Abstract
Bacterial sRNAs are an emerging class of small regulatory RNAs, 40–500 nt in length, which play a variety of important roles in many biological processes through binding to their mRNA or protein targets. A comprehensive database of experimentally confirmed sRNA targets would be helpful in understanding sRNA functions systematically and provide support for developing prediction models. Here we report on such a database—sRNATarBase. The database holds 138 sRNA–target interactions and 252 noninteraction entries, which were manually collected from peer-reviewed papers. The detailed information for each entry, such as supporting experimental protocols, BLAST-based phylogenetic analysis of sRNA–mRNA target interaction in closely related bacteria, predicted secondary structures for both sRNAs and their targets, and available binding regions, is provided as accurately as possible. This database also provides hyperlinks to other databases including GenBank, SWISS-PROT, and MPIDB. The database is available from the web page http://ccb.bmi.ac.cn/srnatarbase/.
Keywords: sRNA, sRNA targets, database, experimental supports
INTRODUCTION
Bacterial sRNAs are an emerging class of small regulatory RNAs, 40–500 nt in length, which play a variety of important roles in many biological processes through binding to their mRNA or protein targets. These processes include regulating the expression of outer membrane proteins (Guillier and Gottesman 2006; Valentin-Hansen et al. 2007), iron homeostasis (Massé et al. 2005, 2007; Vecerek et al. 2007), quorum sensing (Lenz et al. 2005; Tu and Bassler 2007), and bacterial virulence (Romby et al. 2006; Toledo-Arana et al. 2007). For example, it has been found that MicF, a 93-nt sRNA, can inhibit the expression of OmpF, an outer membrane protein (Axmann et al. 2005; Vogel and Papenfort 2006; Prévost et al. 2007; Urban et al. 2007; Song et al. 2008; Desnoyers et al. 2009; Papenfort et al. 2009). Although the functions of some sRNAs have been obtained, there are still many sRNAs with functions waiting to be elucidated. Additionally, more sRNAs have gradually been found using high-throughput experimental technologies and bioinformatics methods (Livny et al. 2006, 2008; Pichon and Felden 2008; Huang et al. 2009; Sharma and Vogel 2009; Backofen and Hess 2010). Determining the functions of bacterial sRNAs will become an important part of sRNA biology.
Bacterial sRNAs can be divided into two classes according to their mode of action (Vogel and Wagner 2007). The first class bindings to protein targets and thereby modifies the activity of their target proteins, while the second class binds to the mRNA targets and regulates expression or stability of their target genes at the post-transcriptional level. Therefore, identification of sRNA targets is very important in determining sRNA functions. Additionally, according to the gene positions of bacterial antisense sRNAs and their targets (Wagner 2009), the sRNAs can also be categorized: cis-encoded sRNAs contain an overlap between the antisense RNA gene and the target gene, and in trans-encoded sRNAs, the antisense RNA gene is separate from the target gene.
In their recent review, Vogel and Wagner (2007) systematically summarized the experimental and bioinformatics approaches for the discovery and validation of sRNA targets. Although sRNA targets should be finally tested by experiments, computational methods still provide a time-saving and less labor-intensive way to identify sRNA targets. For example, in a recently published paper (Richter et al. 2010), Richter and coworkers applied IntaRNA (Busch et al. 2008), a model for prediction of sRNA targets, to identify mRNA targets for the sRNA Yfr1 in the genome Prochlorococcus MED4, which contains more than 1700 mRNA-encoding genes. Initially, the program IntaRNA was used to find the candidate targets for the sRNA Yfr1. Then, the top 10 candidate targets were tested using a GFP-reporter system, and two targets were found. Without a prediction model, however, a large number of experiments would need to be carried out for the identification of Yfr1 targets. This example shows that prediction models for sRNA targets play a key role in elucidating sRNA functions.
Several prediction models have been developed (Zhang et al. 2006; Busch et al. 2008; Tjaden 2008). In principle, the process of developing models is to first extract the rules from a training data set composed of known sRNA targets and then to apply the rules to predict sRNA targets for experimental validation. Therefore, from a machine-learning point of view, it would be better to have as many samples as possible in the training data set. However, the model IntaRNA, containing the largest number of samples in the training data set among the aforementioned models, had only 18 samples for parameters optimization (Busch et al. 2008). Therefore, it is essential to collect as many sRNA targets as possible to construct a comprehensive database of sRNA targets. This will not only be helpful in understanding sRNA functions systematically, but will also provide a benchmark data set for constructing prediction models.
