Abstract
Objective: The aim of this FT-Raman study was to investigate laser-induced compositional changes in enamel after therapy with a low-level infrared diode laser and a photoabsorbing cream, in order to intensify the superficial light absorption before and after cariogenic challenge. Background Data: Dental caries remains the most prevalent disease during childhood and adolescence. Preventive modalities include the use of fluoride, reduction of dietary cariogenic refined carbohydrates, plaque removal and oral hygiene techniques, and antimicrobial prescriptions. A relatively simple and noninvasive caries preventive regimen is treating tooth enamel with laser irradiation, either alone or in combination with topical fluoride treatment, resulting in reduced enamel solubility and dissolution rates. Due to their high cost, high-powered lasers are still not widely employed in private practice in developing countries. Thus, low-power red and near-infrared lasers appear to be an appealing alternative. Materials and Methods: Twenty-four extracted or exfoliated caries-free deciduous molars were divided into six groups: control group (no treatment; n = 8); infrared laser treatment (L; n = 8) (810 nm at 100 mW/cm2 for 90 sec); infrared diode laser irradiation (810 nm at 100 mW/cm2 for 90 sec) and photoabsorbing cream (IVL; n = 8); photoabsorbing cream alone (IV; n = 8); infrared diode laser irradiation (810 nm at 100 mW/cm2 for 90 sec) and fluorinated photoabsorbing agent (IVLF; n = 8); and fluorinated photoabsorbing agent alone (IVF; n = 8). Samples were analyzed using FT-Raman spectroscopy before and after pH cycling cariogenic challenge. Results: There was a significant laser-induced reduction and possible modification of the organic matrix content in enamel treated with the low-level diode laser (the L, IVL, and IVFL groups). Conclusion: The FT-Raman technique may be suitable for detecting compositional and structural changes occurring in mineral phases and organic phases of lased enamel under cariogenic challenge.
Introduction
Dental caries is still considered the most prevalent disease during childhood and adolescence,1–4 and its manifestations are found to be high in some individuals,5 even though a noteworthy decline in their incidence has been documented worldwide in recent decades. The disease has become more selective, with carious disease being seen mostly in certain groups of children who have high carious activity.6 This transmissable bacterial disease affects more children than any other disorder, and it is particularly prevalent in families of low socioeconomic status,7–9 and in immunocompromised children.10 Consequently, the use of combined therapies for this population may be a promising method to prevent and control dental caries.
Caries disease begins in dental enamel, a composite of 85% mineral, 12% water, and 3% protein and lipid by volume. The mineral component is hydroxyapatite, a material with a hexagonal shape with the formula Ca10(PO4)6(OH)2.11 The structure of hydroxyapatite is a combination of PO4 and CaO6 that form polyhedral channels along the crystallographic c-axis, in which hydroxyl groups are placed. The structure of apatite is very adaptive to various types of inclusions. Dental apatite contains a substantial number of carbonate groups, which substitute for the hydroxyl groups (A-type CO32_)11 or for phosphate tetrahedral groups (B-type CO32_),11 and there is a positive correlation between the amount of carbonate and enamel solubility,12 as the lack of carbonate diminishes the crystal's stability.13
White spot lesions are the earliest signs of carious disease, and have the appearance of a chalky white spot on the surface of the tooth, indicating an area of demineralization of enamel, and these are common in populations with high levels of carious disease.
Prevention of the complex multi-factorial disease that is dental caries requires a risk assessment for future caries development and the institution of appropriate preventive modalities and oral hygiene education.14–19 Preventive modalities include the use of fluoride, reduction of dietary cariogenic refined carbohydrates, plaque removal and oral hygiene techniques, and antibiotic prescription. A relatively simple and noninvasive caries preventive regimen is treating primary and permanent tooth enamel with low-level laser irradiation, either alone or in combination with topical fluoride treatment, which results in reduced enamel solubility and dissolution rates.20–23
Since the 1960s, it has been consistently demonstrated that under certain conditions high-powered lasers can reduce the rate of subsurface demineralization of enamel, by altering its crystalline structure, acid solubility, and permeability.24–26 Nevertheless, the real mechanisms of caries inhibition by laser remain unclear.
Due to their high cost, high-powered lasers are not widely employed in private practice in developing countries. The use of low-powered red and near-infrared lasers appears to be an appealing alternative, since reports in the literature suggest that when used alone or with topical fluoride, they may increase the tooth's resistance to dental caries.27–29 Some authors wrote that in order to alter the composition or solubility of dental hard tissues, the laser energy must be strongly absorbed and efficiently converted into heat without damage to underlying or surrounding tissues,30 and that temperatures increases should not exceed 5.6°C as proposed by Zach and Cohen,31 or 50.4°C as proposed by Baldissara et al.32
Recently, the number of studies focusing on the infrared (IR) spectroscopic features of human tissues has increased. The chemical characteristics of tissues following laser irradiation are important, and IR spectroscopy yields information about the chemical structure. Raman spectroscopy enables us to obtain the vibrational (IR and far-IR) spectra of minerals by analyzing scattered light caused by monochromatic laser excitation. This is a versatile and non-destructive spectroscopic technique which allows for simultaneous characterization of the inorganic and organic phases of the tooth. Furthermore, Raman spectra exhibit little interference with water, making Raman spectroscopy advantageous for the study of many biological specimens.33
The aim of this FT-Raman study was to investigate laser-induced compositional changes in enamel after therapy with a low-level infrared diode laser and a photoabsorbing cream, in order to intensify superficial light absorption, both before and after cariogenic challenge.
