Abstract
Metabotropic glutamate receptors type 1 (mGluR1s) and ionotropic AMPA receptors (AMPARs) are colocalized at parallel fibre (PF) to Purkinje cell synapses of the cerebellum. Single stimulation of PFs activates fast AMPAR excitatory postsynaptic currents, whereas the activation of mGluR1s requires burst stimulation. mGluR1s signal through several pathways in Purkinje cells and the most prominent is the activation of a slow EPSC (sEPSC). To separate the two synaptic currents, studies of the sEPSC have commonly been performed in the presence of AMPA/KA receptor antagonists. We show here in rat cerebellar slices that inhibition of the fast EPSC by AMPAR antagonists strongly and selectively potentiates the mGluR1 sEPSC, showing a negative regulation of mGluR1 by AMPAR. This effect is observed with low concentrations of NBQX (300 nm to 1 μm), with the selective AMPAR antagonist GYKI 53655 and also with γ-DGG, a low affinity glutamate receptor antagonist. When photorelease of glutamate from MNI-glutamate was used to study the postsynaptic responses in isolation, AMPAR inhibition produced a similar potentiation of the mGluR1 sEPSC, showing that the interaction is postsynaptic. Finally, perfusion of the postsynaptic cell with PP1, an inhibitor of src-family tyrosine kinase, increased the amplitude of the mGluR1 sEPSC and occluded the effect of AMPAR inhibition. Thus, at PF to Purkinje cell synapses, AMPAR activation inhibits the mGluR1 sEPSC via activation of a src-family tyrosine kinase. Consequently mGluR1 signalling will be more sensitive to spillover of glutamate than to local synaptic release. Furthermore, it will be enhanced at silent PF synapses which are the majority in Purkinje cells.
Introduction
Fast ionotropic receptors of the AMPA/KA type and slow metabotropic glutamate receptors type 1 (mGluR1) coexist at parallel fibre (PF) to Purkinje cell synapses in the cerebellum. Single stimulation of PFs is sufficient to activate postsynaptic AMPARs, but activation of mGluR1 requires burst stimulation to produce sufficient accumulation of the neurotransmitter (Batchelor et al. 1994) and the resulting sEPSC is strongly modulated by glutamate transporters (Brasnjo & Otis, 2001; Reichelt & Knopfel, 2002). mGluR1s are inserted at the periphery of excitatory synapses (Baude et al. 1993; Nusser et al. 1994; Petralia et al. 1998) where they can monitor glutamate released at the same synapse but also that diffusing from nearby synapses.
Activation of mGluR1 in cerebellar Purkinje cells triggers intracellular signalling pathways downstream of the G-protein Gq (Hartmann et al. 2004). These pathways generate two electrophysiological signals: an sEPSC (Batchelor et al. 1994) and a K+ current mediated by BK channels activated by inositol trisphosphate-evoked Ca2+ release (Canepari & Ogden, 2006). mGluR1 activation also stimulates the production of endocannabinoids (Galante & Diana, 2004; Maejima et al. 2005) and potentiates T-type Ca2+ channels (Hildebrand et al. 2009). Burst stimulation of PFs most commonly gives rise to the sEPSC. The underlying conductance change is due to non-selective cation channels permeable to Ca2+ (Canepari et al. 2001, 2004; Tempia et al. 2001), identified as TRPC3 (Hartmann et al. 2008), generating a slow rise of intracellular Ca2+ (Canepari et al. 2004; Canepari & Ogden, 2006). Coupling of the channel to mGluR1 is independent of phospholipase Cβ (PLCβ; Hirono et al. 1998; Canepari et al. 2001; Tempia et al. 2001; Canepari & Ogden, 2006) and mGluR1 signalling is regulated by tyrosine phosphorylation (Canepari & Ogden, 2003, 2006; Hildebrandt et al. 2009).
Evidence from paired granule cell to Purkinje cell recordings suggests that PF synapses are most often silent (Isope & Barbour, 2002) and that the pattern and prevalence of silent synapses contributes to information storage (Brunel et al. 2004). A survey of studies of the sEPSC shows that in all reports experiments were made in the presence of AMPA receptor antagonists (CNQX, NBQX, DNQX or GYKI) a condition that artificially silences the synapse (Batchelor & Garthwaite, 1993, 1997; Brasnjo & Otis, 2001; Canepari et al. 2001, 2004; Tempia et al. 2001; Dzubay & Otis, 2002; Kim et al. 2003; Canepari & Ogden, 2003, 2006; Hartmann et al. 2004; Marcaggi & Attwell, 2005; Wadiche & Jahr, 2005; Jin et al. 2007; see single exception of Tempia et al. 1998, their Fig. 1). Although it is often argued that AMPAR antagonists were required simply to separate the fast and slow EPSCs, we have found evidence that they influence the functional expression of the mGluR1 pathway.
We show here that AMPAR inhibition enhances the mGluR1 sEPSC. Further, the contributions of pre- and postsynaptic mechanisms were investigated by photolytic release of l-glutamate from 4-methoxy-7-nitroindolinyl-l-glutamate (MNI-caged glutamate) and the possible coupling mechanisms between the fast and slow EPSCs were investigated pharmacologically. The results show a negative regulation of mGluR1 signalling by postsynaptic AMPAR activation and provide evidence for the involvement of tyrosine phosphorylation.
Methods
Ethical approval
Sprague–Dawley rats were provided by Janvier (St Berthevin, France) and subsequently housed at the central animal house at University Paris Descartes (centre St Pères, approval number A-750607), approved by the ‘Prefecture de Police’ following inspection by Veterinary Services of the city of Paris, and representatives of the French Ministry of Research and the Ministry for Health, in agreement with the European Directive 86/609/EEC regarding the protection of animals used for experimental and other scientific purposes.
Experimental procedures were approved by the Directorate of Paris Veterinary Services, by the scientific committee of the central animal house of Paris Descartes (centre St Pères) as well as by the ethical committee for animal experimentation of Paris Descartes. We have read the article by Drummond (2008) and the experiments described here comply with journal policies and UK regulations.
Slice preparation
Experiments with electrical stimulation of PFs were performed on transverse slices, 300 μm thick, cut from the cerebellum of 17- to 23-day-old Sprague–Dawley rats. In photolysis experiments, 230 μm-thick sagittal slices were used. Briefly, rats were killed by decapitation under general anaesthesia following inhalation of the volatile anaesthetic isoflurane following the recommendations of the European commission to be used with the Directive 86/609/EEC. The cerebellum was quickly removed and cooled in ice-cold solution. Following removal of the brain stem the tissue was glued to the stage of a vibrotome (Leica VT1200S, Germany). Slices were kept in a vessel bubbled with 95% O2–5% CO2 at 34°C for the rest of the day or alternatively for 1 h and then allowed to cool down to room temperature. These experiments involved the use of 92 animals.
