Abstract
Physical activity enhances hippocampal function but its effects on neuronal structure remain relatively unexplored outside of the dentate gyrus. Using Golgi impregnation and the lipophilic tracer DiI, we show that long-term voluntary running increases the density of dendritic spines in the entorhinal cortex and hippocampus of adult rats. Exercise was associated with increased dendritic spine density not only in granule neurons of the dentate gyrus, but also in CA1 pyramidal neurons, and in layer III pyramidal neurons of the entorhinal cortex. In the CA1 region, changes in dendritic spine density are accompanied by changes in dendritic arborization and alterations in the morphology of individual spines. These findings suggest that physical activity exerts pervasive effects on neuronal morphology in the hippocampus and one of its afferent populations. These structural changes may contribute to running-induced changes in cognitive function.
Keywords: dendritic spine, dentate gyrus, physical exercise, CA1 pyramidal cell, plasticity
Introduction
In humans, exercise is generally believed to enhance learning and memory and delay cognitive decline associated with aging (Colcombe et al., 2004; Lautenschlager and Almeida, 2006). Studies in rodents have also demonstrated that voluntary running improves performance on hippocampus- dependent learning tasks, such as spatial navigation learning and contextual fear conditioning (van Praag et al., 1999; Anderson et al., 2000; Baruch et al., 2004; van Hoomisen et al., 2004; Burghard et al., 2006). These findings suggest that physical activity augments hippocampal function, but the mechanisms which underlie these effects remain to be determined.
The effects of running on neuronal plasticity in the dentate gyrus of the hippocampus have been well-documented. Running has been shown to enhance adult neurogenesis (van Praag et al., 1999, Trejo et al., 2001; Stranahan et al., 2006) as well as to increase dendritic spine density on granule cells of the dentate gyrus (Eadie et al., 2005; Redila and Christie, 2006; Zhao et al., 2006). These effects are consistent with running-induced increases in dentate gyrus activity, indicated by increased expression of the immediate early gene c-fos in granule cells and increased blood flow to the dentate gyrus (Rhodes et al., 2003; Farmer et al., 2004; Pereira et al., 2007). In addition, running has been shown to increase dentate gyrus levels of BdNF, a growth factor associated with synaptic and structural plasticity (Vaynman et al., 2004; Rex et al., 2007).
Running influences other parts of the hippocampal circuitry but the effects in these regions are less well characterized than those of the dentate gyrus. For instance, running increases BdNF expression in the CA1 region (Neeper et al., 1996). Moreover, running increases c-fos expression in the CA1 region and the entorhinal cortex (Oladehin and Waters, 2001; Rhodes et al., 2003). While these results suggest running- induced alterations in a variety of locations along hippocampal circuitry, no studies to date have examined the effects of running on dendritic spine density in the CA fields or within the entorhinal cortex, a primary afferent to the hippocampus. Here we investigated the effects of long term running on a variety of structural measures in the hippocampus and entorhinal cortex. Adult male Sprague Dawley rats (∼ 250 g) were housed individually with (n = 9) or without (n = 8) a running wheel (Lafayette Instruments). Running distance was recorded daily from an optical counter (Supplementary Fig. 1). The animals received food and water ad libitum and the room was maintained on a 12-hr light-dark schedule (lights on at 7 A.M.). Two months after the start of the experiment, the animals were anesthetized with pentobarbital (100 mg/kg) and perfused transcardially with 4% paraformaldehyde in phosphate buffer containing 1.5% picric acid. All animal procedures and protocols were approved by the Princeton University IACUC and followed the NIH Guide for the Care and Use of Laboratory Animals.
