Abstract
No serological method suitable for large screening of antibodies against Histomonas meleagridis in poultry is available so far, the objective targeted in the present investigation. Consequently, an ELISA was developed as a suitable tool for this purpose. Investigating serum samples from non-infected specific pathogen-free (spf) chickens and commercial turkeys a high-background signal was noticed when ELISA plates were directly coated with purified parasitic cells. This signal was significantly reduced by coating the plates with a polyclonal rabbit antibody, raised against histomonads, prior to the addition of the antigen. Adopting this approach five antigen preparations were compared and a high reproducibility could be demonstrated reflected by a very low coefficient of variation of 5.3% and 1.7% for the chicken and turkey sera, respectively. After this initial development all further experiments were carried out with one set of plates and the same antigen preparation. Investigating chicken sera obtained from birds infected at 14 days of life, OD values above a predetermined cut-off value were observed 2 weeks post-infection and a rise of IgG antibodies was noticed until 6 weeks post-infection, when the experiment was terminated. Non-protected turkeys infected at 6 weeks of age displayed an increasing IgG response until 14 days post-infection, prior to the death of animals due to histomonosis. In comparison, the majority of turkeys vaccinated with attenuated histomonads, obtained through prolonged passaging and challenged 4 weeks later with virulent parasites, displayed a demonstrable antibody response after the challenge only. Antibody titres increased until 4 weeks post-challenge when the birds were killed and the study was terminated. Altogether, the developed indirect sandwich ELISA proved to be a quick and efficient method to detect IgG antibodies against H. meleagridis in sera of experimentally infected chickens and turkeys and will be a helpful tool to obtain more insights into the epidemiology of the parasite and the immune response of its hosts.
Keywords: Histomonas meleagridis, Chicken, Turkey, Antibodies, ELISA
1. Introduction
Histomonosis (syn. histomoniasis, blackhead disease) is an important disease in poultry caused by the flagellated protozoon Histomonas meleagridis (Tyzzer, 1920). For a long time the diagnosis of the disease was mainly based on clinical signs and the typical pathomor-phological lesions in the caeca and liver (McDougald, 2005). Direct microscopic investigation of caecal content or excreted faeces and certain tissue staining techniques proved to be very time consuming. Furthermore, specific detection could be hampered due to the presence of unspecific contaminants or other flagellates (Kemp and Reid, 1966).
The ban of all chemotherapeutics followed by the re-emergence of the disease has started the search for new and improved diagnostic methods. Anyhow, most of the currently available diagnostic tools are aiming for detection of the parasite or its DNA, to obtain more information about the pathogenesis of the disease. In this context in situ hybridization and immunohistology were developed to localize parasitic DNA or parasitic cells in tissue samples (Liebhart et al., 2006; Singh et al., 2008). Moreover, the first polymerase chain reaction (PCR) assays for the diagnosis of histomonosis were developed recently, offering the possibility to detect parasitic DNA in all kind of materials including organ samples and faeces (Bleyen et al., 2007; Grabensteiner and Hess, 2006; Hafez et al., 2005; Huber et al., 2005). However, using reisolation intermittent excretion of histomonads in faecal samples was demonstrated (Hess et al., 2006a), which would limit PCR for epidemiological investigations.
Based on the existing literature the lack of diagnostic methods to be applied for diagnosing the infection in live birds is very obvious. The objective of the present investigation was therefore to develop an ELISA which can be used for the screening of sera in order to determine the infectious status of chickens and turkeys according to the antibody response of the animals.
2. Materials and methods
2.1. Rabbit serum
The immunizing antigen for the rabbits was the clonal culture H. meleagridis (Hm/Turkey/Austria/2922-66/C04), originally isolated from a diseased turkey and established through micromanipulation (Hess et al., 2006b). The development of the polyclonal histomonad antiserum in rabbits used for coating the microtitre plates was described in detail previously (Singh et al., 2008). The culture was also used in the animal experiments as described below. Altogether the rabbits were injected four times with 6 weeks intervals. Six weeks after the last injection the animals were euthanized, blood was collected, centrifuged and the serum was stored at −20 °C for further use.
2.2. Chicken and turkey sera from non-infected birds
A total of 37 sera from spf (specific pathogen-free) chickens together with 62 turkey sera, all of them taken from one to 12-week-old non-infected birds from different experimental studies, served as negative controls. Absence of histomonads in these birds was constantly proven by continuous cloacal swabbing and subsequent recultivations.
