SUMMARY
The innate antiviral response is initiated by pattern recognition receptors, which recognize viral pathogen-associated molecular patterns. Here we show that RNA helicase retinoic acid-inducible gene (RIG)-I-like receptors (RLR) in cooperation with Toll-like receptor (TLR)9 is required for expression of type I interferons (IFN)s after infection with herpes simplex virus (HSV). Our work also identified ribonuclease L as a critical component in IFN induction. Moreover, we find that TLR9 and RLRs activate distinct as well as overlapping intracellular signaling pathways. Thus, RLRs are important for recognition of HSV infection, and cooperates with the Toll pathway to induce an antiviral response.
Keywords: Herpes simplex virus, innate immune response, pattern recognition receptors
The early innate host response to viral infections is characterized by production of interferons (IFN)s as well as proinflammatory chemokines and cytokines (21), which exert direct antiviral activity and regulate cellular processes important for antiviral host defense (1,3,7,17).
The antiviral response is initiated by cellular sensor systems that recognize viral molecules, and subsequently activate intracellular signal transduction leading to stimulation of antiviral functions (11,24). Among the pattern recognition receptors (PRR)s, Toll-like receptors (TLR)s are membrane-bound with ligand-binding domains facing towards extracellular or endosomal surfaces, which recognize viral proteins and nucleic acids, respectively (5,6,15,19). The RNA helicases RIG-I and MDA5 (collectively termed the RIG-I like receptors (RLR)s) are located in the cytoplasm and detect intracellular virus through recognition of viral RNA structures (9,10,25,28) or small self-RNAs generated by RNase L (20).
Upon viral recognition, PRRs activate downstream signal transduction in the cell. Three pathways known to be important for antiviral and inflammatory responses are the nuclear factor (NF)-κB pathway, the mitogen-activated protein kinase (MAPK) pathway, and the IFN regulatory factor (IRF) pathway (24), which together coordinate expression of genes with antiviral activity, including type I IFNs (11).
Herpes simplex virus (HSV) type 2 is a DNA virus that can give rise to a number of clinical manifestations, most notably genitial and neonatal herpes. Innate host defense against HSV infections is highly dependent on natural killer cells (4), and the IFN system (7,17), wherefore rapid recognition of the virus by the innate immune system is essential for activation of these early defense systems. TLR2 and TLR9 have been ascribed important functions in this process, and while TLR2 recognizes an unidentified molecular structure on the virion (2,13,14), TLR9 senses HSV infection through recognition of the viral genomes (12,19). However, it is known that in addition to TLRs, other mechanisms of recognition are involved in activating the innate antiviral response against HSV (8,22), and we have recently reported that double-stranded RNA accumulates in HSV-infected cells (27). Recently it has been shown that RNase L-derived, small self-RNA, enhances IFN-β expression in a RLR dependent manner during viral infection (20).
In this study we have examined the role of RLRs in recognition of HSV infections, and its potential action together with TLRs. We demonstrate that RLRs are important for recognition of HSV in fibroblasts and macrophages, and show that simultaneous stimulation through TLR9 and RLRs during HSV-2 infection is required to activate a full signaling response and induce expression of type I IFN.