Single-section Golgi impregnation was carried out as described in Kozorovitskiy et al. (2005). Golgi-impregnated neurons were selected for analysis if they were completely stained without truncated primary dendrites and located in sufficient isolation from neighboring stained cells to trace the dendritic segments back to their parent dendrite. Dendritic spines were sampled from the secondary and tertiary dendrites of each cell type. For dendritic spine density, 5 cells of each type per animal (n = 5–7 animals per cell type, 3 dendritic segments per cell) were averaged to give the mean spine density for each animal. We quantified dendritic arborization and spine density on pyramidal neurons located in layer III of entorhinal cortex—these cells were inferior to the rhinal sulcus on caudal sections (from bregma -5.60 mm to bregma -6.80 mm, approx.; Paxinos and Watson, 1998). Golgi-impregnated neurons were sampled from both the lateral and medial regions because the morphological and electrophysiological properties of layer III neurons are similar in these areas of the entorhinal cortex (Witter and Moser, 2006). Golgi-impregnated neurons in the CA1 region were sampled primarily from bregma -2.30 mm to bregma -6.30 mm. Granule cells were sampled from the suprapyramidal blade. Sampling of this cell type extended from the rostral portion of the granule cell layer (bregma -2.30) to more caudal regions (bregma -6.04). We also measured dendritic spine density on pyramidal neurons in layer II/III of primary visual cortex, sampled from bregma -5.80 mm to bregma -6.80 mm in a subset of animals (n 5 3–4). For dendritic length and branch point analyses, 5 Golgi-impregnated cells per animal were selected for each neuron type. Cells were traced using the 40× objective on an Olympus BX60 microscope with a motorized stage attached to a computer with the aid of StereoInvestigator software (Kozorovitskiy et al., 2005).
Crystals of the lipophilic tracer DiI (Molecular Probes) were inserted at multiple points in the temporal lobe to label neurons in a retrograde manner. Following insertion of crystals, brains were incubated for 3 weeks in PBS at 378, after which tissue blocks were sectioned on a vibratome and imaged as described for analysis of dendritic spine density (Kozorovitskiy et al., 2005). Layer III entorhinal cortex pyramidal cells, CA1 pyramidal cells, and dentate gyrus granule cells were selected for analysis using similar neuroanatomical criteria as described for the Golgi-impregnated tissue (n 5 6–8 animals per neuron type). Differences in the number of animals included in the analysis for each neuron type were the result of variability in the degree to which each brain had a sufficient number of adequately labeled neurons. For analysis of dendritic spine morphology, image stacks (28.0 × 28.0 × 0.5 μm3) were filtered using the lowpass and median functions in Zeiss LSM 510 software (see Supplementary Figs. 2A–B, for comparison of raw capture and filtered images). Image stacks were analyzed using the Autotrace feature in Reconstruct software (http://synapses.bu.edu). First, spines were identified based on the presence of a clearly resolved head and neck. Next, the spine was outlined based on the area of pixels with a grayscale intensity value >50. For analysis of spine length, the full extent of the tracing was determined manually, from the point of contact with the dendritic shaft to the tip of the spine head (Supplementary Fig. 2C). Because the spine head was generally brighter than the spine neck, we outlined the spine head using a brightness criterion of >75. Spine head area measurements were derived from the outlined contour (Supplementary Fig. 2D). We sampled 100 spines per neuron type, per animal, from secondary and tertiary dendrites on 3–5 cells per animal.
Measurements of dendritic shaft diameter were made on the same sets of images used for spine morphology analysis. The diameter of the dendritic shaft was approximated by determining the length of the scanned segment, then dividing the area of the suprathreshold region (pixel intensity >100, Supplementary Fig. 2E) by the length to determine the width. The width of multiple 2D traces taken from serial images along the Z-axis was then averaged to approximate the mean dendritic diameter, as described (Knafo et al., 2004). For all measures, data were compared across runners and controls using two-tailed unpaired student's t-tests using Graphpad Prism software. Relationships between running distance and dendritic spine density were explored using Pearson's correlations.
