Abstract
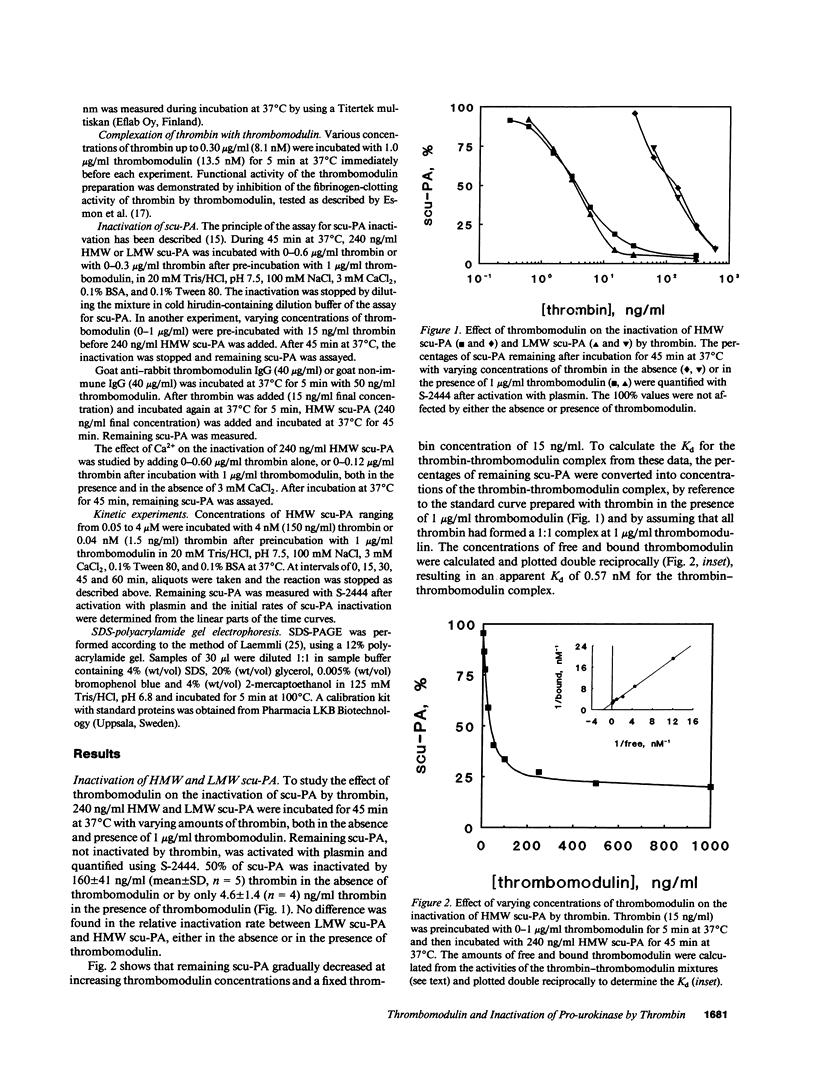
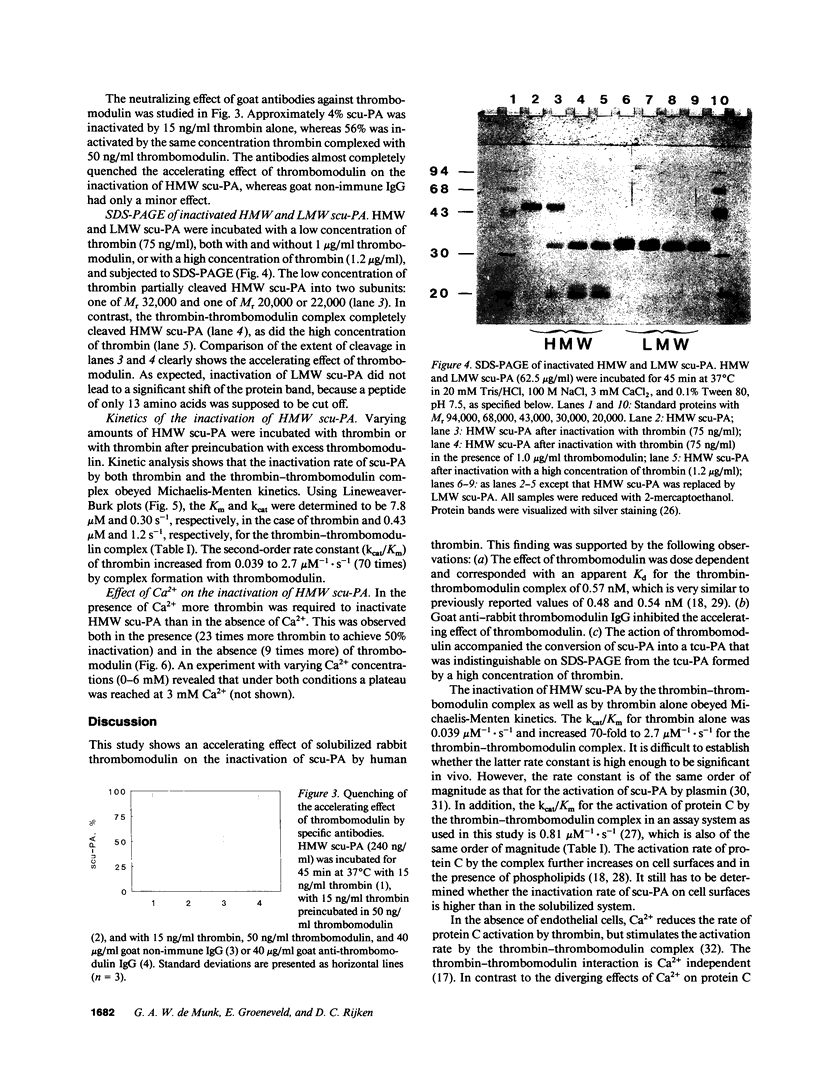
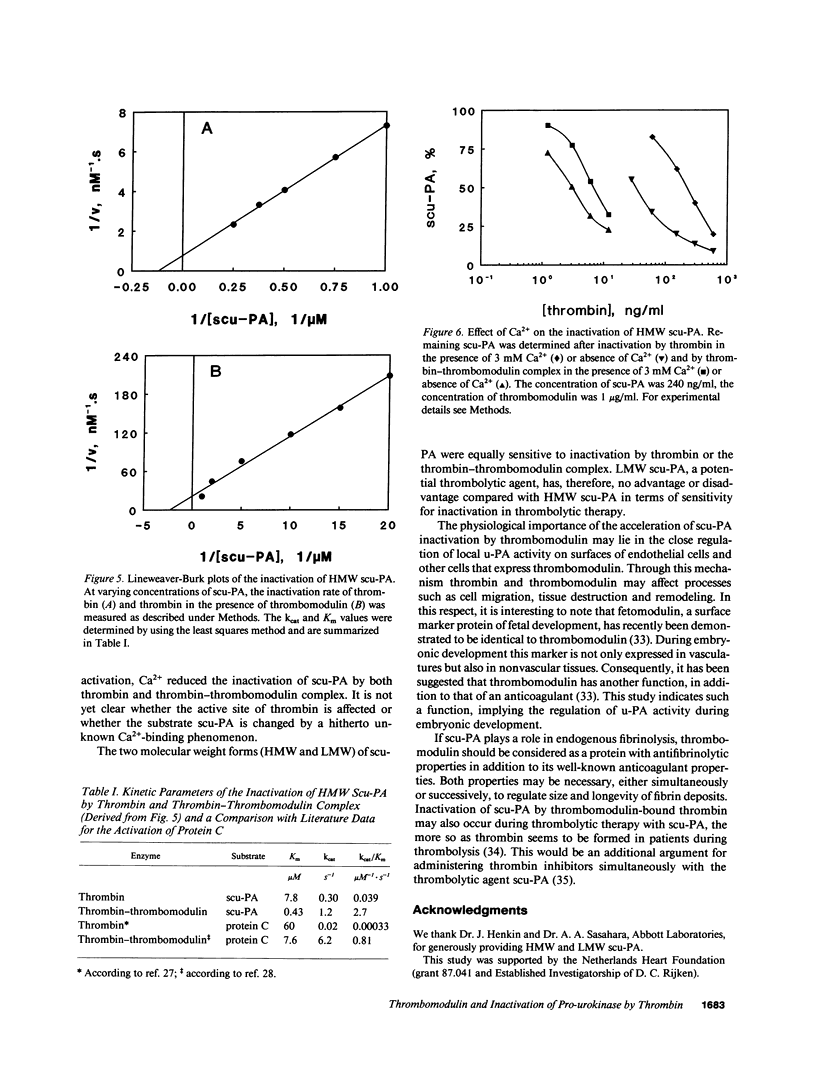
The in vitro effects of thrombomodulin on the inactivation of single chain urokinase-type plasminogen activator (scu-PA) by thrombin were investigated by incubating scu-PA with varying concentrations of human thrombin, in both the absence and presence of soluble rabbit thrombomodulin. 50% inactivation of scu-PA occurred in 45 min at 160 ng/ml thrombin in the absence of thrombomodulin and at 4.6 ng/ml thrombin in the presence of thrombomodulin. No difference was found in either the absence or the presence of thrombomodulin between the inactivation rates of high molecular weight scu-PA, and a low molecular weight scu-PA which lacked the growth factor and kringle domains. Enzyme kinetic experiments with varying concentrations of scu-PA showed that thrombomodulin decreased the Km of thrombin for scu-PA from 7.8 to 0.43 microM and increased the kcat from 0.30 to 1.2 s-1, corresponding to a 70-fold increase in the second-order rate constant kcat/Km. SDS-polyacrylamide gel electrophoresis showed that scu-PA was cleaved into two chains upon inactivation by thrombin, and confirmed the acceleration effect of thrombomodulin on inactivation of scu-PA. Thrombomodulin thus not only has anticoagulant properties but is also antifibrinolytic. The acceleration may imply a new mechanism for the regulation of local plasminogen activator activity on the cell surface.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badylak S. F., Voytik S., Klabunde R. E., Henkin J., Leski M. Bolus dose response characteristics of single chain urokinase plasminogen activator and tissue plasminogen activator in a dog model of arterial thrombosis. Thromb Res. 1988 Nov 15;52(4):295–312. doi: 10.1016/0049-3848(88)90071-0. [DOI] [PubMed] [Google Scholar]
- Bernik M. B. Increased plasminogen activator (urokinase) in tissue culture after fibrin deposition. J Clin Invest. 1973 Apr;52(4):823–834. doi: 10.1172/JCI107246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danø K., Andreasen P. A., Grøndahl-Hansen J., Kristensen P., Nielsen L. S., Skriver L. Plasminogen activators, tissue degradation, and cancer. Adv Cancer Res. 1985;44:139–266. doi: 10.1016/s0065-230x(08)60028-7. [DOI] [PubMed] [Google Scholar]