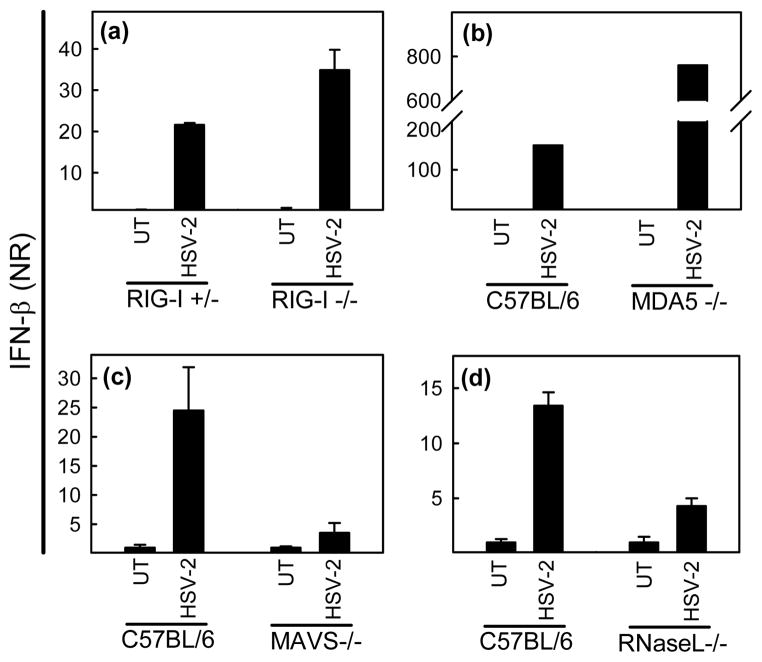
We have previously shown that HSV is recognized through both TLR-dependent and – independent mechanisms (22). In order to examine if the RLRs were involved in this process, we MEFs with targeted deletions in RIG-I, MDA5 or their down-stream adaptor mitochondrial antiviral signaling protein (MAVS)/IFN-β promoter stimulator-1 (IPS-1) were tested. The cells were seeded and treated with HSV-2. Four hours post infection RNA was harvest and levels of IFN-β were measured by Q-PCR and normalized to β-actin. The ability of HSV to induce expression of IFN-β was not impaired in either RIG-I−/− or MDA5 −/− cells (Fig. 1a – 1b), but was, however, strongly reduced in MAVS/IPS-1 −/− cells (Fig. 1c). Interestingly, HSV-2-induced IFN-β expression was also impaired in RNase L −/− fibroblasts (Fig. 1d), previously reported to generate small self-RNAs stimulating the IFN response through both RIG-I and MDA5 (20). Thus, the results suggest that HSV-2 infected is sensed by RLRs, and that RNase L is essential for this process.
Fig. 1.
MAVS/IPS-1 and RNase L are essential for HSV-induced cytokine expression. (a-d) RIG-I −/−, MDA5 −/−, MAVS/IPS-1 −/−, and RNase L −/− MEFs, and their respective heterozygote or wild type cells were seeded at 7×105 cells per 6 well in culture plate and treated with HSV-2, 3 MOI. Four hours post infection RNA was harvest with High Pure RNA Isolation Kit (Roche) and levels of IFN-β were measured by Q-PCR and normalized to β-actin. Data is shown as mean of triplicates +/− SEM.
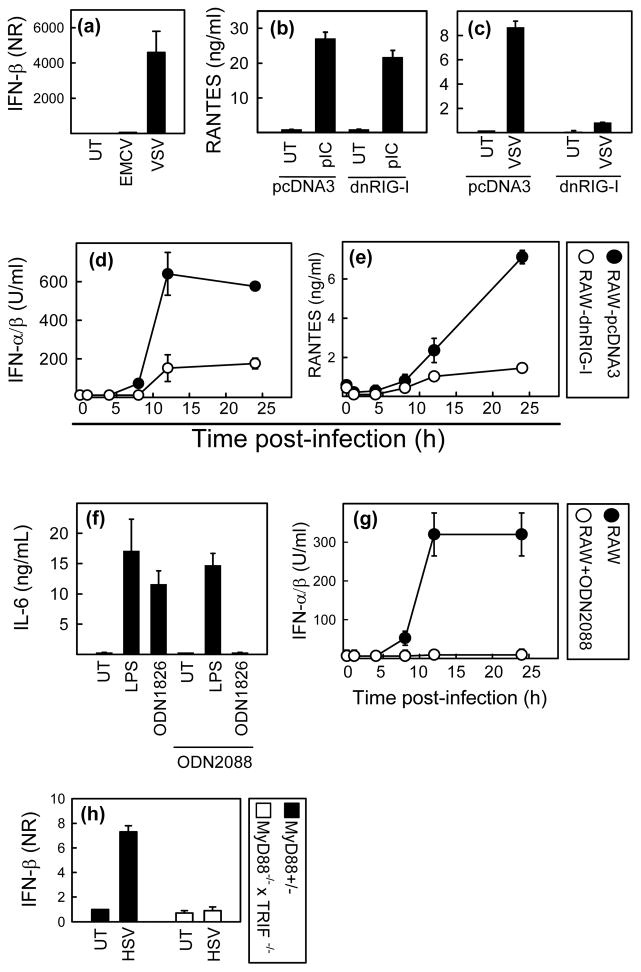
To examine if the role of RLRs in HSV recognition was also apparent in macrophages, we first examined the activity of the RIG-I versus MDA5 pathways in RAW264.7 cells. The cells were seeded and infected with vesicular stomatitis virus (VSV) or encephalomyocarditis virus (EMCV), which activate RIG-I and MDA5, respectively (10). We observed that VSV was able to induce IFN-β production whereas EMCV was not (Fig. 2a), thus indicating that RIG-I but not MDA5 is functional in the RAW264.7 cell line. In the further analysis of the role of RLRs in RAW264.7 cells we therefore focused on RIG-I only. RAW264.7 cells stably transfected with a dominant negative RIG-I mutant (helicase and C-terminal domains) or empty vector (pcDNA3) (23) were seeded and stimulates with PolyIC (Invivogen) or VSV as controls to check specificity. As seen in Fig. 2b, and 2c, expression of dnRIG-I did not affect stimulation through TLR3 but strongly inhibited cellular activation through RIG-I. We next seeded the dnRIG-I-RAW264.7 and pcDNA-RAW264.7 cells and infected with HSV for different time periods, before harvest of supernatants and measurement of cytokines. RANTES was measured by ELISA, and IFN-α/β levels were measured by bioassay (23). HSV-2 induced expression of RANTES and IFN-α/β was strongly reduced in the RAW-dnRIG-I cell line (Fig. 2d–e). Thus, HSV infection is recognized by a RIG-I dependent mechanism in RAW264.7 macrophage-like cells.
