Abstract
To investigate the relationship between angiogenesis and hepatic tumorigenesis, we examined the expression of vascular endothelial growth factor (VEGF) in 8 human colon carcinoma cell lines and in 30 human colorectal cancer liver metastases. Abundant message for VEGF was found in all tumors, localized to the malignant cells within each neoplasm. Two receptors for VEGF, KDR and flt1, were also demonstrated in most of the tumors examined. KDR and flt1 mRNA were limited to tumor endothelial cells and were more strongly expressed in the hepatic metastases than in the sinusoidal endothelium of the surrounding liver parenchyma. VEGF monoclonal antibody administration in tumor-bearing athymic mice led to a dose- and time-dependent inhibition of growth of subcutaneous xenografts and to a marked reduction in the number and size of experimental liver metastases. In hepatic metastases of VEGF antibody-treated mice, neither blood vessels nor expression of the mouse KDR homologue flk-1 could be demonstrated. These data indicate that VEGF is a commonly expressed angiogenic factor in human colorectal cancer metastases, that VEGF receptors are up-regulated as a concomitant of hepatic tumorigenesis, and that modulation of VEGF gene expression or activity may represent a potentially effective antineoplastic therapy in colorectal cancer.
Full text
PDF


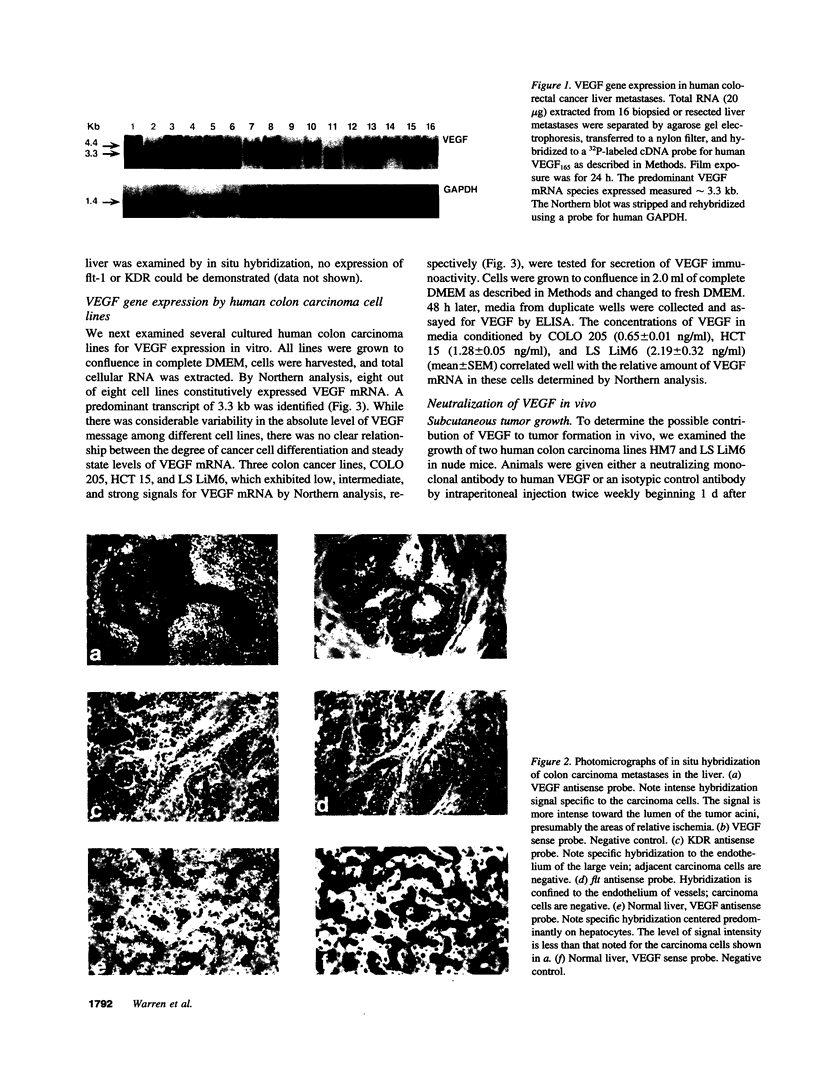
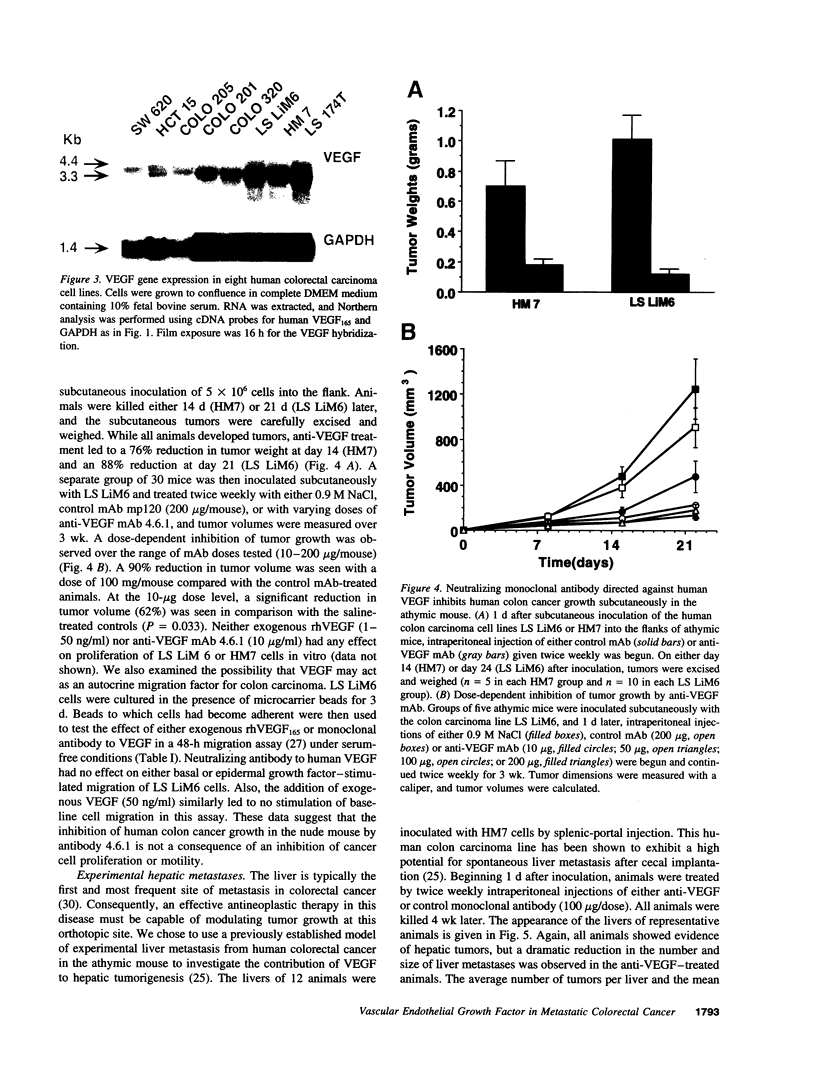
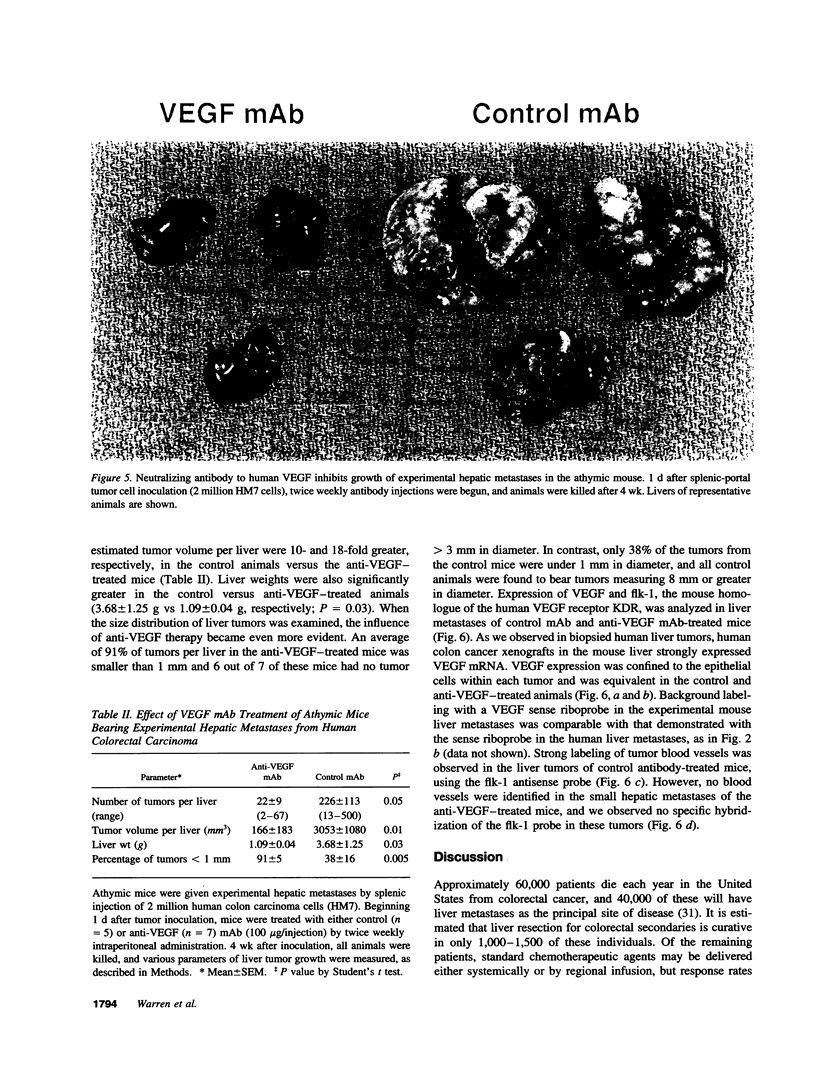



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belloni P. N., Carney D. H., Nicolson G. L. Organ-derived microvessel endothelial cells exhibit differential responsiveness to thrombin and other growth factors. Microvasc Res. 1992 Jan;43(1):20–45. doi: 10.1016/0026-2862(92)90004-9. [DOI] [PubMed] [Google Scholar]
- Bresalier R. S., Niv Y., Byrd J. C., Duh Q. Y., Toribara N. W., Rockwell R. W., Dahiya R., Kim Y. S. Mucin production by human colonic carcinoma cells correlates with their metastatic potential in animal models of colon cancer metastasis. J Clin Invest. 1991 Mar;87(3):1037–1045. doi: 10.1172/JCI115063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresalier R. S., Raper S. E., Hujanen E. S., Kim Y. S. A new animal model for human colon cancer metastasis. Int J Cancer. 1987 May 15;39(5):625–630. doi: 10.1002/ijc.2910390514. [DOI] [PubMed] [Google Scholar]