- Dittman W. A., Majerus P. W. Structure and function of thrombomodulin: a natural anticoagulant. Blood. 1990 Jan 15;75(2):329–336. [PubMed] [Google Scholar]
- Eisenberg P. R., Miletich J. P. Induction of marked thrombin activity by pharmacologic concentrations of plasminogen activators in nonanticoagulated whole blood. Thromb Res. 1989 Sep 1;55(5):635–643. doi: 10.1016/0049-3848(89)90396-4. [DOI] [PubMed] [Google Scholar]
- Esmon C. T., Esmon N. L., Harris K. W. Complex formation between thrombin and thrombomodulin inhibits both thrombin-catalyzed fibrin formation and factor V activation. J Biol Chem. 1982 Jul 25;257(14):7944–7947. [PubMed] [Google Scholar]
- Esmon C. T., Esmon N. L. Protein C activation. Semin Thromb Hemost. 1984 Apr;10(2):122–130. doi: 10.1055/s-2007-1004414. [DOI] [PubMed] [Google Scholar]
- Esmon C. T. The roles of protein C and thrombomodulin in the regulation of blood coagulation. J Biol Chem. 1989 Mar 25;264(9):4743–4746. [PubMed] [Google Scholar]
- Fitzgerald D. J., Fitzgerald G. A. Role of thrombin and thromboxane A2 in reocclusion following coronary thrombolysis with tissue-type plasminogen activator. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7585–7589. doi: 10.1073/pnas.86.19.7585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvin J. B., Kurosawa S., Moore K., Esmon C. T., Esmon N. L. Reconstitution of rabbit thrombomodulin into phospholipid vesicles. J Biol Chem. 1987 Feb 15;262(5):2199–2205. [PubMed] [Google Scholar]
- Gurewich V. Activation of fibrin-bound plasminogen by pro-urokinase and its complementariness with that by tissue plasminogen activator. Enzyme. 1988;40(2-3):97–108. doi: 10.1159/000469151. [DOI] [PubMed] [Google Scholar]
- Gurewich V., Pannell R. Inactivation of single-chain urokinase (pro-urokinase) by thrombin and thrombin-like enzymes: relevance of the findings to the interpretation of fibrin-binding experiments. Blood. 1987 Mar;69(3):769–772. [PubMed] [Google Scholar]