Fig. 2.
RIG-I and TLR9 cooperatively induce expression of cytokines during HSV-2 infection. (a-c) Parental RAW264.7 cells or stably transfected with the empty vector pcDNA3 or a dominant-negative RIG-I construct were seeded at 8×104 cells per well in 96 well culture plates and stimulated with EMCV, 5 MOI; VSV, 1,6 MOI or PolyIC (Invivogen), 25 μg/ml. Four and 16 hours post infection, RNA and cell culture supernatants, respectively, were harvested for measurement of IFN-β (Q-PCR) and RANTES (ELISA, using matched antibody pairs from R&D Systems). (d–e) RAW-pcDNA3 and RAW-dnRIG-I cells were treated with HSV-2, 3 MOI. Supernatants were harvested at indicated time points, and RANTES and IFN-α/β were measured by ELISA and bioassay, respectively. (f) RAW264.7 cells were treated with LPS (100 ng/ml) or ODN1826 (1 μM) in the presence or absence of the TLR9 antagonist ODN2088 (3μM). Supernatants were harvested at 20 h p.i. and IL-6 and IFN-α/β were measured by ELISA and bioassay. (g) RAW264.7 cells incubated in the presence or absence of ODN2088 as indicated were infected with HSV-2 at MOI 2 for the indicated amounts of time. Supernatants were harvested and levels of IFN-α/β were determined by bioassay. (g) MyD88+/− and MyD88−/−xTRIF−/− MEFs were seeded at 7×105 cells per 6 well in culture plate and treated with HSV-2, 3 MOI. Four hours post infection RNA was harvest and levels of IFN-β were measured by Q-PCR and normalized to β-actin.. All data are shown as mean of triplicates +/− SEM.
TLR9 has been reported to recognize HSV infection (12,19). To evaluate the role of TLR9 in HSV-induced cytokine expression in RAW264.7 cells, we used the TLR9 antagonist ODN2088, the specificity of which is shown in Fig. 2f. RAW264.7 cells were incubated in the presence or absence of ODN2088 and infected with HSV-2 for the indicated time intervals, and type I IFN and RANTES were measured in the supernatants. Expression of both cytokines was totally blocked in the presence of ODN2088 regardless the time point (Fig. 2g and data not shown). Thus, TLR9 plays an important role in HSV-induced cytokine expression in RAW264.7 cells.
The role of TLR2, previously shown to recognize HSV (13,14), was examined using RAW264.7 cells stably expressing the a dominant negative mutant of the TLR2 adaptor Mal. However, no role for Mal in HSV-2 induced IFN expression was observed (not shown).