Running increased dendritic spine density on the basal dendrites of pyramidal neurons in layer III of entorhinal cortex (Golgi, t8 = 2.85, P = 0.02, DiI, t14 = 2.26, P = 0.04; Figs. 1 and 2). This occurred without changes in the length or complexity of dendrites in the basal arbor (total dendritic length, t8 = 1.09, P = 0.31; branch points, t8 = 1.24, P = 0.25). No changes in spine density or dendritic length and complexity were observed in the apical dendritic tree of Golgi-impregnated cells in this region (spine density, t8 = 0.41, P = 0.69; total dendritic length, t8 = 1.61, P = 0.15; branch points, t8 = 1.62, P = 0.14). Physical activity also enhanced the number of spines on dendrites of granule neurons in the dentate gyrus, in concurrence with previous reports (Golgi, t12 = 2.47, P = 0.029; DiI, t10 = 5.70, P =0.0002; Figs. 1 and 2; Eadie et al., 2005; Redila and Christie, 2006). In addition, running increased the length and complexity of the dendritic arbor of Golgi-impregnated granule neurons (total dendritic length, t12 = 2.44, P = 0.03; branch points, t12 = 4.82, P = 0.004).
FIGURE 1.
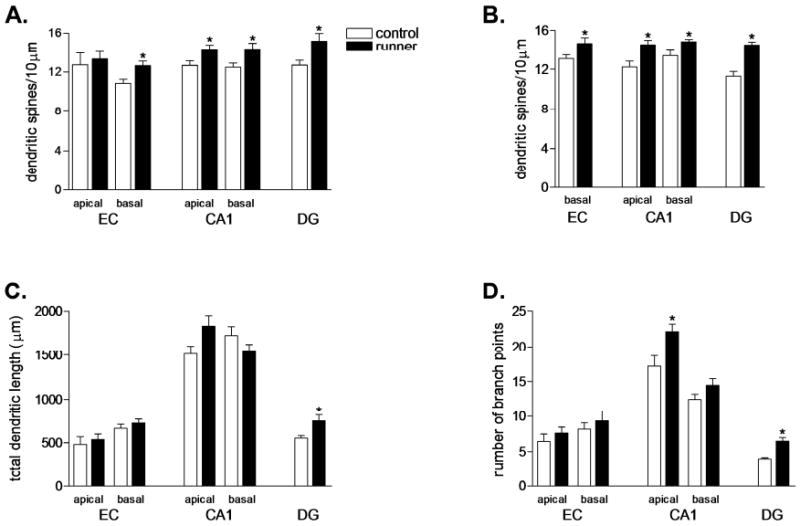
Physical activity increases dendritic spine density and alters dendritic complexity in multiple cell types. (A) Running increased the density of dendritic spines on the basal dendrites of Golgi-impregnated pyramidal cells in the entorhinal cortex (EC); increased spine density was also observed in the basal and apical dendritic trees of neurons in the hippocampal CA1 region. As previously reported (Eadie et al., 2005; Redila et al., 2006), running increased the density of dendritic spines on dentate gyrus granule neurons (DG). (B) We also used the lipophilic tracer DiI to visualize spines within the same group of animals, and observed similar results. DiI-labeled neurons in the brains of runners show increased spine density in the same regions where changes were observed with Golgi impregnation. (C) Running was associated with increased dendritic length in the DG. (D) Physical activity increased the number of branch points in the apical dendritic tree of CA1 pyramidal neurons, and in DG granule neurons. (*) indicates significance (P < 0.05) following 2-tailed unpaired student's t-test. EC, entorhinal cortex; CA1, hippocampal CA1 field; DG, dentate gyrus.
FIGURE 2.