- Brown L. F., Berse B., Jackman R. W., Tognazzi K., Manseau E. J., Dvorak H. F., Senger D. R. Increased expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in kidney and bladder carcinomas. Am J Pathol. 1993 Nov;143(5):1255–1262. [PMC free article] [PubMed] [Google Scholar]
- Brown L. F., Berse B., Jackman R. W., Tognazzi K., Manseau E. J., Dvorak H. F., Senger D. R. Increased expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in kidney and bladder carcinomas. Am J Pathol. 1993 Nov;143(5):1255–1262. [PMC free article] [PubMed] [Google Scholar]
- Brown L. F., Berse B., Jackman R. W., Tognazzi K., Manseau E. J., Senger D. R., Dvorak H. F. Expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in adenocarcinomas of the gastrointestinal tract. Cancer Res. 1993 Oct 1;53(19):4727–4735. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Connolly D. T. Vascular permeability factor: a unique regulator of blood vessel function. J Cell Biochem. 1991 Nov;47(3):219–223. doi: 10.1002/jcb.240470306. [DOI] [PubMed] [Google Scholar]
- Di Renzo M. F., Narsimhan R. P., Olivero M., Bretti S., Giordano S., Medico E., Gaglia P., Zara P., Comoglio P. M. Expression of the Met/HGF receptor in normal and neoplastic human tissues. Oncogene. 1991 Nov;6(11):1997–2003. [PubMed] [Google Scholar]
- Ferrara N., Houck K., Jakeman L., Leung D. W. Molecular and biological properties of the vascular endothelial growth factor family of proteins. Endocr Rev. 1992 Feb;13(1):18–32. doi: 10.1210/edrv-13-1-18. [DOI] [PubMed] [Google Scholar]
- Ferrara N., Leung D. W., Cachianes G., Winer J., Henzel W. J. Purification and cloning of vascular endothelial growth factor secreted by pituitary folliculostellate cells. Methods Enzymol. 1991;198:391–405. doi: 10.1016/0076-6879(91)98040-d. [DOI] [PubMed] [Google Scholar]
