Abstract
A 13-year-old, intact male, mixed-breed dog was evaluated for multiple intradermal nodules around the anus. The nodules were diagnosed as perianal gland adenoma based on histopathologic examination. After therapy with cyclosporin A for 5 wk, the perianal masses were moderately shrunken. The dog’s condition has remained stable over 6 mo.
Résumé
Traitement à la cyclosporine d’un adénome des glandes péri-anales concomitantes avec une hyperplasie prostatique bénigne chez un chien. Un chien mâle intact de race croisée âgé de 13 ans a été évalué pour des nodules intradermiques multiples autour de l’anus. Les nodules ont été diagnostiqués comme un adénome des glandes péri-anales en se basant sur un examen histopathologique. Après une thérapie à la cyclosporin A pendant 5 semaines, les masses péri-anales étaient modérément réduites. L’état du chien est demeuré stable pendant 6 mois.
(Traduit par Isabelle Vallières)
Cyclosporin A is a cyclic oligopeptide macrolide that is extensively employed in organ transplantation, auto-immune disorders, and dermatology of human and veterinary medicine due to its immunosuppressive properties (1). It also has a potential for the treatment of perianal fistulae and epitheliotropic lymphoma in dogs (2,3).
Three types of glandular tumors, namely perianal gland (or circumanal or hepatoid) tumor, apocrine gland tumor of the anal sac, and apocrine gland tumor commonly occur in the perianal area of the dog (4). The biological behavior of each type varies considerably (5). Most perianal gland swellings are focal hyperplasia and the benign proliferative form (adenoma), while their malignant counterparts (adenocarcinoma) are uncommon.
As a therapeutic approach, castration and tumor removal with surgery or cryosurgery is effective for most adenomas (6). Estrogens have been used in the past to reduce tumor volume but carry a significant risk of bone marrow suppression (7). No other pharmacological treatments have been used for hepatoid gland adenoma in dogs. There is only 1 report describing the inhibitory effects of cyclosporin A on proliferation and secretion but in another type of adenoma (pancreatic adenoma) under in vitro conditions (8).
The purpose of this case report is to determine whether administration of cyclosporin A systemically can reduce the proliferative activity of perianal gland tumors in a dog.
Case description
A 13-year-old, intact male, mixed-breed dog was evaluated because of multiple, 0.5 to 1.5 cm in diameter, round, intra-dermal nodules around the anus. There was a history of intermittent hematochezia and perianal nodules causing perianal pain and colorectal obstruction with tenesmus. On physical examination, the lesions of the perianal area were ulcerated and rectal mucous membrane was mildly protruded through the anus (Figure 1A). Hemorrhagic and keratinaceous material from the nodules was extruded with local pressure. Radiography showed prostatomegaly with a mildly compressed rectum (Figure 2). Ultrasonographic examination revealed a diffuse, symmetrically enlarged prostate gland. A punch biopsy (4 mm in diameter) using local anesthesia with lidocaine (Lidocaine HCl Inj. 0.5%; KunWha Pharma, Seoul, Korea) was taken on the right margin of the mass below the anus in the 4 o’clock direction. A perianal gland adenoma was diagnosed based on histopathological examination, which revealed hepatoid epithelial cells with round nuclei with 1 or 2 prominent nucleoli, and moderately dense light eosinophilic granular cytoplasm (Figure 3). The perianal gland adenoma was surrounded by a thin fibrous capsule and a well-developed stroma was noted. According to histopathologic, radiographic and ultrasonographic findings, this case was diagnosed as perianal gland adenoma concurrent with benign prostatic hyperplasia. This patient underwent medical control with cyclosporine (Implanta; Hanmi Pharm, Seoul, Korea), 5 mg/kg, BID, PO, for 5 wk. One week after therapy with cyclosporin A, clinical signs including swelling and exudation had progressively improved without any side effects. After 2 weeks of therapy with cyclosporin A the perianal lesions had partially regressed (0.35 to 1.1 cm in diameter) (Figure 1B). At the completion of the treatment all clinical signs including pain and tenesmus had disappeared. The size of the mass around the anus had remained moderately decreased (0.2 to 0.8 cm in diameter) (Figure 1C) and the prostate seemed to remain mildly shrunken on rectal palpation. Since then the dog has been normal without any side effects or relapses.
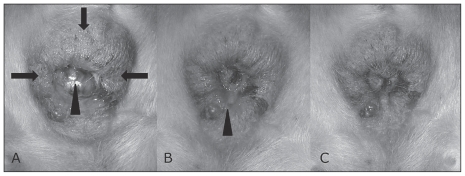
Figure 1.
