Abstract
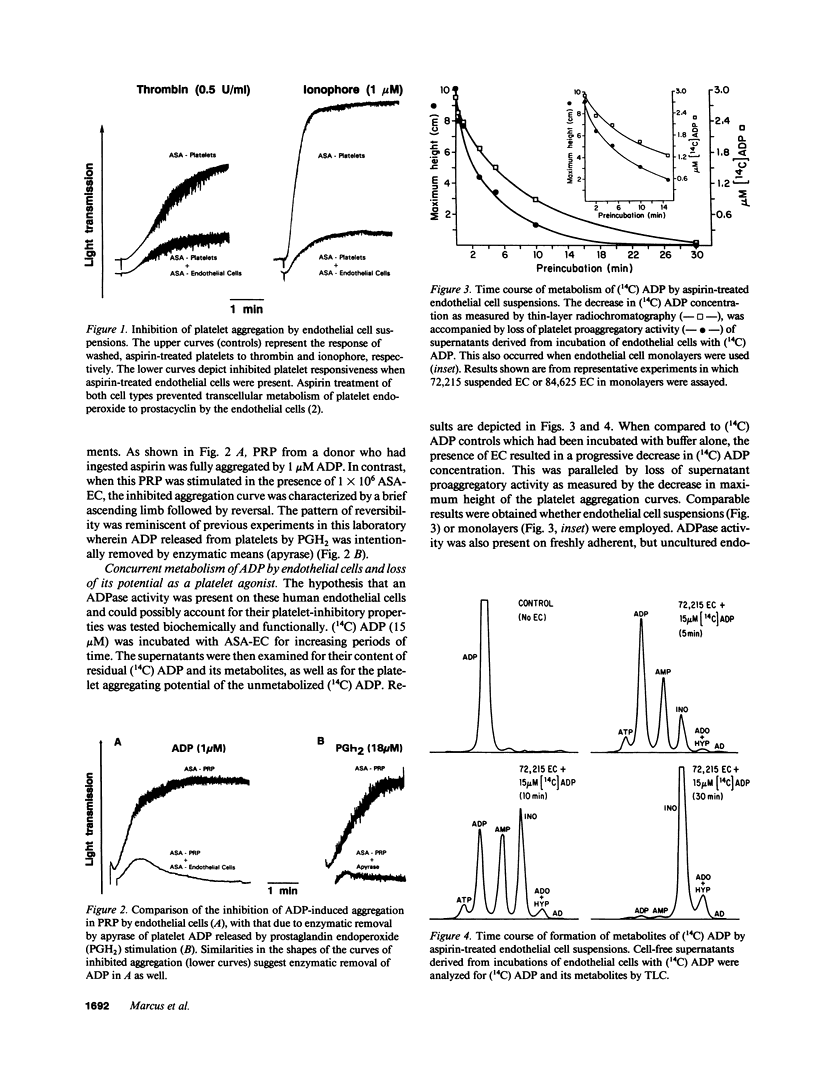
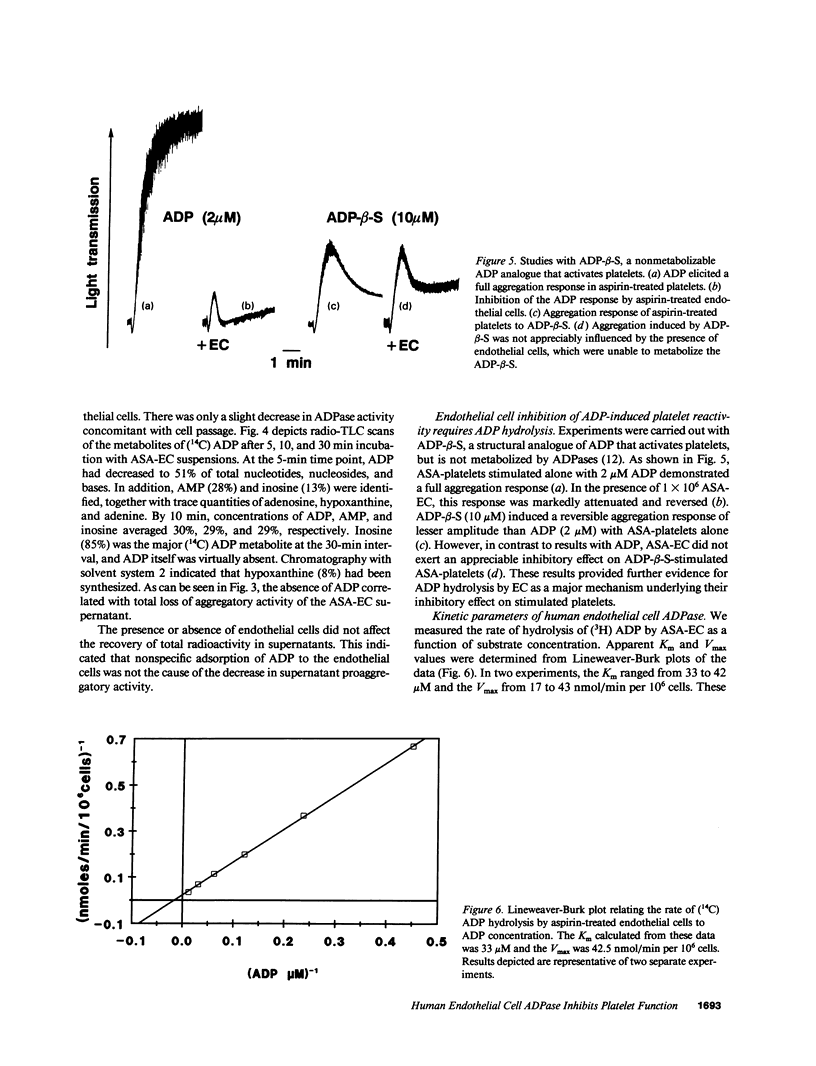
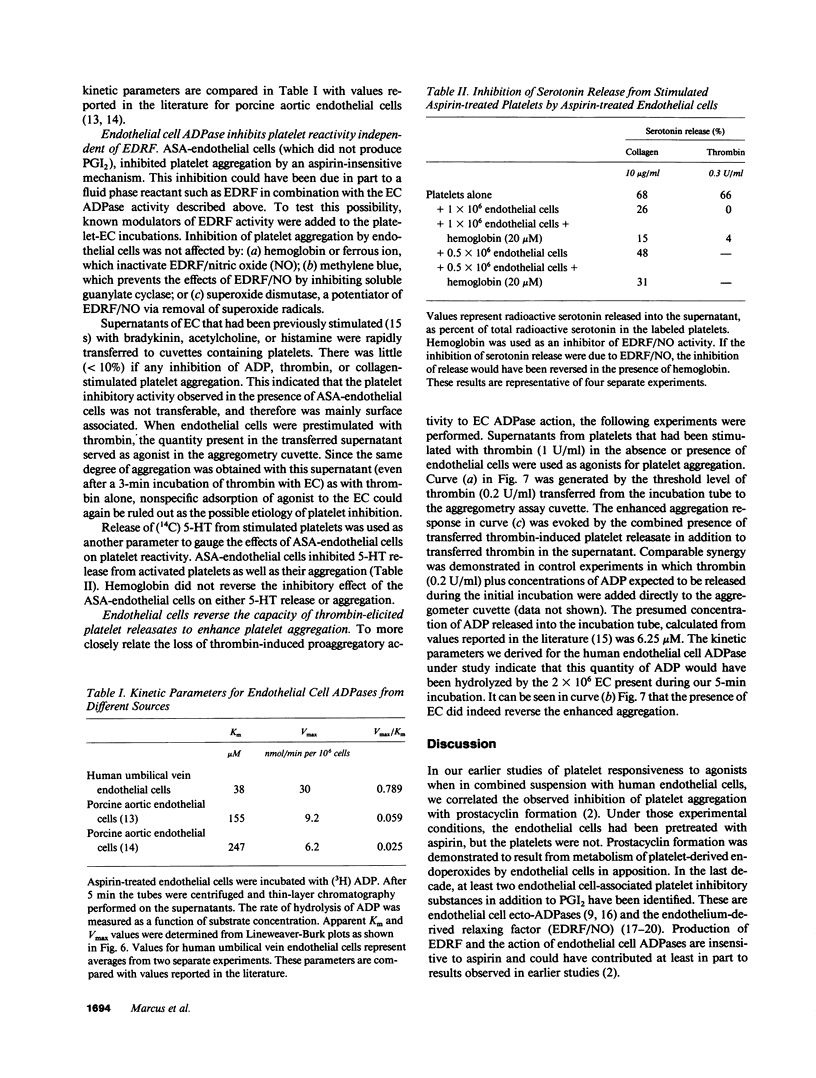
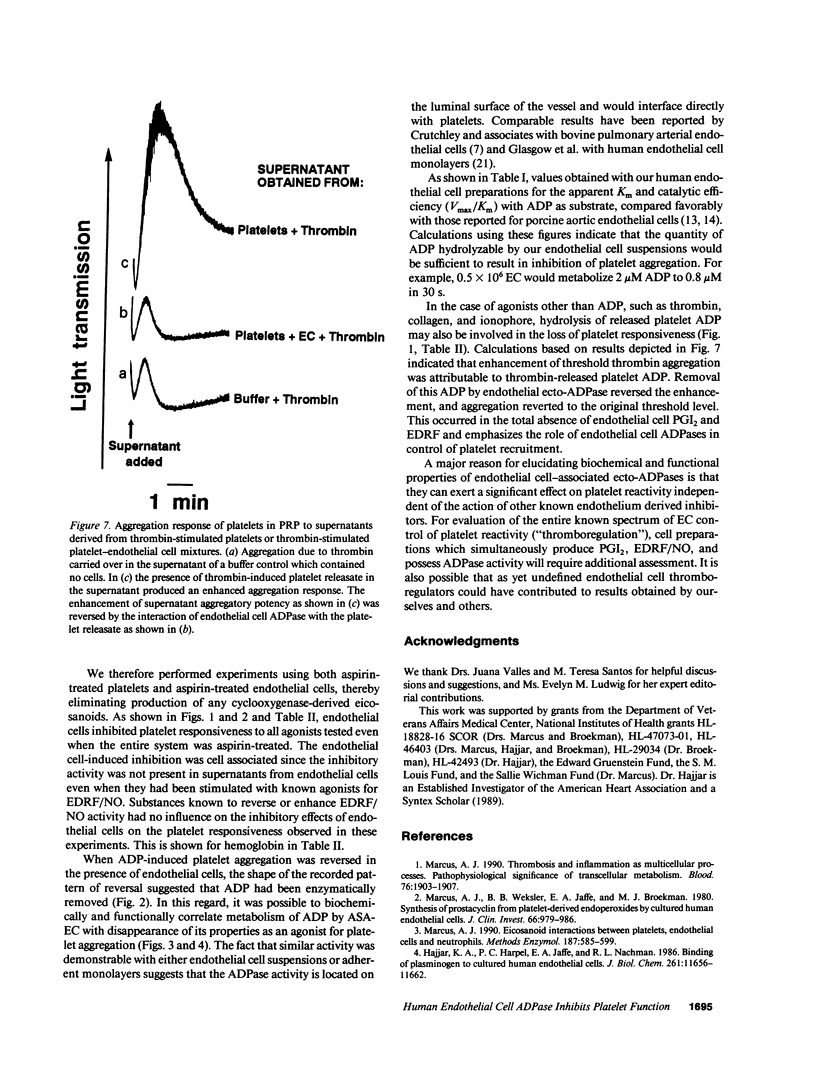
We previously reported that platelets become unresponsive to agonists when stimulated in combined suspension with aspirin-treated human umbilical vein endothelial cells. Inhibition occurred concomitant with metabolism of platelet-derived endoperoxides to prostacyclin by endothelial cells. We now demonstrate that if aspirin-treated platelets which fully respond to appropriate doses of agonists are exposed to aspirin-treated endothelial cells, they remain unresponsive despite absence of prostacyclin. Platelet inhibition is due in large part to ecto-ADPase activity on the endothelial cells. This was established by incubating aspirin-treated endothelial cells with 14C-ADP. Radio-thin layer chromatography and aggregometry demonstrated that 14C-ADP and induction of platelet activation decreased rapidly and concurrently. AMP accumulated transiently, was further metabolized to adenosine, and deaminated to inosine. The apparent Km of the endothelial cell ADPase was 33-42 microM and the Vmax 17-43 nmol/min per 10(6) cells, values in the range of antithrombotic potential. Thus, at least three complementary systems in human endothelial cells control platelet responsiveness: a cell-associated, aspirin-insensitive ADPase which functions in parallel with fluid phase autacoids such as the aspirin-inhibitable eicosanoids, and the aspirin-insensitive endothelium-derived relaxing factor.