Solutions
Slice preparation and recordings in experiments with electrical stimulation were made in a bicarbonate-buffered solution containing (in mm): 115 NaCl, 2.5 KCl, 1.3 NaH2PO4, 26 NaHCO3 and 25 glucose. For slicing the solution contained 4 mm MgCl2 and 0.5 mm CaCl2 and for recovery 1 mm MgCl2 and 2 mm CaCl2. For recording solutions CaCl2 and MgCl2 concentrations were 2.5 and 1 mm, respectively. For experiments with electrical stimulation the bath was continuously perfused at a rate of 1–2 ml min−1 with solution equilibrated with 95% O2–5% CO2 to maintain pH at 7.4. Flash photolysis experiments were done in Hepes-buffered saline without perfusion to minimise cage consumption. This solution contained (in mm): 132 NaCl, 4 KCl, 2.5 NaHCO3, 10 Hepes, 15 glucose and 5 sodium pyruvate (see Holmgren et al. 2009), pH 7.3, supplemented with 1 mm MgCl2 and 2.5 mm CaCl2. Bicarbonate was included in the Hepes-buffered solution to maintain internal pH.
Whole cell patch clamp recordings were made from Purkinje neurons, identified by their size and location at the edge of the molecular and granule cell layers. The internal solution contained (in mm): 140 potassium gluconate, 10 KCl, 10 Hepes, 0.1 EGTA, 4.6 MgCl2, 4 ATPNa2 and 0.4 GTPNa, pH adjusted to 7.3 with KOH and osmolality to 300 mosmol kg−1. When filled with internal solution, recording pipettes had a resistance of between 2.5 and 4.5 MΩ. Membrane currents were recorded at a pipette potential of −60 mV (not corrected for junction potential of approximately −12 mV, pipette – bath). Series resistance was less than 10 MΩ and was 80% compensated. During experiments, the preparation was visualised on an upright microscope (Olympus BFX51; ×60, 0.9 NA water dipping objective). All recordings were made in the presence of 3 μM SR 95531 and 10 μM AP5 to block GABA-A and NMDA receptors.
Parallel fibres were stimulated with a patch pipette positioned at the surface of the molecular layer. Pulses of 100 μs duration and around 40 V from an isolated stimulator were used. Similar values were used in all experiments to achieve comparable numbers and density of active PFs.
For photolysis a xenon arc flashlamp (UV2 filter, Rapp Optoelectronic; Hamburg) generated pulses of 0.5–1 ms with wavelength 330–380 nm. Light was coupled to the epifluorescence condenser of the microscope by a liquid light guide and focused to give a uniform field of 200 μm diameter. Field uniformity was checked with full field CCD images by flashing 6 μm diameter fluorescent beads and recorded with an EMCCD camera (Andor Ixon, Belfast). Light flashes were at maximal energy and applied at 2 min intervals. MNI-caged l-glutamate (kindly provided by Drs John Corrie and George Papageorgiou, MRC-NIMR, London, UK) was used at 270 μm. The cage was added to the bath with mixing and 5 min allowed for equilibration before photolysis. Photolysis was calibrated using small aqueous vesicles suspended in a thin layer of Sylgard resin and containing the caged fluorophore NPE-HPTS (2-nitrophenylethyl ether of pyranine) as described previously (Canepari et al. 2001; Trigo et al. 2009). The conversion of MNI-glutamate relative to NPE-HPTS in this optical arrangement was 4.7:1 and includes a correction for ‘inner filtering’ by the 270 μm MNI-glutamate in the bath between preparation and objective that was determined in separate measurements of the transmission of the photolysis pulse in the bath.
Chemicals
2,3-Dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline-7-sulfonamide (NBQX), d-(–)-2-amino-5-phosphonopentanoic acid (d-AP5), 7-(hydroxyiminocyclopropa[b]chromen-1a-carboxylate ethyl ester (CPCCOEt), γ-d-glutamylglycine (γ-DGG), 7-chlorokynurenic acid (7-Cl-Kyn), 1-(1,1-dimethylethyl)-1-(4-methylphenyl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine (PP1) and TTX were purchased from Tocris Bioscience. CPCCOEt, SR 95531 and d-AP5 were also bought from Ascent Scientific. Bisperoxo(1,10-phenanthroline)oxovanadate (bpv(Phen)) was purchased from Calbiochem. 1-(4-Aminophenyl)-3-methylcarbamyl-4-methyl-7,8-methylenedioxy-3,4-dihydro-5H-2,3-benzodiazepine (GYKI 53655) was a kind gift from Dr Michael Spedding. Stocks of CPCCOEt, PP1 and 7-Cl-Kyn were prepared in DMSO and stored at −20°C. All other stocks were prepared in water. Stock solutions were diluted in saline just before use. All other chemicals were purchased from Sigma.
Results
AMPA receptor activation inhibits the mGluR sEPSC
Train stimulation of PFs (e.g. 10 pulses at 100 Hz) activates a fast AMPA EPSC followed by a slow mGluR EPSC (Batchelor et al. 1994). In a first set of experiments, it was noticed that the mGluR sEPSC was not observed in the absence of antagonists of AMPA/KA receptors. However, it was almost always observed if AMPA/KA receptors were blocked with the antagonist NBQX before recording.
This is illustrated in Fig. 1. PFs were stimulated extracellularly in transverse cerebellar slices by a train of 10 stimuli at 100 Hz activating a fast AMPA EPSC. In most experiments, the stimulation was set to 40 V and 100 μs duration. The position of the pipette was adjusted to elicit an AMPA EPSC of 0.5–1 nA. SR 95531 (3 μM) and D-AP5 (10 μM) were continuously perfused in order to block GABA-A and NMDA receptors respectively. Upon bath application of 1 μm NBQX, the amplitude of the fast EPSC decreased rapidly and a slower synaptic current became visible (Fig. 1A and B). This mGluR1 sEPSC was blocked by addition of the mGluR1 antagonist CPCCOEt (100 μm) as described previously by Batchelor et al. (1994; with MCPG ((RS)-a-Methyl-4-carboxyphenylglycine)) and Canepari et al. (2001). The AMPA EPSC was measured at the peak of the first response of the train but as the AMPA and mGluR currents overlap in control conditions, the analysis window for the sEPSC was chosen based on the current kinetics (see Fig. 1). Although this procedure permitted a good separation of the currents, in some cases it underestimated the peak of the mGluR sEPSC. With a potassium gluconate internal solution and recording at room temperature, the amplitude of the mGluR sEPSC was −30.9 ± 7.7 pA (n = 28) in control, −96.7 ± 17.8 pA (n = 28) in 1 μm NBQX and −29.3 ± 7.8 pA (n = 18) following wash of NBQX.
Figure 1. The mGluR1 sEPSC is enhanced by inhibition of AMPA/KARs.

A, plot of the amplitude of the first AMPA response of the train (/AMPA1, filled symbols) and mGluR sEPSC (open symbols) as a function of time. Bars indicate time of application. B, average of 4 (control) or 5 (NBQX and CPCCOEt) postsynaptic responses to burst stimulation of parallel fibres (10 at 100 Hz) in a transverse slice. Ba, black trace in control, dotted grey trace after addition of 1 μm NBQX and dotted black trace after further addition of 100 μm CPCCOEt. In the presence of NBQX the fast AMPA current is quickly blocked and the mGluR sEPSC becomes visible. Upon application of CPCCOEt the mGluR sEPSC is fully blocked. The open rectangle denotes the time window used to measure the average amplitude of ImGluR plotted in A. Bb, same traces at higher magnification to emphasise the mGluR sEPSC. C, average mGluR sEPSC ±s.e.m.*Data significantly different from control, P < 0.01.