2.3. Chicken sera from infected birds
Chicken sera were taken from a recently described animal trial (Hess et al., 2006a). Briefly, spf chickens (VALO, Lohmann, Cuxhaven, Germany) were infected via the cloaca at 14 days of life using a virulent isolate of H. meleagridis (Hm/Turkey/Austria/2922-66/C04). Birds were bled prior to infection and in weekly intervals up to 6 weeks post-infection when the study was terminated. None of the 10 infected and the 4 in-contact birds displayed any adverse clinical signs throughout the whole experiment.
2.4. Turkey sera from infected birds
Serum samples from a total of 52 turkeys (“B.U.T. 9”) were used from a previously described infection experiment, demonstrating the benefit of vaccination (Hess et al., 2008). All birds were bled prior to vaccination performed via the cloacal route at 14 days of life with attenuated histomonads, achieved by continuous in vitro passaging of a virulent isolate. Investigated sera originated from 14 birds/group of which 10 birds were initially vaccinated either with passage (P) 95 (group I), P215 (group II) or P295 (group III) and from four birds per group which were kept as in-contact animals. Four weeks after vaccination 10 birds/group were challenged cloacally and 4 previously vaccinated birds were kept as in-contact birds. Blood samples from all of the birds were collected weekly until 4 weeks post-challenge. At the same time blood samples were also taken from 10 non-vaccinated but challenged birds which were housed as a separate group IV. With the exception of one bird all birds in this group died or had to be euthanized up to 2 weeks post-challenge allowing only two sampling dates.
Following sample collection blood samples were kept overnight at 4 °C. After 24 h the samples were centrifuged at 3300 × g for 12 min (Hettich Rotanta 460), aliquoted and the sera were stored at −20 °C for further use.
2.5. Preparation of antigen for the ELISA
The clonal culture, which was also used in the animal experiments, was passaged in vitro up to 25 times. The parasites were cultivated in 50 ml sterile FALCON tubes in 9 ml medium 199 HEPES (Gibco™, Invitrogen) containing 15% foetal calf serum (Gibco™, Invitrogen) and 22 mg autoclaved rice starch (Sigma–Aldrich). The cultures were passaged every 48 or 72 h by transferring 1 ml of the culture into 9 ml of fresh medium. For preparation of the antigen, the cultures were purified through several centrifugation steps. First the tubes were placed on a tumbler to dissolve the cell-rice pellet at the bottom of the tube. Then the culture was centrifuged at 1000 × g for 5 min. Afterwards the supernatant was carefully taken off using a sterile transfer pipette and 5 ml of fresh medium was added. The cell pellet was resuspended again using the tumbler and centrifuged at the same velocity as before. These steps were repeated until the culture was washed four times with fresh medium 199. After the last centrifugation step the pellet was dissolved in 1 ml medium 199, transferred to a 2 ml Eppendorf tube and centrifuged at 200 × g for 2 min. After that the supernatant was taken off carefully and the remaining pellet resuspended in phosphate-buffered-saline pH 7.4 plus 0.05% Tween 20 (PBST). The cells were counted in a Neubauer counting chamber and diluted in PBST to reach a final concentration of 10,000 histomonads in 100 μl. The antigen suspensions were aliquoted in 10 ml portions and stored at −20 °C until further use.
