Abstract
Radiocarbon dating is typically an archaeological tool rather than a forensic one. Recently however, we have shown that the amount of radiocarbon present in tooth enamel, as a result of nuclear bomb testing during the cold war, is a remarkably accurate indicator of when a person is born. Enamel isolated from human teeth is processed to form graphite and carbon-14 (14C) levels are measured using accelerator mass spectrometry. Since there is no turnover of enamel after it is formed, 14C levels in the enamel represent 14C levels in the atmosphere at the time of its formation. In this paper we describe the strategy used to determine the date of birth of an individual based on radiocarbon levels in tooth enamel, focusing on the methodology of this strategy. Year of birth information can significantly assist police investigators when the identity of a deceased individual is unknown. In such cases police will try to match particulars of the unidentified individual (which is often only gender and/or an estimate of age), with particulars from missing persons lists.
Introduction
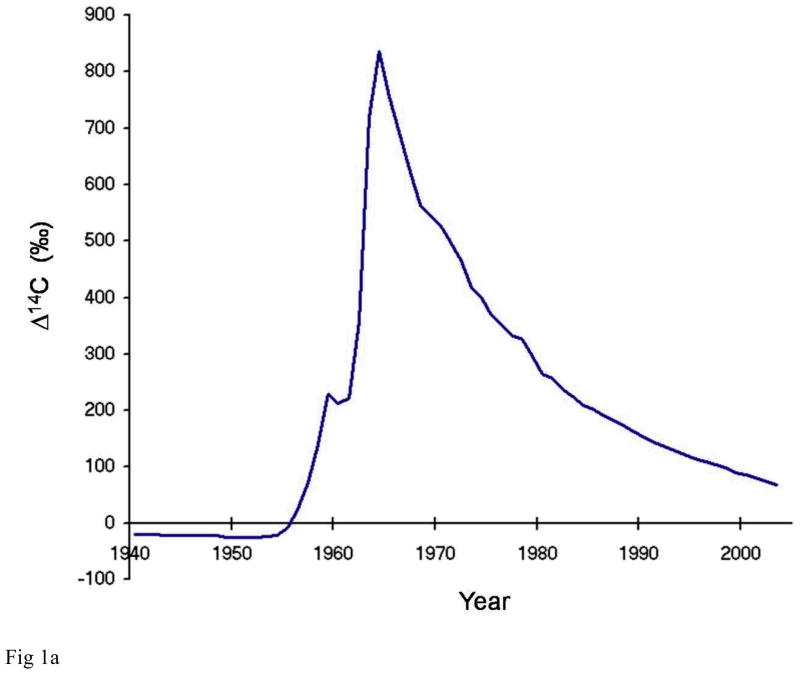
Radiocarbon or carbon-14 (14C) is produced naturally in the atmosphere by cosmic ray interactions with nitrogen-14. Single carbon atoms in the atmosphere are chemically reactive and are quickly oxidized to carbon dioxide CO2. The atmospheric concentration of natural 14C with respect to all carbon has remained relatively stable at about 1.2 parts per trillion over the past several thousand years. Atmospheric detonations of nuclear weapons during the 1950s and early 1960s doubled the concentration of 14C/C in the atmosphere (Figure 1a). From the peak in 1963, the level of 14CO2 has decreased with a mean life of about 16 years. The 14C has not actually disappeared or decayed, it has simply moved out of the atmosphere due to mixing with large marine and terrestrial carbon reservoirs. The temporal variations of artificially high levels of atmospheric radiocarbon have been captured in organic material world-wide and thus offer an opportunity to determine a date of synthesis for biomolecules. Some geographical variation exists because there were a limited number of sources of bomb-pulse 14C. As a result, the upswing and the peak values of the curve do vary with location around the globe[1,2]. However, the pulse of 14CO2 rapidly mixed in the atmosphere with all other CO2 to produce a relatively homogeneous distribution of atmospheric 14CO2 [3]. Since radiocarbon is incorporated into all living things, this pulse is an isotopic chronometer of the past 60 years.
Figure 1.