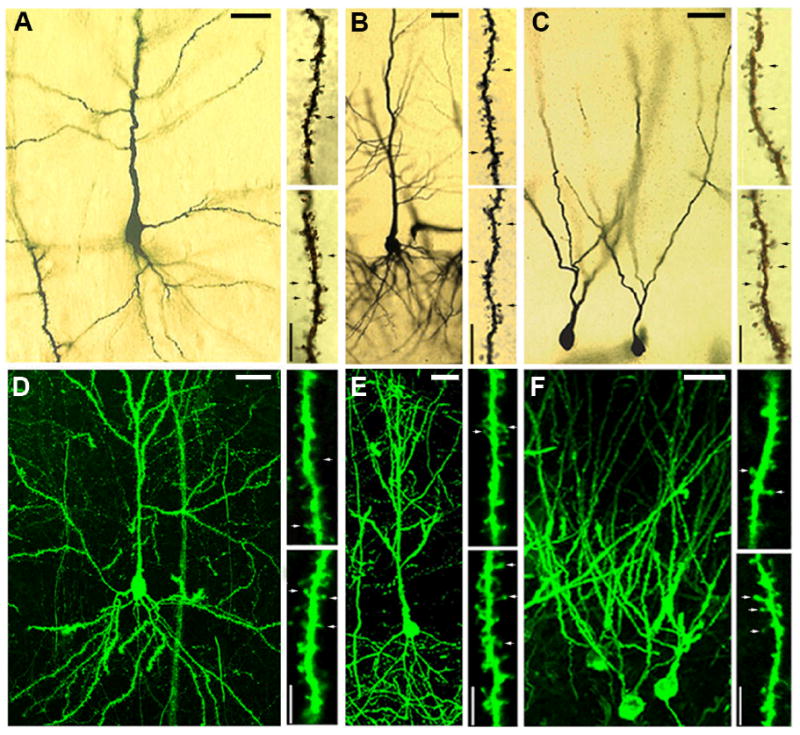
Golgi impregnated and DiI labeled neurons. (A) Golgi-impregnated pyramidal neuron in layer III of entorhinal cortex. (B) Golgi-impregnated pyramidal neuron in the CA1 sub-field of the hippocampus. (C) Golgi-impregnated granule neurons in the dentate gyrus of the hippocampus. (D) DiI-labeled pyramidal neuron in layer III of entorhinal cortex. (E) DiI-labeled pyramidal neuron in the CA1 subfield of the hippocampus. (F) DiIlabeled granule neurons in the dentate gyrus. For each cell type, the upper right panel shows a dendritic segment from a control animal, and the lower right micrograph shows a segment from a runner. Segments shown are from secondary dendrites for all cell types. For pyramidal neurons of the entorhinal cortex and CA1 region, the segments are from the basal dendritic tree. Arrows indicate dendritic spines. Scale bars shown with dendritic segments are 5 μm; scale bars shown with cells are 25 μm.
Additional running-induced changes were observed in the CA1 region of the hippocampus. Running increased the density of dendritic spines on the basal dendrites of pyramidal neurons in this area (Golgi, t10 = 2.61, P = 0.03; DiI, t9 = 2.68, P = 0.03). The basal dendrites of the CA1 pyramidal cells showed no change in dendritic length and branch point numbers with running (total dendritic length, t10 = 0.75, P = 0.46; branch points, t10 = 0.31, P = 0.76). The apical dendrites of CA1 pyramidal neurons also responded to exercise with increased dendritic spine density (Golgi, t10 = 2.24, P = 0.04; DiI, t9 = 3.303, P < 0.01). This change was accompanied by increased complexity, but not length, of the apical dendritic tree (branch points, t10 = 3.13, P = 0.01; total dendritic length, t10 = 1.28, P = 0.23). While it remains uncertain whether the effects of running on dendritic spines are specific to hippocampal circuitry, we observed no such change on the dendrites of pyramidal neurons in layer II/III of primary visual cortex (for the basal dendrites of Golgi-impregnated neurons, t4 = 1.15, P = 0.31; for the apical dendritic tree, t4 = 0.45, P = 0.67, Supplementary Fig. 3).