When we initiated this project, we also noticed two other databases, RegulonDB (Gama-Castro et al. 2008) and sRNAMap (Huang et al. 2009), which include 49 and 60 sRNA–target interactions, respectively. However, the accurate binding regions between sRNAs and their targets, which play a key role in developing models, were not provided (Busch et al. 2008; Tjaden 2008). Additionally, no experimentally confirmed negative samples were included, which are necessary for the construction of prediction models, at least from a machine-learning point of view, To provide detailed information on sRNA targets, we have systematically and manually collected experimental data on sRNA–target interactions from peer-reviewed papers and developed a database for sRNA targets called sRNATarBase. The database contains 10 entries for activated targets, 128 entries for repressed targets, and 252 entries for which no interaction has been reported. The numbers of involved sRNAs, targets, and genomes are 68, 227, and 17, respectively.
RESULTS AND DISCUSSION
Construction of database
After obtaining the detailed information for each experimentally confirmed sRNA–target pair (interaction or noninteraction), we constructed a table file in CSV format that was then imported into a MySql database. To ensure that the data were imported accurately, we performed quality control several times. The template file in CSV format can be downloaded from the database homepage. The Web interface for the database was designed in PHP language.
Database access
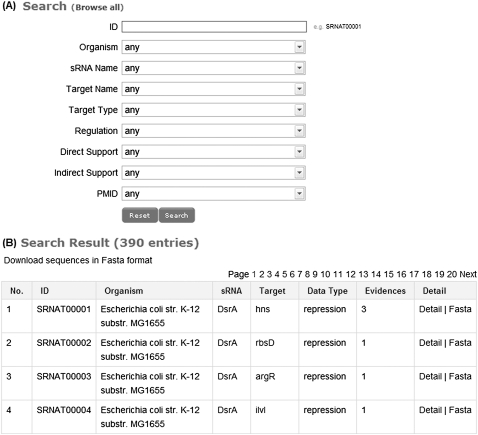
Users can search sRNA targets through the Web interface (Fig. 1A) using the following fields: ID, organism, sRNA name, target name, target type, regulation, direct support, indirect support, PMID, or a combination of fields. The meaning of each field is as follows:
FIGURE 1.
(A) The query interface for the database, from which users can search sRNA targets using a particular field or a combination of fields. (B) The part of the search results using default values, from which users can check detailed information on sRNA targets, such as sRNA sequence, target sequence, and supporting experimental protocols by clicking the hyperlinked “Detail.”
ID
Each database entry is assigned an ID, defined by “SRNAT,” followed by a five-digit number. For example, “SRNAT00001” stands for the interaction of sRNA DsrA and mRNA target hns. There are 390 entries in the database. The IDs were named SRNAT00001, SRNAT00002, …, SRNAT00390.
Organism
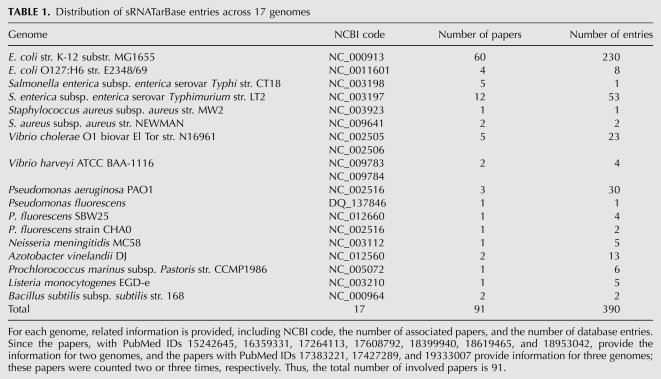
There are 17 genomes involved. The distribution of the database entries across the 17 genomes is given in Table 1. The Escherichia coli str. K-12 genome contains the maximum number of entries, 230.
TABLE 1.