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alheid U., Frölich J. C., Förstermann U. Endothelium-derived relaxing factor from cultured human endothelial cells inhibits aggregation of human platelets. Thromb Res. 1987 Sep 1;47(5):561–571. doi: 10.1016/0049-3848(87)90361-6. [DOI] [PubMed] [Google Scholar]
- Broekman M. J., Eiroa A. M., Marcus A. J. Inhibition of human platelet reactivity by endothelium-derived relaxing factor from human umbilical vein endothelial cells in suspension: blockade of aggregation and secretion by an aspirin-insensitive mechanism. Blood. 1991 Aug 15;78(4):1033–1040. [PubMed] [Google Scholar]
- Cooper D. R., Lewis G. P., Lieberman G. E., Webb H., Westwick J. ADP metabolism in vascular tissue, a possible thrombo-regulatory mechanism. Thromb Res. 1979;14(6):901–914. doi: 10.1016/0049-3848(79)90008-2. [DOI] [PubMed] [Google Scholar]
- Crutchley D. J., Ryan U. S., Ryan J. W. Effects of aspirin and dipyridamole on the degradation of adenosine diphosphate by cultured cells derived from bovine pulmonary artery. J Clin Invest. 1980 Jul;66(1):29–35. doi: 10.1172/JCI109831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusack N. J., Pearson J. D., Gordon J. L. Stereoselectivity of ectonucleotidases on vascular endothelial cells. Biochem J. 1983 Sep 15;214(3):975–981. doi: 10.1042/bj2140975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Glasgow J. G., Schade R., Pitlick F. A. Evidence that ADP hydrolysis by human cells is related to thrombogenic potential1. Thromb Res. 1978 Aug;13(2):255–266. doi: 10.1016/0049-3848(78)90013-0. [DOI] [PubMed] [Google Scholar]