The length of dendritic spines on the basal dendrites of pyramidal neurons in the CA1 field increased with long term running (CA1, t10 = 2.85, P = 0.01, Fig. 3). Runners also exhibited a reduction in spine head area in this region, suggesting a possible increase in the number of filopodia (t10 = 3.12, P = 0.01, Fig. 3). Reduced spine head surface area was also observed on apical dendrites of CA1 pyramidal neurons, with no change in spine length (spine surface area, t10 = 5.33, P = 0.0003; spine length, t10 = 1.54, P = 0.15). No changes in dendritic spine length were observed on the other neuron types examined (EC pyramidal cells, t8 = 0.02, P = 0.97; DG granule cells, t10 = 1.67, P = 0.12). Similarly, no differences in the surface area of the spine head were noted on the other neuron types (EC pyramidal cells, t8 = 0.06, P = 0.95; DG granule cells, t10 = 1.36, P = 0.22). We observed no significant differences in the diameter of the dendritic shaft in any of the neuronal populations examined (EC basal, t8 = 0.01, P = 0.98; CA1 apical, t10 = 0.1852, P = 0.85; CA1 basal, t10 = 1.04, P = 0.32; DG, t10 = 0.22, P = 0.83; Supplementary Table 1). There was no detectable relationship between the average distance run and the density of dendritic spines in any of the regions examined (P > 0.05 for all correlations). Additionally, no correlations were observed between the percent change in running distance from Day 1 to Day 60 and the density of dendritic spines in any of the cell types examined.
FIGURE 3.
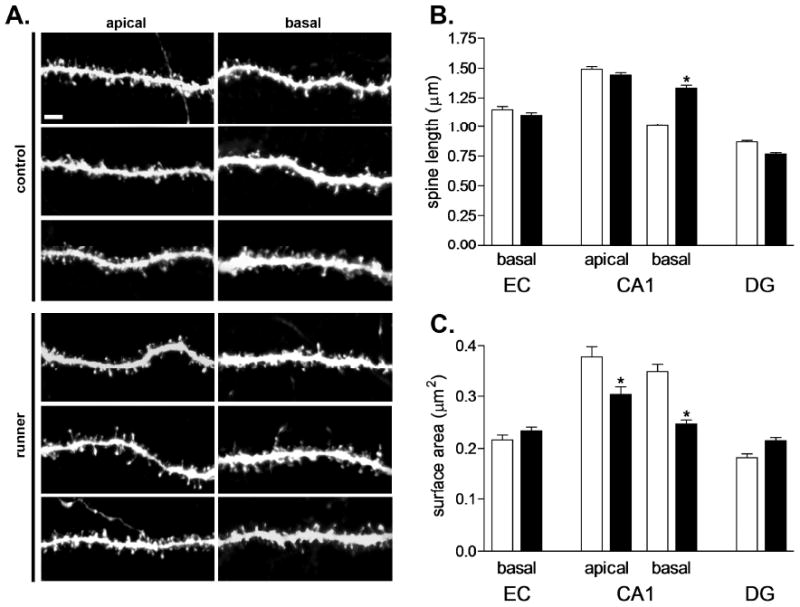
Running alters the morphology of individual dendritic spines in area CA1 of the rat hippocampus. (A) Dendritic segments from the apical and basal trees of CA1 pyramidal neurons in the hippocampus of control animals and runners. Scale bar = 2 μm. (B) Rats that ran for 60 days have longer dendritic spines on CA1 pyramidal cells relative to sedentary controls. No changes in spine length were observed on pyramidal neurons in the entorhinal cortex (EC) or on granule neurons of the dentate gyrus (DG). (C) Running is associated with reduced spine head area in CA1 pyramidal cells, suggestive of an increase in filopodia. No changes in spine head area were observed in other regions. (*) indicates significance (P < 0.05) following 2-tailed unpaired student's t-test.
These results demonstrate that running induces widespread changes in the structure of neurons in both the hippocampus and a major afferent region to the hippocampus, the entorhinal cortex. Two months of running increased dendritic spine density on pyramidal cells of the entorhinal cortex and the CA1 region, as well as on granule cells of the dentate gyrus. In area CA1, increased spine density was accompanied by an overall decrease in spine head diameter, and increase in spine length, suggestive of more filopodia-type spines. Dentate granule neurons and CA1 pyramidal cells also exhibited an increase in the complexity of the dendritic tree. The extent to which these effects are specific to hippocampal circuitry remains unknown—our results leave open the question of the degree of regional specificity in the effects of exercise on the brain.
In apparent contrast to our results, Zhao et al. (2006) reported an increase in the density of mushroom spines specifically on newly generated granule neurons in the dentate gyrus, whereas we found no such change. It should be emphasized, however, that the methods we used labeled a mixed population of newly generated neurons and developmentally generated neurons. If running has a greater influence on immature granule cells than it does on mature granule cells, such effects may be masked when both cell populations are combined.