A — These lesions were ulcerated and the rectal mucous membrane was mildly protruded (arrowhead) due to the masses (arrows). B — Two weeks after cyclosporine therapy. Shrinkage of the lesion and mild exudation are apparent (arrowhead). C — Moderate remission of the perianal lesion 5 wk after cyclosporine therapy. Exudation and protrusion of the rectal mucous membrane have disappeared.
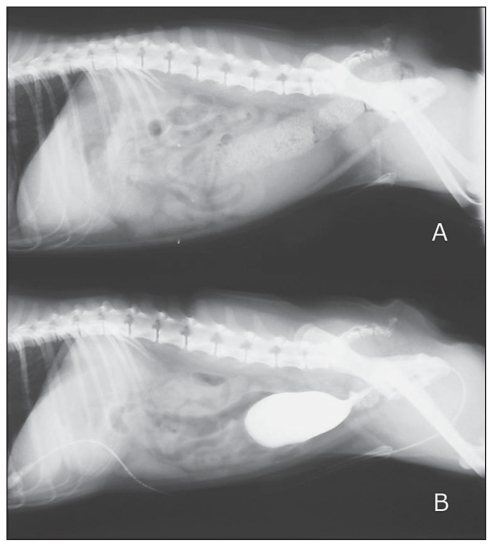
Figure 2.
A — Lateral abdominal radiograph of the dog showing prostatomegaly. B — Retrograde urethrocystography reveals reflux of contrast media into the prostate gland.
Figure 3.
Histopathologically, perianal gland adenoma was clearly observed. In addition, the well-developed stroma surrounding the lobules and hepatoid cells was noted (×100, hematoxylin & eosin).
Discussion
Cyclosporin A is an immunomodulatory/immunosuppressive drug that inhibits T-helper cell activity. Since T-cells orchestrate most chronic immune responses, cyclosporine has broad anti-inflammatory effects (9). Its systemic indications currently include many autoimmune disorders. Recently, cyclosporin A as a calcineurin-inhibitor has been applied successfully to control atopic dermatitis (AD) in dogs (10–13).
The epidemiology of perianal gland neoplasms and their relationship with endogenous sex hormones was described in 472 dogs (4). Neoplasms of the perianal gland are usually slow-growing and adenomas are diagnosed about 4.5 times more often than carcinomas (14). These tumors occur primarily in male dogs and the maintenance of androgen receptors in adenomas of the perianal gland throughout cancer progression provides evidence for the hormone dependency of these tumors (15). Surgical resection including castration in male dogs has most commonly been used to treat perianal gland adenomas; however, fecal incontinence is a common complication following surgical intervention because anal sphincter muscle contiguous to the perianal gland can be damaged during surgery. In the present case, the clients declined to castrate the dog to take further opportunity for breeding; therefore, we decided to provide only medical treatment with cyclosporin A, for its anti-proliferative effects. Previous studies demonstrated that cyclosporin A inhibits testosterone biosynthesis in vivo (16) and in vitro (17). Sikka et al (18) reported that oral administration of cyclosporin A impaired testosterone synthesis mainly by inhibiting the hypothalamopituitary axis function. A decline in testosterone biosynthesis in a dose-dependent manner is attributable to blocking the signal transduction pathway (17). We suggest that testosterone biosynthesis was probably suppressed in this case after using cyclosporin A. A decrease in size of the prostate gland in this dog may be due to the suppression of testosterone, which can regress benign prostatic hyperplasia (BPH). Thus, clinical signs of BPH (tenesmus, persistent or intermittent hematuria, and bleeding) can be improved by reducing the level of testosterone. But there was no change in sexual behavior in this dog. In addition, it is thought that perianal gland adenoma concurrent with rectal compression by BPH caused hematochezia in this dog.
Sjöholm (18) described the inhibitory property of cyclosporin A on rat insulinoma cell proliferation, polyamine content, and insulin secretion. The mechanism of this anti-proliferative effect is suggested to be due to cytotoxicity of cyclosporin A. Cyclosporine induces apoptosis in related cell types by a mechanism similar to inhibition of calcineurin/nuclear factor signaling of activated T-cells (19,20). Mathews and Sukhiani (21) showed that cyclosporin A was effective in dogs with perianal fistulas, which is suggested to be involved with an abnormality in immune function but the exact mechanism is unknown (2,21,22). Hernández et al (23) revealed that cyclosporin A inhibits migration of primary endothelial cells and angiogenesis induced by vascular endothelial growth factor (VEGF), which contributes to tumor neovascularization and is increased in nearly every type of cancer. This growth factor belongs to a family including the placenta growth factor (PGF), VEGF-B, VEGF-C, and VEGF-D, each of which interacts with a different tyrosine kinase receptor. Signaling through these receptors is blocked by cyclosporin A, which results in inhibition of tumor growth (24).