- Goody R. S., Eckstein F., Schirmer R. H. The enzymatic synthesis of thiophosphate analogs of nucleotides. Biochim Biophys Acta. 1972 Jul 13;276(1):155–161. doi: 10.1016/0005-2744(72)90016-2. [DOI] [PubMed] [Google Scholar]
- Gordon E. L., Pearson J. D., Slakey L. L. The hydrolysis of extracellular adenine nucleotides by cultured endothelial cells from pig aorta. Feed-forward inhibition of adenosine production at the cell surface. J Biol Chem. 1986 Nov 25;261(33):15496–15507. [PubMed] [Google Scholar]
- Hajjar K. A., Harpel P. C., Jaffe E. A., Nachman R. L. Binding of plasminogen to cultured human endothelial cells. J Biol Chem. 1986 Sep 5;261(25):11656–11662. [PubMed] [Google Scholar]
- Holmsen H., Setkowsky C. A., Lages B., Day H. J., Weiss H. J., Scrutton M. C. Content and thrombin-induced release of acid hydrolases in gel-filtered platelets from patients with storage pool disease. Blood. 1975 Jul;46(1):131–142. [PubMed] [Google Scholar]
- Ignarro L. J. Biosynthesis and metabolism of endothelium-derived nitric oxide. Annu Rev Pharmacol Toxicol. 1990;30:535–560. doi: 10.1146/annurev.pa.30.040190.002535. [DOI] [PubMed] [Google Scholar]
- Lieberman G. E., Lewis G. P., Peters T. J. A membrane-bound enzyme in rabbit aorta capable of inhibiting adenosine-diphosphate-induced platelet aggregation. Lancet. 1977 Aug 13;2(8033):330–332. doi: 10.1016/s0140-6736(77)91488-x. [DOI] [PubMed] [Google Scholar]
- Lüthje J., Schomburg A., Ogilvie A. Demonstration of a novel ecto-enzyme on human erythrocytes, capable of degrading ADP and of inhibiting ADP-induced platelet aggregation. Eur J Biochem. 1988 Aug 1;175(2):285–289. doi: 10.1111/j.1432-1033.1988.tb14195.x. [DOI] [PubMed] [Google Scholar]