Material and Methods
Tooth selection and grouping
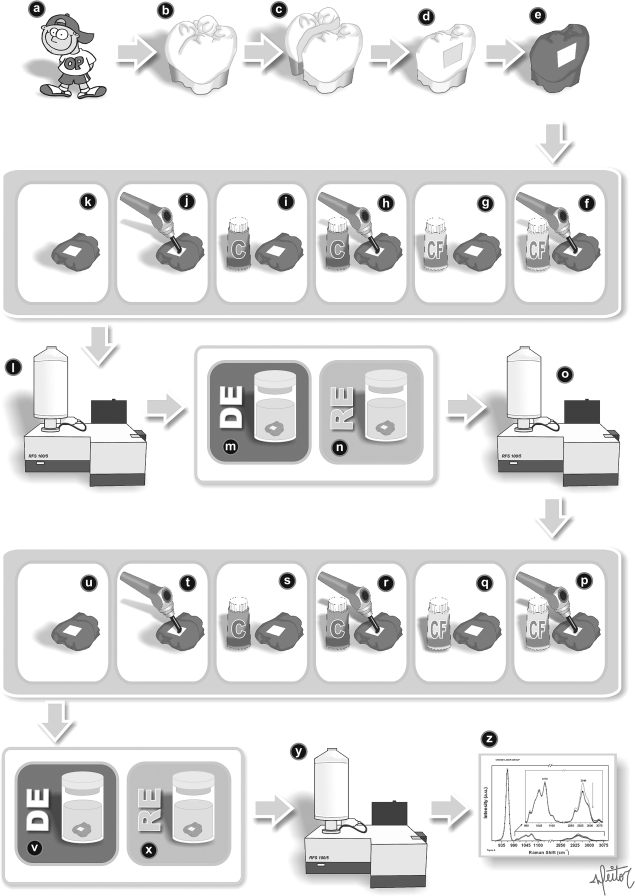
This study had ethical committee approval (CEP–UNICSUL, approval protocol #008/07). Twenty-four extracted or exfoliated caries-free deciduous molars obtained from the Pediatric Dentistry Clinic of Unicsul University/São Paulo-Brazil were divided into six groups as listed in Table 1 and Fig. 1.
Table 1.
Groups, Treatments, and Laser Parameters
| Groups | n | Treatment |
|---|---|---|
| Control group (C) | 8 | No treatment was performed |
| Infrared laser (L) | 8 | Irradiated using only the infrared diode laser; the samples were washed in deionized water |
| Photoabsorbing cream + infrared diode laser irradiation (IVL) | 8 | Green indiocyanine (Acros, , NJ, USA; lot A0232896) gel cream (0.05 g) applied (60 sec); irradiated using the infrared diode laser; the samples were washed in deionized water |
| Photoabsorbing cream (IV) | 8 | Green indiocyanine gel cream (0.05 g) applied (60 sec); the samples were washed in deionized water |
| Fluorinated photoabsorbing cream + infrared diode laser irradiation (IVLF) | 8 | Fluorinated (2% sodium fluoride; ionization coefficient: basic) green indiocyanine gel cream (0.05 g) applied (60 sec); irradiated using the infrared diode laser; the samples were washed in deionized water |
| Fluorinated photoabsorbing agent (IV) | 8 | Fluorinated (2% sodium fluoride; ionization coefficient: basic) green indiocyanine gel cream (0.05 g/Buenos Aires Lab/Brazil) applied (60 sec); the samples were washed in deionized water |
FIG. 1.
(a, b) Posterior caries free deciduous teeth were selected. (c) Samples of buccal and lingual faces were obtained. (d, e) Selected samples prepared with acid–resistance varnish. (f) Fluoride cream group irradiated with laser. (g) Fluoride cream treatment group. (h) Cream group irradiated with laser. (i) Cream treatment group. (j) Laser treatment group. (k) Control group (no treatment). (l) Raman spectroscopy. (m, n) First challenge, pH cycling during 7 days-De (8 h) and Re (16 h). (o) Raman spectroscopy; (p–t) Samples treated again in the same way of first set. (u) Control group received no treatment. (v,x) Second challenge, pH cycling during 7 days-De (8 h) and Re (16 h). (y) Raman spectroscopy. (z) Data treatment and spectras.
The selected deciduous teeth were cut mesiodistally with a low-speed micromotor (LB100; Beltec, Curitiba, Brazil) and diamond disk 540 and 545 (Dremel, Bosch, Corp., Racine, WI, USA) with cooling to obtain two specimens from each tooth. A rectangle of laboratory film (Parafilm®; M Barrier Film, 2 mm wide and 3 mm long) was cut and positioned in the middle third of each specimen, and then the surface was covered with two layers of acid-resistant varnish. After the varnish dried, the rectangles of laboratory film were removed in order to leave a window 2 × 3 mm in size.
Treatments
The specimens of each group were then subjected to the treatments listed in Table 1. The parameters of the infrared diode laser (UltraBlue IV Plus II; DMC Equipaments, Sao Carlos, Brazil) used were 810 nm, 100 mW/cm2, 30 W for 90 sec in continuous mode; input fiber spot size was 9 mm, and output fiber spot size was 6 mm.
After treatment the samples were subjected to FT-Raman spectroscopic analysis.
FT-Raman spectroscopic analysis
The enamel surfaces were analyzed by FT-Raman spectroscopy at three time points: pre-treatment, after the first cariogenic challenge, and after the second cariogenic challenge. An FT-Raman spectrometer (RFS 100/S®; Bruker Inc., Karlsruhe, Germany) with a germanium detector cooled by liquid nitrogen was used to collect the data. The samples were excited by an air-cooled Nd:YAG laser (1064.1 nm).
