Abstract
β-Adrenoceptors (β-ARs) play a critical role in the regulation of cardiovascular function. Intracellular oxygen homeostasis is crucial for the survival of cardiomyocytes. However, it is still unclear whether β-AR activation can modulate intracellular oxygen. Here we used mitochondrial and cytosolic target Renilla luciferase to detect intracellular oxygen concentration. Pharmacological experiments revealed that β2-AR activation specifically regulates intracellular oxygen in cardiomyocytes and COS7 cells. This effect was abrogated by inhibitory G protein (Gi) inhibition, endothelial nitric oxide synthase (eNOS) blockade, and NO scavenging, implicating that the β2-AR–Gi–eNOS pathway is involved in this regulation. β2-AR activation increased the AMP/ATP ratio, AMPK activity, ROS production and prolyl hydroxylase activity. These effects also contribute to the regulation of β2-AR signalling, thus providing an additional layer of complexity to enforce the specificity of β1-AR and β2-AR signalling. Collectively, the study provides novel insight into the modulation of oxygen homeostasis, broadens the scope of β2-AR function, and may have crucial implications for β2-AR signalling regulation.
Introduction
Oxygen homeostasis represents an essential organizing principle of metazoan evolution, development, physiology and pathology (Semenza, 2007a). In the normal physiological state, the majority of cellular oxygen consumption occurs in the mitochondria for the generation of sufficient ATP to satisfy cellular energy requirements. However, there is also a critical level of ‘spare’ oxygen available to facilitate the activity of non-mitochondrial dioxygenases elsewhere in the cell (Taylor, 2008a). A rapid change in cellular oxygen can act as the initial signal to activate the appropriate pathway to combat external stimuli (Taylor, 2008b).
β-Adrenoceptors (β-ARs) are prototypical G protein-coupled receptors (GPCRs) that play a critical role in the regulation of cardiovascular and pulmonary function as well as in other physiological processes (Johnson, 1998; Rockman et al. 2002). β-AR dysfunction is a cause of cardiovascular and respiratory impairment and a consequence of agonist therapy (Insel, 1996). In particular, loss of β-AR responsiveness is causally linked to both asthma (Johnson, 1998) and heart failure (Lefkowitz et al. 2000) as well as to the morbidity and mortality associated with the therapeutic use of β-agonists (Salpeter et al. 2004). Thus, it is necessary to fully understand β-AR function.
β-AR activation can enhance bronchodilatation and alveolar fluid clearance (which increase O2 uptake), increase cardiac output and peripheral vasodilatation (which increase O2 delivery), and enhance cardioprotection and angiogenesis under ischaemic conditions (Lefkowitz et al. 2000; Iaccarino et al. 2002; Mieno et al. 2005; Iaccarino et al. 2005). Such research has mainly focused on β-AR activation at the tissue or organ level. However, oxygen changes that occur at the subcellular level in response to various stimuli have not been studied to date. In this study, we explored the relationship between β-AR activation and intracellular oxygen in cardiomyocytes, revealing that β2-ARs selectively increase intracellular oxygen availability through the β2-AR–Gi–eNOS signalling pathway.
Methods
Plasmids
Mitochondrial targeted Renilla luciferase (MitRLuc) and cytosolic Renilla luciferase (pRL-CMV) plasmids for detecting intracellular oxygen concentration were generously provided by Dr Thilo Hagen and Salvador Moncada (Wolfson Institute for Biomedical Research, University College London, London, UK). The mitochondria-target or cytosol-target Renilla luciferase sequence was obtained from MitRLuc-pcDNA3 or pRL-CMV plasmids, respectively, and then cloned into pLenti6 V5-D to generate pLenti6-V5/MitRLuc or pLenti6-V5/Rluc for cardiomyocyte transfection. The specific protocol for lenti-viral vector construction is shown in the Supplemental material (available online only). β1-AR and β2-AR plasmids were gifts from Dr Kenneth P. Minneman (Emory University School of Medicine, Atlanta, GA, USA). The pST39-HisTrxNVHL-elongin B-elongin C plasmid was kindly provided by Dr S. Tan (Pennsylvania State University, PA, USA).
Neonatal rat cardiomyocyte isolation
All procedures for animals were approved by the Animal Ethics and Experimentation Committee of Tongji University (Shanghai, China) and were performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996) as well as in compliance with the report of the policies and regulations on animal experimentation (Drummond, 2009). One-day-old Sprague–Dawley rats were anaesthetized by inhalation of 2% isoflurane (99.9% from Vedco, St Joseph, MO, USA). The hearts of the neonatal rats were rapidly excised with sharp scissors and then washed with ice-cold PBS (mmol l−1: NaCl 136.9, KCl 2.7, Na2HPO4 8.1 and KH2PO4 1.5, pH 7.3) to remove blood and debris. After removing the connective tissue, blood vessels and the atria, the ventricles were rapidly minced and incubated in a PBS solution containing trypsin (0.2%), collagenase (0.1%) and glucose (0.02%) for 30 min at 37°C. The myocardial cells were then isolated by repeat pipetting of the digested myocardial tissue. The cells in the supernatant were transferred into a tube containing culture medium (Dulbecco's modified Eagle's medium containing 10% fetal bovine serum, 0.1 mmol l−1β-mercaptoethanol, and 100 U ml−1 penicillin and 100 μg ml−1 streptomycin). The tube was centrifuged at 600 g for 5 min at room temperature, and the cell pellet was re-suspended in the culture medium. Isolated cells were purified by pre-plating for 30 min to reduce the number of non-myocytes. Bromodeoxyuridine (0.1 mmol l−1) was also added to prevent the growth of fibroblasts. Cardiomyocyte purity was approximately 95%, as assessed by microscopic observation of cell beating.
Preparation of mitochondrial fractions
Cells were washed in TD buffer (135 mmol l−1 NaCl, 5 mmol l−1 KCl, 25 mmol l−1 Tris-Cl, pH 7.6) and allowed to swell for 10 min in ice-cold hypotonic buffer (10 mmol l−1 NaCl, 1.5 mmol l−1 CaCl2, 10 mmol l−1 Tris-Cl, pH 7.5, protease inhibitors). Cells were Dounce-homogenized (60 strokes), and MS buffer (210 mmol l−1 mannitol, 70 mmol l−1 sucrose, 5 mmol l−1 EDTA, 5 mmol l−1 Tris, pH 7.6) was added to stabilize the mitochondria. After removing nuclear contaminants, the supernatant was layered and spun at 4°C for 30 min at 55 200× g. Mitochondria were collected and resuspended in a final volume of 200 μl of MS buffer for specific experiments. The top layer was collected to obtain cytosolic and leaked nuclear and organelle proteins (free protein fraction).
Detection of oxygen in culture medium
The oxygen concentration of the medium was measured as previously described (Griffiths & Robinson, 1999). A 250 μm diameter fibre-optic sensor for the simultaneous quantification of  (OxyLite, Oxford Optronix, Oxford, UK) was inserted in the culture medium during cell treatment. Short pulses of LED light were transmitted along this fibre-optic sensor to excite a platinum-based fluorophore in the sensor tip. The degree to which this fluorescent light is quenched is directly proportional to the number of oxygen molecules within the surrounding environment. The lifetime of fluorescence is inversely proportional to the concentration of dissolved oxygen and is interpreted to provide an absolute value for
(OxyLite, Oxford Optronix, Oxford, UK) was inserted in the culture medium during cell treatment. Short pulses of LED light were transmitted along this fibre-optic sensor to excite a platinum-based fluorophore in the sensor tip. The degree to which this fluorescent light is quenched is directly proportional to the number of oxygen molecules within the surrounding environment. The lifetime of fluorescence is inversely proportional to the concentration of dissolved oxygen and is interpreted to provide an absolute value for  in mmHg.
in mmHg.
