed for human lamin A/C expression. We tested both the whole cell population and a cell-sorted human CD56+ fraction. The monolayer of cells in 5-cm culture dishes was fixed and permeabilized with methanol at –20 °C for 5 minutes. Immunodetection of human lamin A/C was done as described above for immunohistology. Nuclei were counterstained by incubation with 1 µg/ml Hoechst (Sigma-Aldrich). Incubations were at room temperature.
FISH. Dystrophin immunodetection in muscle sections of DMD patients that received myoblasts from their mother was done as described with NCL-Dys3. Sections were then fixed in Histochoice (Amresco, Solon, OH). Denaturation was done in 70% formamide in saline sodium citrate buffer at 72 °C, followed by dehydration in ethanol. Chromosome X, a satellite probe labeled with a green fluorophore (Cytocell Technologies, Cambridge, UK), was placed and denatured at 75 °C for 2 minutes. Hybridization was completed overnight at 37 °C. Sections were washed in saline sodium citrate buffer pH 7.0 at 72 °C for 2 minutes and in 0.05% Tween-20 in the same buffer at room temperature for 30 seconds. 4′-6-Diamidino-2-phenylindole (Cytocell Technologies) was then applied to detect nuclei.
Microscopic analysis. Hematoxylin–eosin-stained muscle sections, single- and double-immunolabeled muscle sections and cell cultures were analyzed using an Axiophot microscope with epifluorescent and bright field optics (Zeiss, Oberkochen, Germany) and pictures were taken with an A650 IS digital camera (Canon, Tokyo, Japan). Triple-immunolabeled muscle sections were analyzed using an Olympus IX70 microscope with epifluorescent optics (Olympus, Tokyo, Japan) with a FluoView 300 confocal microscopy scanning unit (Olympus) using lasers of 488, 543, and 633 nm. Confocal microscopy images were taken using FluoView 300 software (Olympus).
Acknowledgments
This work was supported by grants from the Association Française contre les Myopathies (AFM), the Canadian Institutes of Health Research, Muscular Dystrophy Canada, the Jesse's Journey Foundation for Gene and Cell Therapy of Canada, and the National Institutes of Health of the USA. J.P.T. has shares in CellGene Inc., a biotechnological company created to accelerate the development of cell therapies. City, state and country in which the work was done: Quebec City, Quebec, Canada.
REFERENCES
- Partridge TA, Grounds M., and, Sloper JC. Evidence of fusion between host and donor myoblasts in skeletal muscle grafts. Nature. 1978;273:306–308. doi: 10.1038/273306a0. [DOI] [PubMed] [Google Scholar]
- Konigsberg IR. The differentiation of cross-striated myofibrils in short term cell culture. Exp Cell Res. 1960;21:414–420. doi: 10.1016/0014-4827(60)90273-1. [DOI] [PubMed] [Google Scholar]
- Watt DJ, Morgan JE., and, Partridge TA. Use of mononuclear precursor cells to insert allogeneic genes into growing mouse muscles. Muscle Nerve. 1984;7:741–750. doi: 10.1002/mus.880070908. [DOI] [PubMed] [Google Scholar]
- Bartlett RJ, Sharp NJ, Hung WY, Kornegay JN., and, Roses AD. Molecular markers for myoblast transplantation in GRMD. Adv Exp Med Biol. 1990;280:273–278. doi: 10.1007/978-1-4684-5865-7_31. [DOI] [PubMed] [Google Scholar]
- Kinoshita I, Vilquin JT, Gravel C, Roy R., and, Tremblay JP. Myoblast allotransplantation in primates. Muscle Nerve. 1995;18:1217–1218. [PubMed] [Google Scholar]
- Boubaker el Andalousi R, Daussin PA, Micallef JP, Roux C, Nougues J, Chammas M, et al. Changes in mass and performance in rabbit muscles after muscle damage with or without transplantation of primary satellite cells. Cell Transplant. 2002;11:169–180. [PubMed] [Google Scholar]
- Holzer N, Hogendoorn S, Zürcher L, Garavaglia G, Yang S, König S, et al. Autologous transplantation of porcine myogenic precursor cells in skeletal muscle. Neuromuscul Disord. 2005;15:237–244. doi: 10.1016/j.nmd.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Skuk D. Myoblast transplantation for inherited myopathies: a clinical approach. Expert Opin Biol Ther. 2004;4:1871–1885. doi: 10.1517/14712598.4.12.1871. [DOI] [PubMed] [Google Scholar]