- Ferrara N., Winer J., Burton T., Rowland A., Siegel M., Phillips H. S., Terrell T., Keller G. A., Levinson A. D. Expression of vascular endothelial growth factor does not promote transformation but confers a growth advantage in vivo to Chinese hamster ovary cells. J Clin Invest. 1993 Jan;91(1):160–170. doi: 10.1172/JCI116166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman J., Shing Y. Angiogenesis. J Biol Chem. 1992 Jun 5;267(16):10931–10934. [PubMed] [Google Scholar]
- Folkman J. The role of angiogenesis in tumor growth. Semin Cancer Biol. 1992 Apr;3(2):65–71. [PubMed] [Google Scholar]
- Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971 Nov 18;285(21):1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- Goto F., Goto K., Weindel K., Folkman J. Synergistic effects of vascular endothelial growth factor and basic fibroblast growth factor on the proliferation and cord formation of bovine capillary endothelial cells within collagen gels. Lab Invest. 1993 Nov;69(5):508–517. [PubMed] [Google Scholar]
- Grant D. S., Kleinman H. K., Goldberg I. D., Bhargava M. M., Nickoloff B. J., Kinsella J. L., Polverini P., Rosen E. M. Scatter factor induces blood vessel formation in vivo. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1937–1941. doi: 10.1073/pnas.90.5.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houck K. A., Ferrara N., Winer J., Cachianes G., Li B., Leung D. W. The vascular endothelial growth factor family: identification of a fourth molecular species and characterization of alternative splicing of RNA. Mol Endocrinol. 1991 Dec;5(12):1806–1814. doi: 10.1210/mend-5-12-1806. [DOI] [PubMed] [Google Scholar]
- Jakeman L. B., Winer J., Bennett G. L., Altar C. A., Ferrara N. Binding sites for vascular endothelial growth factor are localized on endothelial cells in adult rat tissues. J Clin Invest. 1992 Jan;89(1):244–253. doi: 10.1172/JCI115568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly P. A., Djiane J., Postel-Vinay M. C., Edery M. The prolactin/growth hormone receptor family. Endocr Rev. 1991 Aug;12(3):235–251. doi: 10.1210/edrv-12-3-235. [DOI] [PubMed] [Google Scholar]
- Kim K. J., Li B., Houck K., Winer J., Ferrara N. The vascular endothelial growth factor proteins: identification of biologically relevant regions by neutralizing monoclonal antibodies. Growth Factors. 1992;7(1):53–64. doi: 10.3109/08977199209023937. [DOI] [PubMed] [Google Scholar]