2.6. ELISA procedure
The optimal dilutions for the reagents were predetermined through checker-board titrations. For the indirect sandwich ELISA 96-well ELISA plates (Nunc Medisorb®) were coated with 100 μl rabbit anti-histomonas serum in a 1:10,000 dilution in carbonate buffer pH 9.6. The coated plates were left overnight and incubated at 4 °C. The next day the plates were washed with washing buffer (PBS containing 0.05% Tween 20, pH 7.4; 3 times 300 μl/well) using an automated plate washer (IDEXX Columbus D) for removal of all unbound particles. The coated plates were closed with a lid, sealed with laboratory Parafilm® “M” (Pechiney Plastic Packaging, Chicago, USA) and stored at −20 °C until further use. Plates were thawed at room temperature and incubated with 300 μl/well of blocking buffer (Starting Block™ T20 PBS, Pierce) to prevent non specific binding. After 2 min of incubation the buffer was removed through inversion of the plates. The plates were tapped vigorously on absorptive paper to remove the remaining fluid. Afterwards 100 μl of the histomonas antigen diluted in PBST was added to each well and incubated for 1 h at room temperature. Following antigen washing with PBST, 100 μl of the serum samples diluted 1:500 in blocking buffer were added. The plates were incubated again for 1 h at room temperature and then washed as mentioned above. Goat-Anti-Turkey IgG-horseradish-peroxidase (HRP) or Goat-Anti-Chicken-IgG-HRP (Southern Biotechnology, USA) was added in a 1:5000 dilution in PBST and again incubated for 1 h. Washing was once repeated and afterwards tetramethylbenzidine (TMB) substrate (Calbiochem®) was added (100 μl/well) and the plates were incubated for 15 min in the dark. The reaction was stopped with 100 μl 0.5 M sulphuric acid/well. The optical density (OD) of all wells was measured in a spectrophotometer (ELx800, Bio-TEK Instruments Inc.) at a wavelength of 450 nanometers (nm). The same procedure was applied for the indirect ELISA, except that the rabbit antiserum was omitted and the same amount of histomonas cells diluted in carbonate buffer was directly coated on the plates.
Chicken and turkey sera were tested on the same set of microtitre plates coated with the rabbit serum on the same day. After coating the plates they were washed and stored at −20 °C. The used antigen derived of the same passage and it was purified and diluted on the same day, meaning that all tests were done with the same set of plates and antigen. Four wells/plate were used to incorporate always the same two positive and negative control sera in all assays in order to prove the overall test performance. All sera were tested in duplicate.
2.7. Coefficient of variation
To determine the repeatability of the test, five preparations of antigen samples obtained from the same passage (P25), were processed on different occasions and tested accordingly. As primary antibody, 20 sera from chickens and turkeys obtained from non-infected birds were tested on each plate. The mean of every sample set was determined and the standard deviation of the mean values of the five replicates was calculated and put in relation to the overall mean of the replicates. To ensure an adequate repeatability, the allegation was that coefficients of variation should be equal or less than 10% (Crowther, 2000).
2.8. Determination of the cut-off value
The arithmetic mean of all OD values plus three times the standard deviations determined from all non-infected chicken or turkey sera were used to establish the cut-off values. In chickens OD values above 0.54 were considered positive, whereas in turkeys a cut-off value of 0.36 was determined. To demonstrate the benefit of the indirect sandwich ELISA system the same testing was performed in parallel with the indirect ELISA, where the plates were coated directly with the same amount of antigen without the rabbit serum, and the mean values and standard deviations of the results of both tests were compared. All statistics were performed with SPSS 14.0 (SPSS Inc., Chicago, USA).
3. Results
3.1. Test performance of the ELISA
The first experiments were performed with an indirect ELISA by coating the wells directly with the histomonas antigen. The polyclonal rabbit serum raised against H. meleagridis was used as primary antibody in order to control the coating procedure, as high OD values were achieved (data not shown). However, processing turkey and chicken sera from non-infected birds via the indirect ELISA, these control sera showed a high-background signal. To minimize these background signals the plates were first of all coated with the polyclonal anti-histomonas rabbit serum, moving towards an indirect sandwich system. Following this approach the background signals were significantly reduced and the range of values obtained from the negative sera was much smaller (Fig. 1). Comparing five different antigen preparations a coefficient of variation of 5.32% and 1.73% for the tested chicken and turkey sera was obtained.
Fig. 1.
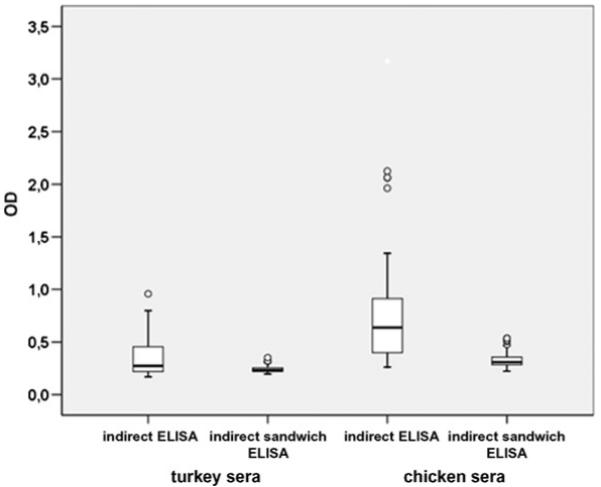
Comparison of the indirect ELISA and the indirect sandwich ELISA measuring sera of non-infected chickens (n = 37) and turkeys (n = 62). Circles represent outliers.