The AMPA EPSC activated by the PF train is large, producing depolarisation of the dendrites. Although AMPARs in Purkinje cells have a very low permeability to Ca2+ (Haüsser & Roth, 1997), the depolarisation due to the EPSC may activate Ca2+ entry into the dendrites and may thereby result in Ca2+-activated K+ conductances. This outward current, which could overlap the inward sEPSC and thereby reduce its apparent amplitude, would be prevented by AMPA receptor antagonists. The possibility that an outward current was generated in this way was tested by blocking the sEPSC with CPCCOEt (see Fig. 2). The current amplitude was measured in control in the absence of NBQX or after estimating the amplitude of the sEPSC in NBQX and washing out the drug to directly compare amplitudes. Data were not significantly different and were pooled together. On average, the mGluR sEPSC was −29.9 ± 16.2 pA in control (n = 12), −113.7 ± 34.7 pA in 1 μm NBQX (n = 7) and −19.4 ± 6.1 pA after 20–25 min wash (n = 7). The outward current measured at the same time point as the sEPSC in 100 μm CPCCOEt was +16.7 ± 3.7 pA (n = 12), too small to explain the sEPSC increase produced by blocking AMPARs. Data are summarised in Fig. 2C.
Figure 2. Overlap with a K+ conductance does not explain the reduced amplitude of the mGluR sEPSC in control conditions.
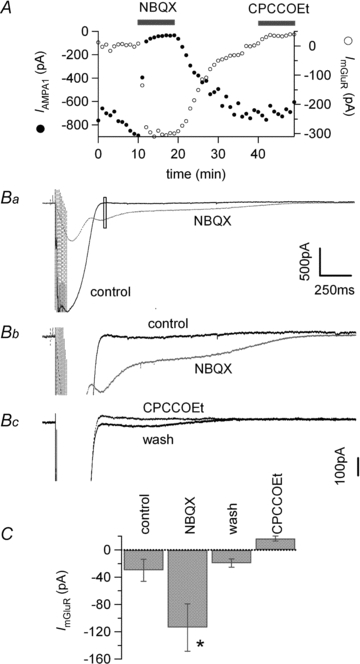
A, plot of the amplitude of the first AMPA response of the train (/AMPA1, filled symbols) and mGluR sEPSC (open symbols) as a function of time. Bars indicate time of application. B, average of 4 (control) or 5 postsynaptic responses to burst stimulation of parallel fibres (10 at 100 Hz) in a transverse slice. Ba, black trace in control, dotted grey trace after addition of 1 μm NBQX and dotted black trace after addition of 100 μm CPCCOEt. In the presence of NBQX the fast AMPA EPSC is quickly blocked and the mGluR sEPSC becomes visible. NBQX, 1 μm, increased the amplitude of the mGluR sEPSC from +2.7 pA to −301.7 pA. This effect was reversed upon 20 min washout of the drug and the mGluR sEPSC measured at the same time point was −6.3 pA. Therefore, the wash should have restored the K+ outward current if it is the source of the apparent inhibition of the mGluR sEPSC. Upon inhibition of mGluR1 by 100 μm CPCCOEt, the train activated an outward current of +36.9 pA. This outward current, however, is not sufficient to account for the decrease of the mGluR sEPSC during wash of NBQX. The open rectangle denotes the time window used to measure the amplitude of ImGluR plotted in A. Bb, same traces at higher magnification to emphasise the mGluR sEPSC. Bc, same for wash and CPCCOEt applications. C, average mGluR sEPSC ±s.e.m.*Data significantly different from control, P < 0.02.
Selectivity of the effect of NBQX on the sEPSC
NBQX is a competitive antagonist of both AMPA and KA receptors. The potentiation of the mGluR sEPSC by NBQX could therefore be mediated by a block of either AMPA or KA receptors. Experiments were performed to study the specificity of the effect.
First, experiments presented above were made with 1 μm NBQX, blocking on average 96.3% of the AMPA EPSC. Although this is a low concentration for KA receptors (see Bureau et al. 1999), reducing the NBQX concentration to 300 nm, blocking 72.6% of the AMPA EPSC, was also sufficient to increase the amplitude of the mGluR sEPSC. The mGluR sEPSC was −30.2 ± 10.5 pA (n = 3) in control and −88.5 ± 9.7 pA (n = 3) in the presence of 300 nm NBQX. Although NBQX is an AMPA/KA receptor antagonist, the high sensitivity to NBQX suggests that the modulation of the sEPSC is mediated by AMPARs rather than kainate receptors (KARs).
Second, the effect of GYKI 53655, a selective non-competitive AMPAR antagonist (Paternain et al. 1995) was tested. Figure 3A shows that inhibition of AMPARs by GYKI 53655 also increased the amplitude of the mGluR sEPSC. Upon application of 5 μm GYKI 53655, the fast EPSC was rapidly inhibited and the mGluR sEPSC amplitude increased. This is seen both on average traces in Fig. 3Aa and b, and in the amplitude plot of Fig. 3Ac. Both effects were reversible upon washout of the drug. The mGluR sEPSC was −25.2 ± 5.6 pA in control (n = 24) and −75.3 ± 11.6 pA (n = 24) in 5 μm GYKI 53655 (see summary in Fig. 3C). Addition of 1 μm NBQX further depressed the AMPA EPSC but there was no additional effect on the mGluR sEPSC (see Fig. 3C). On average the mGluR sEPSC was −60.1 ± 13.9 pA (n = 7 cells) in GYKI 53655 plus 1 μm NBQX.
Figure 3. Selective inhibition of AMPARs enhances mGluR sEPSC.

Aa, average postsynaptic currents following burst stimulation (10 at 100 Hz) in control (average of 3 traces, black), 5 μm GYKI 53655 (average of 5 traces, grey) and wash (average of 5 traces, dotted line). Ab, same but at higher magnification to emphasise the mGluR sEPSC. GYKI increased the mGluR sEPSC reversibly. Ac, amplitude plot of the first AMPA response in the train (/AMPA1, filled symbols) and the mGluR sEPSC (open symbols). Ba and b, average postsynaptic currents following 10 stimulations at 100 Hz in control (average of 4 traces) and 1 μm NBQX (average of 5 traces) in the continued presence of 50 μm d-AP5 and 25 μm 7-Cl-Kyn. Block of NMDARs does not affect the potentiation of mGluR sEPSC by NBQX. Bc, amplitude plot of the first AMPA response (filled symbols) in the train and the mGluR sEPSC (open symbols) in the presence of 50 μm d-AP5 and 25 μm 7-Cl-Kyn. Bars indicate application time. C, bar graph of the average amplitude of mGluR sEPSC in control, 5microM GYKI, GYKI + 1 NBQX, 1 or 2 mM γ-DGG and DGG + 1 NBQX. *Data significantly different from control, P < 0.01.
We checked that the current potentiated by GYKI 53655 was identified correctly as being due to mGluR1. It was blocked by 100 μm CPCCOEt (n = 5). The mGluR sEPSC was −27.9 ± 7.6 pA in control, −74.9 ± 17.9 pA in GYKI 53655 and +3.4 ± 0.3 pA in GYKI 53655 + CPCCOEt.