- Skuk D., and, Tremblay JP. Myoblast transplantation: the current status of a potential therapeutic tool for myopathies. J Muscle Res Cell Motil. 2003;24:285–300. [PubMed] [Google Scholar]
- Skuk D, Roy B, Goulet M, Chapdelaine P, Bouchard JP, Roy R, et al. Dystrophin expression in myofibers of Duchenne muscular dystrophy patients following intramuscular injections of normal myogenic cells. Mol Ther. 2004;9:475–482. doi: 10.1016/j.ymthe.2003.11.023. [DOI] [PubMed] [Google Scholar]
- Skuk D, Goulet M, Roy B, Piette V, Côté CH, Chapdelaine P, et al. First test of a “high-density injection” protocol for myogenic cell transplantation throughout large volumes of muscles in a Duchenne muscular dystrophy patient: eighteen months follow-up. Neuromuscul Disord. 2007;17:38–46. doi: 10.1016/j.nmd.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Skuk D, Goulet M, Roy B, Chapdelaine P, Bouchard JP, Roy R, et al. Dystrophin expression in muscles of duchenne muscular dystrophy patients after high-density injections of normal myogenic cells. J Neuropathol Exp Neurol. 2006;65:371–386. doi: 10.1097/01.jnen.0000218443.45782.81. [DOI] [PubMed] [Google Scholar]
- Alameddine HS, Louboutin JP, Dehaupas M, Sébille A., and, Fardeau M. Functional recovery induced by satellite cell grafts in irreversibly injured muscles. Cell Transplant. 1994;3:3–14. doi: 10.1177/096368979400300103. [DOI] [PubMed] [Google Scholar]
- Wernig A, Zweyer M., and, Irintchev A. Function of skeletal muscle tissue formed after myoblast transplantation into irradiated mouse muscles. J Physiol (Lond) 2000;522:333–345. doi: 10.1111/j.1469-7793.2000.t01-2-00333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernig A, Irintchev A., and, Lange G. Functional effects of myoblast implantation into histoincompatible mice with or without immunosuppression. J Physiol (Lond) 1995;484:493–504. doi: 10.1113/jphysiol.1995.sp020681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irintchev A, Langer M, Zweyer M, Theisen R., and, Wernig A. Functional improvement of damaged adult mouse muscle by implantation of primary myoblasts. J Physiol (Lond) 1997;500:775–785. doi: 10.1113/jphysiol.1997.sp022057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irintchev A, Rosenblatt JD, Cullen MJ, Zweyer M., and, Wernig A. Ectopic skeletal muscles derived from myoblasts implanted under the skin. J Cell Sci. 1998;111:3287–3297. doi: 10.1242/jcs.111.22.3287. [DOI] [PubMed] [Google Scholar]
- Kinoshita I, Vilquin JT., and, Tremblay JP. Mechanism of increasing dystrophin-positive myofibers by myoblast transplantation: study using mdx/β-galactosidase transgenic mice. Acta Neuropathol. 1996;91:489–493. doi: 10.1007/s004010050456. [DOI] [PubMed] [Google Scholar]
- Yao SN., and, Kurachi K. Implanted myoblasts not only fuse with myofibers but also survive as muscle precursor cells. J Cell Sci. 1993;105:957–963. doi: 10.1242/jcs.105.4.957. [DOI] [PubMed] [Google Scholar]
- Gross JG., and, Morgan JE. Muscle precursor cells injected into irradiated mdx mouse muscle persist after serial injury. Muscle Nerve. 1999;22:174–185. doi: 10.1002/(sici)1097-4598(199902)22:2<174::aid-mus5>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Heslop L, Beauchamp JR, Tajbakhsh S, Buckingham ME, Partridge TA., and, Zammit PS. Transplanted primary neonatal myoblasts can give rise to functional satellite cells as identified using the Myf5nlacZl+ mouse. Gene Ther. 2001;8:778–783. doi: 10.1038/sj.gt.3301463. [DOI] [PubMed] [Google Scholar]
- Brimah K, Ehrhardt J, Mouly V, Butler-Browne GS, Partridge TA., and, Morgan JE. Human muscle precursor cell regeneration in the mouse host is enhanced by growth factors. Hum Gene Ther. 2004;15:1109–1124. doi: 10.1089/hum.2004.15.1109. [DOI] [PubMed] [Google Scholar]
- Ehrhardt J, Brimah K, Adkin C, Partridge T., and, Morgan J. Human muscle precursor cells give rise to functional satellite cells in vivo. Neuromuscul Disord. 2007;17:631–638. doi: 10.1016/j.nmd.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Negroni E, Riederer I, Chaouch S, Belicchi M, Razini P, Di Santo J, et al. In vivo myogenic potential of human CD133+ muscle-derived stem cells: a quantitative study. Mol Ther. 2009;17:1771–1778. doi: 10.1038/mt.2009.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellaire G, Eskiw CH, Dehghani H, Ching RW., and, Bazett-Jones DP. Mitotic accumulations of PML protein contribute to the re-establishment of PML nuclear bodies in G1. J Cell Sci. 2006;119:1034–1042. doi: 10.1242/jcs.02817. [DOI] [PubMed] [Google Scholar]