Distribution of sRNATarBase entries across 17 genomes
sRNA name
The number of involved sRNAs is 68. Users can select any one of them to search the database. For example, if sRNA RybB is selected and other fields use the default values, then five entries—SRNAT00038, SRNAT00040, SRNAT00041, SRNAT00268, and SRNAT00320—will be obtained.
Target name
The number of involved targets is 227. Users can select any one of them to search the database. For example, if target mRNA OmpF is selected and other fields use the default values, one interaction entry, SRNAT00014 (MicF-OmpF), and eight no-interaction entries will be obtained.
Target type
Two kinds of sRNA targets, mRNAs and proteins, are provided in our database because present studies show that sRNAs function primarily by binding mRNAs or proteins. The numbers of entries for mRNA and protein targets are 379 and 11, respectively.
Regulation
Three regulation types are provided for the interaction of sRNA–mRNA or protein targets, including repression, activation, and no interaction. “Repression” means that the expression of mRNA targets was repressed, the stability of mRNA targets was decreased, or the activity of protein targets was down-regulated (Altuvia et al. 1998; Romeo 1998; Massé et al. 2005). “Activation” indicates that the expression of mRNA targets was activated, the stability of mRNA targets was increased, or the activity of protein targets was up-regulated (Majdalani et al. 1998; Prévost et al. 2007). “No interaction” shows that the expression level of mRNA targets, the stability of mRNA targets, or the activity of protein targets was basically not affected by sRNAs.
Direct support
Only those targets that were confirmed by at least one of the following experimental protocols (Vogel and Wagner 2007; Frohlich and Vogel 2009; Sharma and Vogel 2009): point mutation of mRNA, point mutation of sRNA, mRNA reporter gene, sRNA reporter gene, sRNA deletion, sRNA knockout, and in vitro footprinting, were considered to be true targets. Because these protocols provided direct support for sRNA–target interaction, these data can be applied to construct models for the prediction of sRNA targets.
Indirect support
Many experimental protocols, such as 2D-PAGE and microarray, can be used to detect gene expression level. These can also be used to decipher possible sRNA targets. However, the distinction between primary targets and secondary targets cannot be determined by gene expression only (Vogel and Wagner 2007). Therefore, all experimental protocols listed in this search field provide only indirect support for sRNA targets.
PMID
PMIDs are the indexes of papers stored in the PubMed database. Here we provide a PMID field for a user to quickly determine which entries in the sRNATarBase are associated with the given PMID. For example, one entry, SRNAT00001, can be obtained by setting up the PMID field as “10954740” and other fields by their default values. The number of involved papers is 91. The other role of the PMID is to let a user easily find the papers related to some particular database entry.
If a user searches the database using default values, all entries will be displayed and sorted by sRNA target ID (Fig. 1B). Additionally, all database entries can be downloaded by clicking “Download sequences in Fasta format” under “(B) Search Result (390 entries)” (Fig. 1B). Each entry in Fasta format is depicted by a description line containing the sRNA target ID, organism name, sRNA name, target name, and regulation type separated by the sign “|”, followed by sRNA sequence, hyphens “--”, and target sequence. The detailed information as follows for each entry can also be obtained by clicking on the hyperlinked “Detail” (Fig. 1B).
