Abstract
The death of over 300 sows in 2 months on a 3000 sow farrow-to-isowean operation in Manitoba was attributed to infection with Actinobacillus equuli. This pathogen commonly infects foals, and is rarely reported in swine. Our report is the second recently published case of this pathogen in North American swine.
Résumé
Actinobacillus equuli comme agent pathogène primaire chez les truies de reproduction et les porcelets. La mort de plus de 300 truies en 2 mois dans une exploitation de 3000 truies de la mise bas au sevrage en isolement au Manitoba a été attribuée à l’infection par Actinobacillus equuli. Cet agent pathogène infecte communément les poulains et est rarement signalé chez les porcs. Notre rapport est le deuxième cas à être récemment publié pour cet agent pathogène chez les porcs nord-américains.
(Traduit par Isabelle Vallières)
Actinobacillus equuli, a gram-negative, rod-shaped bacterium that naturally occurs as a commensal on the mucosal membranes of adult horses, is commonly the cause of fatal septicemia in newborn foals (1–6). Foals are thought to be directly infected by the mare through the mouth or respiratory tract during or immediately following birth (6).
On rare occasions, A. equuli has been reported to be an opportunistic pathogen of pigs (1). The infection of pigs is typically associated with abortion, septicemia, and polyarthritis (1,2,7,8). Recently, this bacterium was isolated from a Manitoba sow herd that experienced the death of more than 300 animals over the span of approximately 2 mo. This case is unique in that it featured peracute clinical disease progression and abnormally high mortality.
Case description
Seven sows died suddenly on a farm in Manitoba that had previously tested positive for porcine reproductive and respiratory syndrome (PRRS) virus and Mycoplasma hyopneumoniae. Clinically affected individuals ceased feeding and died rapidly, often within 6 to 12 h. Other clinical signs included lethargy, recumbency, and hyperesthesia, particularly of the limbs.
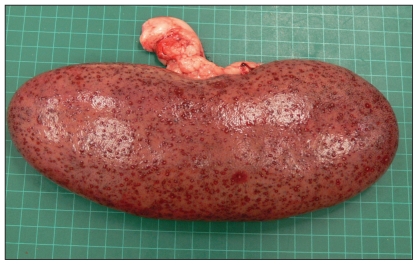
Necropsies were performed at the farm and at the Veterinary Diagnostic Services Laboratory (VDS) at Manitoba Agriculture, Food and Rural Initiatives, and all postmortem examinations revealed consistent findings. Carcasses were in good body condition but eyes were sunken into the orbit, consistent with dehydration. Multifocal subcutaneous hemorrhages were observed throughout the carcass, along with epicardial and endocardial hemorrhages. The pericardial sac contained a small volume of clear serosanguineous fluid that was mixed with fibrin strands. The lungs were congested and edematous with foam in the trachea lumen. The liver was also congested. Kidneys had multiple spots on the surface (Figure 1), and bilaterally, within the renal cortex of the kidneys, there were multifocal embolic infarcts (1 to 3 mm) characterized by a pale necrotic center surrounded by a ring of hemorrhage. One kidney had several large renal cysts, an incidental finding. The spleen was also diffusely congested and enlarged. The serosal surfaces of the intestines in 1 carcass were lackluster with a ground glass appearance.
Figure 1.
Sow kidney with embolic nephritis resulting from septicemia caused by Actinobacillus equuli.
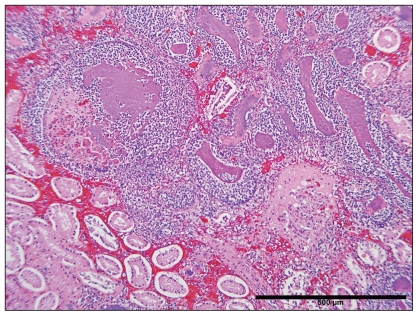
Histopathological examination of tissues revealed congested kidneys with multifocal, randomly distributed, and often coalescing emboli (Figure 2). Emboli were composed primarily of fibrin, as well as degenerate and non-degenerate neutrophils embedded with basophilic bacilli that were 1 μm wide and 2 μm long. Rare bacterial emboli were also observed within the interstitium of the grossly hemorrhagic skeletal muscle where they were often associated with scattered muscle fiber necrosis. Proteinaceous fluid was observed expanding the epicardium of the heart along with multifocal subendocardial hemorrhages. Small blood vessels within the myocardium also contained large colonies of bacilli. The liver and lungs were diffusely congested and fibrin thrombi, associated with similar bacterial emboli, were observed in the alveolar capillaries of the lungs. Bacterial emboli were also identified in various tissues including the spleen and adrenal gland.