The volitional nature and intensity of exercise could modulate effects on neuronal structure. Briones et al. (2005) reported no change in synaptic ultrastructure in the dentate molecular layer with exercise; however, this exercise protocol consisted of 5 minutes per day on a treadmill for 2 weeks, while our animals ran voluntarily in a wheel for 2 months. Given previous studies suggesting that there may be a time-dependent component for the effects of exercise on adult neurogenesis (Holmes et al., 2004; Stranahan et al., 2006), it is possible that long-term exercise might be necessary in order to increase the number of dendritic spines and synapses. Alternatively or in addition, the stress of involuntary treadmill running in combination with the exercise-induced elevation of adrenal steroid hormones could inhibit formation of new dendritic spines.
Our findings of increased dendritic spine density and enhanced dendritic complexity in the dentate gyrus are in concurrence with previous studies (Eadie et al., 2005; Redila and Christie, 2006). The finding that running enhances dendritic architecture in CA1 contrasts with a previous report by Faherty et al. (2003), which showed no change in dendritic complexity following 4–5 months of exercise, in both CA1 pyramidal cells and dentate granule neurons. One possibility is that the effects of exercise on neuronal structure in the hippocampus may be transient, with a homeostatic decrease in dendritic branching following the initial extension of new processes. Future studies will be needed to determine the time course of these effects.
The results of the present report demonstrating dendritic alterations in several neuronal populations of the hippocampal circuitry following running present the possibility that these structural changes may contribute to running-induced alterations in hippocampal function. Changes in dendritic spines and dendritic complexity on mature neurons, in combination with enhanced production of new granule cells in the dentate gyrus (van Praag et al., 1999), may contribute to the improvements in learning and memory performance on hippocampusdependent tasks (Leuner et al., 2003, 2006). However, it is important to note that the behavioral effects of exercise include improvements in performance on tasks which do not require the hippocampus, such as conditioned place preference and rotarod training (Buitrago et al., 2004; Eisenstein and Holmes, 2007). In humans, physical fitness is associated with improvements in a wide range of capabilities, including recall memory, reaction time and motor coordination (Baylor and Spirduso,1988; Colcombe et al., 2004; Lautenschlager and Almeida, 2006; Pereira et al, 2007). If the effects of exercise on behavior are not exclusive to hippocampus-dependent tasks, then it is possible that the effects of exercise on brain structure may also extend beyond the hippocampal circuitry.
Supplementary Material
Supplementary Figure 1. All animals given access to running wheels ran extensively.
Supplementary Figure 2. Image preparation and measurement of individual dendritic spines. (A), Raw-capture image from a single plane of the z-stack. (B), Same image after median and low-pass filtering in Zeiss LSM510 software. (C) Areas outlined in red show tracings of individual dendritic spines, defined by our criterion of pixel intensity >50, using Reconstruct software. The yellow lines demonstrate measurements of spine length, from the point of contact with the shaft to the tip of the spine. (D) Areas outlined in blue depict regions containing spine heads exceeding our criterion (pixel intensity >75). Surface area measurements were derived from the outlined contour. (E). The region shown in yellow shows the area where pixel intensity is >100, our threshold for measuring the dendritic shaft. Scalebar in (A) = 2μm.
Supplementary Figure 3. Running does not influence dendritic spine density in pyramidal neurons from layer II/III of visual cortex. There were no differences between runners and controls in dendritic spine density on Golgi-impregnated visual cortex pyramidal neurons, (sampled from bregma -5.80 mm to bregma -6.80 mm, defined with reference to Paxinos and Watson 1998).
Acknowledgments
The authors thank Dr. John C. Fiala for valuable communications regarding the Reconstruct measurement program. Authors also thank Dr. Benedetta Leuner, Dr. Erica R. Glasper, and Dr. Yevgenia Kozorovitskiy for comments on the manuscript.