Tests were also made with the non-specific low affinity glutamate receptor antagonist γ-DGG. γ-DGG at 1 or 2 mm increased the amplitude of the mGluR sEPSC from −30.0 ± 11.5 pA (n = 10) to −72.1 ± 21.3 pA (n = 10) (see Fig. 3C). No further increase of the mGluR sEPSC was seen with addition of 1 μm NBQX. The mGluR EPSC was −62.2 ± 16.9 pA (n = 10) in NBQX +γ-DGG.
Experiments presented so far were performed in the presence of 10 μm d-AP5, a specific NMDA receptor antagonist. However, to test whether NMDA receptors might be involved some experiments were performed in the continued presence of 50 μm d-AP5, an antagonist of the glutamate binding site, and 25 μm 7-chlorokynurenic acid (7-Cl-Kyn), an antagonist of the glycine binding site of the NMDA receptor. In Fig. 2B, the amplitude of the mGluR sEPSC was −25 pA in d-AP5 + 7-Cl-Kyn and −99.8 pA after addition of 1 μm NBQX. On average the mGluR sEPSC was −50.8 ± 13.5 pA (n = 8) in the presence of d-AP5 + 7-Cl-Kyn and −153.3 ± 41.0 pA (n = 8) after addition of 1 μm NBQX (P < 0.02, Wilcoxon signed rank test).
To summarise, the selective inhibition of AMPARs by the non-competitive antagonist GYKI 53655 increased the mGluR sEPSC and occluded the effect of NBQX. Also, NBQX was effective at low concentrations (300 nm to 1 μm). Together, these observations suggest that the selective inhibition of AMPARs rather than KARs is responsible for the increase of mGluR sEPSC.
Pre- or postsynaptic AMPARs?
In the experiments presented above with PF stimulation, the glutamate released could activate both presynaptic AMPARs, regulating release, and postsynaptic AMPARs, regulating the mGluR pathway in the postsynaptic cell. To study the postsynaptic processes selectively we used flash photolysis of MNI-glutamate to release glutamate independently of presynaptic PFs. Experiments were done in the presence of TTX (1 μm), SR 95531 (10 μm) and d-AP5 (50 μm). Glutamate was shown to be released uniformly over the whole somatic and dendritic field in a 0.5–1 ms flash (see Methods). The results obtained in a representative experiment are shown in Fig. 4. MNI-glutamate was present at a concentration of 270 μm in Hepes-buffered solution without perfusion. In these experiments the temperature was raised to 30°C in order to favour the mGluR pathway and recapture of glutamate by glutamate transporters, two mechanisms with a high temperature dependence. This may help clear the glutamate released in the slice in the absence of perfusion. Under these conditions, stable responses could be elicited in Purkinje cells, with activation of AMPA/KA currents followed by the mGluR current. The mGluR currents observed were much larger than those recorded with PF stimulation because they are evoked in the whole dendritic tree.
Figure 4. The mGluR current elicited by flash photorelease of glutamate is also facilitated by AMPAR inhibition.
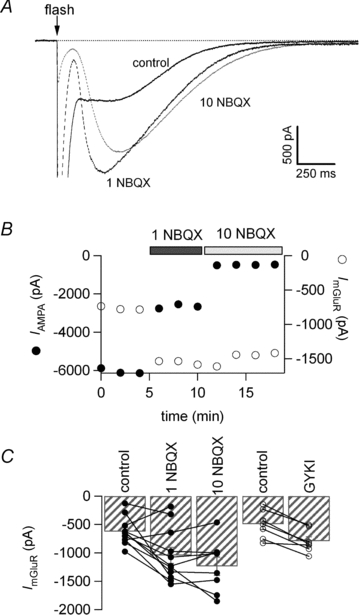
A, flash photolysis of MNI-glutamate at the time indicated by the arrow activated a fast AMPA/KA current and a slow mGluR current (control trace in black, average of 3 traces). Upon partial block of the AMPA response by 1 μm NBQX (dotted black trace, average of 3 traces) the peak mGluR current increased from −807 pA to −1624 pA. Further block of the AMPA current following addition of 10 μm NBQX (dotted grey trace, average of 4 traces) failed to potentiate the mGluR amplitude further. B, amplitude plot of the AMPA/KA current and mGluR current. C, bar graph of the average amplitude ±s.e.m. for the mGluR current and individual data points. Both NBQX and GYKI increased the amplitude of the current significantly. Wilcoxon signed ranks test, P < 0.01 and P < 0.02, respectively.
Figure 4A shows the current recorded after release of approximately 70 μm l-glutamate in a 1 ms flash in the absence (control) and the presence of 1 μm or 10 μm NBQX. The amplitude of the early, rapidly decaying current was reduced in 1 μm NBQX due to block of AMPA/KA receptors, and the amplitude of the late current simultaneously increased. The peak of the late current seen in NBQX was sufficiently delayed with respect to the early current for it to be readily distinguished. Figure 4B shows how the amplitudes of the early NBQX-sensitive AMPA receptor-mediated currents and of the late mGluR-mediated currents evolved with time. In this example the amplitude of the mGluR1-mediated current was −0.81 nA in the absence of NBQX. The amplitude increased to −1.62 nA as 1 μm NBQX was applied and declined slightly during the continued presence of 10 μm NBQX. As reported in previous studies (Canepari et al. 2001; Canepari & Ogden, 2003, 2006) the slow component of current evoked by photolytic glutamate release was blocked by the mGluR1 antagonist CPCCOEt (100 μm; n = 3) and the non-selective group I and II mGluR antagonist RS-MCPG (1 mm, n = 5).
On average, the peak mGluR1-mediated current was −626 ± 76 pA in control (n = 11 cells), −1044 ± 141 pA in 1 μm NBQX (n = 11; P < 0.01) and −1237 ± 162 pA in 10 μm NBQX (n = 8; P < 0.01; see Fig. 4C). A similar increase was seen with the AMPAR selective antagonist GYKI 53655. The mGluR current was −496 ± 95 pA (n = 7) in control and −793 ± 77 pA (n = 7) in 5 or 10 μm GYKI 53655. Data are summarised in Fig. 4C.
Photolytic glutamate release induced very large AMPA currents in the dendrites of Purkinje cells and gave rise to dendritic calcium spikes. The possibility exists that the amplitude of the mGluR slow current was underestimated because of a Ca2+-activated K+ outward current arising from dendritic Ca2+ influx during the calcium spikes produced by AMPAR activation. This was tested by experiments done in the presence of CPCCOEt. Application of CPCCOEt (100 μm) in the absence of NBQX blocked the mGluR current but did not uncover a contaminating outward K+ current that might explain the effect of AMPAR inhibition. Furthermore, the photolysis records show that a proportion of the current attributed to mGluR1 in the absence of AMPAR antagonists is contaminated with a component of AMPAR current, leading to an underestimation of the mGluR1 potentiation attributed to AMPAR block.
The experiments described above with photolytic release of l-glutamate indicate that the facilitation of the mGluR1 current seen with AMPA receptor antagonists at the PF–Purkinje cell synapse is a postsynaptic phenomenon, involving cross-talk between AMPARs and the mGluR signalling pathway.