In our case, there was no evidence of inflammation in the peripheral region of the adenoma. At its early presentation the lesion was ulcerated probably as a consequence of the pressure produced by the space-occupying mass. The biopsy sample was taken from a non-ulcerated area of the mass and inflammatory cells were not detected. The presence of an inflammatory component, however, cannot be ruled out since not all of the mass was excised. Cyclosporine may contribute to resolution of the inflammation not only by anti-inflammatory action but also by decrease of the mass probably by apoptosis, but the actual mechanism is not understood.
Although dogs are less susceptible to the side effects of cyclosporin A than other animals based on toxicity studies in rats, mice, rabbits, and dogs (25), dogs may uncommonly experience gingival hyperplasia and papillomatosis, vomiting, diarrhea, bacteriuria, bacterial skin infections, anorexia, hirsuitism, involuntary shaking, nephropathy, bone marrow suppression, and lymphoplasmatoid dermatosis at daily doses of 20 to 30 mg/kg (26).
Potential side effects in animals due to cyclosporin A therapy can be expected at the level of various side effects reported in humans. Hypertrichosis occurs in 50% to 80% of human transplant recipients taking cyclosporine (27). Acute bone pain, joint swelling, and limb paraesthesia have been reported in humans taking cyclosporine after renal transplantation or for immune-mediated diseases (28). Patricelli et al (29) reported that lameness in dogs receiving cyclosporine has been associated with septic arthritis and possibly the recurrence of panosteitis. Neuropsychological effects, including tremor, headaches, depression, anxiety, confusion, and somnolence, have been reported in humans receiving cyclosporine (30).
Because there have never been studies on cyclosporine effects on perianal gland adenoma in animals, the authors were guided by dosage and duration reported in research on perianal fistula in dogs. The optimal dosage and therapy period for perianal adenoma, therefore, should be further studied. However, we may consider various therapeutic dosing plans of cyclosporine based on previous studies. First, cyclosporin A is most commonly administered orally at a daily dose of 5 mg/kg to treat atopic dermatitis in dogs (31,32). Despite the beneficial effect of cyclosporin A, the high cost of cyclosporin A prohibits its use in many cases requiring long-term management. Application of low dose cyclosporin A combined with ketoconazole has been studied in dogs with anal fistula (33). Ketoconazole is reported to extend the half-life of cyclosporin A in a dose-dependent manner by reducing the dose of cyclosporin A required to maintain therapeutic serum concentrations by 56% to 90% (34).
Estrogen can be considered as a medical treatment for suppressing testosterone in perianal gland adenoma when the owners refuse castration. However, bone marrow suppression and fatal anaplastic anemia are serious side effects of estrogen therapy. Recently, zinc gluconate/arginine, has become available for chemical castration of 3- to 10-month-old male puppies. Zinc causes testicular atrophy and decreases spermatogenesis with concurrent reduction of androgen concentration when injected in the testicular parenchyma (35).
Cyclosporin A was administered for 5 wk in the present case. This dog showed moderately improved perianal lesion and complete remission of concurrent clinical signs. The absence of clinical signs remained stable for 6 mo, possibly due to the anti-proliferative effects and anti-inflammatory action of cyclosporin A as described above. Information on mechanism and action of cyclosporine on perianal gland adenoma and other forms of canine tumors is required in large scale studies.