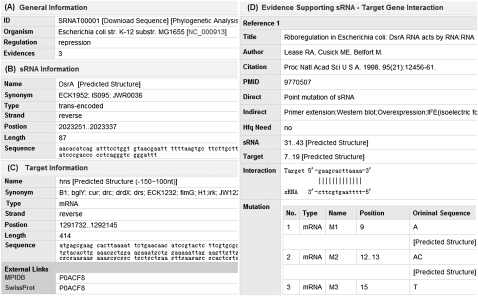
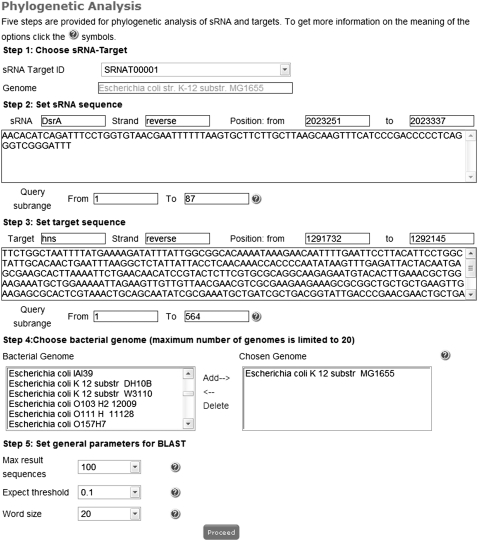
First, general information is provided (Fig. 2A), including entry ID, links to BLAST-based phylogenetic analysis, and GenBank for genome information (Benson et al. 2009) and regulation type. According to Zhang et al. (2006), Busch et al. (2008), and Tjaden (2008), a particular sRNA–mRNA target interaction is often conserved in closely related bacteria. To provide comprehensive support for the sRNA research community, we present the BLAST-based procedure for conservation analysis of sRNA–mRNA target interaction. When a user clicks the hyperlinked “Phylogenetic analysis,” a new web page will be displayed (Fig. 3). This page contains the corresponding entry ID, genome name, sRNA information, mRNA information, options for the user to choose closely related bacteria, and parameters for BLAST comparison. Here we want to emphasize that the sequences for both sRNA and mRNA cannot be changed, but the user can set up the range for BLAST analysis. Because the binding region on the mRNA target is often located in the flanking region around the initial start codon, we include the upstream 150 nt of the mRNA target. Additionally, the maximum allowed number of closely related genomes is 20. Finally, the parsed BLAST results will be provided for each selected genome, from which the user can check the upstream, downstream, and overlapping genes of the entry.
FIGURE 2.
Detailed information is displayed for a particular database entry, including general information (A), sRNA information (B), target information (C), and supporting information (D). Additionally, the links to the databases NCBI (A,B,D), MPID (C), and SWISS-PROT (C) are provided from which further information on the sRNA and its target can be accessed.
FIGURE 3.
The interface of BLAST-based phylogenetic analysis of an sRNA–mRNA target interaction is provided, through which the conservation of sRNA–mRNA target interaction or their binding regions can be checked among the closely related bacteria.
Second, sRNA information is provided (Fig. 2B), which involves name, link to the predicted secondary structure, type (trans-, cis-encoded, or protein binding), strand, position on the genome, and sequence.
Third, target information, including target name, link to its predicted secondary structure, strand, position on the genome, and external links to the databases MPIDB (Goll et al. 2008) and SWISS-PROT (UniProt Consortium 2010) is provided (Fig. 2C). Here the secondary structure of the flanking region −150–100 around the initial start codon of the target is predicted because in the sRNATarBase, of the 95 entries containing binding regions, 91 of them are located in this region.
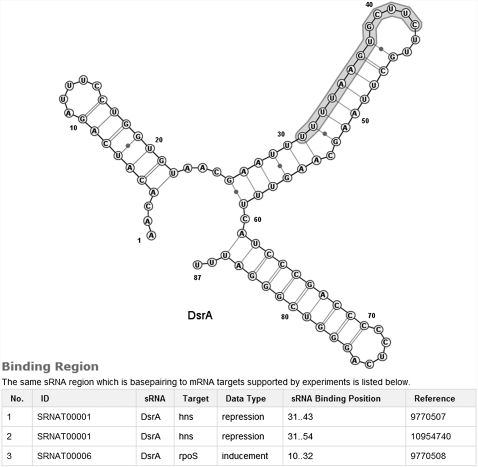
Fourth, the evidence supporting the sRNA–target interaction is listed (Fig. 2D). This includes information on references, direct support, indirect support, binding positions, and their mapping on the predicted secondary structures, interaction regions, and available mutations. Here the RNAfold program is used to predict secondary structures of both sRNA and mRNA (Hofacker 2003), and VARNA is applied to draw RNA secondary structure. VARNA is a comprehensive and flexible tool to display RNA secondary structure (Darty et al. 2009). Many functions, such as rotation, linear drawing, and circular drawing, can be selected on the right-click menu. For example, Figure 4 shows the binding region of sRNA DsrA with its target hns on the predicted secondary structure. From Figure 4, the users can also check the binding information of the sRNA with other available targets.
FIGURE 4.
The predicted secondary structure of sRNA DsrA and its binding information with target hns, as well as the binding region list of sRNA DsrA with other targets are displayed, from which the structure features of the binding regions between an sRNA and its targets can be explored.