- Kim K. J., Li B., Winer J., Armanini M., Gillett N., Phillips H. S., Ferrara N. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993 Apr 29;362(6423):841–844. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- Kondo S., Asano M., Matsuo K., Ohmori I., Suzuki H. Vascular endothelial growth factor/vascular permeability factor is detectable in the sera of tumor-bearing mice and cancer patients. Biochim Biophys Acta. 1994 Mar 31;1221(2):211–214. doi: 10.1016/0167-4889(94)90016-7. [DOI] [PubMed] [Google Scholar]
- Kondo S., Asano M., Suzuki H. Significance of vascular endothelial growth factor/vascular permeability factor for solid tumor growth, and its inhibition by the antibody. Biochem Biophys Res Commun. 1993 Aug 16;194(3):1234–1241. doi: 10.1006/bbrc.1993.1955. [DOI] [PubMed] [Google Scholar]
- Kuan S. F., Byrd J. C., Basbaum C. B., Kim Y. S. Characterization of quantitative mucin variants from a human colon cancer cell line. Cancer Res. 1987 Nov 1;47(21):5715–5724. [PubMed] [Google Scholar]
- Levitan N. Chemotherapy in colorectal carcinoma. Surg Clin North Am. 1993 Feb;73(1):183–198. doi: 10.1016/s0039-6109(16)45936-0. [DOI] [PubMed] [Google Scholar]
- Malden L. T., Novak U., Burgess A. W. Expression of transforming growth factor alpha messenger RNA in the normal and neoplastic gastro-intestinal tract. Int J Cancer. 1989 Mar 15;43(3):380–384. doi: 10.1002/ijc.2910430305. [DOI] [PubMed] [Google Scholar]
- McGuire R. F., Bissell D. M., Boyles J., Roll F. J. Role of extracellular matrix in regulating fenestrations of sinusoidal endothelial cells isolated from normal rat liver. Hepatology. 1992 Jun;15(6):989–997. doi: 10.1002/hep.1840150603. [DOI] [PubMed] [Google Scholar]
- Millauer B., Wizigmann-Voos S., Schnürch H., Martinez R., Møller N. P., Risau W., Ullrich A. High affinity VEGF binding and developmental expression suggest Flk-1 as a major regulator of vasculogenesis and angiogenesis. Cell. 1993 Mar 26;72(6):835–846. doi: 10.1016/0092-8674(93)90573-9. [DOI] [PubMed] [Google Scholar]