The power of the incident Nd:YAG laser beam used on the sample was 150 mW. The spectral resolution was set to 4 cm–1, and for each specimen three spectra for each time point were collected, with 250 scans for a total of 432 spectra. The averages of the three spectra per specimen for each period were calculated, resulting in 144 spectra. For the qualitative and semi-quantitative spectral analysis, the average spectra were baseline corrected and then normalized to the 960 cm–1 peak.25,36,37
The changes in the organic and inorganic enamel components were analyzed by comparing the integrated areas of the Raman peaks centered at 1071 cm–1 (p1) and 2940 cm–1 (p2), to the peak at 961 cm–1 (p3). The integrated areas of the peaks were calculated with Microcal Origin5.0® software (Microcal Software, Inc., Northampton, MA, USA).
Artificial caries development
All the samples were subjected to the process of superficial induction of carious lesion formation (using the pH cycling model of ten Cate and Duijsters34 modified by Mendes and Nicolau35). In this experimental model, the samples were subjected to alternating solutions of demineralization and remineralization for 7 uninterrupted days at room temperature without agitation. The specimens were placed individually into plastic pots containing 8 mL of demineralization solution (DE) for 8 h, and then in 8 mL of remineralization solution (RE) for 16 h, in order to simulate the light-dark cycle of daily life.
Daily solution changes were performed and maintained at room temperature. The solutions were prepared with distilled and deionized water.
The samples were subjected to the first cariogenic challenge, analyzed by FT-Raman spectroscopy, then treated as described in Table 1, and then subjected to the second cariogenic challenge, and finally again analyzed by FT-Raman spectroscopy.
Statistical analysis
A paired samples t-test was used to analyze the changes seen before and after treatment. A 95% confidence interval was used to evaluate the statistical significance using Instat software (GraphPad Software Inc., San Diego, CA, USA).
Results
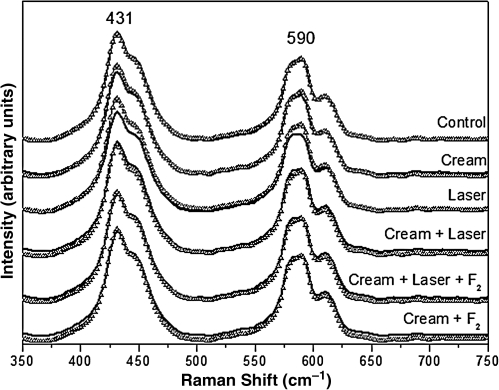
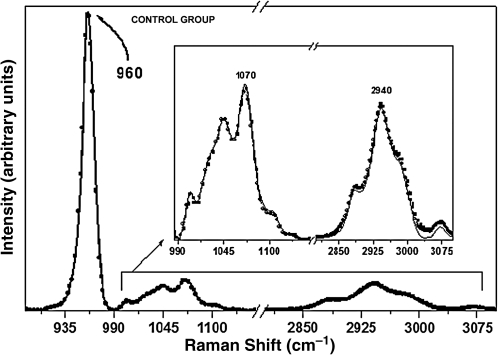
The Raman spectra of the organic and inorganic components of the enamel are shown in Figs. 2–8. The Raman signals have been vertically shifted for clarity. The peak at 1071 cm–1 is attributed to the type B carbonate vibrational mode. The peak at 2940 cm–1 is related to the organic component of enamel (i.e., CH2 stretching vibrations). The intensity of the organic peak was reduced after the first and second cariogenic challenges compared to the control group, in which the intensity was not affected. For the carbonate content, the intensity was not affected except for the group treated with cream + laser + fluoride (Fig. 5).
FIG. 2.
Spectra of the mineral components.
FIG. 8.
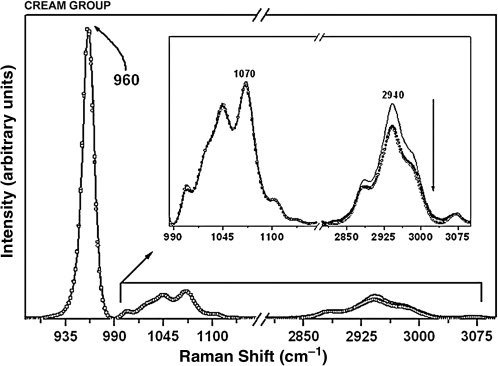
Raman spectra of the enamel with the phosphate peak at 960 cm−1, the carbonate peak at 1070 cm−1, and organic matter peak at 2940 cm−1 for the specimens in the cream-only group at pre-treatment (solid black line), after the first cariogenic challenge (circles), and after the second cariogenic challenge (squares). The arrow indicates intensity reduction. (Inset) Raman spectra in the 990–3100 cm−1 range, showing the components associated with the carbonate and organic sample content.
FIG. 5.
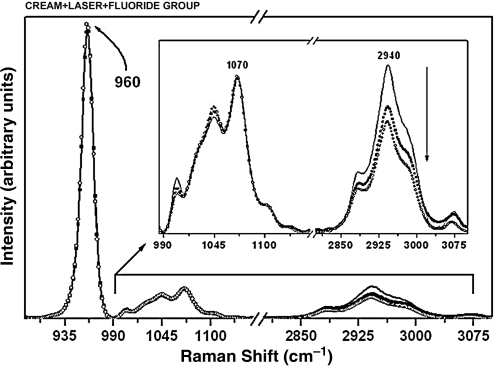
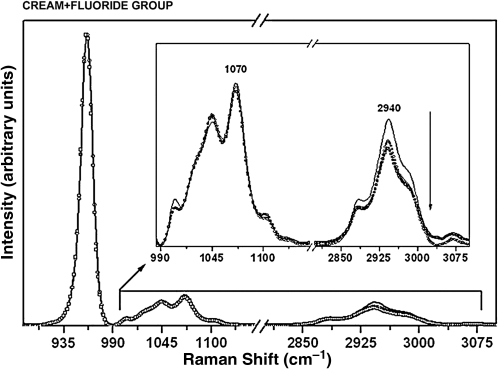
Raman spectra of the enamel with the phosphate peak at 960 cm−1, the carbonate peak at 1070 cm−1, and organic matter peak at 2940 cm−1 for the specimens from the cream + laser + fluoride group at pre-treatment (solid black line), after the first cariogenic challenge (circles), and after the second cariogenic challenge (squares). The arrow indicates intensity reduction. (Inset) Raman spectra in the 990–3100 cm−1 range, showing the components associated with the carbonate and organic sample content.