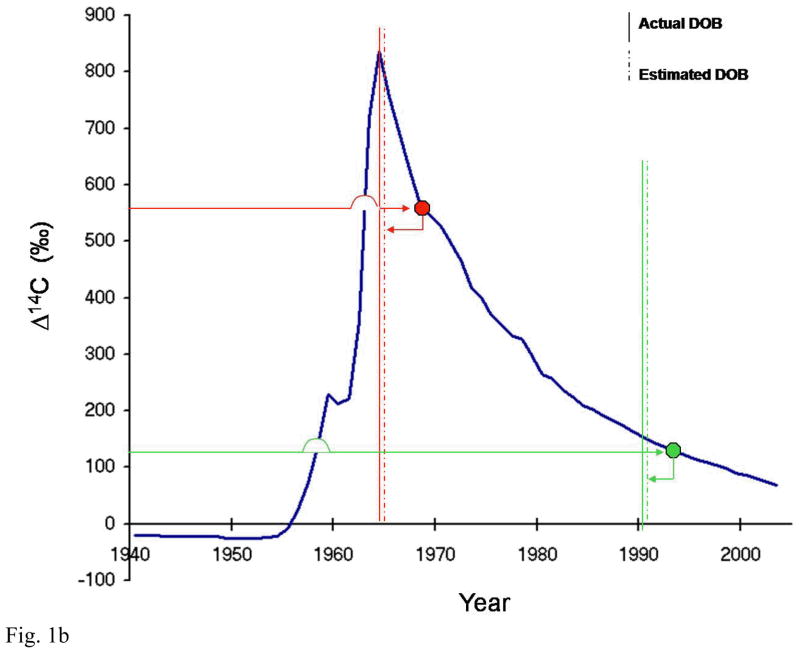
Northern hemisphere atmospheric 14C concentration as a function of time and strategy behind radiocarbon tooth dating (a) Northern hemisphere growing season average of atmospheric 14C concentration in CO2 from 1940–2007. It is constructed using several different data sets that used tree rings, unpublished recent plant growth, and direct atmospheric sampling to provide carbon samples[1,2,30,31]. The vertical axis uses the Δ4C nomenclature defined by [27] (b) To estimate an individual’s date of birth, the level of 14C measured in tooth enamel is plotted on to the curve of atmospheric 14C against time (blue) to find the year of enamel synthesis (right-pointing arrows). The known age at enamel formation for individual teeth is then subtracted from the year obtained to give the date of birth (left-pointing arrows; dashed vertical lines). Two representative cases are shown (red and green); two teeth were analysed for the case depicted in red, allowing one to determine if the person was born on the ascending or descending –slope of the bomb curve. Solid vertical lines represent the actual date of birth.
The carbon isotopic content of new plant growth reflects the atmospheric radiocarbon concentration. New leaves are produced in a matter of weeks while larger fruit and vegetables form over the period of a month or two. Herbivores lag the atmosphere slightly because their primary carbon source is weeks to months removed from the atmosphere. Omnivores and carnivores lag the atmosphere further because their carbon sources are another step removed. The date of formation of a tissue or specific biomolecule can be estimated from the bomb-curve by considering these lags in incorporation and relating the 14C concentration with the date. Enamel formation can occur over several years in humans[4]. For radiocarbon analysis of tooth age, we use the upper limit of enamel formation (i.e. the time of enamel laydown completion), which balances out lag periods of 14C incorporation from the atmosphere to the body.
Rational for using enamel rather than collagen
Bone is a preferred sample matrix for traditional radiocarbon dating. Bone’s ability to resist rapid decay while containing a relatively high concentration of carbon makes it a desirable material for traditional dating[5]. Traditional radiocarbon bone dating dissolves the mineral component of bone in acid and retains collagen to avoid potential complications with mineral exchange of carbonates in the environment over thousands of years. Collagen is a protein and is not affected by environmental carbonate exchange like the mineral component of bone.
Attempts to use bone and cartilage for bomb-pulse dating however, have had limited success. Bone and cartilage are living tissues and exhibit low but variable turnover, depending on age, activity and type of bone[6–11]. Older individuals tend to lose more bone than they replace during the bone recycling process. In general, bomb pulse dating of bone collagen can be used to determine if someone was alive during the period of the pulse, but cannot determine a date of birth or death[12]. Cartilage has similar limitations as bone[13]. Like bone, cartilage integrates a lifetime of 14C exposure with variable turnover into the tissue. The slow, but variable turnover of cartilage makes it an unsuitable tissue for age determination.