The above data suggest that both RLRs and TLRs are required for HSV-induced IFN expression in RAW264.7 macrophages. To examine if this was also the case in fibroblasts, we examined the ability of MyD88−/−xTRIF−/− MEFs to induce expression of IFN-β in response to HSV infection. As shown in Fig. 2h, the ability of HSV infection to trigger IFN-β expression was totally abrogated in MyD88−/−xTRIF−/− MEFs. This finding together with the data shown in Fig. 1C, support the conclusion that signaling through both TLR and RLRs is required to stimulate IFN expression in fibroblasts in response to HSV infection.
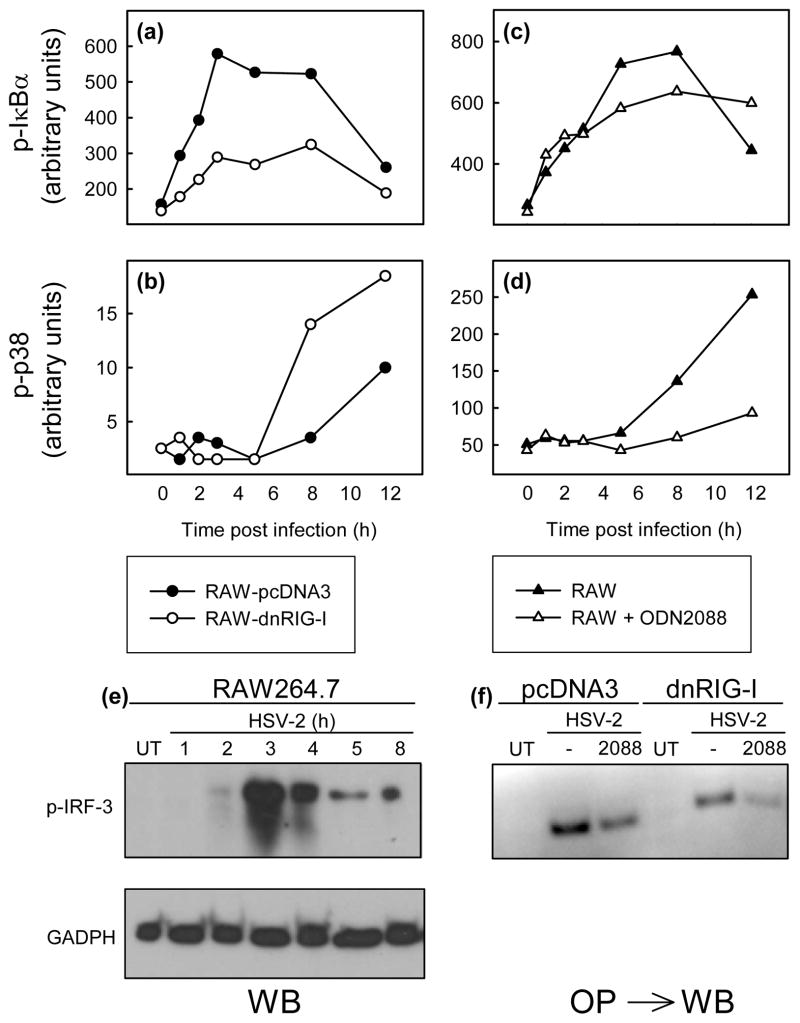
To look further into the mechanism underlying the observation that RIG-I and TLR9 cooperatively induce expression of cytokines, we examined how these PRRs affected the ability of HSV-2 infection to activate signal transduction. RAW-pcDNA3 and RAW-dnRIG-I cells as well as RAW264.7 cells incubated in the presence or absence of ODN2088 were infected with HSV-2 for the indicated time intervals before harvest of cell lysates (Bio-Rad). Phosphorylation of IκBα and MAPK p38 was determined by Luminex Technology (Bio-Rad). Activation of the NF-κB pathway was reduced in cells expressing dnRIG-I, whereas p38 was activated to even higher levels in RAW-dnRIG-I cells as compared to RAW-pcDNA3 cells (Fig. 3a–3b). Interestingly, when signaling through TLR9 was blocked, we observed the opposite picture, i.e. unaltered activation of NF-κB but reduced activation of p38 (Fig. 3c–3d). Finally, we examined the involvement of TLR9 and RIG-I in activation of IRF-3 by HSV-2, which peaked at 3 h p.i (Fig. 3e). Inhibition of either RIG-I or TLR9 led to reduced activation of IRF-3 after HSV infection, and combined inhibition of both PRRs led to nearly total abrogation of HSV-2-activated phosphorylation of IRF-3 (Fig. 3f). Thus, RIG-I and TLR9 control activation of overlapping, yet distinct, sets of signaling pathways in response to HSV-2 infection in RAW264.7 cells.