FIG. 7.
Raman spectra of the enamel with the phosphate peak at 960 cm−1, the carbonate peak at 1070 cm−1, and organic matter peak at 2940 cm−1 for the specimens in the cream + fluoride group at pre-treatment (solid black line), after the first cariogenic challenge (circles), and after the second cariogenic challenge (squares). The arrow indicates intensity reduction. (Inset) Raman spectra in the 990–3100 cm−1 range, showing the components associated with the carbonate and organic sample content.
The analysis of the integrated area of the carbonate content (1071/960 cm–1) showed a statistically significant reduction in the integrated area ratio only in the group treated with cream + laser + fluoride, between the pre-treatment and after the first challenge (p = 0.0354), and after the second challenge (p = 0.0260) (Tables 2 and 4).
Table 2.
Mean and Standard Deviations (SD) of Integrated Areas of Carbonate/PO4 Enamel Content for Each Group and Period of Treatment
| Group | Pre-treatment | First cariogenic challenge | Second cariogenic challenge |
|---|---|---|---|
| Control | 0.21 (0.03) | 0.22 (0.04) | 0.21 (0.05) |
| Cream | 0.20 (0.04) | 0.20 (0.04) | 0.19 (0.03) |
| Laser | 0.22 (0.05) | 0.20 (0.03) | 0.21 (0.06) |
| Cream + laser | 0.21 (0.04) | 0.20 (0.04) | 0.21 (0.05) |
| Cream + laser + fluoride | 0.22* (0.07) | 0.19* (0.06) | 0.19* (0.06) |
| Cream + fluoride | 0.20 (0.05) | 0.20 (0.05) | 0.19 (0.04) |
Asterisks denote statistically significant differences between treatments.
Table 4.
Statistically Significant Results of the Comparisons of Spectral Integrated Areas of Carbonate and Organic Components
| Component | Group | Comparison | p Value |
|---|---|---|---|
| Carbonate | Cream + laser + fluoride | Pre-treatment vs. first challenge | 0.0354 |
| Pre-treatment vs. second challenge | 0.0260 | ||
| Cream + laser + fluoride | Pre-treatment vs. first challenge | 0.0313 | |
| Pre-treatment vs. second challenge | 0.0106 | ||
| Organic | Cream + laser | Pre-treatment vs. first challenge | 0.0296 |
| Laser | Pre-treatment vs. first challenge | 0.0159 | |
| Pre-treatment vs. second challenge | 0.0072 |
A paired-samples t-test was used.
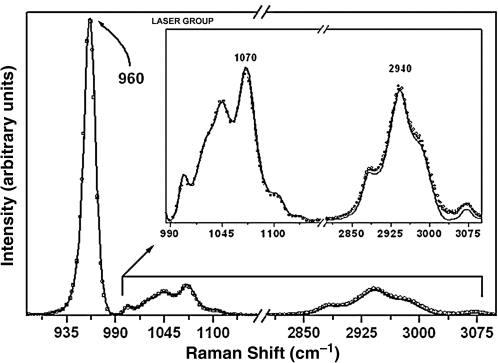
The analysis of the integrated area of the organic content (2940/960 cm–1) showed a statistically significant reduction in the integrated area ratio of the specimens treated only with the laser (Fig. 4) These differences were found between pre-treatment and after the first challenge (p = 0.0159), and after the second challenge (p = 0.0072) (Tables 3 and 4).
FIG. 4.
Raman spectra of the enamel with the phosphate peak at 960 cm−1, the carbonate peak at 1070 cm−1, and organic matter peak at 2940 cm−1 for the specimens from laser-treated group at pre-treatment (solid black line), after the first cariogenic challenge (circles), and after the second cariogenic challenge (squares). The arrow indicates intensity reduction. (Inset) Raman spectra in the 990–3100 cm−1 range, showing the components associated with the carbonate and organic sample content.
Table 3.
Mean and Standard Deviations (SD) of Integrated Areas of Organic/PO4 Enamel Content for Each Group and Period of Treatment
| Group | Pre-treatment | First cariogenic challenge | Second cariogenic challenge |
|---|---|---|---|
| Control | 0.46 (0.11) | 0.51 (0.20) | 0.48 (0.22) |
| Cream | 0.45 (0.15) | 0.35 (0.16) | 0.39 (0.13) |
| Laser | 0.61* (0.26) | 0.42* (0.17) | 0.32* (0.10) |
| Cream + laser | 0.42* (0.17) | 0.34* (0.16) | 0.39 (0.21) |
| Cream + laser + fluoride | 0.52* (0.30) | 0.43* (0.26) | 0.39* (0.23) |
| Cream + fluoride | 0.46 (0.24) | 0.39 (0.14) | 0.35 (0.22) |
Asterisks denote statistically significant differences between treatments.
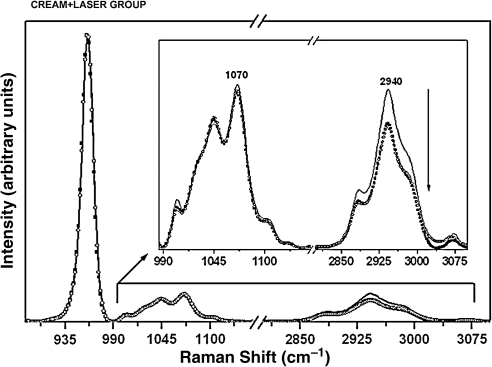
Significant differences for the organic matrix were also observed in the specimens treated with cream + laser (Fig. 6). These differences were found between the pre-treatment and after the first challenge (p = 0.0296) (Tables 3 and 4). Other significant differences were found in the specimens treated with cream + laser + fluoride (Fig. 5). These differences were found between the pre-treatment and after the first challenge (p = 0.0313), and after the second challenge (p = 0.0106) (Tables 3 and 4).