Oxygen availability assay
Oxygen availability assays were conducted as previously described (Hagen et al. 2003). In brief, cardiomyocytes or transformed African green monky kidney fibroblast cells were transfected with oxygen detection plasmids. Following 48 h transfection, the cells were subjected to specific treatments, as indicated in the figure legends. The Renilla luciferase substrate, coelenterazine-hcp, was added 5 min prior to the luminescence measurement at a final concentration of 5 μg ml−1. Luminescence was measured by a microplate reader (Synergy 4, BioTek Instruments).
Nitric oxide (NO) measurement
NO was detected by a fluorescent NO-sensitive dye, 4,5-diaminofluorescein (DAF-2). Treated cells were incubated with DAF-2 diacetate (5 μm, Calbiochem) for 10 min at 37°C and subsequently washed. Single cell fluorescence was detected by a laser confocal microscope (A1R, Nikon, Tokyo, Japan). DAF-2 fluorescence was excited at 480 nm, and emitted fluorescence was measured at 540 nm.
Measurement of intracellular ATP and AMP levels
Cells were lysed with 500 μl of 0.2 mol l−1 HClO4 and centrifuged at 5000 g for 10 min. The supernatant was neutralized with KOH on ice, centrifuged and filtered through a 10 kDa, followed by a 3 kDa, cutoff filter (Millipore, Billerica, MA, USA). The resulting filtrate was diluted 1:1 in 100 mmol l−1 phosphate buffer (pH 7.0) and analysed by HPLC (Waters, Milford, MA, USA) with an LC-18T reverse-phase column (Supelco, Bellefonte, PA, USA) at a flow rate of 1 ml min−1, and the absorbance at 254 nm was recorded. Each elution peak was compared with AMP and ATP standards (Sigma) to confirm its identity (Dasgupta & Milbrandt, 2007).
AMPK activity assay
AMPK activity was determined as previously described (Kim et al. 2001). Cells were lysed with digitonin buffer (50 mmol l−1 Tris-HCl, pH 7.3, 50 mmol l−1 NaF, 30 mmol l−1 glycerol phosphate, 250 mmol l−1 sucrose, 1 mmol l−1 sodium metavanadate, and 0.4 mg ml−1 digitonin) on ice for 2 min. AMPK was partially purified from cell lysates by adding saturated ammonium sulfate to a final concentration of 35% (v/v) on ice for 15 min. AMPK activity was determined with these fractionated proteins in kinase assay buffer (62.5 mmol l−1 Hepes, pH 7.0, 62.5 mmol l−1 NaCl, 62.5 mmol l−1 NaF, 6.25 mmol l−1 sodium pyrophosphate, 1.25 mmol l−1 EDTA, 1.25 mmol l−1 EGTA, and 1 mmol l−1 dithiothreitol) containing 200 μmol l−1 AMP and an ATP mixture (200 μmol l−1 ATP and 1.5 μCi of [γ-32P]ATP), with or without 250 μmol l−1 SAMS peptide (HMRSAMSGLHLVKRR) at 30°C for 10 min. The reaction was terminated by spotting the reaction mixture on phosphocellulose paper (P81), and the paper was extensively washed with 150 mmol l−1 phosphoric acid. The radioactivity was measured with a scintillation counter.
Reactive oxygen species (ROS) measurement
Intracellular ROS was detected with a fluorescent probe, dichlorodihydrofluorescein diacetate (H2DCFDA) (Molecular Probes). Cardiomyocytes were cultured in 24-well plates to 80–90% confluence and dyed with a 1 mg ml−1 probe for 30 min in the dark at 37°C. Cells were then subjected to treatments as described in the figure legends. Fluorescence was measured at a 450 nm excitation and 530 nm emission wavelength by a microplate reader.
PHD activity assay
Prolyl hydroxylase (PHD) activity was determined as previously described (Oehme et al. 2004). Cell lysates were used as the source of prolyl hydroxylases. Hydroxylase reactions were carried out by using 1 μg (μl protein)−1 of whole cell lysates in the presence of 100 μmol l−1 2-oxoglutarate, 1 μmol l−1 FeSO4 and 2 mmol l−1 ascorbate for 1 h at room temperature. After washing, 0.022 μg μl−1 thioredoxin-tagged pVHL in complex with elongins B and C (VBC) was allowed to bind to the hydroxylated peptide. Bound VBC complex was detected by rabbit anti-thioredoxin antibodies and secondary horseradish peroxidase-coupled anti-rabbit antibodies (Sigma) using the 3,3′,5,5′-tetramethyl-benzidine substrate kit (Pierce). The peroxidase reaction was stopped by adding H2SO4, and absorbance was determined at 450 nm in a microplate reader. Each experiment was calibrated to an internal standard curve using hydroxyproline (Pro564)-containing HIF-1α peptide (amino acids 556–574) and VBC complex.
Statistical analysis
Data are shown as mean ± s.e.m. Multiple group comparison was performed by one-way ANOVA followed by the Bonferroni procedure for comparison of means. Comparison between two groups was analysed by the two-tailed Student's t test or two-way ANOVA. Values of P < 0.05 were considered statistically significant.
Results
Intracellular oxygen assay
The MitRLuc-pcDNA3 plasmid was constructed by fusing the mitochondrial target sequence of MnSOD to the N-terminus of Renilla luciferase. The plasmid has been previously used to assay mitochondrial oxygen concentration by Drs Thilo Hagen (Hagen et al. 2003) and Jacques E. Riby (Riby et al. 2008). We performed relevant experiments to further validate this oxygen detection method.
The cell line derived from cervical cancer cells transfected with Mito Rluc-pcDNA3 were used to conduct an immunofluorescence experiment. The results suggested that the MitoRLuc protein was primarily localized in the mitochondria (Fig. 1A). Mit RLuc expression was nearly completely colocalized with the NDUFS2 and cytochrome c protein which are important components of the mitochondrial membrane respiratory chain (Procaccio et al. 1998; Loeffen et al. 2001; Waterhouse et al. 2001) (Fig. 1B, Supplemental Fig. S1 (available online only)). Moreover, Western blot analysis revealed that MitRLuc protein was only detected in the mitochondria fraction (Fig. 1C). Renilla luciferase activity was dependent on its substrates and O2. At a given substrate concentration, Renilla luciferase activity merely correlates with O2 availability. When HeLa cells were cultured in decreasing oxygen, Renilla luciferase activity was found to be decreased but up-regulation of HIF-1α expression (as a marker for the oxygen state) was detected simultaneously (Fig. 1D and E). This finding is also consistent with a previous report that Renilla luciferase activity reflects oxygen levels both in immunoprecipitated proteins and in intact cells (Hagen et al. 2003). Thus, we employed a Renilla luciferase reporter to detect intracellular oxygen availability.
Figure 1. Evaluation of the method for intracellular oxygen detection.