Inhibition of src-family tyrosine kinases
Specific inhibitors have previously been used to investigate the signalling pathway initiated by activation of mGluR1 by photolytic glutamate release (Canepari & Ogden, 2003, 2006). These experiments showed that inhibition of protein tyrosine phosphatase inhibited the mGluR1 current and inhibition of tyrosine kinase potentiated submaximal mGluR1 currents. The known selectivity of the most active inhibitory ligands indicated that src-family tyrosine kinase and tyrosine phosphatase are a regulatory pathway. Furthermore, it has been reported that GluR2 AMPAR activation in Purkinje cells activates an associated Lyn tyrosine kinase by a metabotropic mechanism that does not require channel permeation (Hayashi et al. 1999). Consequently, a src-family protein tyrosine kinase (PTK) inhibitor, PP1, and a protein tyrosine phosphatase inhibitor, bpv(Phen), were used here to test the involvement of tyrosine phosphorylation/dephosphorylation in the regulation of the mGluR1 current by AMPARs in Purkinje cells.
Addition of 10 μm PP1 in the patch pipette produced a potentiation of the sEPSC compared with control recordings. PP1 was dissolved initially in DMSO which was present at a final concentration of 0.1% v/v. This concentration of DMSO was present in interleaved control recordings. With PP1 in the recording pipette, the sEPSC was −58.45 ± 9.4 pA (n = 9 cells and measured at 20 min whole cell recording). On addition of 1 μm NBQX there was a non-significant increase to −63.3 ± 15.1 pA and subsequently a decline to −41.8 ± 12.5 pA after 20 min in NBQX (n = 9 cells). In interleaved controls, infusion of 0.1% DMSO alone in the patch pipette had no effect on the initial mGluR sEPSC amplitude and furthermore the potentiating effect of adding 1 μm NBQX was seen as described earlier (see Fig. 5A, B and E). On average, the mGluR sEPSC was −12.2 ± 5.8 pA in control, −42.1 ± 11.3 pA in 1 μm NBQX (at the beginning of AMPA block) and −31.0 ± 8.2 pA at 20 min NBQX (n = 7 cells). Figure 5A and C present averages of five (control) and nine (PP1) cells over time in whole cell. Two cells were excluded from the control average as they showed no mGluR sEPSC under either set of conditions and could not be included in the normalisation of data.
Figure 5. The src-family tyrosine kinase inhibitor PP1 occludes the effect of AMPAR inhibition.
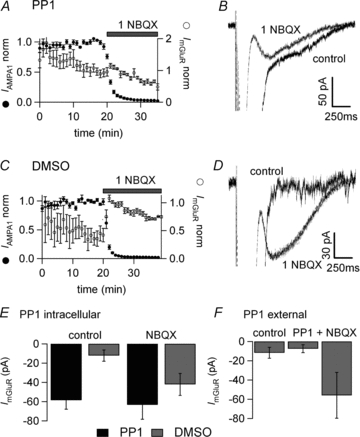
A and B, 10 μm PP1 (+0.1% DMSO) was added to the internal solution and allowed to diffuse for 20 min into the postsynaptic Purkinje cell via the patch pipette. C and D, interleaved control experiments with 0.1% DMSO added to the internal solution. A and C, average amplitude plot of first AMPA EPSC and mGluR EPSC normalised to the response at the beginning of AMPA block. A, with PP1, there is no significant increase of the sEPSC amplitude following NBQX application. C, with DMSO only, there is a large increase of the mGluR sEPSC following NBQX application. B and D show average traces with no significant increase when PP1 is infused into the cell and a large increase for the DMSO-only experiment. E, bar graph of average amplitude ±s.e.m. of mGluR sEPSC for cells recorded with PP1 supplemented internal (black) and control with DMSO (grey). Control values were taken at 20 min whole cell recording and NBQX values at the peak of the effect. With PP1 the control value was increased and there was no further effect of NBQX whereas in interleaved DMSO recordings there was a potentiation of the sEPSC. The increased sEPSC in PP1 was significantly different to DMSO controls, P < 0.01. NBQX significantly increased the sEPSC in DMSO control experiments (P < 0.05) but not with internal PP1. F, bar graph of average amplitude ±s.e.m. of mGluR sEPSC in control, during extracellular application of 10 μm PP1 and addition of 1 μm NBQX. Extracellular application of PP1 had no effect on the sEPSC.
The potentiation of the mGluR sEPSC by PP1 was apparent very quickly on establishing the whole cell recordings. For this reason the possibility that it was acting externally after ejection from the pipette was tested by applying PP1 extracellularly at 10 μm using a local application pipette or simple bath perfusion. No effect was seen on the mGluR sEPSC. The sEPSC was −11.8 ± 5.7 pA without PP1 (n = 9), −7.5 ± 4.2 pA in the presence of external PP1 (n = 11) and −55.9 ± 23.8 pA in 1 μm NBQX (n = 10) (Fig. 5F). This suggests that PP1 acts intracellularly as it diffuses into the postsynaptic Purkinje cell.
Thus, intracellularly applied tyrosine kinase inhibitor PP1 increased the amplitude of the mGluR sEPSC, mimicking the effect seen when AMPARs were blocked. Furthermore, PP1 also occluded the enhancing effect of NBQX block of AMPAR on the mGluR1 sEPSC. These results suggest that activation of synaptic AMPARs inhibits the mGluR1 sEPSC via the postsynaptic activation of a protein tyrosine kinase. The requirement for postsynaptic intracellular application of PP1 and the ineffectiveness of extracellular application also indicates that PTK signalling from AMPARs to mGluR1 is postsynaptic.
The tyrosine phosphatase inhibitor bpv(Phen) (Posner et al. 1994) applied at 100 μm to the bath inhibited the mGluR sEPSC, as previously reported for photolytically released l-glutamate (Canepari & Ogden, 2003; 2006;). Data averaged from nine cells showed a mGluR sEPSC of −31.1 ± 7.9 pA in control, −77.6 ± 14.7 pA in 1 μm NBQX or 10 μm GYKI, and −17.9 ± 6.2 pA after 10 min in 100 μm bpv(Phen), 20.8 ± 5.3% of the value in NBQX or GYKI (n = 9 cells). (The data can be found in Supplemental Fig. S1, available online only.) The possibility that bpv(Phen) was acting presynaptically in these experiments was tested. In the absence of AMPAR antagonist the AMPA EPSC was 98.3 ± 7.2% (n = 4) of the control value after 10 min application of 100 μm bpv(Phen), indicating that bpv(Phen) did not affect presynaptic release and was acting postsynaptically.
Discussion
We have shown in cerebellar Purkinje cells that AMPAR inhibition facilitates the mGluR1 sEPSC. This regulation does not involve either kainate (KA) or NMDA receptors, being specific to AMPAR activation. It is also observed when receptors are activated by photorelease of glutamate, indicating that it is a postsynaptic phenomenon. Finally, it is occluded by inhibition of src-family protein tyrosine kinases by PP1 applied intracellularly and blocked by the tyrosine phosphatase inhibitor bpv(Phen). This suggests that AMPARs can regulate the mGluR1 pathway postsynaptically via protein tyrosine phosphorylation.