3.2. Analysis of the chicken and turkey sera from experimentally infected birds by the indirect sandwich ELISA
3.2.1. Chicken sera
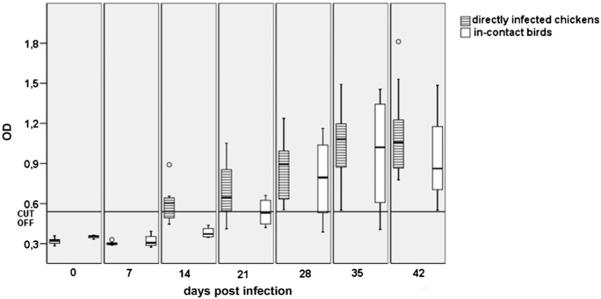
Testing of sera started prior to the infection (day 0). An antibody titre above the determined cut-off value was first noticed on the 14th day post-infection (d.p.i.) in some of the directly infected animals whereas antibodies in the in-contact birds stayed below the cut-off value (Fig. 2). A rise of antibodies was noticed at 21 d.p.i. and all directly infected birds were above the cut-off value at 28 d.p.i. The in-contact birds showed first positive titres at 21 d.p.i. and until the end of the experiment all in-contact birds displayed a clear positive antibody titre.
Fig. 2.
OD values of sera obtained from 14-day-old chickens which were infected either directly (n = 10) or kept as in-contact (n = 4) birds. The sera were tested weekly over 6 weeks beginning with the day of the infection (day 0). Circles represent outliers.
3.2.2. Turkey sera
First measurement started prior to vaccination of birds at 14 days of life (day 0). Within the next 2 weeks all birds vaccinated with one of the higher passages (P215 or P295) did not develop an antibody response above the cut-off value. In comparison, birds from group I vaccinated with the lowest passage (P95) reacted earlier to the antigen, with the first noticeable OD values above the cut-off on the 14th day post-vaccination (p.v.). All birds in group I developed a positive antibody reaction 1 week post-challenge and the OD values increased only slightly until the termination of the experiment. In group II vaccinated with P215 first OD values above the cut-off were noticed 3 weeks p.v. Furthermore, it took up to day 49 p.v. which is 3 weeks post-challenge (p.c.) until all birds were tested positive, displaying a somewhat delayed reaction in comparison with group I. Birds from group III infected with the highest passage number (P295) showed a similar reaction pattern as described for the birds in group II and the first positive samples were noticed on day 21 p.v. At the end of the experiment and 4 weeks p.c. the turkeys of this group displayed the highest antibody titres (Fig. 3).
Fig. 3.
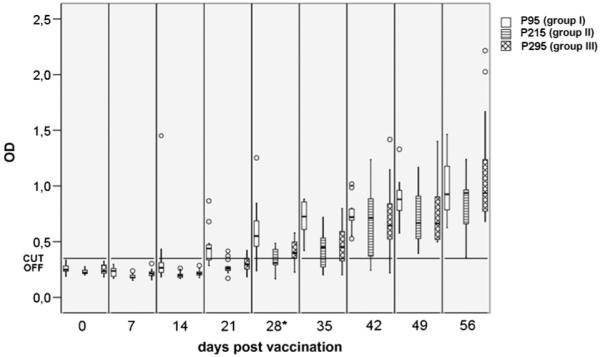
IgG antibody levels determined in turkey sera collected from birds vaccinated at 14 days of life with histomonads resembling different levels of attenuation (P95, P215 and P295) obtained by continuous passaging. The birds were challenged 4 weeks later with virulent Histomonas meleagridis. Testing started at day of vaccination (day 0) and was performed until 4 weeks post-challenge. *Day of challenge with virulent parasites. Circles represent outliers.
An antibody response above the cut-off value was also noticed in all non-vaccinated birds of group IV which survived up to 14 d.p.i. (Fig. 4). No further measurement was possible as all birds died by histomonosis during the next few days. Exceptional to this, one bird (no. 5) survived until day 23 displaying a further rise of antibodies.
Fig. 4.