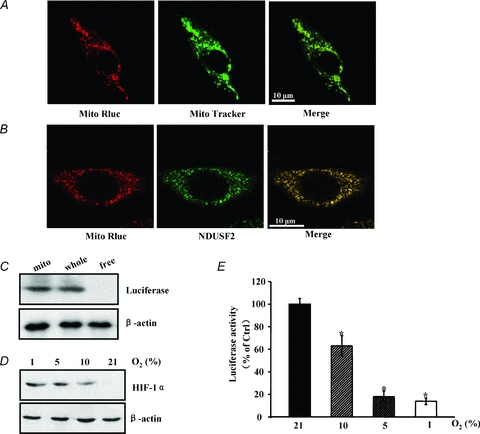
A, HeLa cells were transfected with the MitRLuc-pcDNA3 plasmid. After 48 h, the cells were stained with Mito-Tracker Green FM (Molecular Probes) to identify the localization of the mitochondria. Following mitochondrial labelling, cells were fixed with 4% paraformaldehyde, permeabilized with 0.1% Triton X-100 for 15 min, immunoblotted with luciferase antibody (Santa Cruz), and stained with Cy3-conjugated second antibody (Invitrogen). The sample was detected by confocal laser scanning microscopy. Merged images show the colocalization of the Mit-RLuc protein in mitochondria. B, HeLa cells were transfected with the MitRLuc-pcDNA3 plasmid. Forty-eight hours after transfection, the cells were fixed with 4% paraformaldehyde and permeabilized with 0.1% Triton X-100 for 15 min. After blocking with PBS supplemented with 5% bovine serum albumin for 30 min, the cells were immunolabelled with luciferase antibody followed by a Cy3-conjugated second antibody (Invitrogen). These cells were then immunoblotted with NDUFS2 (Santa Cruz) and FITC-labelled second antibody (Invitrogen). The samples were analysed using confocal laser microscopy. A representative image is shown. C, HeLa cells were transfected with MitRLuc-pcDNA3 for 48 h. Whole cell lysate, mitochondria (Mito) and free protein (whole cell lysate except the mitochondrial fraction) were prepared for the analysis of luciferase expression. D, HeLa cells were incubated at 21%, 10%, 5% and 1% O2 for 8 h, and the induction of the HIF-1α protein was estimated by Western blot. β-Actin was used as a loading control. A representative image is shown. E, HeLa cells expressing MitRLuc-pcDNA3 were treated as in D. Luciferase activity was detected with a microplate reader. Decreased oxygen caused a concentration-dependent reduction in luciferase activity.
β2-AR stimulation increases intracellular oxygen availability in cardiomyocytes
In cardiomyocytes expressing the MitRLuc protein, the non-selective β-AR agonist isoproterenol (isoprenaline (ISO)) caused a rapid and significant increase in mitochondrial oxygen in a dose- and time-dependent manner. The maximal effect appeared at 1–10 μmol l−1 after 8 min of ISO stimulation (Fig. 2A). To determine which β-AR subtype is responsible for this effect, we used selective stimuli to activate either β1-AR (combination of ISO with the β2-AR antagonist ICI118551) or β2-AR (ISO with the β1-AR antagonist CGP20712A). ICI118551 (ICI) or CGP20712A (CGP) alone did not result in alteration of intracellular oxygen levels (data not shown). Selective stimulation of β2-AR increased mitochondrial oxygen to a degree similar to ISO stimulation alone. Furthermore, the concentration of mitochondrial oxygen in β2-AR-stimulated cells was significantly higher than that in unstimulated cells (Ctrl) (Fig. 2B). However, selective β1-AR stimulation did not change mitochondrial oxygen. To exclude the possibility that ICI may exert an effect on β1-AR, we also investigated the contraction amplitude of cardiomyocytes in response to β-AR stimulation. The result showed that β1-AR inhibition by CGP remarkably decreased the contraction amplitude of cardiomyocytes compared with ISO treatment. However, ISO plus different concentrations of ICI did not affect the contraction amplitude compared with ISO stimulation alone, implicating that ICI treatment only inhibited β2-AR-selective events but not β1-AR selective events (Supplemental Fig. S2).
Figure 2. β2-AR activation increases mitochondrial oxygen availability in cardiomyocytes.
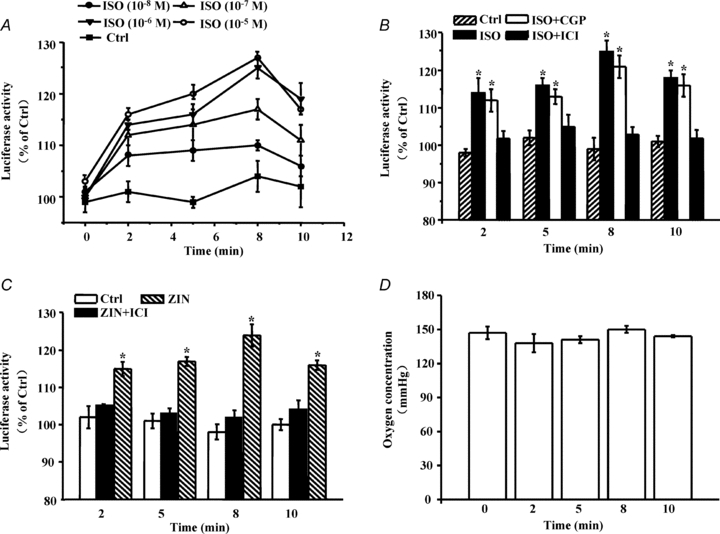
A, cardiomyocytes expressing the MitRLuc protein were treated with different concentrations of isoproterenol (isoprenaline, ISO) for 2, 5, 8 and 10 min. Following addition of the substrate coelenterazine (5 μg ml−1), luminescence was determined with a microplate reader. Renilla luciferase activity was used to reflect the changes in intracellular oxygen. ISO regulated intracellular oxygen in a concentration- and time-dependent manner. B and C, cardiomyocytes expressing the MitRLuc protein were subjected to different pharmacological interventions to identify which receptor subtype was responsible for the change in intracellular oxygen. The drugs and doses were as follows: CGP20712A (CGP), a selective β1-AR inhibitor, 300 nmol l−1; ICI118551 (ICI), a selective β2-AR inhibitor, 100 nmol l−1; ISO, 1 μmol l−1; Zinterol (ZIN), a selective β2-AR agonist, 1 μmol l−1. Cells were pretreated with antagonists for 15 min before the addition of agonists. Luminescence was determined as depicted in Methods. Values are expressed as a percentage compared to untreated cells. Asterisks (*) indicate P < 0.05 versus untreated cells (Ctrl). Error bars represent standard deviations. All data shown represent the mean ±s.e.m. for three separate experiments in duplicate. D, cardiomyocytes were treated with ISO (1 μmol l−1) for the indicated time. The oxygen concentration in the culture medium was measured with a 250 μm-diameter fibre-optic sensor (OxyLite, Oxford Optronix, Oxford, UK). Data represent the means of three independent experiments. Error bars represent standard deviation.
To further confirm this finding, we also stimulated cardiomyocytes using a selective β2-AR agonist Zinterol (ZIN). This treatment resulted in an increase in mitochondrial oxygen similar to that of ISO stimulation, and the increase could be eliminated by ICI pretreatment (Fig. 2C). Through similar experiments, we also found that cytosolic oxygen was specifically regulated by β2-AR activation (data not shown).
Because oxygen might diffuse from the culture medium into the cells, the possibility that the increase in intracellular oxygen is a result of diffusion from the extracellular environment was considered. OxyLite, a fibre-optic oxygen sensor, is a sensitive apparatus for the estimation of oxygen content (Griffiths & Robinson, 1999). It is sensitive to oxygen levels and, unlike 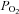 electrodes, does not consume oxygen, which could result in an artificially low
electrodes, does not consume oxygen, which could result in an artificially low 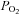 reading. We used the OxyLite method to assay oxygen concentration in culture medium. The result suggested that oxygen concentration in the culture medium remained unchanged at different time points (Fig. 2D), implicating that the change in intracellular oxygen is not due to diffusion from the extracellular environment. Taken together, β2-AR activation specifically modulates intracellular oxygen availability.
reading. We used the OxyLite method to assay oxygen concentration in culture medium. The result suggested that oxygen concentration in the culture medium remained unchanged at different time points (Fig. 2D), implicating that the change in intracellular oxygen is not due to diffusion from the extracellular environment. Taken together, β2-AR activation specifically modulates intracellular oxygen availability.