Grant sponsors: National Institutes of Health (NIH), NRSA; Grant numbers: F31 AG024690-03, MH059740
References
- Anderson BJ, Rapp DN, Baek DH, McCloskey DP, Coburn-Litvak PS, Robinson JK. Exercise influences spatial learning in the radial arm maze. Physiol Behav. 2000;70:425–429. doi: 10.1016/s0031-9384(00)00282-1. [DOI] [PubMed] [Google Scholar]
- Baruch DE, Swain RA, Helmstetter FJ. Effects of exercise on Pavlovian fear conditioning. Behav Neurosci. 2004;118:1123–1127. doi: 10.1037/0735-7044.118.5.1123. [DOI] [PubMed] [Google Scholar]
- Baylor AM, Spirduso WW. Systematic aerobic exercise and components of reaction time in older women. J Gerontol. 1988;43:121–126. doi: 10.1093/geronj/43.5.p121. [DOI] [PubMed] [Google Scholar]
- Briones TL, Suh E, Jozsa L, Rogozinska M, Woods J, Wadowska M. Changes in number of synapses and mitochondria in presynaptic terminals in the dentate gyrus following cerebral ischemia and rehabilitation training. Brain Res. 2005;1033:51–57. doi: 10.1016/j.brainres.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Buitrago MM, Schulz JB, Dichgans J, Luft AR. Short and longterm motor skill learning in an accelerated rotarod training paradigm. Neurobiol Learning Mem. 2004;81:211–216. doi: 10.1016/j.nlm.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Burghardt PR, Pasumarthi RK, Wilson MA, Fadel J. Alterations in fear conditioning and amygdalar activation following chronic wheel running in rats. Pharmacol Biochem Behav. 2006;84:306–312. doi: 10.1016/j.pbb.2006.05.015. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Kramer AF, Erickson KI, Scalf P, McAuley E, Cohen NJ, Webb A, Jerome GJ, Marquez DX, Elavsky S. Cardiovascular fitness, cortical plasticity, and aging. Proc Natl Acad Sci USA. 2004;101:3316–3321. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eadie BD, Redila VA, Christie BR. Voluntary exercise alters the cytoarchitecture of the adult dentate gyrus by increasing cellular proliferation, dendritic complexity, and spine density. J Comp Neurol. 2005;486:39–47. doi: 10.1002/cne.20493. [DOI] [PubMed] [Google Scholar]
- Eisenstein SA, Holmes PV. Chronic and voluntary exercise enhances learning of conditioned place preference to morphine in rats. Pharmacol Biochem Behav. 2007;86:607–615. doi: 10.1016/j.pbb.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Faherty CJ, Kerley D, Smeyne RJ. A Golgi-Cox morphological analysis of neuronal changes induced by environmental enrichment. Brain Res Dev Brain Res. 2003;141:55–61. doi: 10.1016/s0165-3806(02)00642-9. [DOI] [PubMed] [Google Scholar]
- Holmes MM, Galea LA, Mistlberger RE, Kempermann G. Adult hippocampal neurogenesis and voluntary running activity: Circadian and dose-dependent effects. J Neurosci Res. 2004;76:216–222. doi: 10.1002/jnr.20039. [DOI] [PubMed] [Google Scholar]
- Knafo S, Ariav G, Barkai E, Libersat F. Olfactory learning-induced increase in spine density along the apical dendrites of CA1 hippocampal neurons. Hippocampus. 2004;14:819–825. doi: 10.1002/hipo.10219. [DOI] [PubMed] [Google Scholar]
- Kozorovitskiy Y, Gross CG, Kopil C, Battaglia L, McBreen M, Stranahan AM, Gould E. Experience induces structural and biochemical changes in the adult primate brain. Proc Natl Acad Sci USA. 2005;102:17478–17482. doi: 10.1073/pnas.0508817102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lautenschlager NT, Almeida OP. Physical activity and cognition in old age. Curr Opin Psychiatry. 2006;19:190–193. doi: 10.1097/01.yco.0000214347.38787.37. [DOI] [PubMed] [Google Scholar]
- Leuner B, Falduto J, Shors TJ. Associative memory formation increases the observation of dendritic spines in the hippocampus. J Neurosci. 2003;23:659–665. doi: 10.1523/JNEUROSCI.23-02-00659.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Gould E, Shors TJ. Is there a link between adult neurogenesis and learning? Hippocampus. 2006;16:216–224. doi: 10.1002/hipo.20153. [DOI] [PubMed] [Google Scholar]