Although dental enamel is the hardest substance in the body, teeth are not routinely used in traditional radiocarbon dating due to fear of carbonate mineral exchange during centuries of burial. After being produced in childhood or adolescence, there is no turnover of enamel throughout life, and the 14C concentration of the enamel reflects the level in food sources at the time of enamel formation. Since teeth are formed at distinct, well-documented ages during childhood[4,14], the 14C concentration in dental enamel can be used to determine an approximate date of birth[15,16]. The absence of bomb-pulse carbon from enamel places date of birth no more recent than the early 1950’s or 40’s (depending on the tooth number analysed). The technique reports accurate determination of teeth of known age with precision ± 1.5 years, a significant improvement over previous techniques[15].
Processing of enamel samples is different than soft tissues because the carbon resides in a mineral matrix rather than protein. Enamel samples must be dissolved in acid and the liberated CO2 must be trapped for isotopic analysis. In collagen separations, the mineral phase is dissolved and discarded and the protein not affected by the acid is retained for isotopic analysis. The live part of the tooth, principally dentin, is similar to bone, with high collagen content and slow turnover and recycling of the carbon. Its collagen provides little information from 14C other than whether an individual was alive during the pulse[10].
Enamel preparation
The crown of the tooth is cut away from the root at the level of the cervical line. The crown is then immersed in 10N NaOH (Merck >99%) and placed in a water-bath sonicator overnight (Branson 150). The temperature of the water in the sonicator bath warms up gradually over time, to a maximum temperature of 70° C. Approximately every 24 hrs the NaOH is replaced and the soft non-enamel structures etched away using blunt dissection. The enamel is then washed in DDH2O, re-submersed in 10N NaOH, and returned to the sonicator. This procedure is repeated daily until all dentin and soft structures are stripped from the enamel. The enamel is then rinsed several times in DDH2O and dried at 65° C overnight. Enamel is weighed to the nearest 0.1 mg and kept sealed in a glass tube ready for accelerator mass spectrometer (AMS) pre-treatment.
AMS sample preparation
Aliquots of the enamel samples are placed in culture tubes for pre-treatment to remove the surface carbon that may have contaminated the enamel between formation and analysis. Since the carbon content of enamel is 0.5–0.6%, 80–150 mg aliquots are typically used to get full sized samples for 14C analysis. Enamel samples are immersed in 1.0N HCl at room temperature for 1.5 hr, rinsed 3 times with 18.2 MΩ DDH2O and placed on a heating block at 95° C to dry overnight. The acid pre-treatment is designed to etch the outer surface of the enamel that was exposed to the harsh alkali environment earlier without dissolving too much of the enamel. Base always contains some carbonate that can potentially exchange with the enamel during the enamel preparation step. Furthermore, alkali solutions remove CO2 from the atmosphere and produce carbonate and bicarbonate in solution that can precipitate. Each dried enamel sample is broken into 5–10 pieces and placed in an individual single-use reactor and again weighed to the nearest 0.1 mg. The harsher acid etching method removes a couple milligrams of exterior enamel surface in a 100 mg enamel sample. Enamel splits are hydrolyzed to CO2 in individual reaction chambers, evacuated, heated and acidified with concentrated orthophosphoric acid at 90° C. The evolved CO2 is purified, trapped, and reduced to graphite in the presence of iron catalyst in individual reactors[17,18]. With the aliquots used, nearly all CO2 samples are > 500μg C. CO2 is split and δ13C measured by stable isotope ratio mass spectrometry. A δ13C correction of −15 +/−2 is typically used for samples that are too small to obtain a CO2 split. Background values are controlled by consistently following procedures, frequently baking sample tubes and CuO oxidizer, and maintaining a clean lab[19].
AMS Sample measurement and analysis
Graphite targets have previously been measured using the 10-MV HVEE FN-class tandem electrostatic AMS system at the Center for Accelerator Mass Spectrometry at LLNL. The general operation is similar to that described by reference 19 when performing high-precision measurements of 18,000 year old turbidites used as secondary standards. Details on the design of the AMS system and its operation can be found in the literature[20–23]. The system employs a LLNL designed high-output solid graphite Cs-sputter source[21] which emits ~300 μA of 12C− from a full-sized sample, corresponding to ~900 14C counts per second from a contemporary sample. The FN AMS system routinely achieves 15% total system efficiency for C[23]. Enamel samples are usually full sized and contemporary, so analysis times are relatively rapid, generally about 5 minutes. The enamel samples are measured for 30,000 14C counts per cycle for 4–7 cycle repetitions and achieve standard deviations of 0.3–0.8%.