Fig. 3.
RIG-I and TLR9 activate distinct signaling pathways and co-ordinately stimulate activation of IRF-3. (a-d) RAW-pcDNA3 and RAW-dnRIG-I cells (a-b) or RAW264.7 cells incubated in the presence of 3 μM ODM2088 (c-d) were infected with HSV-2 (MOI 3), and total cell lysates (Bio-Plex cell lysis kit, Bio-Rad) were harvested after the indicated amounts of time. Phosphorylation of P-IκBα, (a, c), and p38 (b, d) was measured by Luminex Technology, using a kit from Bio-Rad. (e) RAW264.7 cells were infected with HSV-2 at MOI 3, and total cell lysates were made at the indicated time points p.i. Phospho-IRF-3 and GAPDH were detected by Western Blotting. (f) RAW-pcDNA3 and RAW-dnRIG-I cells were incubated in the presence of 3 μM ODN2088 and infected with HSV-2 (MOI 3) for 3 h. Total cell lysates were made, and proteins bound to the IFN-β promoter PRD I-III region were precipitated. Phospho-IRF-3 was detected by Western Blotting. Similar results were obtained in at least 3 independent experiments.
In this work we show that type I IFN production during HSV-2 infection, is dependent on RLRs and RNase L. We also show that RLRs and TLR9 cooperatively induce expression of cytokines during HSV-2 infection. These findings thus identify RLRs as PRRs that recognizes HSV-2 infection, and also show that these cytosolic receptors act in concert with TLR9 to induce cytokine expression. Our results also identify RNase L as an important part of this induction.
Until recently it was believed that RIG-I only recognize 5′-triphosphate end of RNA (9,25), a structure assumed not to be present during HSV replication. It is now known that RIG-I also activates antiviral immune responses upon binding to dsRNA (26), and we have previously shown that dsRNA indeed does accumulate during HSV infection (27). In addition, RNase L augments IFN-β expression through the RLRs and MAVS/IPS-1, by generating small self-RNAs (20). The results presented here show that HSV-induced IFN expression is dependent on RNase L. Thus, it is possible that both viral and self RNAs stimulate IFN induction in an RLR-dependent manner during HSV infection.
Another important finding is that RIG-I cooperates with TLR9 in induction of cytokine expression, and that the two PRRs differentially stimulate signaling pathways. Two studies have previously shown that RIG-I and TLR3 coordinately activate the host response against Influenza A virus and Respiratory syncytial virus infections (16,18), and our work further supports the idea that a full innate antiviral response is induced through activation of several PRRs which act in concert to mediate host defense.
Collectively, our work has identified RLRs as PRRs recognizing HSV infection, and shows that simultaneous stimulation through two or more PRRs impacts on the nature of the antiviral response.
Acknowledgments
This work was supported by research grants from Danish Medical Research Council (grant no 271-06-0438), The Danish Natural Science Research Council (grant no 272-05-0222), The Lundbeck Foundation (grant nos 104/04 and 116/06), Aarhus University (AU) Research Foundation, the research program in Molecular Medicine, The Faculty of Health Science, AU, and The Beckett Foundation and NIH/NCI grant CA044059 (to RHS). The technical assistance of Kirsten Stadel Petersen and Birthe Søby is greatly appreciated.