To keep the database updated for the sRNA research community, sRNATarBase allows users to submit newly identified sRNA targets to the database in two ways. The first is to enter the information for each sRNA–target entry individually in the submission form. The second is to submit many sRNA–target entries by filling in the related information in a template file in CSV format, which can be downloaded from our web page. After passing examination by the Web Administrator, the related data will be put into the database. At the same time, we will also continue to scan the literature to update the database.
Other functions
To provide better support for the sRNA research community, we also provide the following functions, including BLAST comparison, browsing and downloading the database, and prediction of sRNA targets. For BLAST comparison, the BLAST database includes all sRNA sequences from the sRNATarBase. When a query sRNA sequence is compared with the BLAST database, all hits will be listed, including the sRNA name, sRNA length, BLAST score, identity, E-value, and Link containing detailed comparison information. The database entry can be obtained by clicking the hyperlinked sRNA name. Additionally, all database entries can be browsed individually or downloaded entirely. We presently provide two formats, CSV and Fasta, for a user to download the data. And because the prediction of sRNA targets plays a key role in elucidating sRNA functions, we integrated the web server developed by our laboratory, sRNATarget, into the database interface (Zhao et al. 2008; Cao et al. 2009) for prediction of sRNA targets.
Future directions
Here we describe the database, sRNATarBase, for sRNA targets verified by experiments. Compared to other databases (Gama-Castro et al. 2008; Huang et al. 2009) in sRNA targets, the characteristics of sRNATarBase are as follows: First, our database not only contains more entries but also provides available binding regions between sRNAs and their targets as well as available mutation information. Second, we also provide no-interaction entries. From a machine-learning point of view, both positive samples (activation and repression) and negative samples (no interaction) are necessary, which can be used to construct models directly. In fact, we have even applied part entries of the database to develop two prediction models, sRNATargetNB and sRNATargetSVM (Zhao et al. 2008; Cao et al. 2009). The classification accuracy, sensitivity, and specificity were 93.03%, 40.90%, and 93.71% for sRNATargetNB; and 80.55%, 72.73%, and 80.65% for sRNATargetSVM, respectively. Obviously, to provide better support for the prediction of sRNA targets, the accuracy, sensitivity, and specificity should be improved. Third, BLAST-based phylogenetic analysis of sRNA–mRNA target interaction is provided; this can be used to check the conservation of sRNA–mRNA targets or their binding regions in closely related bacteria. Fourth, the mapping of binding regions on the predicted secondary structures of both sRNAs and mRNA targets is provided, which can be used to explore the structure characteristics of binding regions. In summary, the above features make sRNATarBase a comprehensive database for sRNA targets.
In the future, we will focus on three points. The first is to continue collecting sRNA targets from the literature so that more entries can be included in our database. The second is to develop more accurate prediction models using the database. The third is to incorporate the predicted targets of all known sRNAs into the database so that we can provide comprehensive support for the sRNA research community.
MATERIALS AND METHODS
To construct the sRNATarBase database, we queried PubMed using the related keywords, such as “sRNA targets” or “sRNA,” and read the resultant papers (before May 2010). Finally, 91 papers were chosen for extraction of sRNA targets, and 390 entries of sRNA–target interaction were obtained. Table 1 summarizes the distribution of these 390 entries among 17 genomes. The detailed information for these entries, such as supporting information, sRNA sequences and their secondary structures, mRNA target sequences and their secondary structures, available binding regions, and BLAST-based phylogenetic analysis, has been provided in our database.
ACKNOWLEDGMENTS
This work was supported by the National High Technology Development Program of China under Grant No. 2006AA02Z323. We are grateful to Zhao Zhongming from Vanderbilt University Medical Center for his careful revision in English.
Footnotes
Article published online ahead of print. Article and publication date are at http://www.rnajournal.org/cgi/doi/10.1261/rna.2193110.