Corrections for background contamination introduced during AMS sample preparation are made following the procedures of [24]. All data are normalized with six identically prepared NIST SRM 4990B (Oxalic Acid I) primary standards. NIST SRM 4990C (Oxalic Acid II), IAEA-C6 (formerly known as ANU sucrose)[25], and TIRI wood[26] are used as secondary standards and quality controls to monitor spectrometer performance. The ratio of NIST SRM 4990C to NIST SRM 4990B (Oxalic Acid II/Oxalic Acid I) measured between February 2005 and October 2008 on 18 different sample wheels containing enamel samples had an average value of 1.291±0.002 (1 SD), in agree with the certified value of 1.293±0.001. The measured value of IAEA-C6 on the same sample wheels was 1.503±0.004 fraction modern, also in agreement with the recommended certificate value of 1.506±0.001 fraction modern. 14C-free calcite serves as background material for processing the enamel samples. The enamel samples are organized in groups of 10–14 unknowns bracketed by primary standards with one primary standard in the middle of the group. The secondary standards, primary standards and group of unknowns are measured consecutively as a cycle. Upon completion of a cycle the set of primary standards, secondary standards and unknown samples are measured again until desired precision is achieved. A typical group of 14 enamel samples is measured completely in 2–3 h. The measurement error is determined for each sample and generally ranges between ± 0.2 – 0.8% (1 SD). All 14C data are reported as decay corrected Δ14C following the dominant convention of [27]. This convention established for reporting radiocarbon data in chronological and geophysical studies was not developed to deal with post-bomb data, but it is the most common pending the adoption of a standard nomenclature for post-bomb data[28]. Δ14C was calculated using the following formula:
F14C is defined in Eq. 2 of [28]. It is enrichment or depletion of 14C relative to oxalic acid standard normalized for isotope fractionation. λ = 1/8267 yr−1. y = year of measurement after 1950 A.D.
From 14C to year of birth
The average age at which enamel formation is completed for each specific tooth has been determined previously and is dependent on the tooth number and gender of the person[4,15]. In cases where the sex of a person is unknown, the average time for enamel completion for males and females can be calculated. To estimate an individual’s date of birth (i) 14C concentration measured in the tooth enamel is plotted on to a curve of atmospheric 14C against time (for northern atmospheric 14C levels, see Figure 1a) to determine the year of enamel synthesis and (ii) the time (in years) taken for the enamel to form is subtracted from the year obtained (see Figure 1b). This gives an estimated date of birth.
If it is not obvious whether an individual is born before or after the peak of the bomb tests, then two teeth with different enamel laydown times need to be analysed – this will distinguish whether the 14C measurement relates to the rising or falling part of the curve[15].
Conclusions
Radiocarbon dating of human tooth enamel provides a reliable and accurate dating strategy for determining the date of birth of an individual. Traditional methods used for age determination in adults can yield error margins of +/− 10 years[29]. Such estimates offer little help to police authorities who as a first step towards making an identification try to match unidentified bodies/remains to missing persons lists. Given the significantly improved precision in age determination using radiocarbon dating, and the increasing availability (especially of smaller ‘table-top’ AMS models) and precision of AMS, application of the outlined dating method offers considerable potential for routine forensic application.
Acknowledgments
This work was supported by grants from the Human Frontiers Science Program and by NIH/NCRR (RR13461). This work was performed in part under the auspices of the U.S. Department of Energy by Lawrence Livermore National Laboratory under Contract DE-AC52-07NA27344.
References
- 1.Hua Q, Barbetti M. Radiocarbon. 2004;46:1273. [Google Scholar]
- 2.Levin I, Kromer B. Radiocarbon. 2004;46:1261. [Google Scholar]
- 3.Ubelaker DH, Buchholz BA. Forensic Science Communications. 2006;8 online journal. http://www2.fbi.gov/hq/lab/fsc/backissu/jan2006/research/2006_01_research01.htm.