β2-AR stimulation specifically increases intracellular oxygen availability in another type of mammalian cell
In addition, we used another cell line to determine whether β2-AR specifically regulates intracellular oxygen. COS7 cells were co-transfected with the oxygen assay plasmid and β1-AR or β2-AR. Immunofluorescent staining revealed that β1-AR and β2-AR were mainly expressed on cell membranes (Fig. 3A). Endogenous expression of β1-AR and β2-AR proteins was difficult to detect in COS7 cells by Western blot; however, stable expression was found in transfected COS7 cells (Fig. 3B). ISO stimulation caused a significant increase in mitochondrial and cytosolic oxygen concentrations in cells expressing β2-AR but not in cells expressing β1-AR (Fig. 3C, Supplemental Fig. S3). This finding is consistent with the observed result in cardiomyocytes, and further confirms that β2-AR stimulation plays a major role in the regulation of intracellular oxygen availability.
Figure 3. β2-AR activation regulates mitochondrial oxygen availability in COS7 cells.
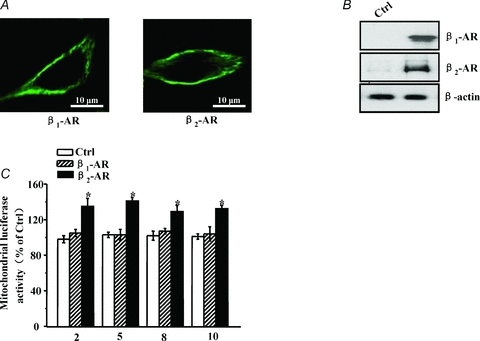
A, COS7 cells were transfected with HA-tagged β1-AR or β2-AR plasmid for 48 h and then fixed with 4% paraformaldehyde and permeabilized with 0.1% Triton X-100 for 15 min. After blocking with PBS supplemented with 5% bovine serum for 30 min, cells were incubated with an HA primary antibody for 1 h at room temperature followed by an FITC-coupled secondary antibody for 30 min. Immunofluorescent staining was detected by confocal microscopy. A representative image is shown. B, COS7 cells were treated as in A, and untreated cells served as a control group (Ctrl). β1-AR and β2-AR expression was estimated by Western blot. β-Actin was used as a loading control. A representative experiment is shown. C, COS7 cells co-expressed the MitRLuc and β1-AR or β2-AR proteins for 48 h and were then stimulated with ISO for the indicated time to detect Renilla luciferase activity. Values are expressed as a percentage compared to unstimulated cells (Ctrl). Asterisks (*) indicate P < 0.05 versus unstimulated cells.
β2-AR regulates oxygen availability through the β2-AR–Gi–eNOS pathway
In the hearts of mammals including humans, stimulation of native cardiac β2-AR activates both the stimulatory G protein and pertussis toxin (PTX)-sensitive Gi signalling pathways (Kuschel et al. 1999; Jo et al. 2002). Gs signalling leads to protein kinase A activation, phosphorylates the receptor and switches β2-AR coupling from Gs to Gi (Tong et al. 2005). PTX, an inhibitor of Gi signalling, is a valuable tool to dissect Gi-dependent components of β2-AR signalling (Danson et al. 2005). We found that PTX treatment completely abolished β2-AR-mediated intracellular oxygen change, suggesting that Gi proteins are involved in the regulation of intracellular oxygen availability (Fig. 4A).
Figure 4. β2-AR regulates oxygen availability through the β2-AR–Gi–eNOS pathway.
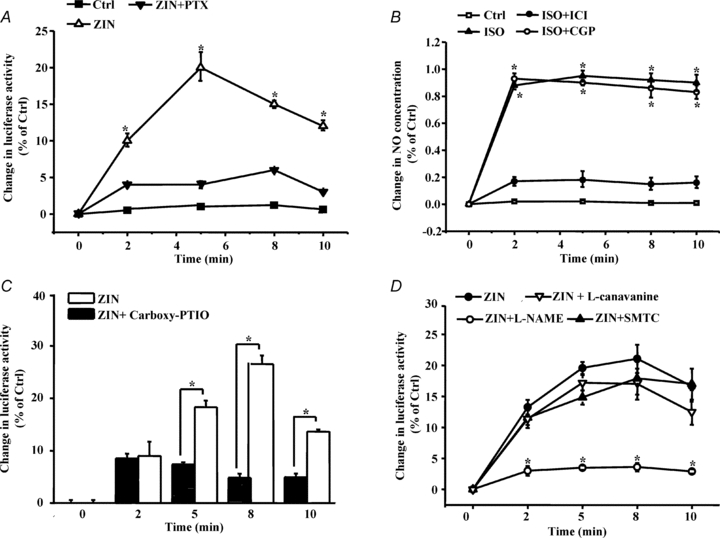
A, C and D, cardiomyocytes expressing the MitRLuc protein were pretreated with PTX (0.5 μg ml−1, pretreated for 3 h at 37°C), carboxy-PTIO, and NOS inhibitors (pretreated for 30 min at 37°C), including l-NAME (500 nmol l−1), SMTC (100 nmol l−1) and l-canavanine (1 μmol l−1). β-AR was stimulated by different treatments, and luciferase activity was then determined as described in Methods. The data are displayed as the degree of change relative to unstimulated cells (Ctrl). B, cardiomyocytes were loaded with a DAF-2 diacetate probe, pretreated with CGP or ICI for 15 min, and then stimulated with ZIN for the indicated time. DAF-2 fluorescence was excited at 480 nm, and emitted fluorescence was measured at 540 nm. Values are expressed as the relative change compared to untreated cells (Ctrl). Single cell fluorescence was detected by laser confocal microscopy. The data represent the means of three independent experiments, and each point represents the average of ten cells.
In addition, we found that ISO treatment caused rapid NO release in cardiomyocytes in a concentration-dependent manner (Supplemental Fig. S4), and NO release was specifically mediated by β2-AR, but not β1-AR activation (Fig. 4B). We asked whether NO generation is tightly associated with intracellular oxygen regulation. Carboxy-PTIO was employed to eliminate β2-AR-mediated NO generation (Goldstein et al. 2003). Meanwhile, β2-AR-mediated change in intracellular oxygen was nearly blocked by NO scavenging (Fig. 4C).
NO in the heart is generated by endothelial NO synthase (eNOS) in the endothelium and caveolae of cardiomyocytes, by neuronal NO synthase (nNOS) in the sarcoplasmic reticulum and possibly mitochondria, and by inducible NO synthase (iNOS) in the sarcoplasm under pathological situations (Moncada & Erusalimsky, 2002). Inhibition of nNOS or iNOS by S-methylthiocitrulline (SMTC, 100 nm) (Seddon et al. 2008) or l-canavanine (1 μm) (Mansart et al. 2006) had no effect on intracellular oxygen availability. However, inhibition of eNOS by N-nitro-l-arginine methyl ester (l-NAME, 500 nm) (Laursen et al. 2001) almost abolished the β2-AR effect on intracellular oxygen, suggesting that eNOS-mediated NO release is responsible for the observed changes in intracellular oxygen (Fig. 4D, Supplemental Fig. S5).
β2-AR stimulation increases the AMP/ATP ratio, AMPK activity, ROS production, and PHD activity
At steady state, approximately 90% of available oxygen is consumed by the mitochondria to produce ATP through oxidative phosphorylation (Rolfe & Brown, 1997). Cells have evolved protective mechanisms to enhance survival through the initiation of signalling pathways that sense alterations in cellular energy status. In cardiomyocytes, β2-AR stimulation resulted in a reduction in ATP production but was accompanied by a rise in its precursor, AMP (Supplemental Fig. S6A and B). The AMP/ATP ratio was enhanced (Fig. 5A), which subsequently led to the activation of AMP-activated kinase (AMPK) (Fig. 5B). AMPK helps to maintain cellular energy homeostasis and ATP levels (Hardie, 2008). In addition, ROS generation is also a significant consequence of oxidative phosphorylation. Excessive or discontinuous oxygen consumption has significant pathological implications through ROS generation. We found that β2-AR activation caused the inhibition of mitochondrial respiration and the increase in ROS production, implying that oxygen regulation and ROS production are encountered in physiological states (Fig. 5C).