- Neeper SA, Gomez-Pinilla F, Choi J, Cotman CW. Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Res. 1996;726:49–56. [PubMed] [Google Scholar]
- Oladehin A, Waters RS. Location and distribution of Fos protein expression in rat hippocampus following acute moderate aerobic exercise. Exp Brain Res. 2001;137:26–35. doi: 10.1007/s002210000634. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. California: Academic Press; 1998. [Google Scholar]
- Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM, Sloan R, Gage FH, Brown TR, Small SA. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci USA. 2007;104:5638–5643. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redila VA, Christie BR. Exercise-induced changes in dendritic structure and complexity in the adult hippocampal dentate gyrus. Neuroscience. 2006;137:1299–1307. doi: 10.1016/j.neuroscience.2005.10.050. [DOI] [PubMed] [Google Scholar]
- Rex CS, Lin CY, Kramar EA, Chen LY, Gall CM, Lynch G. Brain-derived neurotrophic factor promotes long-term potentiation-related cytoskeletal changes in adult hippocampus. J Neurosci. 2007;27:3017–3029. doi: 10.1523/JNEUROSCI.4037-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes JS, Garland T, Jr, Gammie SC. Patterns of brain activity associated with variation in voluntary wheel-running behavior. Behav Neurosci. 2003;117:1243–1256. doi: 10.1037/0735-7044.117.6.1243. [DOI] [PubMed] [Google Scholar]
- Stranahan AM, Khalil D, Gould E. Social isolation delays the positive effects of running on adult neurogenesis. Nat Neurosci. 2006;9:526–533. doi: 10.1038/nn1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trejo JL, Carro E, Torres-Aleman I. Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J Neurosci. 2001;21:1628–1634. doi: 10.1523/JNEUROSCI.21-05-01628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hoomissen JD, Holmes PV, Zellner AS, Poudevigne A, Dishman RK. Effects of b-adrenoreceptor blockade during chronic exercise on contextual fear conditioning and mRNA for galanin and brain-derived neurotrophic factor. Behav Neurosci. 2004;118:1378–1390. doi: 10.1037/0735-7044.118.6.1378. [DOI] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci USA. 1999;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci. 2004;20:2580–2590. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- Witter M, Moser E. Spatial representation and the architecture of the entorhinal cortex. Trends Neurosci. 2006;29:671–678. doi: 10.1016/j.tins.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Zhao C, Teng EM, Summers RG, Jr, Ming GL, Gage FH. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci. 2006;26:3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. All animals given access to running wheels ran extensively.
Supplementary Figure 2. Image preparation and measurement of individual dendritic spines. (A), Raw-capture image from a single plane of the z-stack. (B), Same image after median and low-pass filtering in Zeiss LSM510 software. (C) Areas outlined in red show tracings of individual dendritic spines, defined by our criterion of pixel intensity >50, using Reconstruct software. The yellow lines demonstrate measurements of spine length, from the point of contact with the shaft to the tip of the spine. (D) Areas outlined in blue depict regions containing spine heads exceeding our criterion (pixel intensity >75). Surface area measurements were derived from the outlined contour. (E). The region shown in yellow shows the area where pixel intensity is >100, our threshold for measuring the dendritic shaft. Scalebar in (A) = 2μm.
Supplementary Figure 3. Running does not influence dendritic spine density in pyramidal neurons from layer II/III of visual cortex. There were no differences between runners and controls in dendritic spine density on Golgi-impregnated visual cortex pyramidal neurons, (sampled from bregma -5.80 mm to bregma -6.80 mm, defined with reference to Paxinos and Watson 1998).