- 4.Nolla CM. J Dent Child. 1960;27:254. [Google Scholar]
- 5.Taylor RE, Suchey JM, Payen LA, Slota PJ. J Forensic Sciences. 1989;34:1196–1205. [PubMed] [Google Scholar]
- 6.Jackson SH, Heininger JA. Biochim Biophys Acta. 1975;381:359. doi: 10.1016/0304-4165(75)90241-x. [DOI] [PubMed] [Google Scholar]
- 7.Manolagas SC, Jilka RL. N Engl J Med. 1995;332:305. doi: 10.1056/NEJM199502023320506. [DOI] [PubMed] [Google Scholar]
- 8.Parfitt AM. Bone. 2002;30:809. [Google Scholar]
- 9.Babraj JA, Smith K, Cuthbertson DJ, Rickhuss P, Dorling JS, Rennie MJ. J Bone Miner Res. 2005;20:930. doi: 10.1359/JBMR.050201. [DOI] [PubMed] [Google Scholar]
- 10.Ubelaker DH, Buchholz BA, Stewart J. J Forensic Sciences. 2006;51:488. doi: 10.1111/j.1556-4029.2006.00125.x. [DOI] [PubMed] [Google Scholar]
- 11.Hedges REM, Clement JG, Thomas CDL, O’Connell TC. Am J Phys Anthropol. 2007;133:808. doi: 10.1002/ajpa.20598. [DOI] [PubMed] [Google Scholar]
- 12.Wild E, Golser R, Hille P, Kutschera W, Priller A, Puchegger S, Rom W, Steier P, Vycudilik W. Radiocarbon. 1998;40(1):273–281. [Google Scholar]
- 13.Libby WF, Berger R, Mead JF, Alexander GV, Ross JF. Science. 1964;146:1172. doi: 10.1126/science.146.3648.1170. [DOI] [PubMed] [Google Scholar]
- 14.Bolanos MV, Manrique MC, Bolanos MJ, Briones MT. Forensic Sci Int. 2000;110:97. doi: 10.1016/s0379-0738(00)00154-7. [DOI] [PubMed] [Google Scholar]
- 15.Spalding KL, Buchholz BA, Druid H, Bergman LE, Frisén J. Nature. 2005;437:333. doi: 10.1038/437333a. [DOI] [PubMed] [Google Scholar]
- 16.Cook GT, Dunbar E, Black SM, Xu S. Radiocarbon. 2006;48:305. [Google Scholar]
- 17.Vogel JS, Southon JR, Nelson DE. Nucl Instrum Methods Phys Res Sect B. 1987;29:50. [Google Scholar]
- 18.Santos GM, Southon JR, Druffel-Rodriguez KC, Griffin S, Mazon M. Radiocarbon. 2004;46:165. [Google Scholar]
- 19.Zermeño P, Kurdyla DK, Buchholz BA, Heller SJ, Kashgarian M, Frantz BR. Nucl Instrum Methods Phys Res Sect B. 2004;223:293. [Google Scholar]
- 20.Guilderson TP, Southon JR, Brown TA. Radiocarbon. 2003;45:75. [Google Scholar]
- 21.Davis JC, Proctor ID, Southon JR, Caffee MW, Heikkinen DW, Roberts ML, Moore TL, Turteltaub KW, Nelson DE, Lloyd DH, Vogel JS. Nucl Instrum Methods Phys Res, Sect B. 1990;52:269. [Google Scholar]
- 22.Southon JR, Roberts ML. Nucl Instrum Methods Phys Res, Sect B. 2000;172:257. [Google Scholar]
- 23.Fallon SJ, Guilderson TP, Brown TA. Nucl Instrum Methods Phys Res Sect B. 2007;259:106. [Google Scholar]
- 24.Brown TA, Southon JR. Nucl Instrum Methods Phys Res Sect B. 1997;123:208. [Google Scholar]
- 25.Rozanski K, Stichler W, Gonfiantini R, Scott EM, Beukens RP, Kromer B, van der Plicht J. Radiocarbon. 1992;34:506. [Google Scholar]
- 26.Scott EM. Radiocarbon. 2003;45:135. [Google Scholar]
- 27.Stuiver M, Polach HA. Radiocarbon. 1977;19:355. [Google Scholar]
- 28.Reimer PJ, Brown TA, Reimer RW. Radiocarbon. 2004;46:1299. [Google Scholar]
- 29.Ritz-Timme S, Cattaneo C, Collins MJ, Waite ER, Schütz HW, Kaatsch HJ, Borrman HI. Int J Legal Med. 2000;113(3):129–36. doi: 10.1007/s004140050283. [DOI] [PubMed] [Google Scholar]
- 30.Stuiver M, Reimer PJ, Bard E, Beck JW, Burr GS, Hughen KA, Kromer B, McCormac G, Van der Plicht J, Spurk M. Radiocarbon. 1998;40:1041. [Google Scholar]
- 31.Stuiver M, Reimer PJ, Baziunas TF. Radiocarbon. 1998;40:1127. [Google Scholar]