- Monacci W. T., Merrill M. J., Oldfield E. H. Expression of vascular permeability factor/vascular endothelial growth factor in normal rat tissues. Am J Physiol. 1993 Apr;264(4 Pt 1):C995–1002. doi: 10.1152/ajpcell.1993.264.4.C995. [DOI] [PubMed] [Google Scholar]
- Ohtani H., Nakamura S., Watanabe Y., Mizoi T., Saku T., Nagura H. Immunocytochemical localization of basic fibroblast growth factor in carcinomas and inflammatory lesions of the human digestive tract. Lab Invest. 1993 May;68(5):520–527. [PubMed] [Google Scholar]
- Olander J. V., Connolly D. T., DeLarco J. E. Specific binding of vascular permeability factor to endothelial cells. Biochem Biophys Res Commun. 1991 Feb 28;175(1):68–76. doi: 10.1016/s0006-291x(05)81201-x. [DOI] [PubMed] [Google Scholar]
- Olson T. A., Mohanraj D., Carson L. F., Ramakrishnan S. Vascular permeability factor gene expression in normal and neoplastic human ovaries. Cancer Res. 1994 Jan 1;54(1):276–280. [PubMed] [Google Scholar]
- Paku S., Lapis K. Morphological aspects of angiogenesis in experimental liver metastases. Am J Pathol. 1993 Sep;143(3):926–936. [PMC free article] [PubMed] [Google Scholar]
- Park J. E., Keller G. A., Ferrara N. The vascular endothelial growth factor (VEGF) isoforms: differential deposition into the subepithelial extracellular matrix and bioactivity of extracellular matrix-bound VEGF. Mol Biol Cell. 1993 Dec;4(12):1317–1326. doi: 10.1091/mbc.4.12.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plate K. H., Breier G., Millauer B., Ullrich A., Risau W. Up-regulation of vascular endothelial growth factor and its cognate receptors in a rat glioma model of tumor angiogenesis. Cancer Res. 1993 Dec 1;53(23):5822–5827. [PubMed] [Google Scholar]
- Plate K. H., Breier G., Weich H. A., Risau W. Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature. 1992 Oct 29;359(6398):845–848. doi: 10.1038/359845a0. [DOI] [PubMed] [Google Scholar]
- Rosen E. M., Meromsky L., Setter E., Vinter D. W., Goldberg I. D. Quantitation of cytokine-stimulated migration of endothelium and epithelium by a new assay using microcarrier beads. Exp Cell Res. 1990 Jan;186(1):22–31. doi: 10.1016/0014-4827(90)90205-o. [DOI] [PubMed] [Google Scholar]