Figure 5. β2-AR activation increases the AMP/ATP ratio, AMPK activity, ROS production and PHD activity.
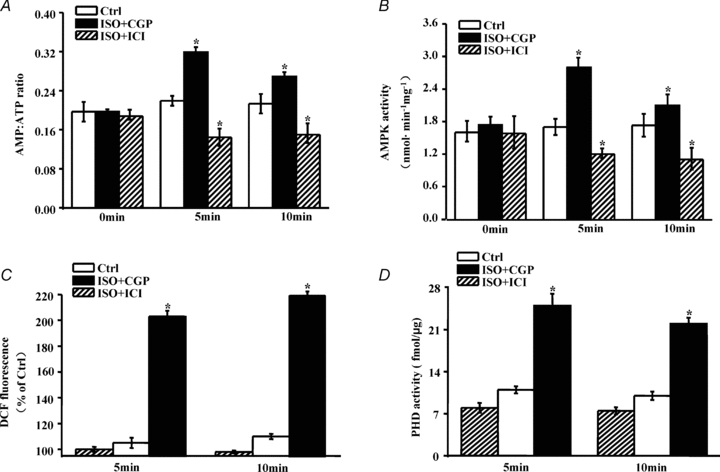
A, B and C, cardiomyocytes were pretreated with ICI or CGP for 15 min and then stimulated with ISO for the indicated time. Intracellular ATP and AMP levels were measured by HPLC. Results are expressed as AMP/ATP ratios at different time points. AMPK was purified from cell lysates. AMPK activity was determined as described in Methods through measurement of the radioactivity of an enzymatic reaction. Intracellular ROS levels were measured using a fluorescent probe H2DCFDA. The data are expressed as a percentage compared to the untreated group (Ctrl). D, cardiomyocytes were treated as in A, and whole cell lysates were subjected to an in vitro PHD activity assay using the wild-type HIF-1α (556–574) peptide. The specific procedure is described in Methods. All data shown represent the mean ±s.e.m. for three separate experiments in duplicate. Asterisks (*) indicate P < 0.05 versus untreated cells (Ctrl) for the corresponding time point.
In addition to its role in normal metabolism, oxygen is a crucial substrate for numerous enzymes, such as dioxygenase. Prolyl hydroxylase (PHD) is a dioxygenase that uses O2 and α-ketoglutarate as substrates and generates CO2 and succinate as by-products; this enzyme is sensitive to oxygen state. We found that PHD activity was specifically up-regulated by β2-AR, but not β1-AR stimulation (Fig. 5D). Alterations in PHD activity may transmit O2-responsive signals to regulate cellular physiological events.
Discussion
Recent evidence suggests that as molecular oxygen became integral to biochemical pathways, many enzymatic reactions central to anoxic metabolism were effectively replaced in aerobic organisms (Raymond & Segre, 2006). Oxygen is required not only for metabolism processes but also for other biological functions. The vast majority of oxygen consumed is used by mitochondria for ATP production to facilitate normal physiological function at steady state. The remaining ‘spare’ oxygen is used for non-mitochondrial processes and is consumed by non-mitochondrial dioxygenases (Taylor, 2008). Thus, precise and rapid redistribution of intracellular oxygen is crucial for cell survival in response to sudden stimuli. β2-AR activation specifically results in an increase in intracellular oxygen availability. The phenomenon occurs within 10 min and can be considered as an acute response.
β2-AR is a prototypical GPCR with distinct localization within membrane microdomains (DiPilato & Zhang, 2009) and is coupled with both the Gi and Gs proteins. Its activation increases cardiac contraction through Gs-mediated signalling and reduces cardiac contraction or initiates anti-apoptotic signalling via the Gi-coupled signalling pathway (Tong et al. 2005). In the study, we found that the Gi protein plays an important role in the regulation of intracellular oxygen. This finding further extends the function of the Gi protein in cells.
Lipid rafts and caveolae are typical membrane microdomains that spatially organize GPCR signalling molecules to promote kinetically favourable interactions to facilitate transduction (Ostrom & Insel, 2004). G proteins undergo signal-dependent trafficking into or out of lipid rafts. Rafts function as sites for G-protein trafficking and internalization as well as platforms for signalling (Allen et al. 2006). In addition, caveolae are reported to regulate the physiological signalling of β2-AR subtypes in cardiomyocytes and may influence the coupling of Gαi (Rybin et al. 2000; Xiang et al. 2002). We suppose that this membrane microdomain also acts in intracellular oxygen regulation. In a preliminary experiment, we found that once caveolae were destroyed by methyl-β-cyclodextrin (MβC, 1 mm), β2-AR stimulation did not change the level of intracellular oxygen, implicating that localization of β2-AR to caveolae is required. Furthermore, the concentration of intracellular oxygen was not different among PTX-pretreated, MβC-pretreated, or PTX plus MβC-pretreated cells in response to β2-AR stimulation (Supplemental Fig. S7). These data also suggest that caveolae and the Gi protein co-exist within the same pathway to regulate intracellular oxygen. In addition, other studies report that Gαs is also related to lipid rafts/caveolae in cardiomyocytes, and this localization might modulate Gαs-coupled GPCR signalling (Rybin et al. 2000; Head et al. 2006). Allen et al. first used a simplified model system in C6 glioma cells to clarify the underlying mechanisms (Allen et al. 2009). They revealed that caveolin-1 and lipid microdomains act in Gαs trafficking and signalling. Gαs in lipid rafts/caveolae is removed from membrane signalling cascades, and caveolins might dampen global Gαs–adenylyl cyclase–cAMP signalling. This finding indirectly supports our finding: in response to β2-AR stimulation, lipid rafts/caveolae are involved in Gs inhibition, which may aid in the β2-AR coupling switch from Gs to Gi protein. Gi proteins then participate in the regulation of intracellular oxygen and in turn, initiate other signalling pathways.
ATP-dependent signalling is a protective mechanism for cell survival (Towler & Hardie, 2007). β2-AR activation causes a decrease in ATP and an increase in AMP. An increase in the AMP/ATP ratio leads to the activation of AMP-activated kinase (AMPK). The AMPK pathway is involved in the beneficial effects of exercise and represents a new therapeutic target for metabolic diseases, such as diabetes. AMPK activation promotes catabolic pathways, including glucose transport, gluconeogenesis, respiration and the use of alternative energy sources to oxygen, and downregulates anabolic pathways. These events are critical in maintaining cellular ATP levels. Furthermore, rapid activation of AMPK occurs in conditions of myocardial ischaemia. AMPK activation increases energy production and inhibits apoptosis, thereby protecting the heart during ischaemic stress (Dyck & Lopaschuk, 2006). In summary AMPK activation plays a beneficial role in cardiovascular protection.
The efficient respiratory chain functions within a narrow range of O2 concentrations (Semenza, 2007b). Oxygen changes trigger mitochondrial respiratory chain dysfunction. A fraction of electrons escape the respiratory chain and combine with O2 prematurely, resulting in the generation of ROS. β2-AR activation causes the inhibition of the respiratory chain and an increase in ROS production. ROS has been reported to transmit intracellular signalling events (Ushio-Fukai, 2006) and leads to the modulation of protein function and protein–protein interactions (Stadtman, 2006). Moreover, ROS formation is required for β2-AR signal transduction and stabilizes the active receptor conformations by redox-mediated modification of cysteine residues of the receptor and/or Gα protein (Moniri & Daaka, 2007). Thus, ROS generation is also helpful for fine-tuning β2-AR signalling.