- Bornemann A., and, Schmalbruch H. Immunocytochemistry of M-cadherin in mature and regenerating rat muscle. Anat Rec. 1994;239:119–125. doi: 10.1002/ar.1092390202. [DOI] [PubMed] [Google Scholar]
- Irintchev A, Zeschnigk M, Starzinski-Powitz A., and, Wernig A. Expression pattern of M-cadherin in normal, denervated, and regenerating mouse muscles. Dev Dyn. 1994;199:326–337. doi: 10.1002/aja.1001990407. [DOI] [PubMed] [Google Scholar]
- Borisov AB. Regeneration of skeletal and cardiac muscle in mammals: do nonprimate models resemble human pathology. Wound Repair Regen. 1999;7:26–35. doi: 10.1046/j.1524-475x.1999.00026.x. [DOI] [PubMed] [Google Scholar]
- Cossu G., and, Molinaro M. Cell heterogeneity in the myogenic lineage. Curr Top Dev Biol. 1987;23:185–208. doi: 10.1016/s0070-2153(08)60625-0. [DOI] [PubMed] [Google Scholar]
- Lee-Pullen TF, Bennett AL, Beilharz MW, Grounds MD., and, Sammels LM. Superior survival and proliferation after transplantation of myoblasts obtained from adult mice compared with neonatal mice. Transplantation. 2004;78:1172–1176. doi: 10.1097/01.tp.0000137936.75203.b4. [DOI] [PubMed] [Google Scholar]
- Huard J, Verreault S, Roy R, Tremblay M., and, Tremblay JP. High efficiency of muscle regeneration after human myoblast clone transplantation in SCID mice. J Clin Invest. 1994;93:586–599. doi: 10.1172/JCI117011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skuk D, Furling D, Bouchard JP, Goulet M, Roy B, Lacroix Y, et al. Transplantation of human myoblasts in SCID mice as a potential muscular model for myotonic dystrophy. J Neuropathol Exp Neurol. 1999;58:921–931. doi: 10.1097/00005072-199909000-00003. [DOI] [PubMed] [Google Scholar]
- Quinlan JG, Lyden SP, Cambier DM, Johnson SR, Michaels SE., and, Denman DL. Radiation inhibition of mdx mouse muscle regeneration: dose and age factors. Muscle Nerve. 1995;18:201–206. doi: 10.1002/mus.880180209. [DOI] [PubMed] [Google Scholar]
- Quinlan JG, Cambier D, Lyden S, Dalvi A, Upputuri RK, Gartside P, et al. Regeneration-blocked mdx muscle: in vivo model for testing treatments. Muscle Nerve. 1997;20:1016–1023. doi: 10.1002/(sici)1097-4598(199708)20:8<1016::aid-mus12>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Wakeford S, Watt DJ., and, Partridge TA. X-irradiation improves mdx mouse muscle as a model of myofiber loss in DMD. Muscle Nerve. 1991;14:42–50. doi: 10.1002/mus.880140108. [DOI] [PubMed] [Google Scholar]
- Wirtz P, Loermans H., and, Rutten E. Effects of irradiation on regeneration in dystrophic mouse leg muscles. Br J Exp Pathol. 1982;63:671–679. [PMC free article] [PubMed] [Google Scholar]
- Beauchamp JR, Heslop L, Yu DS, Tajbakhsh S, Kelly RG, Wernig A, et al. Expression of CD34 and Myf5 defines the majority of quiescent adult skeletal muscle satellite cells. J Cell Biol. 2000;151:1221–1234. doi: 10.1083/jcb.151.6.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit PS, Golding JP, Nagata Y, Hudon V, Partridge TA., and, Beauchamp JR. Muscle satellite cells adopt divergent fates: a mechanism for self-renewal. J Cell Biol. 2004;166:347–357. doi: 10.1083/jcb.200312007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham M., and, Montarras D. Skeletal muscle stem cells. Curr Opin Genet Dev. 2008;18:330–336. doi: 10.1016/j.gde.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Zammit PS, Relaix F, Nagata Y, Ruiz AP, Collins CA, Partridge TA, et al. Pax7 and myogenic progression in skeletal muscle satellite cells. J Cell Sci. 2006;119:1824–1832. doi: 10.1242/jcs.02908. [DOI] [PubMed] [Google Scholar]
- Yablonka-Reuveni Z, Day K, Vine A., and, Shefer G. Defining the transcriptional signature of skeletal muscle stem cells. J Anim Sci. 2008;86 14 Suppl:E207–E216. doi: 10.2527/jas.2007-0473. [DOI] [PMC free article] [PubMed] [Google Scholar]