- Siegrist W., Stutz S., Eberle A. N. Homologous and heterologous regulation of alpha-melanocyte-stimulating hormone receptors in human and mouse melanoma cell lines. Cancer Res. 1994 May 15;54(10):2604–2610. [PubMed] [Google Scholar]
- Sladowski D., Steer S. J., Clothier R. H., Balls M. An improved MTT assay. J Immunol Methods. 1993 Jan 4;157(1-2):203–207. doi: 10.1016/0022-1759(93)90088-o. [DOI] [PubMed] [Google Scholar]
- Stagg R. J., Venook A. P., Chase J. L., Lewis B. J., Warren R. S., Roh M., Mulvihill S. J., Grobman B. J., Rayner A. A., Hohn D. C. Alternating hepatic intra-arterial floxuridine and fluorouracil: a less toxic regimen for treatment of liver metastases from colorectal cancer. J Natl Cancer Inst. 1991 Mar 20;83(6):423–428. doi: 10.1093/jnci/83.6.423. [DOI] [PubMed] [Google Scholar]
- Terman B. I., Dougher-Vermazen M., Carrion M. E., Dimitrov D., Armellino D. C., Gospodarowicz D., Böhlen P. Identification of the KDR tyrosine kinase as a receptor for vascular endothelial cell growth factor. Biochem Biophys Res Commun. 1992 Sep 30;187(3):1579–1586. doi: 10.1016/0006-291x(92)90483-2. [DOI] [PubMed] [Google Scholar]
- Waltenberger J., Claesson-Welsh L., Siegbahn A., Shibuya M., Heldin C. H. Different signal transduction properties of KDR and Flt1, two receptors for vascular endothelial growth factor. J Biol Chem. 1994 Oct 28;269(43):26988–26995. [PubMed] [Google Scholar]
- Weiss L., Grundmann E., Torhorst J., Hartveit F., Moberg I., Eder M., Fenoglio-Preiser C. M., Napier J., Horne C. H., Lopez M. J. Haematogenous metastatic patterns in colonic carcinoma: an analysis of 1541 necropsies. J Pathol. 1986 Nov;150(3):195–203. doi: 10.1002/path.1711500308. [DOI] [PubMed] [Google Scholar]
- Zetter B. R. The cellular basis of site-specific tumor metastasis. N Engl J Med. 1990 Mar 1;322(9):605–612. doi: 10.1056/NEJM199003013220907. [DOI] [PubMed] [Google Scholar]
- de Vries C., Escobedo J. A., Ueno H., Houck K., Ferrara N., Williams L. T. The fms-like tyrosine kinase, a receptor for vascular endothelial growth factor. Science. 1992 Feb 21;255(5047):989–991. doi: 10.1126/science.1312256. [DOI] [PubMed] [Google Scholar]