References
- 1.Ank N, West H, Bartholdy C, Eriksson K, Thomsen AR, Paludan SR. Lambda interferon (IFN-{lambda}), a type III IFN, is induced by viruses and IFNs and displays potent antiviral activity against select virus infections in vivo. J Virol. 2006;80:4501–4509. doi: 10.1128/JVI.80.9.4501-4509.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aravalli RN, Hu S, Rowen TN, Palmquist JM, Lokensgard JR. Cutting edge: TLR2-mediated proinflammatory cytokine and chemokine production by microglial cells in response to herpes simplex virus. J Immunol. 2005;175:4189–4193. doi: 10.4049/jimmunol.175.7.4189. [DOI] [PubMed] [Google Scholar]
- 3.Beutler B. Inferences, questions and possibilities in Toll-like receptor signalling. Nature. 2004;430:257–263. doi: 10.1038/nature02761. [DOI] [PubMed] [Google Scholar]
- 4.Biron CA, Byron KS, Sullivan JL. Severe herpesvirus infections in an adolescent without natural killer cells. N Engl J Med. 1989;320:1731–1735. doi: 10.1056/NEJM198906293202605. [DOI] [PubMed] [Google Scholar]
- 5.Boehme KW, Guerrero M, Compton T. Human Cytomegalovirus Envelope Glycoproteins B and H Are Necessary for TLR2 Activation in Permissive Cells. J Immunol. 2006;177:7094–7102. doi: 10.4049/jimmunol.177.10.7094. [DOI] [PubMed] [Google Scholar]
- 6.Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303:1529–1531. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- 7.Dupuis S, Jouanguy E, Al Hajjar S, Fieschi C, Al Mohsen IZ, Al Jumaah S, Yang K, Chapgier A, Eidenschenk C, Eid P, Al Ghonaium A, Tufenkeji H, Frayha H, Al Gazlan S, Al Rayes H, Schreiber RD, Gresser I, Casanova JL. Impaired response to interferon-alpha/beta and lethal viral disease in human STAT1 deficiency. Nat Genet. 2003;33:388–391. doi: 10.1038/ng1097. [DOI] [PubMed] [Google Scholar]
- 8.Hochrein H, Schlatter B, O’Keeffe M, Wagner C, Schmitz F, Schiemann M, Bauer S, Suter M, Wagner H. Herpes simplex virus type-1 induces IFN-{alpha} production via Toll-like receptor 9-dependent and -independent pathways. Proc Natl Acad Sci U S A. 2004;101:11416–11421. doi: 10.1073/pnas.0403555101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hornung V, Ellegast J, Kim S, Brzozka K, Jung A, Kato H, Poeck H, Akira S, Conzelmann KK, Schlee M, Endres S, Hartmann G. 5′-Triphosphate RNA is the ligand for RIG-I. Science. 2006;314:994–997. doi: 10.1126/science.1132505. [DOI] [PubMed] [Google Scholar]
- 10.Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K, Uematsu S, Jung A, Kawai T, Ishii KJ, Yamaguchi O, Otsu K, Tsujimura T, Koh CS, Reis e Sousa, Matsuura Y, Fujita T, Akira S. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441:101–105. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- 11.Kawai T, Akira S. Innate immune recognition of viral infection. Nat Immunol. 2006;7:131–137. doi: 10.1038/ni1303. [DOI] [PubMed] [Google Scholar]
- 12.Krug A, Luker GD, Barchet W, Leib DA, Akira S, Colonna M. Herpes simplex virus type 1 activates murine natural interferon-producing cells through toll-like receptor 9. Blood. 2004;103:1433–1437. doi: 10.1182/blood-2003-08-2674. [DOI] [PubMed] [Google Scholar]
- 13.Kurt-Jones EA, Belko J, Yu C, Newburger PE, Wang J, Chan M, Knipe DM, Finberg RW. The role of toll-like receptors in herpes simplex infection in neonates. J Infect Dis. 2005;191:746–748. doi: 10.1086/427339. [DOI] [PubMed] [Google Scholar]
- 14.Kurt-Jones EA, Chan M, Zhou S, Wang J, Reed G, Bronson R, Arnold MM, Knipe DM, Finberg RW. Herpes simplex virus 1 interaction with Toll-like receptor 2 contributes to lethal encephalitis. Proc Natl Acad Sci U S A. 2004;101:1315–1320. doi: 10.1073/pnas.0308057100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurt-Jones EA, Popova L, Kwinn L, Haynes LM, Jones LP, Tripp RA, Walsh EE, Freeman MW, Golenbock DT, Anderson LJ, Finberg RW. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nature Immunol. 2000;1:398–401. doi: 10.1038/80833. [DOI] [PubMed] [Google Scholar]