FIG. 6.
Raman spectra of the enamel with the phosphate peak at 960 cm−1, the carbonate peak at 1070 cm−1, and organic matter peak at 2940 cm−1 for the specimens from the cream + laser-treated group at pre-treatment (solid black line), after the first cariogenic challenge (circles), and after the second cariogenic challenge (squares). The arrow indicates intensity reduction. (Inset) Raman spectra in the 990–3100 cm−1 range, showing the components associated with the carbonate and organic sample content.
Discussion
In this study, the effectiveness of the low-power infrared laser (LPIRL) and photoabsorbing cream in altering enamel composition after demineralization was investigated. We compared the use of LPIRL alone, the topical application of fluorinated cream alone, the combination of the topical fluorinated cream with LPIRL, topical fluorinated photoabsorbing cream alone, or the combination of the cream and LPIRL. After cariogenic challenge some changes were seen in the enamel post-treatment.
Enamel is almost entirely mineral by weight (96%), but only 87% mineral by volume. Thus 13% of the space in the enamel is filled with water and soluble and insoluble proteins. The organic and water components of enamel allow diffusion of ions from plaque and saliva into the mineral elements, causing caries to form.13
The laser's effects on dental hard tissues depends on the irradiation parameters used, such as wavelength, pulsed or continuous emission, pulse duration, pulse energy, repetition rate, beam spot size, delivery method, laser beam characteristics, and optical properties of the tissue such as its refractive index, scattering coefficient (μs), absorption coefficient (μa), as well as scattering anisotropy.3 Consequently, the transmission, scattering, and absorption by target tissues must be taken into consideration, along with thermal propagation in order to assess the effects of laser therapy.
For caries prevention, in order to alter the composition of dental hard tissues, the laser energy must be strongly absorbed and converted efficiently to heat, but the effects must be restricted to the most superficial tissues, without damaging underlying or surrounding tissues.3 Therefore, wavelengths must be chosen for which absorption is high for hydroxyapatite and water, which is true when enamel is irradiated with high-powered lasers as CO2 and erbium lasers. The absorption coefficient of enamel for lasers emitting at 450–800 nm, such as those used in this study, is approximately 1.030, and to promote the induction of chemical changes in the enamel, the laser energy must be strongly absorbed. In this study we used photoabsorbing cream in order to increase the absorption of infrared laser energy by the enamel, in an effort to induce photochemical and thermal changes that make it more resistant to cariogenic challenge.
Some researchers suggest that the clinical efficacy of fluoride may be enhanced through improved delivery or by its use in combination with laser energy. Indeed, CO2, Nd:YAG, argon, and other high-powered lasers have shown efficacy in preventing dental caries in vitro and in vivo.4,20,21,38–44
Some reports have shown that the low-power red laser can induce caries prevention,27–29 and as it does not promote heating, the mechanism of action must be different. Our study proposes a new approach using photoabsorbing cream so that the tissues may more strongly absorb the low-power laser energy. Chromophores such as those used in the cream are useful for enhancing photodynamic and photothermal killing of microorganisms, as well as for tooth whitening and brightening. Chromophores include intrinsic or extrinsic light acceptors that induce and/or enhance photochemical reactions, leading to the generation of nitrogen oxide, singlet oxygen, and other free radicals within tissues.23 Indocyanine green (ICG), such as that used in this study, is clinically used as a fluorescent dye for imaging purposes. Its rapid circulation kinetics and minimal toxicity have prompted investigation into its utility as a photosentitizer for therapeutic applications such as those proposed by McNally et al., including ablation of carious tissue.45
The spectra of the demineralized tissue appeared similar to those seen pre-treatment, indicating that the low-energy laser treatment performed between the first and second cariogenic challenges only slightly affected the enamel's apatite and caused no structural damage. As there was also no alteration of the bandwidth of phosphate identified, this indicates that the laser treatments we used did not damage the crystalline phase of the tissues.
Carbonate,12 although it is a precursor of hydroxyapatite, may cause crystal defects, fits poorly into the lattice, and generates more of the acid-soluble apatite.46 In human enamel, the carbonate content consists of 10% type A and 90% type B carbonate. The total carbonate content was found to be significantly higher in deciduous teeth (2.23%) than in permanent enamel (2.15%),47 indicating the higher vulnerability of deciduous teeth to demineralization. Carbonate loss after argon laser irradiation (67 J/cm2), Er:YAG laser irradiation (5.1 J/cm2), and CO2 laser treatment (3–5 J/cm2) has also been observed.24,47,48
The band at 1070 cm–1 shown in Fig. 3 and Table 4 assigned to type B carbonate in this FT-Raman study demonstrated that the intensity and spectral integrated area of BCO32– peak related to 960 cm–1 (PO43– peak) decreased significantly only after the treatment with cream + laser (p = 0.0354 for after the first challenge; p = 0.026 after the second challenge). Liu et al.,25 using Er:YAG laser treatment (2 Hz, 5.1 J/cm2, spot size 1 mm, 5 sec irradiation/spot), observed a decrease in type B carbonate post-irradiation.
FIG. 3.
Raman spectra of the enamel with the phosphate peak at 960 cm−1, the carbonate peak at 1070 cm−1, and organic matter peak at 2940 cm−1 for the specimens from the control group pre-treatment (solid black line), after the first cariogenic challenge (circles), and after the second cariogenic challenge (squares). The arrow indicates intensity reduction. (Inset) Raman spectra in the 990–3100 cm−1 range, showing the components associated with the carbonate and organic sample content.