Figure 2.
Bacterial emboli in the kidney of a sow infected with Actinobacillus equuli (hematoxylin and eosin). Bar = 500 μm.
The large numbers of gram-negative bacilli observed in the lung and kidney tissue were identified as A. equuli by bacteriology. This initial classification was based on a Gram stain (gram-negative rods), oxidase test (positive), and urease test (positive) that, together, identified the organism as A. equuli. The organism was not beta hemolytic on tryptic soy agar (TSA) with 5% sheep blood. The bacterium was later more specifically identified as A. equuli subsp. equuli by the Animal Health Centre (Abbotsford, British Columbia) using a bacterial identification system (Biolog Microlog Version 4; Biolog, Hayward, California, USA). In addition, test results were negative for bile esculin. The 16S ribosomal RNA gene was sequenced as described elsewhere (9) and was found to be 100% identical with that of A. equuli subsp. equuli.
Samples were negative when tested by polymerase chain reaction (PCR) for PRRS and H1N1 and H3N2 swine influenza viruses, but positive when tested by PCR for porcine circovirus in 1 set of lungs, although there was no histological evidence of circovirus-associated disease. Thus, the final diagnosis was A. equuli septicemia with embolic nephritis. Other differentials included systemic disease due to porcine circovirus, classical swine fever, Salmonella, Erysipelothrix rhusiopathiae, Actinobacillus suis, Leptospira sp., and Streptococcus suis.
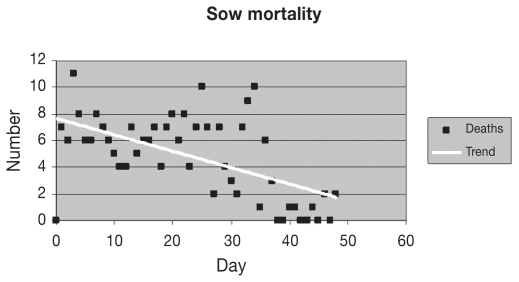
Antibiotic susceptibility tests indicated that the bacterium identified as A. equuli was sensitive to tetracycline, neomycin, trimethoprin, ceftiofur, spectinomycin, and ampicillin. Preventative antibiotic therapy was aggressively administered throughout the 2-month course of the outbreak using both feed- and water-based antibiotics that were indicated in the bacterial sensitivity testing. Individual sows were also treated with long-acting injectable antibiotics and anti-inflammatory therapy. Farm staff used clinical signs (failure to rise when touched) and body temperature readings to screen sows requiring individual treatment. There was some response to injectable antibiotics and anti-inflammatory drugs, although some of the sows that survived would eventually waste and require euthanasia or culling. Daily sow mortality was highly variable; however, mortality levels declined with time (Figure 3).
Figure 3.
Daily sow mortality in a herd (initial population of 3000) infected with Actinobacillus equuli.
An autogenous killed vaccine was developed and, within 6 wk following the onset of the clinical outbreak, the entire sow herd was vaccinated with 1 dose of vaccine and boosted with a second dose 2 to 3 wk later. In order to prevent clinical disease in replacement animals, gilts were vaccinated twice prior to entry into the herd.
Approximately 1 mo after the initial case, 2 sows from the same barn were presented for complete necropsy. Necropsy and histological lesions were consistent with the previous cases described, although A. equuli was now found to be resistant to tetracycline with in vitro antibiotic sensitivity testing.