- 16.Le Goffic R, Pothlichet J, Vitour D, Fujita T, Meurs E, Chignard M, Si-Tahar M. Cutting Edge: Influenza A virus activates TLR3-dependent inflammatory and RIG-I-dependent antiviral responses in human lung epithelial cells. J Immunol. 2007;178:3368–3372. doi: 10.4049/jimmunol.178.6.3368. [DOI] [PubMed] [Google Scholar]
- 17.Leib DA, Harrison TE, Laslo KM, Machalek MA, Moorman NJ, Virgin HW. Interferons regulate the phenotype of wild-type and mutant herpes simplex viruses in vivo. J Exp Med. 1999;189:663–672. doi: 10.1084/jem.189.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu P, Jamaluddin M, Li K, Garofalo RP, Casola A, Brasier AR. Retinoic acid-inducible gene I mediates early antiviral response and Toll-like receptor 3 expression in respiratory syncytial virus-infected airway epithelial cells. J Virol. 2007;81:1401–1411. doi: 10.1128/JVI.01740-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lund J, Sato A, Akira S, Medzhitov R, Iwasaki A. Toll-like receptor 9-mediated recognition of Herpes simplex virus-2 by plasmacytoid dendritic cells. J Exp Med. 2003;198:513–520. doi: 10.1084/jem.20030162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malathi K, Dong B, Gale M, Jr, Silverman RH. Small self-RNA generated by RNase L amplifies antiviral innate immunity. Nature. 2007;448:816–819. doi: 10.1038/nature06042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malmgaard L. Induction and regulation of IFNs during viral infections. J Interferon Cytokine Res. 2004;24:439–454. doi: 10.1089/1079990041689665. [DOI] [PubMed] [Google Scholar]
- 22.Malmgaard L, Melchjorsen J, Bowie AG, Mogensen SC, Paludan SR. Viral activation of macrophages through TLR-dependent and -independent pathways. J Immunol. 2004;173:6890–6898. doi: 10.4049/jimmunol.173.11.6890. [DOI] [PubMed] [Google Scholar]
- 23.Melchjorsen J, Jensen SB, Malmgaard L, Rasmussen SB, Weber F, Bowie AG, Matikainen S, Paludan SR. Activation of innate defense against a paramyxovirus is mediated by RIG-I and TLR7 and TLR8 in a cell-type-specific manner. J Virol. 2005;79:12944–12951. doi: 10.1128/JVI.79.20.12944-12951.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mogensen TH, Paludan SR. Reading the viral signature by Toll-like receptors and other pattern recognition receptors. J Mol Med. 2005;83:180–192. doi: 10.1007/s00109-004-0620-6. [DOI] [PubMed] [Google Scholar]
- 25.Pichlmair A, Schulz O, Tan CP, Naslund TI, Liljestrom P, Weber F, Reis e Sousa RIG-I-Mediated Antiviral Responses to Single-Stranded RNA Bearing 5′ Phosphates. Science. 2006;314:997–1001. doi: 10.1126/science.1132998. [DOI] [PubMed] [Google Scholar]
- 26.Takahasi K, Yoneyama M, Nishihori T, Hirai R, Kumeta H, Narita R, Gale M, Jr, Inagaki F, Fujita T. Nonself RNA-Sensing Mechanism of RIG-I Helicase and Activation of Antiviral Immune Responses. Mol Cell. 2008;29:428–440. doi: 10.1016/j.molcel.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 27.Weber F, Wagner V, Rasmussen SB, Hartmann R, Paludan SR. Double-stranded RNA is produced by positive-strand RNA viruses and DNA viruses but not in detectable amounts by negative-strand RNA viruses. J Virol. 2006;80:5059–5064. doi: 10.1128/JVI.80.10.5059-5064.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoneyama M, Kikuchi M, Natsukawa T, Shinobu N, Imaizumi T, Miyagishi M, Taira K, Akira S, Fujita T. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat Immunol. 2004;5:730–737. doi: 10.1038/ni1087. [DOI] [PubMed] [Google Scholar]