In conclusion, this is the first report which describes management and prognosis of perianal gland adenoma with long-term administration of cyclosporin A without radiation therapy and surgical resection. The purpose of this study was to determine if the administration of cyclosporine systemically could reduce the proliferative activity of perianal gland adenoma in a dog. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Robson DC, Burton GG. Cyclosporin: Applications in small animal dermatology. Vet Derm. 2003;14:1–9. doi: 10.1046/j.1365-3164.2003.00317.x. [DOI] [PubMed] [Google Scholar]
- 2.Mathews KA, Sukhiani HR. Randomized controlled trial of cyclosporine for treatment of perianal fistulas in dogs. J Am Vet Med Assoc. 1997;211:1249–1253. [PubMed] [Google Scholar]
- 3.Rosenkrantz WS, Griffin CE, Barr RJ. Clinical evaluation of cyclosporine in animal models with cutaneous immune mediated disease and epitheliotropic lymphoma. J Am Anim Hosp Assoc. 1989;25:377–384. [Google Scholar]
- 4.Hayes HM, Jr, Wilson GP. Hormone-dependent neoplasms of the canine perianal gland. Cancer Res. 1977;37:2068–2071. [PubMed] [Google Scholar]
- 5.Morris J, Dobson J. Small Animal Oncology. 1st ed. UK: Blackwell Science; 2001. Perianal tumors; pp. 135–137. [Google Scholar]
- 6.Liska WD, Withrow SJ. Cryosurgical treatment of perianal gland adenomas in the dog. J Am Anim Hosp Assoc. 1978;14:457–463. [Google Scholar]
- 7.Withrow SJ. Perianal tumors. In: Withrow SJ, MacEwen EG, editors. Small Animal Clinical Oncology. 3rd ed. Philadelphia: WB Saunders; 2001. pp. 346–353. [Google Scholar]
- 8.Sjöholm A. Inhibitory effects of cyclosporin A on rat insulinoma cell proliferation, polyamine content and insulin secretion. Mol Cell Endocrinol. 1994;99:21–24. doi: 10.1016/0303-7207(94)90141-4. [DOI] [PubMed] [Google Scholar]
- 9.Halloran PF, Kung L, Noujaim J. Calcineurin and the biological effect of cyclosporine and tacrolimus. Transplant Proceedings. 1998;30:2167–2170. doi: 10.1016/s0041-1345(98)00577-6. [DOI] [PubMed] [Google Scholar]
- 10.Fontaine J, Olivry T. Treatment of canine atopic dermatitis with cyclosporine: A pilot clinical study. Vet Rec. 2001;148:662–663. doi: 10.1136/vr.148.21.662. [DOI] [PubMed] [Google Scholar]
- 11.Olivry T, Rivierre C, Jackson HA, Murphy KM, Davidson G, Sousa CA. Cyclosporine decreases skin lesions and pruritus in dogs with atopic dermatitis: A blind randomized prednisolone-controlled trial. Vet Derm. 2002;13:77–87. doi: 10.1046/j.1365-3164.2002.00283.x. [DOI] [PubMed] [Google Scholar]
- 12.Olivry T, Steffan J, Fisch RD, et al. Randomized controlled trial of the efficacy of cyclosporine in the treatment of atopic dermatitis in dogs. J Am Vet Med Assoc. 2002;22:370–377. doi: 10.2460/javma.2002.221.370. [DOI] [PubMed] [Google Scholar]
- 13.Steffan J, Alexander D, Brovedani F, Fisch RD. Comparison of cyclosporine A with methylprednisolone for treatment of canine atopic dermatitis: A parallel blinded randomized controlled trial. Vet Derm. 2003;14:11–22. doi: 10.1046/j.1365-3164.2003.00318.x. [DOI] [PubMed] [Google Scholar]
- 14.Evans ER, Pierrepoint CG. Tissue-steroid interactions in canine hormone-dependent tumors. Vet Rec. 1975;97:464–467. [PubMed] [Google Scholar]
- 15.Pisani G, Millanta F, Lorenzi D, Vannozzi I, Poli A. Androgen receptor expression in normal, hyperplastic and neoplastic hepatoid glands in the dog. Res Vet Sci. 2006;8:231–236. doi: 10.1016/j.rvsc.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Seethalakshmi L, Menon M, Malhotra RK, Diamond DA. Effect of cyclosporine A on male reproduction in rats. J Urol. 1987;138:991–995. doi: 10.1016/s0022-5347(17)43479-3. [DOI] [PubMed] [Google Scholar]
- 17.Seethalakshmi L, Flores C, Malhotra RK, et al. The mechanism of cyclosporine’s action in the inhibition of testosterone biosynthesis by rat leydig cells in vitro. Transplantation. 1992;53:190–195. doi: 10.1097/00007890-199201000-00037. [DOI] [PubMed] [Google Scholar]
- 18.Sikka SC, Bhasin S, Coy DC, Koyle MA, Swerdloff RS, Rajfer J. Effects of cyclosporine A on the hypothalamic-pituitary-gonadal axis in the male rat: Mechanism of action. Endocrinology. 1988a;123:1069. doi: 10.1210/endo-123-2-1069. [DOI] [PubMed] [Google Scholar]