The inhibition of mGluR1 signalling described here can be compared with other reports of synaptically induced mGluR1 regulation. Batchelor & Garthwaite (1997) described a facilitation of the PF sEPSP by climbing fibre (CF) stimulation. The facilitation lasted for about 2 min and the effect of CF stimulation was mimicked by depolarization sufficient to fire Ca2+ spikes or by photorelease of Ca2+ from the caged Ca2+ chelator NPE-EGTA. This differs from the present observations in enhancement rather than depression of mGluR1 by prior AMPAR activation (with CF in their case), the long duration of the effect, and that the experiments were done with low NBQX concentrations present throughout. In contrast to the results of Batchelor and Garthwaite, a study by Jin et al. (2007) showed a long-lasting irreversible depression of the mGluR1 sEPSC following repeated burst stimulation of CF or by strong depolarization (5 s at 0 mV), the latter with NBQX present. The results differ from those reported here in not being observed with PF burst stimulation and were irreversible. The present observations show a different phenomenon, an inhibition of mGluR1 signalling that is prevented by block of AMPA receptors and is readily reversible.
The possible involvement of presynaptic AMPA/KA receptors may also be considered. Presynaptic KA receptors regulate release at PF synapses, facilitating release onto Purkinje cells during bursts (Delaney & Jahr, 2002). In their study Delaney & Jahr (2002) showed that application of NBQX at 10 μm decreased release. Here we show that low concentrations of NBQX are sufficient to enhance the mGluR sEPSC, and the similar action of the specific AMPAR antagonist GYKI 53655 indicates that the effect here is mediated by AMPARs. Further, we show that it is not a presynaptic effect. It is blocked by postsynaptic infusion of PP1 and seen with direct activation of postsynaptic AMPAR by flash photolysis. The effect of inhibiting postsynaptic AMPARs we describe here and that of inhibiting presynaptic KARs described by Delaney & Jahr (2002) are therefore clearly distinguishable.
The possibility that potentiation by AMPA receptor block might be indirect should be considered. AMPA receptor activation will have produced dendritic depolarization of the dendrites and subthreshold Ca2+ entry because of the poor space clamp even in the absence of Ca2+ spiking. High intracellular Ca2+ has been shown to increase the amplitude of the sEPSC (Batchelor & Garthwaite, 1997; Dzubay & Otis, 2002) and sequestering Ca2+ with high intracellular BAPTA concentrations blocks the sEPSC (Dzubay & Otis, 2002; Hildebrandt et al. 2009). Reducing the activation of AMPA receptors is expected to decrease Ca2+ entry and therefore the mGluR1 sEPSC by this mechanism, opposite to the observations reported here.
An earlier systematic pharmacological dissection of the coupling of mGluR1 activation to the sEPSC showed evidence of regulation only by protein tyrosine kinase and phosphatase (PTK/PTP) and G-protein, serine/threonine kinase inhibition showing no effect (Canepari & Ogden, 2003). Evidence of metabotropic actions of AMPA receptors themselves has been shown for the activation of the G-protein Gi (GluR1; Wang et al. 1997) and the activation of the src-family non-receptor PTK Lyn (Hayashi et al. 1999). Lyn was shown to co-precipitate with GluR2 in cerebellar Purkinje cells and to be activated by high but not by low concentrations of the agonist AMPA (Hayashi et al. 1999). These observations were made in tissue culture, necessitating long exposures to AMPA. Here, in contrast, we have shown that the potentiation of mGluR currents by block of AMPA receptors can be seen with brief exposure to synaptically released l-glutamate. If the effect is mediated by Lyn this indicates that the kinase can be activated by a much shorter activation of AMPA receptors, on the time scale of synaptic transmission. The ability of tyrosine phosphatase inhibition to suppress the sEPSC, the PLC-mediated Ca2+ release and the enhancement of T-type Ca2+ channels suggests that the target of tyrosine phosphorylation is mGluR1 or the G-protein Gq.
The increase in amplitude of the mGluR1 current and the block of the AMPAR current occur in parallel, within the time of the response to each synaptic burst stimulation. No delay was seen in the development of the mGluR current. The inhibitory effect of AMPAR activation therefore takes place before the observed rise of the mGluR sEPSC, i.e. within about 250 ms.
Implications for Ca2+ signalling
mGluR1 receptors in Purkinje cells have been shown to act through three pathways that are influenced by PTK/PTP. All three pathways may alter excitability and intracellular Ca2+ concentrations. These are as follows. First, the sEPSC described here is partly carried by Ca2+ ions and has been shown to contribute to a slow Ca2+ rise (Canepari et al. 2004). Second, Ca2+ release from intracellular stores mediated by PLC generates a transient Ca2+ concentration that activates an inhibitory Ca2+-dependent ‘BK’ K+ conductance (Canepari & Ogden, 2006). Although activated at an approximately 2-fold lower l-glutamate concentration than the sEPSC, this pathway additionally requires priming by Ca2+ influx in P/Q channels and its contribution may be modified by local activity. Furthermore, it is activated more quickly than the sEPSC, with a delay of 100 ms, and we do not know whether the regulation by AMPA receptors is fast enough in this case. Because of the shorter delay it is difficult to separate the transient K+ current from the sEPSC in electrical recordings; however, this might be achieved with Ca2+ indicators in future experiments. Finally, Hildebrand et al. (2009) have shown that mGluR1 activation potentiates T-type Ca2+ channels in Purkinje cells and that this is also potentiated by tyrosine kinase inhibitors. All three PTK/PTP-regulated pathways will be expected to result in an increase of intracellular Ca2+ on reducing AMPA receptor activation, although the effect on the Ca2+ release pathway is less predictable because of the requirement for priming by Ca2+ influx.
Functional implications
Glutamate receptor antagonists have been used to better visualise and isolate the mGluR sEPSC. In fact, all studies of the mGluR current using synaptic stimulation that we have found in the literature have been done in the presence of AMPAR antagonists. The present work shows that the mGluR1 pathway is facilitated in these conditions and that we have not fully understood the conditions required for physiological activation of the mGluR1 sEPSC.
Immunogold labelling shows mGluR1 localised at the edge and AMPA receptors centrally at PF–Purkinje cell synapses (Baude et al. 1993). The inhibition of mGluR1 signalling by AMPAR activation will be greatest when the synapse operates with glutamate at high concentration released directly from the presynaptic terminal. On the other hand, if glutamate diffuses from nearby synapses or from ectopic release sites (Matsui & Jahr, 2005), it will reach the mGluR1 first and the AMPARs at a lower concentration. In this configuration the inhibitory cascade would be less active. Thus, the gain of the mGluR1 signal will be set by activation of local AMPARs: it will be high for glutamate diffusing from nearby synapses which encounters first the mGluRs, and low for glutamate released at the same synapse which first rapidly activates the AMPARs. This type of regulation would allow the synapse to distinguish between local or spillover glutamate. Indeed, similar concentrations reached by local release or spillover do not have the same implications for the synapse. Locally, this might correspond to limited release, whereas the same concentration for spillover glutamate might correspond to massive remote synaptic release.
Much evidence shows that mGluR1s are required for motor learning and for long term depression (LTD) at PF synapses (Aiba et al. 1994; Conguet et al. 1994; Coesmans et al. 2003). However, it is less clear that they are essential for the induction of experimental LTD (Biderot et al. 2009). LTD is thought to result from net loss of AMPA receptors by enhanced endocytosis from PF synapses (Wang & Linden, 2000). Once LTD is induced, thereby reducing the postsynaptic activation of AMPARs, mGluR1 signalling will be selectively enhanced. As a potential correlate, Isope & Barbour (2002) showed that in the adult rat, the fraction of silent synapses between PF and Purkinje neuron is very high (approximately 85%). Both at depressed synapses and silent synapses, mGluR1 signalling is expected to be more prominent.