REFERENCES
- Altuvia S, Zhang A, Argaman L, Tiwari A, Storz G 1998. The Escherichia coli OxyS regulatory RNA represses fhlA translation by blocking ribosome binding. EMBO J 17: 6069–6075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axmann IM, Kensche P, Vogel J, Kohl S, Herzel H, Hess WR 2005. Identification of cyanobacterial non-coding RNAs by comparative genome analysis. Genome Biol 6: R73 doi: 10.1186/gb-2005-6-9-r73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backofen R, Hess WR 2010. Computational prediction of sRNAs and their targets in bacteria. RNA Biol 7: 1–10 [DOI] [PubMed] [Google Scholar]
- Benson DA, Karsch-Mizrachi I, Lipman DJ, Ostell J, Sayers EW 2009. GenBank. Nucleic Acids Res 37: D26–D31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch A, Richter AS, Backofen R 2008. IntaRNA: Efficient prediction of bacterial sRNA targets incorporating target site accessibility and seed regions. Bioinformatics 24: 2849–2856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Zhao Y, Cha L, Ying X, Wang L, Shao N, Li W 2009. sRNATarget: A web server for prediction of bacterial sRNA targets. Bioinformation 3: 364–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darty K, Denise A, Ponty Y 2009. VARNA: Interactive drawing and editing of the RNA secondary structure. Bioinformatics 25: 1974–1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desnoyers G, Morissette A, Prévost K, Massé E 2009. Small RNA-induced differential degradation of the polycistronic mRNA iscRSUA. EMBO J 28: 1551–1561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohlich KS, Vogel J 2009. Activation of gene expression by small RNA. Curr Opin Microbiol 12: 674–682 [DOI] [PubMed] [Google Scholar]
- Gama-Castro S, Jiménez-Jacinto V, Peralta-Gil M, Santos-Zavaleta A, Peñaloza-Spinola MI, Contreras-Moreira B, Segura-Salazar J, Muñiz-Rascado L, Martínez-Flores I, Salgado H, et al. 2008. RegulonDB (version 6.0): Gene regulation model of Escherichia coli K-12 beyond transcription, active (experimental) annotated promoters and Textpresso navigation. Nucleic Acids Res 36: D120–D124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goll J, Rajagopala SV, Shiau SC, Wu H, Lamb BT, Uetz P 2008. MPIDB: The microbial protein interaction database. Bioinformatics 24: 1743–1744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillier M, Gottesman S 2006. Remodelling of the Escherichia coli outer membrane by two small regulatory RNAs. Mol Microbiol 59: 231–247 [DOI] [PubMed] [Google Scholar]
- Hofacker IL 2003. Vienna RNA secondary structure server. Nucleic Acids Res 31: 3429–3431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HY, Chang HY, Chou CH, Tseng CP, Ho SY, Yang CD, Ju YW, Huang HD 2009. sRNAMap: Genomic maps for small non-coding RNAs, their regulators and their targets in microbial genomes. Nucleic Acids Res 37: D150–D154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz DH, Miller MB, Zhu J, Kulkarni RV, Bassler BL 2005. CsrA and three redundant small RNAs regulate quorum sensing in Vibrio cholerae. Mol Microbiol 58: 1186–1202 [DOI] [PubMed] [Google Scholar]
- Livny J, Brencic A, Lory S, Waldor MK 2006. Identification of 17 Pseudomonas aeruginosa sRNAs and prediction of sRNA-encoding genes in 10 diverse pathogens using the bioinformatic tool sRNAPredict2. Nucleic Acids Res 34: 3484–3493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livny J, Teonadi H, Livny M, Waldor MK 2008. High-throughput kingdom-wide prediction and annotation of bacterial non-coding RNAs. PLoS ONE 3: e3197 doi: 10.1371/journal.pone.0003197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majdalani N, Cunning C, Sledjeski D, Elliott T, Gottesman S 1998. DsrA RNA regulates translation of RpoS message by an anti-antisense mechanism independent of its action as an antisilencer of transcription. Proc Natl Acad Sci 95: 12462–12467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massé E, Vanderpool CK, Gottesman S 2005. Effect of RyhB small RNA on global iron use in Escherichia coli. J Bacteriol 187: 6962–6971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massé E, Salvail H, Desnoyers G, Arguin M 2007. Small RNAs controlling iron metabolism. Curr Opin Microbiol 10: 140–145 [DOI] [PubMed] [Google Scholar]