Organic matrix at very low concentrations (1%), is also present in dental enamel. This takes the form of very small peptides and amino acids distributed throughout the tissue and presumably represents the remnants of the original developmental matrix, perhaps retained by binding to the hydroxyapatite crystals.49,50 It provides the template for enamel mineralization and continues to be the means of transporting certain substances within the enamel. It may have great potential to control the diffusion pathway in enamel and thus may play a significant role in laser-induced caries prevention.26 However, quantification of the organic phase remains a problem, since it is only a tiny component of dental enamel.
In Raman studies26,51 the 2940-cm–1 band is used to quantify the relative organic change, since it is sharper and stronger than the amide bands. The organic peaks at 1200–1700 cm–1 displayed a broader configuration because some portions may remain as a hybrid and partially amorphous phase.52 Our data demonstrate that the band's intensity reduction was seen after all laser treatment types at all time points, indicating a decrease in organic matter. These results are in agreement with those of a previous study, which revealed a laser-induced decrease in band intensity at 1200–1600 and 2800–3200 cm–1.53 Our data show that this was the spectral region with the widest difference in intensity, and thus Raman spectroscopy was a useful tool to analyze this tiny component.
In contrast to the breakdown of the protein matrix by proteolysis, the interaction between laser energy, the chromophores, and the enamel can cause protein denaturation that results in blockage of the diffusion pathway in the enamel.25 Indeed, the consolidation of the protein component of the organic matrix after denaturation can produce a significant reduction in the crystal surface area available to acid decalcification.50
Currently, it is believed that the decrease in enamel solubility after laser treatment is due to changes in its structure, such as reductions in water and carbonate content, increases in the amount of hydroxyl ions, pyrophospate formation, and protein decomposition.20,21,24 Another possible effect may be changes and perhaps destruction of the organic material in the interprismatic spaces.
For low-energy lasers such as argon lasers, some authors suggest that they have photochemical effects, inducing changes in the polarization of some components of the enamel.
It is well known that low-level laser therapy does not lead to negative thermal effects, but the cream used in our study for photoabsorption may cause temperature increases capable of changing the enamel's organic matter.
The results of this study of the use of FT-Raman spectroscopy suggest that the laser treatment types we tested may induce photochemical effects and cause minor thermal alterations that induce decomposition of the enamel's organic matter, which although it is only present in small quantities, may play a significant role in inhibiting ionic diffusion through the surface, and thus preventing enamel demineralization.25,26,27,50–54
Conclusion
Laser-induced reductions and modifications in the organic matrix were observed in this study, indicating that further studies are needed of the mechanisms of caries prevention in enamel treated with low-level laser therapy. Indeed, our study proposes that FT-Raman spectroscopy may be suitable for detecting compositional and structural changes in both the mineral and organic phases of lased enamel under cariogenic challenge.
Acknowledgments
This work was supported by FAPESP (01/14384-8 and 05/50811-9) and CNPq (National Council of Technical and Scientific Development) (grant no. 302393/2003-0). The authors wish to thank DMC Equipamentos Ltda. for supplying the laser equipment used in this study.
Disclosure Statement
No conflicting financial interests exist.
References
- 1.U.S. Department of Health and Human Services, National Institute of Dental and Craniofacial Research, and National Institutes of Health 2000J. Calif. Dent. Assoc. 2868511324049 [Google Scholar]
- 2.Lima Y.B.O. Cury J.A. Seasonal variation of fluoride intake by children in a subtropical region. Caries Res. 2003;37:335–338. doi: 10.1159/000072164. [DOI] [PubMed] [Google Scholar]
- 3.Ana P.A. Bachmann L. Zezell D.M. Lasers effects on enamel for caries prevention. Laser Physics. 2006;16:865–875. [Google Scholar]
- 4.Rodrigues L.K.A. Santos M.N. Pereira D. Assaf A.V. Pardi V. Carbon dioxide laser in dental caries prevention. J. Dent. 2004;32:531–540. doi: 10.1016/j.jdent.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 5.Hugo F.N. Vale G.C. Ccahuana-Vásquez R.A. Cypriano S. de Sousa M.L. Polarization of dental caries among individuals aged 15 to 18 years. J. Appl. Oral Sci. 2007;15:253–258. doi: 10.1590/S1678-77572007000400003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tayanin G.L. Petersson G.H. Bratthall D. Caries risk profiles of 12- and 13-year-old children in Laos and Sweden. Oral Health Prev. Dent. 2005;3:15–23. [PubMed] [Google Scholar]