Acknowledgments
We thank Alain Marty, Isabel Llano, Jacsue Kehoe and Philippe Ascher for discussion and comments on the manuscript. This work was supported by the Centre National de la Recherche Scientifique, the Agence Nationale pour la Recherche, the Fondation pour la Recherche Medicale, and EU (PHOTOLYSIS-LSHM-CT-2007-037765).
Glossary
Abbreviations
- AMPAR
AMPA receptor
- bpv(Phen)
a protein tyrosine phosphatase inhibitor
- d-AP5
d-(–)-2-amino-5-phosphonopentanoic acid
- 7-Cl-Kyn
7-chlorokinurenic acid
- CPCCOEt
an mGluR1 antagonist
- γ-DGG
γ-d-glutamylglycine
- GYKI 53655
an AMPA receptor antagonist
- KAR
kainate receptor
- LTD
long-term depression
- mGluR1
metabotropic glutamate receptor type 1
- MNI-glutamate
4-methoxy-7-nitroindolinyl-l-glutamate
- NBQX
an AMPA/KA receptor antagonist
- NPE-HPTS
2-nitrophenylethyl ether of pyranine
- PF
parallel fibre
- PP1
an src-family protein tyrosine kinase inhibitor
- PTK
protein tyrosine kinase
- sEPSC
slow EPSC
Author contributions
Both authors contributed to the conception and design of the experiments, drafting and revising the article and approved the final version to be published. The analysis and interpretation of the data were done by C.A. The whole study was performed at the Laboratoire de Physiologie cérébrale, Université Paris Descartes.
Supplemental material
Figure S1
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors
References
- Aiba A, Kano M, Chen C, Stanton ME, Fox GD, Herrup K, Zwingman TA, Tonegawa S. Deficient cerebellar long-term depression and impaired motor learning in mGluR1 mutant mice. Cell. 1994;79:377–388. [PubMed] [Google Scholar]
- Batchelor AM, Garthwaite J. Novel synaptic potentials in cerebellar Purkinje cells: probable mediation by metabotropic glutamate receptors. Neuropharmacology. 1993;32:11–20. doi: 10.1016/0028-3908(93)90124-l. [DOI] [PubMed] [Google Scholar]
- Batchelor AM, Garthwaite J. Frequency detection and temporally dispersed synaptic signal associated through a metabotropic glutamate receptor pathway. Nature. 1997;385:74–77. doi: 10.1038/385074a0. [DOI] [PubMed] [Google Scholar]
- Batchelor AM, Madge DJ, Garthwaite J. Synaptic activation of metabotropic glutamate receptors in the parallel fibre-Purkinje cell pathway in rat cerebellar slices. Neuroscience. 1994;63:911–915. doi: 10.1016/0306-4522(94)90558-4. [DOI] [PubMed] [Google Scholar]
- Baude A, Nusser Z, Roberts JDB, Mulvihill E, Mcllhinney RAJ, Somogyi P. The metabotropic glutamate receptor (mGluR1α) is concentrated at perisynaptic membrane of neuronal subpopulations as detected by immunogold reaction. Neuron. 1993;11:771–787. doi: 10.1016/0896-6273(93)90086-7. [DOI] [PubMed] [Google Scholar]
- Bidoret C, Ayon A, Barbour B, Casado M. Presynaptic NR2A-containing NMDA receptors implement a high-pass filter synaptic plasticity rule. Proc Natl Acad Sci U S A. 2009;106:14126–14131. doi: 10.1073/pnas.0904284106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasnjo G, Otis TS. Neuronal glutamate transporters control activation of postsynaptic metabotropic glutamate receptors and influence cerebellar long-term depression. Neuron. 2001;31:607–616. doi: 10.1016/s0896-6273(01)00377-4. [DOI] [PubMed] [Google Scholar]
- Brunel N, Hakim V, Isope P, Nadal JP, Barbour B. Optimal information storage and the distribution of synaptic weights: perceptron versus Purkinje cell. Neuron. 2004;43:745–757. doi: 10.1016/j.neuron.2004.08.023. [DOI] [PubMed] [Google Scholar]
- Bureau I, Bischoff S, Heinemann SF, Mulle C. Kainate receptor-mediated responses in the CA1 field of wild-type and GluR6-deficient mice. J Neurosci. 1999;19:653–663. doi: 10.1523/JNEUROSCI.19-02-00653.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canepari M, Auger C, Ogden D. Ca2+ion permeability and single-channel properties of the metabotropic slow EPSC of rat Purkinje neurons. J Neurosci. 2004;24:3563–3573. doi: 10.1523/JNEUROSCI.5374-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canepari M, Ogden D. Evidence for protein tyrosine phosphatase, tyrosine kinase, and G-protein regulation of the parallel fiber metabotropic slow EPSC of rat cerebellar Purkinje neurons. J Neurosci. 2003;23:4066–4071. doi: 10.1523/JNEUROSCI.23-10-04066.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canepari M, Ogden D. Kinetic, pharmacological and activity-dependent separation of two Ca2+ signalling pathways mediated by type 1 metabotropic glutamate receptors in rat Purkinje neurones. J Physiol. 2006;573:65–82. doi: 10.1113/jphysiol.2005.103770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canepari M, Papageorgiou G, Corrie JET, Watkins C, Ogden D. The conductance underlying the parallel fibre slow EPSP in rat cerebellar Purkinje neurones studied with photolytic release of l-glutamate. J Physiol. 2001;533:765–772. doi: 10.1111/j.1469-7793.2001.00765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coesmans M, Smitt PA, Linden DJ, Shigemoto R, Hirano T, Yamakawa Y, van Alphen AM, Luo C, Van Der Geest JN, Kros JM, Gaillard CA, Frens MA, de Zeeuw CI. Mechanisms underlying cerebellar motor deficits due to mGluR1-autoantibodies. Ann Neurol. 2003;53:325–336. doi: 10.1002/ana.10451. [DOI] [PubMed] [Google Scholar]
- Conquet F, Bashir ZI, Davies CH, Daniel H, Ferraguti F, Bordi F, Franz-Bacon K, Reggiani A, Matarese V, Condé F, Collingridge GL, Crépel F. Motor deficit and impairment of synaptic plasticity in mice lacking mGluR1. Nature. 1994;372:237–243. doi: 10.1038/372237a0. [DOI] [PubMed] [Google Scholar]
- Delaney AJ, Jahr CE. Kainate receptors differentially regulate release at two parallel fiber synapses. Neuron. 2002;36:475–482. doi: 10.1016/s0896-6273(02)01008-5. [DOI] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2008;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzubay JA, Otis TS. Climbing fiber action of metabotropic glutamate receptors on cerebellar Purkinje neurons. Neuron. 2002;36:1159–1167. doi: 10.1016/s0896-6273(02)01052-8. [DOI] [PubMed] [Google Scholar]