In addition, β2-AR activation specifically enhances the activity of PHD proteins. More importantly, this trend in PHD activity is consistent with changes in intracellular oxygen. PHD proteins are essential sensors of cellular oxygen state and mediate O2-responsive signals through the modification of the target proteins with which they interact. One subtype, EGLN3, is most abundant in cardiac and smooth muscle (Xie et al. 2009). EGLN3 interacts directly with β2-AR to serve as an endogenous β2-AR prolyl hydroxylase and consequently affects receptor degradation and down-regulation. β2-AR signalling modulates intracellular oxygen availability, and the altered oxygen level triggers a change in PHD activity. PHD activity has feedback effects on the responsiveness of β2-AR signalling. β2-AR, O2 and PHD constitute a rapid, accurate and complicated signalling network that responds to various stimuli, and oxygen acts as a mediator to regulate this signalling network.
In conclusion, β2-AR activation specifically modulates intracellular oxygen availability. This regulation is mediated by the β2-AR–Gi–eNOS pathway, which consequently affects AMPK activity, ROS generation and PHD activity. These findings provide insight into the regulation of oxygen homeostasis, broaden the scope of β2-AR functions, and may have crucial implications for β2-AR regulation.
Acknowledgments
This work was supported by the National Science Fund of China (30425016, 30330290 and 30470961), the ‘973’ Program Fund of China (2007CB512100), the ‘863’ Program Fund of China (2007AA02Z438), the Program Fund for Shanghai Subject Chief Scientists, the Program Fund for Innovative Research Teams by the Ministry of Education of China (Y.-H.C.), the Shanghai Pujiang Program Fund (07PJ14058, L.P.), the ‘973’ Program Fund of China (2006CB504100, Z.-N.Z.), and the National Science Fund of China (30528011, J.C.). The authors have no conflicts of interest to disclose.
Glossary
Abbreviations
- AMPK
AMP-activated kinase
- β-AR
β-adrenoceptor
- DAF-2
4,5-diaminofluorescein
- eNOS
endothelial nitric oxide synthase
- Gi
inhibitory G protein
- GPCRs
G protein-coupled receptors
- H2DCFDA
dichlorodihydrofluorescein diacetate
- iNOS
inducible NO synthase
- ISO
isoproterenol (isoprenaline)
- nNOS
neuronal NO synthase
- NO
nitric oxide
- PHD
prolyl hydroxylase
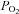
partial pressure of oxygen
- PTX
pertussis toxin
- ROS
reactive oxygen species
Author contributions
Y.-H.C. designed the research; J.L., B.Y., Z.H., Y.L., J.X., Y.S., Y.L., D.L., Y.Z. and J.S. performed the research; J.L., B.Y., Z.H., L.P., Z.-N.Z. and J.C. analysed the data; J.L., B.Y., Z.H. and Y.-H.C. wrote the paper. All authors read and approved the manuscript for publication.
Supplemental material
Figure S1. The colocalization of MitRLuc and Cyt C protein
HeLa cells were transfected with the MitRLuc-pcDNA3 plasmid. Forty-eight hours after transfection, the cells were fixed with 4% paraformaldehyde and permeabilized with 0.1% Triton X-100 for 15 min. After blocking with PBS supplemented with 5% bovine serum albumin for 30 min, the cells were immunolabeled with luciferase antibody followed by a Cy3-conjugated second antibody (Invitrogen). These cells were then immunoblotted with Cyt C (Santa Cruz) and FITC-labeled second antibody (Invitrogen). The samples were analyzed using confocal laser microscopy. A representative image is shown.
Figure S2. The effect of β1-AR or β2-AR stimulation on cardiomyocytes contractile response
Cardiomyocytes were treated as shown. For the measurement of contractility, the treated cells were mounted on the stage of an inverted fluorescence microscope (Zeiss, Axiovert S 100) equipped with a 40× oil-immersion objective in a circular temperature-controlled chamber (Intracell, Cambridge, UK) at 37°C, pH 7.4. Light-dark contrast at the cell edges provided a marker for measurement of amplitude of cell shortening monitored with a video motion analyzer. All measurements were processed by the SoftEdge software (IonOptix, Milton, MA, USA). The data represent the means of three independent experiments. The error bars represent standard deviation.
Figure S3. β2-AR stimulation specifically regulates cytosolic oxygen
COS7 cells were cotransfected with Mit-RLuc and β1-AR or β2-AR plasmids for 48 h, and these cells were then stimulated with ISO for indicated time to detect Renilla luciferase activity. Luminescence was determined with a microplate reader. Values are expressed as the percentage compared to unstimulated cells (Ctrl). Asterisks (*) indicate p < 0.05 versus unstimulated cells.
Figure S4. ISO causes rapid NO release in a concentration-dependent manner
Cardiomyocytes were loaded with a DAF-2 diacetate probe and stimulated with different concentrations of ISO for indicated time. DAF-2 fluorescence was excited at 480 nm, and emitted fluorescence was measured at 540 nm. Values are expressed as the relative change compared to unstimulated cells (Ctrl). The data represent the means of three independent experiments. The error bars represent standard deviation.
Figure S5. The effects of NOS inhibition on cytosolic oxygen availability
Cardiomyocytes expressing cytosolic luciferase were pretreated with NOS inhibitors, including L-NAME (500 nmol/L), SMTC (100 nmol/L), and L-canavanine (1 μmol/L), for 30 min at 37 °C. After the addition of the substrate coelenterazine (5 μg/ml), luminescence was determined with a microplate reader. The data are expressed as the degree of change relative to unstimulated cells (Ctrl). The means ± SEM of three independent experiments are shown.
Figure S6. β2-AR stimulation increases AMP but decreases ATP production
Cardiomyocytes were pretreated with ICI or CGP for 15 min, and then stimulated with ISO for the indicated time. Intracellular ATP and AMP levels were measured by HPLC (Waters, Milford, MA) with an LC-18T reverse-phase column (Supelco, Bellefonte, PA) at a flow rate of 1 ml/min, and the absorbance at 254 nm was recorded. Each elution peak was compared with AMP and ATP standards (Sigma) to confirm its identity. The data are expressed as a percentage compared to the untreated group (Ctrl). All data shown represent the means ± SEM for three separate experiments in duplicate. Asterisks indicate p < 0.05 versus unstimulated cells at the corresponding time point.
Figure S7. Effect of PTX and MβC on β2-AR-mediated mitochondrial oxygen change
Cardiomyocytes expressing Mit-RLuc protein were pretreated with PTX, MβC, PTX plus MβC or left untreated. Then these cells were stimulated with ZIN for 5 or 10 min. Renilla luciferase activity was determined by a microplate reader. The data are expressed as a percentage compared to the unstimulated group (Ctrl), and represents the means ± SEM for three separate experiments in duplicate. Asterisks (*) indicate p < 0.05 versus unstimulated cells at the corresponding time.
HeLa or COS7 cells transfection
One day before transfection, HeLa or COS7 cells were plated in the appropriate amount of growth medium without antibiotics so that they will be 80–90% confluent at the time of transfection. For each transfection sample, prepare DNA-Lipofectamine™ 2000 complexes as follows, (1) Dilute the DNA molecule in the appropriate amount of Opti-MEM Medium without serum. (2) Mix Lipofectamine™ 2000 gently before use, then dilute the appropriate amount in Opti-MEM Medium without serum. Mix gently and incubate for 5 minutes at room temperature. (3)After the 5 minute incubation, combine the diluted DNA with the diluted Lipofectamine™ 2000. Finally,the DNA-Lipofectamine™ 2000 complex was added into cell medium. Thirty-six hours after transfection, cells were harvested for specific experiment.