In addition to sow mortality, 1- to 4-day-old piglets from rooms that had been populated by sows originating from areas of the barn affected by A. equuli suffered pyrexia, arthritis, and mortality. Six of the affected piglets were submitted to VDS for complete necropsy. Bilaterally, kidneys had multifocal pinpoint red foci scattered throughout the renal cortices. Within the joint spaces, increased volumes of pale yellow serosanguineous fluid with fibrin were observed. One-day-old piglets had periarticular fibrin and edema, while older piglets had small accumulations of fibrin within the thoracic and abdominal cavities. Histologically, there was evidence of embolic nephritis, although only rare bacterial colonies could be identified within the lesion. Fibrinous peritonitis, periarthritis, and arthritis were also confirmed. Escherichia coli, Staphylococcus aureus, and other bacteria in mixed cultures were isolated from tissues. Actinobacillus equuli organisms were only cultured from the joint of 1 piglet, but animals had been treated with antibiotics prior to euthanasia. Results were PCR negative for PRRS and classical swine fever, and positive for porcine circovirus. Thus, bacteriology results were inconclusive; however, infection with A. equuli in utero or immediately following birth was considered a distinct possibility.
Discussion
Two subspecies of A. equuli, namely, A. equuli subsp. haemolyticus and A. equuli subsp. equuli, have been identified (2,4,10). Sequencing of the 16S rRNA was performed to confirm the identity of the isolate, but sequencing alone is not sufficient to differentiate between the 2 subtypes (11). The subspecies are distinguishable by their contrasting hemolytic and CAMP reactions. Actinobacillus equuli subsp. haemolyticus is hemolytic and CAMP-positive, while A. equuli subsp. equuli is non-hemolytic and CAMP-negative (10). To date, A. equuli subsp. haemolyticus has only been isolated from horses, whereas A. equuli subsp. equuli has been isolated from pigs and horses, and has been implicated in both sleepy foal disease and piglet septicemia (2,10). Results from this case study support these findings as the pathogenic species from the affected sows was subtyped as A. equuli subsp. equuli. Our tests simultaneously ruled out the possibility of A. suis, another pathogen of pigs causing similar lesions, given that this bacterium, unlike A. equuli subsp. equuli, is hemolytic (1).
As previously noted, the mare is the primary source of infection in foals, and there is conflicting evidence concerning the role of the adult horse as the source of infection in pigs as well (1,6,7). One confirmed outbreak occurred on a farm that housed numerous horses over several years (12), while another case is known to have occurred on a farm where the previous owner was a horse dealer, though horses had not been on the premises for at least 2 y prior to the infection (7). A recent case in the United States, however, could not attribute the disease to transmission from horses as the affected pig had no known contact with horses (1). Thus, the source appears to remain unknown, as is the case with the current outbreak. The farm had excellent biosecurity standards. The infected sow herd had no direct contact with horses, although several barn employees were known to be in contact with horses. Water, feed, and mice in the area, as well as another 3000 sow farrowing unit located 900 m from the infected unit, all tested negative by culture for the bacterium. A pasture with grazing cattle surrounded the barn, but these were not tested for the organism. Further investigation on potential sources of infection of pigs with A. equuli is essential for establishing appropriate management and preventative measures.
The first recorded report of A. equuli infection in pigs in the United States was documented in 1941 and there have been a limited number of cases subsequently (7). Recently, however, a 1-year-old gilt died as a result of disease caused by A. equuli, representing what is thought to be the first case in the United States in 30 y (1). The outbreak of A. equuli in this report represents the second case in pigs within a relatively short time frame. The case itself is unique as it documents the infection and death of over 300 sows from an initial population of 3000 sows. To our knowledge, such a high level of mortality in adult pigs resulting from septicemia caused by A. equuli has never been documented. There have been other cases of A. equuli in Canada, but these cases occurred in piglets and did not manifest in sows, and were easily controlled using antibiotics (Dr. Neil Shantz, Warman Veterinary Services, Warman, Saskatchewan, personal communication). These cases demonstrate that A. equuli can act as a secondary pathogen.