- Galante M, Diana M. Group I metabotropic glutamate receptors inhibit GABA release at interneuron-Purkinje cell synapses through endocannabinoid production. J Neurosci. 2004;24:4865–4874. doi: 10.1523/JNEUROSCI.0403-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann J, Blum R, Kovalchuk Y, Adelsberger H, Kuner R, Durand GM, Miyata M, Kano M, Offermanns S, Konnerth A. Distinct roles of Gαq and G11 for Purkinje cell signalling and motor behavior. J Neurosci. 2004;24:5119–5130. doi: 10.1523/JNEUROSCI.4193-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann J, Dragicevic E, Adelsberger H, Henning HA, Sumser M, Abramowitz J, Blum R, Dietrich A, Freichel M, Flockerzi V, Birnbaumer L, Konnerth A. TRPC3 channels are required for synaptic transmission and motor coordination. Neuron. 2008;59:392–398. doi: 10.1016/j.neuron.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häusser M, Roth A. Dendritic and somatic glutamate receptor channels in rat cerebellar Purkinje cells. J Physiol. 1997;501:77–95. doi: 10.1111/j.1469-7793.1997.077bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Umemori H, Mishina M, Yamamoto T. The AMPA receptor interacts with and signals through the protein tyrosine kinase Lyn. Nature. 1999;397:72–76. doi: 10.1038/16269. [DOI] [PubMed] [Google Scholar]
- Hildebrand ME, Isope P, Miyazaki T, Nakaya T, Garcia E, Feltz A, Schneider T, Hescheler J, Kano M, Sakimura K, Watanabe M, Dieudonné S, Snutch TP. Functional coupling between mGluR1 and Cav3.1 T-type calcium channels contributes to parallel fibre-induced fast calcium signaling within Purkinje cell dendritic spines. J Neurosci. 2009;29:9668–9682. doi: 10.1523/JNEUROSCI.0362-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirono M, Konishi S, Yoshioka T. Phospholipase C-independent group I metabotropic glutamate receptor-mediated inward current in mouse Purkinje cells. Biochem Biophys Res Commun. 1998;251:753–758. doi: 10.1006/bbrc.1998.9465. [DOI] [PubMed] [Google Scholar]
- Holmgren CD, Mukhtarov M, Rheims S, Malkov AE, Popova IY, Khalilov I, Zilberter T, Bregestovski P, Zilberter Y. Energy substrate availability as a determinant of neuronal resting potential, GABA signaling and spontaneous network activity in the neonatal cortex. J Neurochem. 2009;112:900–912. doi: 10.1111/j.1471-4159.2009.06506.x. [DOI] [PubMed] [Google Scholar]
- Isope P, Barbour B. Properties of unitary granule cell→Purkinje cell synapses in adult rat cerebellar slices. J Neurosci. 2002;22:9668–9678. doi: 10.1523/JNEUROSCI.22-22-09668.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Kim SJ, Kim J, Worley PF, Linden DJ. Long-term depression of mGluR1 signalling. Neuron. 2007;55:277–287. doi: 10.1016/j.neuron.2007.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Kim YS, Yuan JP, Petralia RS, Worley PF, Linden DJ. Activation of the TRPC1 cation channel by metabotropic glutamate receptor mGluR1. Nature. 2003;426:285–291. doi: 10.1038/nature02162. [DOI] [PubMed] [Google Scholar]
- Maejima T, Oka S, Hashimotodani Y, Ohno-Shosaku T, Aiba A, Wu D, Waku K, Sugiura T, Kano M. Synaptically driven endocannabinoid release requires Ca2+-assisted metabotropic glutamate receptor subtype 1 to phospholipase Cβ4 signaling cascade in the cerebellum. J Neurosci. 2005;25:6826–6835. doi: 10.1523/JNEUROSCI.0945-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcaggi P, Attwell D. Endocannabinoid signalling depends on the spatial pattern of synapse activation. Nat Neurosci. 2005;8:1–6. doi: 10.1038/nn1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui K, Jahr CE. Ectopic release of synaptic vesicles. Neuron. 2005;40:1173–1183. doi: 10.1016/s0896-6273(03)00788-8. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Mulvihill E, Streit P, Somogyi P. Subsynaptic segregation of metabotropic and ionotropic glutamate receptors as revealed by immunogold localization. Neuroscience. 1994;61:421–427. doi: 10.1016/0306-4522(94)90421-9. [DOI] [PubMed] [Google Scholar]
- Paternain AV, Morales M, Lerma J. Selective antagonism of AMPA receptors unmasks kainate receptor-mediated responses in hippocampal neurons. Neuron. 1995;14:185–189. doi: 10.1016/0896-6273(95)90253-8. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Zhao H-M, Wang Y-X, Wenthold RJ. Variations in the tangential distribution of postsynaptic glutamate receptors in Purkinje cell parallel and climbing fiber synapses during development. Neuropharmacology. 1998;37:1321–1334. doi: 10.1016/s0028-3908(98)00118-x. [DOI] [PubMed] [Google Scholar]
- Posner BI, Faure R, Burgess JW, Bevan AP, Lachance D, Zhang-Sun G, Fantus G, Ng JB, Hall DA, Lum BS, Shaver A. Peroxovanadium compounds. J Biol Chem. 1994;269:4596–4604. [PubMed] [Google Scholar]
- Reichelt W, Knopfel T. Glutamate uptake controls the expression of a slow postsynaptic current mediated by mGluRs in cerebellar Purkinje cells. J Neurophysiol. 2002;87:1974–1980. doi: 10.1152/jn.00704.2001. [DOI] [PubMed] [Google Scholar]
- Tempia F, Alojado ME, Strata P, Knöpfel T. Characterization of the mGluR1-mediated electrical and calcium signaling in Purkinje cells of mouse cerebellar slices. J Neurophysiol. 2001;86:1389–1397. doi: 10.1152/jn.2001.86.3.1389. [DOI] [PubMed] [Google Scholar]
- Tempia F, Miniaci MC, Anchisi D, Strata P. Postsynaptic current mediated by metabotropic glutamate receptors in cerebellar Purkinje cells. J Neurophysiol. 1998;80:520–528. doi: 10.1152/jn.1998.80.2.520. [DOI] [PubMed] [Google Scholar]
- Trigo FF, Corrie JE, Ogden D. Laser photolysis of caged compounds at 405 nm: photochemical advantages, localisation, phototoxicity and methods for calibration. J Neurosci Methods. 2009;180:9–21. doi: 10.1016/j.jneumeth.2009.01.032. [DOI] [PubMed] [Google Scholar]
- Wadiche JI, Jahr CE. Patterned expression of Purkinje cell glutamate transporters controls synaptic plasticity. Nat Neurosci. 2005;8:1329–1334. doi: 10.1038/nn1539. [DOI] [PubMed] [Google Scholar]
- Wang Y, Small DL, Stanimirovic DB, Morley P, Durkin JP. AMPA receptor-mediated regulation of a Gi-protein in cortical neurons. Nature. 1997;389:502–504. doi: 10.1038/39062. [DOI] [PubMed] [Google Scholar]
- Wang YT, Linden DJ. Expression of cerebellar long-term depression requires postsynaptic clathrin-mediated endocytosis. Neuron. 2000;25:635–647. doi: 10.1016/s0896-6273(00)81066-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