Lentiviral vector construction and infection
Virus was produced using the ViraPower lentiviral expression system (Invitrogen) according to the manufacturer's protocol. Briefly, Mitochondria-target or cytosol-target Renilla luciferase sequence was obtained from MitRLuc-pcDNA3 or pRL-CMV plasmid respectively, and then cloned into pLenti6 V5-D for to generate pLenti6-V5/MitRLuc or pLenti6-V5/Rluc expression plasmid. The day before transfection, 293FT cells were plated into a 10 cm tissue culture plate to 90%–95% confluence. One day later, 9 μg of ViraPower packaging mix and 3 μg of pLenti6-V5/MitRLuc or pLenti6-V5/Rluc plasmid were co-transfected into the 293FT cells using 36 μl of Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer's instructions. Forty-eight hours after co-transfection, the viral supernatant was collected by centrifugation at 3,000 rpm for 15 min. The viral titer was determined by transduction of RKO cells with serial dilutions of the viral supernatant and colony counting after blasticidin selection (8 μg/ml). The viral supernatant was added to RKO cells (1×105 cells/well in a six-well plate) at a multiplicity of infection of 5 with 6 μg/ml Polybrene. After 24 h, the medium was replaced with fresh DMEM medium with 10% fetal bovine serum (FBS). After an additional 24 h, the cells were collected for functional assays or selected by blasticidin, collected and concentrated, and the titer was determined.
Cardiomyocytes were infected 36 h after plating by using the above lentiviruse at a moi of 50 in maintenance medium containing 5% horse serum and 8 μg/ml of polybrene. Twenty-four hours after infection, cardiomyocytes were incubated in maintenance medium for an additional 24 h for corresponding experiment.
References
- Allen JA, Halverson-Tamboli RA, Rasenick MM. Lipid raft microdomains and neurotransmitter signaling. Nat Rev Neurosci. 2006;8:128–140. doi: 10.1038/nrn2059. [DOI] [PubMed] [Google Scholar]
- Allen JA, Yu JZ, Dave RH, Bhatnagar A, Roth BL, Rasenick MM. Caveolin-1 and lipid microdomains regulate Gs trafficking and attenuate Gs/adenylyl cyclase signaling. Mol Pharmacol. 2009;76:1082–1093. doi: 10.1124/mol.109.060160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danson EJF, Zhang YH, Sears CE, Edwards AR, Casadei B, Paterson DJ. Disruption of inhibitory G-proteins mediates a reduction in atrial β-adrenergic signaling by enhancing eNOS expression. Cardiovasc Res. 2005;67:613–623. doi: 10.1016/j.cardiores.2005.04.034. [DOI] [PubMed] [Google Scholar]
- Dasgupta B, Milbrandt J. Resveratrol stimulates AMP kinase activity in neurons. Proc Natl Acad Sci U S A. 2007;104:7217–7222. doi: 10.1073/pnas.0610068104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPilato LM, Zhang J. The role of membrane microdomains in shaping β2-adrenergic receptor-mediated cAMP dynamics. Mol Biosyst. 2009;5:832–837. doi: 10.1039/b823243a. [DOI] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyck JRB, Lopaschuk GD. AMPK alterations in cardiac physiology and pathology: enemy or ally? J Physiol. 2006;574:95–112. doi: 10.1113/jphysiol.2006.109389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein S, Russo A, Samuni A. Reactions of PTIO and carboxy-PTIO with ·NO, ·NO2, and O2−·. J Biol Chem. 2003;278:50949–50955. doi: 10.1074/jbc.M308317200. [DOI] [PubMed] [Google Scholar]
- Griffiths JR, Robinson SP. The OxyLite: a fibre-optic oxygen sensor. Brit J Radiol. 1999;72:627–630. doi: 10.1259/bjr.72.859.10624317. [DOI] [PubMed] [Google Scholar]
- Hagen T, Taylor CT, Lam F, Moncada S. Redistribution of intracellular oxygen in hypoxia by nitric oxide: effect on HIF1α. Science. 2003;302:1975–1978. doi: 10.1126/science.1088805. [DOI] [PubMed] [Google Scholar]
- Hardie DG. AMPK: a key regulator of energy balance in the single cell and the whole organism. Int J Obes. 2008;4:S7–S12. doi: 10.1038/ijo.2008.116. [DOI] [PubMed] [Google Scholar]
- Head BP, Patel HH, Roth DM, Murray F, Swaney JS, Niesman IR, Farquhar MG, Insel PA. Microtubules and actin microfilaments regulate lipid raft/caveolae localization of adenylyl cyclase signaling components. J Biol Chem. 2006;281:26391–26399. doi: 10.1074/jbc.M602577200. [DOI] [PubMed] [Google Scholar]
- Iaccarino G, Ciccarelli M, Sorriento D, Galasso G, Campanile A, Santulli G, Cipolletta E, Cerullo V, Cimini V, Altobelli GG, Piscione F, Priante O, Pastore L, Chiariello M, Salvatore F, Koch WJ, Trimarco B. Ischemic neoangiogenesis enhanced by β2-adrenergic receptor overexpression: a novel role for the endothelial adrenergic system. Circ Res. 2005;97:1182–1189. doi: 10.1161/01.RES.0000191541.06788.bb. [DOI] [PubMed] [Google Scholar]
- Iaccarino G, Cipolletta E, Fiorillo A, Annecchiarico M, Ciccarelli M, Cimini V, Koch WJ, Trimarco B. β2-Adrenergic receptor gene delivery to the endothelium corrects impaired adrenergic vasorelaxation in hypertension. Circulation. 2002;106:349–355. doi: 10.1161/01.cir.0000022690.55143.56. [DOI] [PubMed] [Google Scholar]
- Insel PA. Adrenergic receptors – evolving concepts and clinical implications. N Engl J Med. 1996;334:580–585. doi: 10.1056/NEJM199602293340907. [DOI] [PubMed] [Google Scholar]
- Johnson M. The β-adrenoceptor. Am J Respir Crit Care Med. 1998;158:S146–S153. doi: 10.1164/ajrccm.158.supplement_2.13tac110. [DOI] [PubMed] [Google Scholar]
- Jo SH, Leblais V, Wang PH, Crow MT, Xiao RP. Phosphatidylinositol 3-kinase functionally compartmentalizes the concurrent Gs signaling during β2-adrenergic stimulation. Circ Res. 2002;91:46–53. doi: 10.1161/01.res.0000024115.67561.54. [DOI] [PubMed] [Google Scholar]
- Kim J, Yoon MY, Choi SL, Kang I, Kim SS, Kim YS, Choi YK, Ha J. Effects of stimulation of AMP-activated protein kinase on insulin-like growth factor 1- and epidermal growth factor-dependent extracellular signal-regulated kinase pathway. J Biol Chem. 2001;276:19102–19110. doi: 10.1074/jbc.M011579200. [DOI] [PubMed] [Google Scholar]
- Kuschel M, Zhou YY, Cheng H, Zhang SJ, Chen Y, Lakatta EG, Xiao RP. Gi protein-mediated functional compartmentalization of cardiac β2-adrenergic signaling. J Biol Chem. 1999;274:22048–22052. doi: 10.1074/jbc.274.31.22048. [DOI] [PubMed] [Google Scholar]
- Laursen JB, Somers M, Kurz S, McCann L, Warnholtz A, Freeman BA, Tarpey M, Fukai T, Harrison DG. Endothelial regulation of vasomotion in apoE-deficient mice: implications for interactions between peroxynitrite and tetrahydrobiopterin. Circulation. 2001;103:1282–1288. doi: 10.1161/01.cir.103.9.1282. [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ, Rockman HA, Koch WJ. Catecholamines, cardiac β-adrenergic receptors, and heart failure. Circulation. 2000;101:1634–1637. doi: 10.1161/01.cir.101.14.1634. [DOI] [PubMed] [Google Scholar]