- Marcus A. J. Eicosanoid interactions between platelets, endothelial cells, and neutrophils. Methods Enzymol. 1990;187:585–599. doi: 10.1016/0076-6879(90)87066-c. [DOI] [PubMed] [Google Scholar]
- Marcus A. J. Stratton lecture 1989. Thrombosis and inflammation as multicellular processes: pathophysiologic significance of transcellular metabolism. Blood. 1990 Nov 15;76(10):1903–1907. [PubMed] [Google Scholar]
- Marcus A. J., Weksler B. B., Jaffe E. A., Broekman M. J. Synthesis of prostacyclin from platelet-derived endoperoxides by cultured human endothelial cells. J Clin Invest. 1980 Nov;66(5):979–986. doi: 10.1172/JCI109967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin W., Villani G. M., Jothianandan D., Furchgott R. F. Selective blockade of endothelium-dependent and glyceryl trinitrate-induced relaxation by hemoglobin and by methylene blue in the rabbit aorta. J Pharmacol Exp Ther. 1985 Mar;232(3):708–716. [PubMed] [Google Scholar]
- Norman G. A., Follett M. J., Hector D. A. Quantitative thin-layer chromatography of ATP and the products of its degradation in meat tissue. J Chromatogr. 1974 Mar 13;90(1):105–111. doi: 10.1016/s0021-9673(01)94779-x. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Pearson J. D., Carleton J. S., Gordon J. L. Metabolism of adenine nucleotides by ectoenzymes of vascular endothelial and smooth-muscle cells in culture. Biochem J. 1980 Aug 15;190(2):421–429. doi: 10.1042/bj1900421. [DOI] [PMC free article] [PubMed] [Google Scholar]