- 19.Eckstein LA, Van Quill KR, Bui SK, Uusitalo MS, O’Brien JM. Cyclosporine A inhibits calcineurin/nuclear factor of activated T-cells signaling and induces apoptosis in retinoblastoma cells. Invest Ophthalmol Vis Sci. 2005;46:782–790. doi: 10.1167/iovs.04-1022. [DOI] [PubMed] [Google Scholar]
- 20.Mosieniak G, Figiel I, Kaminska B. Cyclosporin A, an immuno-suppressive drug, induces programmed cell death in rat C6 glioma cells by a mechanism that involves the AP-1 transcription factor. J Neurochem. 1997;68:1142–1149. doi: 10.1046/j.1471-4159.1997.68031142.x. [DOI] [PubMed] [Google Scholar]
- 21.Mathews KA, Ayres SA, Tano CA, Riley SM, Sukhiani HR, Adams C. Cyclosporin treatment of perianal fistulas in dogs. Can Vet J. 1997;38:39–41. [PMC free article] [PubMed] [Google Scholar]
- 22.Killingsworth CR, Walshaw R, Reimann KA, Rosser EJ., Jr Thyroid and immunologic status of dogs with perianal fistula. Am J Vet Res. 1988;49:1742–1746. [PubMed] [Google Scholar]
- 23.Hernández GL, Volpert OV, Íñiguez MA, et al. Selective inhibition of vascular endothelial growth factor-mediated angiogenesis by cyclosporine A. J Exp Med. 2001;193:607–620. doi: 10.1084/jem.193.5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cockerill GW, Bert AG, Ryan GR, Gamble JR, Vadas MA, Cockerill PN. Regulation of granulocyte-macrophage colony-stimulating factor and E-selectin expression in endothelial cells by cyclosporine A and the T-cell transcription factor NFAT. Blood. 1995;86:2689–2698. [PubMed] [Google Scholar]
- 25.Gregory CR. Immunosuppressive agents. In: Bonagura J, editor. Kirk’s Current Veterinary Therapy XIII: Small Animal Practice. Philadelphia: WB Saunders; 1999. pp. 509–513. [Google Scholar]
- 26.Scott DW, Miller R, Griffin CE. Muller and Kirk’s Small Animal Dermatology. 6th ed. Philadelphia: WB Saunders; 2001. Dermatologic therapy; pp. 243–244. [Google Scholar]
- 27.Gafter-Gvili A, Sredni B, Gal R, Gafter U, Kalechman Y. Cyclosporine A-induced hair growth in mice is associated with inhibition of calcineurin-dependent activation of NFAT in follicular keratinocytes. Am J Physiol Cell Physiol. 2003;284:1593–1603. doi: 10.1152/ajpcell.00537.2002. [DOI] [PubMed] [Google Scholar]
- 28.Kart-Koseoglu H, Yucel AE, Isyklar I, Turker I, Akcaly Z, Haberal M. Joint pain and arthritis in renal transplant recipients and correlation with cyclosporine therapy. Proceedings of the Tissue Typing and Immunosuppression Symposium. 2002;555 doi: 10.1007/s00296-002-0283-y. [DOI] [PubMed] [Google Scholar]
- 29.Patricelli AJ, Hardie RJ, McAnulty JF. Cyclosporine and ketoconazole for the treatment of perianal fistulas in dogs. J Am Vet Med Assoc. 2002;220:1009–1016. doi: 10.2460/javma.2002.220.1009. [DOI] [PubMed] [Google Scholar]
- 30.Gijtenbeek JM, van den Bent MJ, Vecht CJ. Cyclosporine neurotoxicity: A review. J Neurol. 1999;246:339–346. doi: 10.1007/s004150050360. [DOI] [PubMed] [Google Scholar]
- 31.Burton G, Burrows A, Walker R, et al. Efficacy of cyclosporine in the treatment of atopic dermatitis in dogs-combined results from two veterinary dermatology referral centers. Aust Vet J. 2004;82:681–685. doi: 10.1111/j.1751-0813.2004.tb12153.x. [DOI] [PubMed] [Google Scholar]
- 32.Steffan J, Parks C, Seewald W. North American Veterinary Dermatology Cyclosporine Study Group. J Am Vet Med Assoc. 2005;226:1855–1863. doi: 10.2460/javma.2005.226.1855. [DOI] [PubMed] [Google Scholar]
- 33.O’Neill T, Edwards GA, Holloway S. Efficacy of combined cyclosporine A and ketoconazole treatment of anal furunculosis. J Small Anim Pract. 2004;45:238–243. doi: 10.1111/j.1748-5827.2004.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 34.Dahlinger J, Gregort C, Bea J. Effect of ketoconazole on cyclosporine dose in healthy dogs. Vet Surg. 1998;27:64–68. doi: 10.1111/j.1532-950x.1998.tb00099.x. [DOI] [PubMed] [Google Scholar]
- 35.Bloomberg MS. Surgical neutering and non-surgical alternatives. J Am Vet Med Assoc. 1996;208:517–519. [PubMed] [Google Scholar]