- Loeffen J, Elpeleg O, Smeitink J, Smeets R, Stöckler-Ipsiroglu S, Mandel H, Sengers R, Trijbels F, van den Heuvel L. Mutations in the complex I NDUFS2 gene of patients with cardiomyopathy and encephalomyopathy. Ann Neurol. 2001;49:195–201. doi: 10.1002/1531-8249(20010201)49:2<195::aid-ana39>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Mansart A, Bollaert PE, Giummelly P, Capdeville-Atkinson C, Atkinson J. Effects of dexamethasone and L-canavanine on the intracellular calcium-contraction relation of the rat tail artery during septic shock. Am J Physiol Heart Circ Physiol. 2006;291:H1177–H1182. doi: 10.1152/ajpheart.00997.2005. [DOI] [PubMed] [Google Scholar]
- Mieno S, Watanabe F, Sawa Y, Horimoto H. Gene transfer of β2 adrenergic receptor enhances cardioprotective effects of ischemic preconditioning in rat hearts after myocardial infarction. Interact Cardiovasc Thorac Surg. 2005;4:163–167. doi: 10.1510/icvts.2004.096792. [DOI] [PubMed] [Google Scholar]
- Moncada S, Erusalimsky JD. Does nitric oxide modulate mitochondrial energy generation and apoptosis? Nat Rev Mol Cell Biol. 2002;3:214–220. doi: 10.1038/nrm762. [DOI] [PubMed] [Google Scholar]
- Moniri NH, Daaka Y. Agonist-stimulated reactive oxygen species formation regulates 2-adrenergic receptor signal transduction. Biochem Pharmacol. 2007;74:64–73. doi: 10.1016/j.bcp.2007.03.016. [DOI] [PubMed] [Google Scholar]
- Oehme F, Jonghaus W, Narouz-Ott L, Huetter J, Flamme I. A nonradioactive 96-well plate assay for the detection of hypoxia-inducible factor prolyl hydroxylase activity. Anal Biochem. 2004;330:74–80. doi: 10.1016/j.ab.2004.03.066. [DOI] [PubMed] [Google Scholar]
- Ostrom RS, Insel PA. The evolving role of lipid rafts and caveolae in G protein-coupled receptor signaling: implications for molecular pharmacology. Br J Pharmacol. 2004;143:235–245. doi: 10.1038/sj.bjp.0705930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Procaccio V, de Sury R, Martinez P, Depetris D, Rabilloud T, Soularue P, Lunardi J, Issartel JP. Mapping to 1q23 of the human gene (NDUFS2) encoding the 49-kDa subunit of the mitochondrial respiratory Complex I and immunodetection of the mature protein in mitochondria. Mamm Genome. 1998;9:482–484. doi: 10.1007/s003359900803. [DOI] [PubMed] [Google Scholar]
- Raymond J, Segre D. The effect of oxygen on biochemical networks and the evolution of complex life. Science. 2006;311:1764–1767. doi: 10.1126/science.1118439. [DOI] [PubMed] [Google Scholar]
- Riby JE, Firestone GL, Bjeldanes LF. 3,3-Diindolylmethane reduces levels of HIF-1 and HIF-1 activity in hypoxic cultured human cancer cells. Biochem Pharmacol. 2008;75:1858–1867. doi: 10.1016/j.bcp.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockman HA, Koch WJ, Lefkowitz RJ. Seven-transmembrane-spanning receptors and heart function. Nature. 2002;415:206–212. doi: 10.1038/415206a. [DOI] [PubMed] [Google Scholar]
- Rolfe DF, Brown GC. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol Rev. 1997;77:731–758. doi: 10.1152/physrev.1997.77.3.731. [DOI] [PubMed] [Google Scholar]
- Rybin VO, Xu X, Lisanti MP, Steinberg SF. Differential targeting of β-adrenergic receptor subtypes and adenylyl cyclase to cardiomyocyte caveolae. A mechanism to functionally regulate the cAMP signaling pathway. J Biol Chem. 2000;275:41447–41457. doi: 10.1074/jbc.M006951200. [DOI] [PubMed] [Google Scholar]
- Salpeter SR, Ormiston TM, Salpeter EE. Cardiovascular effects of β-agonists in patients with asthma and COPD. Chest. 2004;125:2309–2321. doi: 10.1378/chest.125.6.2309. [DOI] [PubMed] [Google Scholar]
- Seddon MD, Chowienczyk PJ, Brett SE, Casadei B, Shah AM. Neuronal nitric oxide synthase regulates basal microvascular tone in humans in vivo. Circulation. 2008;117:1991–1996. doi: 10.1161/CIRCULATIONAHA.107.744540. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Life with oxygen. Science. 2007a;318:62–64. doi: 10.1126/science.1147949. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Oxygen-dependent regulation of mitochondrial respiration by hypoxia-inducible factor 1. Biochem J. 2007b;405:1–10. doi: 10.1042/BJ20070389. [DOI] [PubMed] [Google Scholar]
- Stadtman ER. Protein oxidation and aging. Free Radical Res. 2006;40:1250–1258. doi: 10.1080/10715760600918142. [DOI] [PubMed] [Google Scholar]
- Taylor CT. Mitochondria, oxygen sensing, and the regulation of HIF-2α. Focus on “Induction of HIF-2α is dependent on mitochondrial O2 consumption in an O2-sensitive adrenomedullary chromaffin cell line”. Am J Physiol Cell Physiol. 2008a;294:C1300–C1302. doi: 10.1152/ajpcell.00206.2008. [DOI] [PubMed] [Google Scholar]
- Taylor CT. Mitochondria and cellular oxygen sensing in the HIF pathway. Biochem J. 2008b;409:19–26. doi: 10.1042/BJ20071249. [DOI] [PubMed] [Google Scholar]
- Tong H, Bernstein D, Murphy E, Steenbergen C. The role of β-adrenergic receptor signaling in cardioprotection. FASEB J. 2005;19:983–985. doi: 10.1096/fj.04-3067fje. [DOI] [PubMed] [Google Scholar]
- Towler MC, Hardie DG. AMP-activated protein kinase in metabolic control and insulin signaling. Circ Res. 2007;100:328–341. doi: 10.1161/01.RES.0000256090.42690.05. [DOI] [PubMed] [Google Scholar]
- Ushio-Fukai M. Localizing NADPH oxidase-derived ROS. Sci STKE. 2006;22:re8. doi: 10.1126/stke.3492006re8. [DOI] [PubMed] [Google Scholar]
- Waterhouse NJ, Goldstein JC, Von Ahsen O, Schuler M, Newmeyer DD, Green DR. Cytochrome c maintains mitochondrial transmembrane potential and ATP generation after outer mitochondrial membrane permeabilization during the apoptotic process. J Cell Biol. 2001;153:319–328. doi: 10.1083/jcb.153.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Y, Rybin VO, Steinberg SF, Kobilka B. Caveolar localization dictates physiologic signaling of β2-adrenoceptors in neonatal cardiac myocytes. J Biol Chem. 2002;277:34280–34286. doi: 10.1074/jbc.M201644200. [DOI] [PubMed] [Google Scholar]
- Xie L, Xiao K, Whalen EJ, Forrester MT, Freeman RS, Fong G, Gygi SP, Lefkowitz RJ, Stamler JS. Oxygen-regulated β2-adrenergic receptor hydroxylation by EGLN3 and ubiquitylation by pVHL. Sci Signal. 2009;2:ra33. doi: 10.1126/scisignal.2000444. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