- Papenfort K, Said N, Welsink T, Lucchini S, Hinton JC, Vogel J 2009. Specific and pleiotropic patterns of mRNA regulation by ArcZ, a conserved Hfq-dependent small RNA. Mol Microbiol 74: 139–158 [DOI] [PubMed] [Google Scholar]
- Pichon C, Felden B 2008. Small RNA gene identification and mRNA target predictions in bacteria. Bioinformatics 24: 2807–2813 [DOI] [PubMed] [Google Scholar]
- Prévost K, Salvail H, Desnoyers G, Jacques JF, Phaneuf E, Massé E 2007. The small RNA RyhB activates the translation of shiA mRNA encoding a permease of shikimate, a compound involved in siderophore synthesis. Mol Microbiol 64: 1260–1273 [DOI] [PubMed] [Google Scholar]
- Richter AS, Schleberger C, Backofen R, Steglich C 2010. Seed-based INTARNA prediction combined with GFP-reporter system identifies mRNA targets of the small RNA Yfr1. Bioinformatics 26: 1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romby P, Vandenesch F, Wagner EG 2006. The role of RNAs in the regulation of virulence-gene expression. Curr Opin Microbiol 9: 229–236 [DOI] [PubMed] [Google Scholar]
- Romeo T 1998. Global regulation by the small RNA-binding protein CsrA and the non-coding RNA molecule CsrB. Mol Microbiol 29: 1321–1330 [DOI] [PubMed] [Google Scholar]
- Sharma CM, Vogel J 2009. Experimental approaches for the discovery and characterization of regulatory small RNA. Curr Opin Microbiol 12: 536–546 [DOI] [PubMed] [Google Scholar]
- Song T, Mika F, Lindmark B, Liu Z, Schild S, Bishop A, Zhu J, Camilli A, Johansson J, Vogel J, et al. 2008. A new Vibrio cholerae sRNA modulates colonization and affects release of outer membrane vesicles. Mol Microbiol 70: 100–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjaden B 2008. TargetRNA: A tool for predicting targets of small RNA action in bacteria. Nucleic Acids Res 36: W109–W113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo-Arana A, Repoila F, Cossart P 2007. Small noncoding RNAs controlling pathogenesis. Curr Opin Microbiol 10: 182–188 [DOI] [PubMed] [Google Scholar]
- Tu KC, Bassler BL 2007. Multiple small RNAs act additively to integrate sensory information and control quorum sensing in Vibrio harveyi. Genes Dev 21: 221–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- UniProt Consortium 2010. The Universal Protein Resource (UniProt) in 2010. Nucleic Acids Res 38: D142–D148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban JH, Papenfort K, Thomsen J, Schmitz RA, Vogel J 2007. A conserved small RNA promotes discoordinate expression of the glmUS operon mRNA to activate GlmS synthesis. J Mol Biol 373: 521–528 [DOI] [PubMed] [Google Scholar]
- Valentin-Hansen P, Johansen J, Rasmussen AA 2007. Small RNAs controlling outer membrane porins. Curr Opin Microbiol 10: 152–155 [DOI] [PubMed] [Google Scholar]
- Vecerek B, Moll I, Blasi U 2007. Control of Fur synthesis by the non-coding RNA RyhB and iron-responsive decoding. EMBO J 26: 965–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel J, Papenfort K 2006. Small non-coding RNAs and the bacterial outer membrane. Curr Opin Microbiol 9: 605–611 [DOI] [PubMed] [Google Scholar]
- Vogel J, Wagner EG 2007. Target identification of small noncoding RNAs in bacteria. Curr Opin Microbiol 10: 262–270 [DOI] [PubMed] [Google Scholar]
- Wagner EG 2009. Kill the messenger: Bacterial antisense RNA promotes mRNA decay. Nat Struct Mol Biol 16: 804–806 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Sun S, Wu T, Wang J, Liu C, Chen L, Zhu X, Zhao Y, Zhang Z, Shi B, et al. 2006. Identifying Hfq-binding small RNA targets in Escherichia coli. Biochem Biophys Res Commun 343: 950–955 [DOI] [PubMed] [Google Scholar]
- Zhao Y, Li H, Hou Y, Cha L, Cao Y, Wang L, Ying X, Li W 2008. Construction of two mathematical models for prediction of bacterial sRNA targets. Biochem Biophys Res Commun 372: 346–350 [DOI] [PubMed] [Google Scholar]