- Günzler W. A., Steffens G. J., Otting F., Kim S. M., Frankus E., Flohé L. The primary structure of high molecular mass urokinase from human urine. The complete amino acid sequence of the A chain. Hoppe Seylers Z Physiol Chem. 1982 Oct;363(10):1155–1165. doi: 10.1515/bchm2.1982.363.2.1155. [DOI] [PubMed] [Google Scholar]
- Haley P. E., Doyle M. F., Mann K. G. The activation of bovine protein C by factor Xa. J Biol Chem. 1989 Sep 25;264(27):16303–16310. [PubMed] [Google Scholar]
- Hauert J., Nicoloso G., Schleuning W. D., Bachmann F., Schapira M. Plasminogen activators in dextran sulfate-activated euglobulin fractions: a molecular analysis of factor XII- and prekallikrein-dependent fibrinolysis. Blood. 1989 Mar;73(4):994–999. [PubMed] [Google Scholar]
- Ichinose A., Fujikawa K., Suyama T. The activation of pro-urokinase by plasma kallikrein and its inactivation by thrombin. J Biol Chem. 1986 Mar 15;261(8):3486–3489. [PubMed] [Google Scholar]
- Imada S., Yamaguchi H., Nagumo M., Katayanagi S., Iwasaki H., Imada M. Identification of fetomodulin, a surface marker protein of fetal development, as thrombomodulin by gene cloning and functional assays. Dev Biol. 1990 Jul;140(1):113–122. doi: 10.1016/0012-1606(90)90058-q. [DOI] [PubMed] [Google Scholar]
- Johnson A. E., Esmon N. L., Laue T. M., Esmon C. T. Structural changes required for activation of protein C are induced by Ca2+ binding to a high affinity site that does not contain gamma-carboxyglutamic acid. J Biol Chem. 1983 May 10;258(9):5554–5560. [PubMed] [Google Scholar]
- Kekwick R. A. The serum proteins in multiple myelomatosis. Biochem J. 1940 Sep;34(8-9):1248–1257. doi: 10.1042/bj0341248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lijnen H. R., Stump D. C., Collen D. C. Single-chain urokinase-type plasminogen activator: mechanism of action and thrombolytic properties. Semin Thromb Hemost. 1987 Apr;13(2):152–159. doi: 10.1055/s-2007-1003486. [DOI] [PubMed] [Google Scholar]
- Lijnen H. R., Van Hoef B., Collen D. Activation with plasmin of tow-chain urokinase-type plasminogen activator derived from single-chain urokinase-type plasminogen activator by treatment with thrombin. Eur J Biochem. 1987 Dec 1;169(2):359–364. doi: 10.1111/j.1432-1033.1987.tb13620.x. [DOI] [PubMed] [Google Scholar]