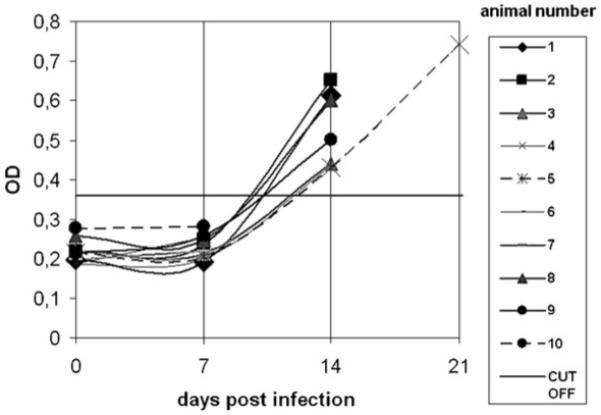
OD values measured in turkey sera of non-vaccinated turkeys infected as challenge control at 6 weeks of age. Data are shown until day 21 p.i., the final day for blood sampling. Day 0 = day of infection.
4. Discussion and conclusion
Since some decades the ELISA can be considered as the predominant serological assay in biomedical diagnosis and research, mainly due to the high flexibility and the large sample size to be processed at the same time (Crowther, 2000). However, for H. meleagridis such a system is still not available, a gap to be closed with the present study in which an ELISA is described for the first time capable to measure an antibody response against H. melegaridis in poultry.
In order to set up such a system, microtitre plates were coated with purified histomonads as antigen fraction. However, it turned out that supposed negative sera processed with this type of ELISA showed a very high-background signal. This problem was significantly minimized using a polyclonal rabbit serum as coating antibody in the assay, moving from the indirect ELISA to an indirect sandwich ELISA. Furthermore, it was possible to increase the precision of the OD values using this system, displayed in a far lower standard deviation within the tested negative control chicken and turkey sera.
In a second step serum samples were tested, obtained from experimentally infected spf chickens and commercial turkeys.
Investigating the chicken sera antibody titres above the cut-off value were noticed 14 d.p.i. These results are in agreement with the findings reported by Clarkson (1963) who noticed precipitating antibodies 11 d.p.i. by applying the agar-gel diffusion technique. In this study he tested 14 8-week-old turkeys and 10 6-week-old chickens which he infected via the cloaca with homogenised tissue samples containing histomonas cells and treated the birds afterwards with acinitrazol in order to guarantee survival of the animals. Nevertheless, most of the turkeys died from histomonosis. The surviving six birds were re-infected several times and followed up to more than 15 month after the initial infection. Precipitating antibodies in chickens and turkeys were first noticed 14 days after infection, similar to the observation noticed in the present study. At much later stages the author found very inconclusive results which he attributed to the way immunity was induced and the low number of animals. Overall he concluded that the infection must progress to a certain extent in order to obtain a measurable level of precipitating antibodies. In the present study higher mean titres were obtained from the birds vaccinated with the least attenuated protozoa, supporting this assumption. Such histomonads have obviously kept the ability to penetrate the intestinal mucosal barrier, an obvious distinction between virulent and attenuated isolates (Lund, 1959). The fact that all non-vaccinated birds which died after the second blood sampling at 14 days post-infection expressed an antibody titre above the cut-off value is also an indicator for the relationship between virulence and antibody response.
The increase of antibodies following challenge was most severe in the two groups vaccinated with the more attenuated histomonads. Interestingly, in these groups some birds were noticed which did not display an antibody response above the cut-off level prior to challenge. Anyhow, following challenge none of these birds suffered or died from histomonosis indicating that humoral antibodies are of limited value for protection. This is also in agreement with earlier findings reported by Clarkson (1963), who failed to protect turkeys by transferring serum from immune to susceptible birds.
Although IgG antibodies may not play a major role in the development of a protective immunity against the protozoan parasite, they present a good option to determine the infection status of poultry, especially chickens as the development of clinical signs and post-mortem lesions is much less severe in these birds (Hess et al., 2006a). Based on the presented data clinical asymptomatic, infected chickens can be identified starting 14 days post-infection.
Therefore, future investigations will be concentrated to demonstrate the benefit of such an ELISA under field conditions as an important diagnostic tools to identify carriers and infected chicken flocks, especially those housed in alternative housing systems as they are more prone to the infection (Esquenet et al., 2003; Hafez et al., 2001; Homer and Butcher, 1991).