It is crucial that practitioners and laboratory diagnosticians become aware of the potential for A. equuli to cause disease in pigs, considering that the number and severity of cases in North America may be on the rise. Producers should also be aware of the possibility of infection, particularly where pigs are housed with horses or in areas where horses were previously kept. This knowledge will allow for appropriate and timely biosecurity measures to be undertaken to minimize transmission and serious disease outbreaks. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Ramos-Vara JA, Wu CC, Mitsui I, Lin TL, Miller MA. Metritis, valvular endocarditis, and septicemia by Actinobacillus equuli in a gilt in the United States. Vet Pathol. 2008;45:495–499. doi: 10.1354/vp.45-4-495. [DOI] [PubMed] [Google Scholar]
- 2.Aalbaek B, Ostergaard S, Buhl R, Jensen HE, Christensen H, Bisgaard M. Actinobacillus equuli subsp. equuli associated with equine valvular endocarditis. APMIS. 2007;115:1437–1442. doi: 10.1111/j.1600-0463.2007.00768.x. [DOI] [PubMed] [Google Scholar]
- 3.Sternberg S, Johannisson A, Magnusson U, Jensen-Waern M. Effects of Actinobacillus equuli culture supernatants on equine neutrophil functions and survival. J Vet Med B. 1999;46:595–602. doi: 10.1046/j.1439-0450.1999.00285.x. [DOI] [PubMed] [Google Scholar]
- 4.Holyoak GR, Smith CM, Boyette R, et al. Serum antibodies in mares and foals to Actinobacillus equuli whole cells, outer membrane proteins, and Aqx toxin. Vet Immunol Immunopath. 2007;118:310–316. doi: 10.1016/j.vetimm.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 5.Pusterla N, Jones MEB, Mohr FC, et al. Fatal pulmonary hemorrhage with RTX toxin-producing Actinobacillus equuli subspecies haemolyticus infection in an adult horse. J Vet Diagn Invest. 2008;20:118–121. doi: 10.1177/104063870802000127. [DOI] [PubMed] [Google Scholar]
- 6.Rycroft AN, Garside LH. Actinobacillus species and their role in animal disease. Vet J. 2000;159:18–36. doi: 10.1053/tvjl.1999.0403. [DOI] [PubMed] [Google Scholar]
- 7.Windsor RS. Actinobacillus equuli infection in a litter of pigs and a review of previous reports on similar infections. Vet Rec. 1973;92:178–180. doi: 10.1136/vr.92.7.178. [DOI] [PubMed] [Google Scholar]
- 8.Moyaert H, Decostere A, Baele M, et al. An unusual Actinobacillus equuli strain isolated from a rabbit with Tyzzer’s disease. Vet Microbiol. 2007;124:184–186. doi: 10.1016/j.vetmic.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 9.Cai H, Archambault M, Prescott JF. 16S ribosomal RNA sequence–based identification of veterinary clinical bacteria. J Vet Diagn Invest. 2003;15:465–469. doi: 10.1177/104063870301500511. [DOI] [PubMed] [Google Scholar]
- 10.Christensen H, Bisgaard M, Olsen JE. Reclassification of equine isolates previously reported as Actinobacillus equuli, variants of A. equuli, Actinobacillus suis or Bisgaard taxon 11 and proposal of A. equuli subsp. equuli subsp. nov. and A. equuli subsp. haemolyticus subsp. nov. Int J Syst Evol Microbiol. 2002;52:1569–1576. doi: 10.1099/00207713-52-5-1569. [DOI] [PubMed] [Google Scholar]
- 11.Aalbaek B, Ostergaard S, Buhl R, Jensen HE, Christensen H, Bisgaard M. Actinobacillus equuli subsp. equuli associated with equine valvular endocarditis. APMIS. 2007;115:1437–42. doi: 10.1111/j.1600-0463.2007.00768.x. [DOI] [PubMed] [Google Scholar]
- 12.Edwards PR, Taylor EL. Shigella equirulis infection in a sow. Cornell Vet. 1941;31:392–393. [Google Scholar]