- 7.Gillcrist J.A. Brumley D.E. Blackford J.U. Community socioeconomic status and children's dental health. J. Am. Dent. Assn. 2001;132:216–222. doi: 10.14219/jada.archive.2001.0158. [DOI] [PubMed] [Google Scholar]
- 8.Tinanoff N. Introduction to the Early Childhood Caries Conference: initial description and current understanding. Comm. Dent. Oral Epidemiol. 1998;26(Suppl. 1):5–7. doi: 10.1111/j.1600-0528.1998.tb02089.x. [DOI] [PubMed] [Google Scholar]
- 9.Vargas C.M. Crall J.J. Schneider D.A. Sociodemographic distribution of pediatric dental caries: NHANES III, 1988–1994. J. Am. Dent. Assn. 1998;129:1229–1238. doi: 10.14219/jada.archive.1998.0420. [DOI] [PubMed] [Google Scholar]
- 10.Hicks M.J. Flaitz C.M. Carter A.B., et al. Dental caries in HIV-infected children: a longitudinal study. Pediatr. Dent. 2000;22:359–364. [PubMed] [Google Scholar]
- 11.White T. Ferraris C. Kim J. Madhavi S. Apatite—an adaptive framework structure. Rev. Mineral Geochem. 2005;57:307–401. [Google Scholar]
- 12.Sojun Clasen A.B. Ruyter I.E. Quantitative determination of type A and type B carbonate in human deciduous and permanent enamel by means of Fourier transform infrared spectrometry. Adv. Dent. Res. 1997;11:523–527. doi: 10.1177/08959374970110042101. [DOI] [PubMed] [Google Scholar]
- 13.Zero D.T. Dental caries process. Dent. Clin. North Am. 1999;43:635–663. [PubMed] [Google Scholar]
- 14.Featherstone J.D. The caries balance: contributing factors and early detection. J. Calif. Dent. Assoc. 2003;31:129–133. [PubMed] [Google Scholar]
- 15.Featherstone J.D. The science and practice of caries prevention. J. Am. Dent. Assn. 2000;131:887–899. doi: 10.14219/jada.archive.2000.0307. [DOI] [PubMed] [Google Scholar]
- 16.Featherstone J.D. Prevention and reversal of dental caries: role of low level fluoride. Comm. Dent. Oral Epidemiol. 1999;27:31–40. doi: 10.1111/j.1600-0528.1999.tb01989.x. [DOI] [PubMed] [Google Scholar]
- 17.Hicks J. Garcia-Godoy F. Flaitz C. Biological factors in dental caries: role of saliva and dental plaque in the dynamic process of demineralization and remineralization (part 1) J. Clin. Pediatr. Dent. 2003;28:47–51. doi: 10.17796/jcpd.28.1.yg6m443046k50u20. [DOI] [PubMed] [Google Scholar]
- 18.Hicks J. Garcia-Godoy F. Flaitz C. Biological factors in dental caries: role of saliva and dental plaque in the dynamic process of demineralization and remineralization (part 2) J. Clin. Pediatr. Dent. 2003;28:119–124. doi: 10.17796/jcpd.28.1.yg6m443046k50u20. [DOI] [PubMed] [Google Scholar]
- 19.Hicks J. Garcia-Godoy F. Flaitz C. Biological factors in dental caries: role of saliva and dental plaque in the dynamic process of demineralization and remineralization (part 3) J. Clin. Pediatr. Dent. 2004;28:203–214. doi: 10.17796/jcpd.28.3.w0610427l746j34n. [DOI] [PubMed] [Google Scholar]
- 20.Hicks J. Flaitz C. Ellis R. Westerman G. Powell L. Primary tooth enamel surface topography with in vitro argon laser irradiation alone and combined fluoride and argon laser treatment: scanning electron microscopic study. Pediatr. Dent. 2003;25:491–496. [PubMed] [Google Scholar]
- 21.Westerman G.H. Hicks M.J. Flaitz C. Powell G.L. In vitro enamel caries formation: argon laser, light-emitting diode and APF treatment effect. Am. J. Dent. 17:383–387. [PubMed] [Google Scholar]
- 22.Anderson J.R. Ellis R.W. Blankenau R.J. Beiraghi S.M. Westerman G.H. Caries resistance in enamel by laser irradiation and topical fluoride treatment. J. Clin. Laser Med. Surg. 2000;18:33. doi: 10.1089/clm.2000.18.33. [DOI] [PubMed] [Google Scholar]
- 23.De Sant'Anna G.R. Paleari G.S.L. Duarte D.A. Brugnera A., Jr. Pacheco-Soares C. Surface morphology of sound deciduous tooth enamel after application of a photo-absorbing cream and infrared low-level laser irradiation: An in vitro scanning electron microscopy study. Photomed Laser Surg. 2007;25:500–507. doi: 10.1089/pho.2007.2088. [DOI] [PubMed] [Google Scholar]
- 24.Featherstone J.B.D. Fried D. Eric R.B. Mechanism of laser induced solubility reduction of dental enamel. Proc. SPIE. 1997;2973:112–116. [Google Scholar]
- 25.Liu Y. Hsu C.Y. Laser-induced compositional changes on enamel: A FT-Raman study. J. Dent. 2007;35:226–230. doi: 10.1016/j.jdent.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 26.Hsu C.Y. Jordan T.H. Dederich D.N. Wefel J.S. Effects of low energy CO2 laser irradiation and the organic matrix on inhibition of enamel demineralization. J. Dent. Res. 2000;79:1725–1730. doi: 10.1177/00220345000790091401. [DOI] [PubMed] [Google Scholar]
- 27.Slujáiev I.F. Pak A.N. He-Ne laser effect on dental enamel solubility in health and caries. Stomatology. 1990;5:6–9. [Google Scholar]
- 28.Andreu M.I.G. Zaldivar C.V. Dben A.G. Influencia de la radiación láser de baja potencia em molares permanente inmaduros. Rev. Cub. Estomatol. 1996;33:1–4. [Google Scholar]
- 29.Fagnoni V. Sapino S. Zulian P. Iemma D. Ionofluor + laser = prevenzione. Min. Stomatol. 1989;38:769–772. [PubMed] [Google Scholar]
- 30.Featherstone J.D.B. Nelson D.G.A. Laser effects on dental hard tissue. Adv. Dent. Res. 1987;1:21–26. doi: 10.1177/08959374870010010701. [DOI] [PubMed] [Google Scholar]