- Lijnen H. R., Van Hoef B., Collen D. Comparative kinetic analysis of the activation of human plasminogen by natural and recombinant single-chain urokinase-type plasminogen activator. Biochim Biophys Acta. 1986 Dec 10;884(3):402–408. doi: 10.1016/0304-4165(86)90190-x. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Nielsen L. S., Hansen J. G., Skriver L., Wilson E. L., Kaltoft K., Zeuthen J., Danø K. Purification of zymogen to plasminogen activator from human glioblastoma cells by affinity chromatography with monoclonal antibody. Biochemistry. 1982 Dec 7;21(25):6410–6415. doi: 10.1021/bi00268a014. [DOI] [PubMed] [Google Scholar]
- Owen W. G., Esmon C. T. Functional properties of an endothelial cell cofactor for thrombin-catalyzed activation of protein C. J Biol Chem. 1981 Jun 10;256(11):5532–5535. [PubMed] [Google Scholar]
- Rijken D. C., Binnema D. J., Los P. Specific fibrinolytic properties of different molecular forms of pro-urokinase from a monkey kidney cell culture. Thromb Res. 1986 Jun 15;42(6):761–768. doi: 10.1016/0049-3848(86)90112-x. [DOI] [PubMed] [Google Scholar]
- Scully M. F., Ellis V., Watahiki Y., Kakkar V. V. Activation of pro-urokinase by plasmin: non-Michaelian kinetics indicates a mechanism of negative cooperativity. Arch Biochem Biophys. 1989 Feb 1;268(2):438–446. doi: 10.1016/0003-9861(89)90311-1. [DOI] [PubMed] [Google Scholar]
- Stump D. C., Lijnen H. R., Collen D. Purification and characterization of a novel low molecular weight form of single-chain urokinase-type plasminogen activator. J Biol Chem. 1986 Dec 25;261(36):17120–17126. [PubMed] [Google Scholar]
- Stump D. C., Stassen J. M., Demarsin E., Collen D. Comparative thrombolytic properties of single-chain forms of urokinase-type plasminogen activator. Blood. 1987 Feb;69(2):592–596. [PubMed] [Google Scholar]
- Wijngaards G., Rijken D. C., van Wezel A. L., Groeneveld E., van der Velden C. A. Characterization and fibrin-binding properties of different molecular forms of pro-urokinase from a monkey kidney cell culture. Thromb Res. 1986 Jun 15;42(6):749–760. doi: 10.1016/0049-3848(86)90111-8. [DOI] [PubMed] [Google Scholar]