- 31.Zach L. Cohen G. Pulp response to externally applied heat. Oral. Surg. Oral Med. Oral Pathol. 1965;19:515–530. doi: 10.1016/0030-4220(65)90015-0. [DOI] [PubMed] [Google Scholar]
- 32.Baldissara P. Catapano S. Scotti R. Clinical and histological evaluation of thermal injury thresholds in human teeth: a preliminary study. J. Oral Rehabil. 1997;24:791–801. doi: 10.1046/j.1365-2842.1997.00566.x. [DOI] [PubMed] [Google Scholar]
- 33.Carden A. Morris M.D. Application of vibrational spectroscopy to the study of mineralized tissues. J. Biomed. Optics. 2000;5:259–268. doi: 10.1117/1.429994. [DOI] [PubMed] [Google Scholar]
- 34.Ten Cate J.M. Duijsters P.P. Alternating demineralization and remineralization of artificial enamel lesions. Caries Res. 1982;16:201–210. doi: 10.1159/000260599. [DOI] [PubMed] [Google Scholar]
- 35.Mendes F.M. Nicolau J. Utilization of laser fluorescence to monitor caries lesions development in primary teeth. J. Dent. Child. 2004;71:139–142. [PubMed] [Google Scholar]
- 36.Tsuda H. Ruben J. Arends J. Raman spectra of human dentine mineral. Eur. J. Oral Sci. 1996;104:123–131. doi: 10.1111/j.1600-0722.1996.tb00056.x. [DOI] [PubMed] [Google Scholar]
- 37.Ko A.C. Choo-Smith L.P. Hewko M., et al. Ex vivo detection and characterization of early dental caries by optical coherence tomography and Raman spectroscopy. J. Biomed. Opt. 2005;10:031118. doi: 10.1117/1.1915488. [DOI] [PubMed] [Google Scholar]
- 38.Stern R.H. Sognnaes R.F. Goodman F. Laser effect on in vitro enamel permeability and solubility. J. Am. Dent. Assoc. 1966;73:838–843. doi: 10.14219/jada.archive.1966.0319. [DOI] [PubMed] [Google Scholar]
- 39.Yamamoto H. Ooya K. Potential of yttrium-aluminum-garnet laser in caries prevention. J. Oral Pathol. Med. 1974;3:7–15. doi: 10.1111/j.1600-0714.1974.tb01693.x. [DOI] [PubMed] [Google Scholar]
- 40.Cecchini R.C.M. Zezell D.M. Oliveira E. Freitas P.M. Eduardo C.P. Effect of Er:YAG laser on enamel acid resistance: morphological and atomic spectrometry analysis. Lasers Surg. Med. 2005;37:373–380. doi: 10.1002/lsm.20247. [DOI] [PubMed] [Google Scholar]
- 41.Chin-Ying S.H. Gao X.L. Pan J.S. Wefel J.S. Effects of CO2 laser on fluoride uptake in enamel. J. Dent. 2004;32:161–167. doi: 10.1016/j.jdent.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 42.Morioka T. Tagomori S. Combined effects of laser and fluoride on acid resistance of human dental enamel. Caries Res. 1989;23:225–231. doi: 10.1159/000261182. [DOI] [PubMed] [Google Scholar]
- 43.Nelson D.G.A. Jongebloed W. Featherstone J.D.B. Laser irradiation of human dental enamel and dentin. N.Z. Dental J. 1986;82:74–77. [PubMed] [Google Scholar]
- 44.Delbem A.C.B. Cury J.A. Nakassima C.K. Gouveia V.G. Theodoro L.H. Effect of Er:YAG laser on CaF2 formation and its anticariogenic action on human enamel: an in vitro study. J. Clin. Laser Med. Surg. 2003;21:197–201. doi: 10.1089/104454703768247765. [DOI] [PubMed] [Google Scholar]
- 45.McNally K.M. Gillings B.R.D. Dawes J.M. Dye-assisted diode laser ablation of carious enamel and dentine. Aust. Dent. J. 2008;44:169–175. doi: 10.1111/j.1834-7819.1999.tb00218.x. [DOI] [PubMed] [Google Scholar]
- 46.Nelson D.G.A. Wefel J.S. Jongebloed W.L. Featherstone J. Morphology, histology and crystallography of human dental enamel treated with pulsed low-energy infrared laser irradiation. Caries Res. 1987;21:411–426. doi: 10.1159/000261047. [DOI] [PubMed] [Google Scholar]
- 47.Nelson D.G.A. Shariati M. Glena R. Shields C.P. Featherstone J.D. Effect of pulsed low energy infrared laser irradiation on artificial caries-like lesion formation. Caries Res. 1986;20:289–299. doi: 10.1159/000260948. [DOI] [PubMed] [Google Scholar]
- 48.Deng Y. Hsu C.Y.S. Combined effect of fluoride and laser on the crystalline structure of human enamel—a pilot study. Proc. SPIE. 2005;5687:43–49. [Google Scholar]
- 49.Oho T. Morioka T. A possible mechanism of acquired acid resistance of human dental enamel by laser irradiation. Caries Res. 1990;24:86–92. doi: 10.1159/000261245. [DOI] [PubMed] [Google Scholar]
- 50.Fleming S. Tawashi R. Dissolution retardation of dental enamel with special reference to the protein matrix. Can. J. Pharm. Sci. 1977;12:55–59. [Google Scholar]
- 51.Hsu C.Y. Jordan T.H. Dederich D.N. Wefel J.S. Laser-matrix fluoride effects on enamel demineralization. J. Dent. Res. 2001;80:1797–1801. doi: 10.1177/00220345010800090501. [DOI] [PubMed] [Google Scholar]
- 52.Kirchner M.T. Edwards H.G.M. Ancient and modern specimens of human teeth: a Fourier transform Raman spectroscopic study. J. Raman Spectrosc. 1997;28:171–178. [Google Scholar]
- 53.Camerlingo C. Lepore M. Gaeta G.M. Riccio R. Riccio C. De Rosa A. De Rosa M. Er:YAG laser treatments on dentine surface: Micro-Raman spectroscopy and SEM analysis. J. Dentistry. 2004;32:399–405. doi: 10.1016/j.jdent.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 54.Gerard D.E. Fried D. Featherstone J.D.B. Nancollas G.H. Influence of laser irradiation on the constant composition kinetics of enamel dissolution. Caries Res. 2005;39:387–392. doi: 10.1159/000086845. [DOI] [PubMed] [Google Scholar]