For turkeys the ELISA is probably less relevant because the clinical picture is very often characterized by high mortality and post-mortem lesions are very characteristic for the infection with H. meleagridis. Anyhow, recently published data indicate that histomonosis in standard turkey farms is most frequently associated with mortality below 10% (Callait-Cardinal et al., 2007).
Regardless of epidemiological investigations the established indirect sandwich ELISA is a valuable tool in experimental studies to control the progress of the infection with H. meleagridis in chickens and turkeys as described in the present study.
References
- Bleyen N, De Gussem K, De Gussem J, Goddeeris BM. Specific detection of Histomonas meleagridis in turkeys by a PCR assay with an internal amplification control. Vet. Parasitol. 2007;143:206–213. doi: 10.1016/j.vetpar.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Callait-Cardinal MP, Leroux S, Venereau E, Chauve CM, Le Pottier G, Zenner L. Incidence of histomonosis in turkeys in France since the bans of dimetridazole and nifursol. Vet. Rec. 2007;161:581–585. doi: 10.1136/vr.161.17.581. [DOI] [PubMed] [Google Scholar]
- Clarkson MJ. Immunological responses to Histomonas meleagridis in the turkey and fowl. Immunology. 1963;6:156–168. [PMC free article] [PubMed] [Google Scholar]
- Crowther JR. The ELISA Guidebook. Humana Press; Totowa, New Jersey: 2000. p. 423. ( Methods in Molecular Biology 149). [DOI] [PubMed] [Google Scholar]
- Esquenet C, De Herdt P, De Bosschere H, Ronsmans S, Ducatelle R, Van Erum J. An outbreak of histomoniasis in free-range layer hens. Avian Pathol. 2003;32:305–308. doi: 10.1080/0307945031000097903. [DOI] [PubMed] [Google Scholar]
- Grabensteiner E, Hess M. PCR for the identification and differentiation of Histomonas meleagridis, Tetratrichomonas gallinarum and Blastocystis sp. Vet. Parasitol. 2006;142:223–230. doi: 10.1016/j.vetpar.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Hafez HM, Hauck R, Luschow D, McDougald L. Comparison of the specificity and sensitivity of PCR, nested PCR, and real-time PCR for the diagnosis of histomoniasis. Avian Dis. 2005;49:366–370. doi: 10.1637/7341-020805R.1. [DOI] [PubMed] [Google Scholar]
- Hafez HM, Mazaheri A, Prusas C, Böhland K, Pöppel M, Schulze D. Actual infectious diseases in layer flocks kept in alternative rearing systems. Tieraerztl. Prax. 2001;29:168–174. [Google Scholar]
- Hess M, Grabensteiner E, Liebhart D. Rapid transmission of the protozoan parasite Histomonas meleagridis in turkeys and specific pathogen-free chickens following cloacal infection with a monoeukaryotic culture. Avian Pathol. 2006a;35:280–285. doi: 10.1080/03079450600815507. [DOI] [PubMed] [Google Scholar]
- Hess M, Kolbe T, Grabensteiner E, Prosl H. Clonal cultures of Histomonas meleagridis, Tetratrichomonas gallinarum and a Blastocystis sp. established through micromanipulation. Parasitology. 2006b;133:547–554. doi: 10.1017/S0031182006000758. [DOI] [PubMed] [Google Scholar]
- Hess M, Liebhart D, Grabensteiner E, Singh A. Cloned Histomonas meleagridis passaged in vitro resulted in reduced pathogenicity and is capable of protecting turkeys from histomonosis. Vaccine. 2008;26:4187–4193. doi: 10.1016/j.vaccine.2008.05.071. [DOI] [PubMed] [Google Scholar]
- Homer BL, Butcher GD. Histomoniasis in Leghorn pullets on a Florida farm. Avian Dis. 1991;35:621–624. [PubMed] [Google Scholar]
- Huber K, Chauve C, Zenner L. Detection of Histomonas meleagridis in turkeys cecal droppings by PCR amplification of the small subunit ribosomal DNA sequence. Vet. Parasitol. 2005;131:311–316. doi: 10.1016/j.vetpar.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Kemp RL, Reid WM. Staining techniques for differential diagnosis of histomoniasis and mycosis in domestic poultry. Avian Dis. 1966;10:357–363. [Google Scholar]
- Liebhart D, Weissenbock H, Hess M. In-situ hybridization for the detection and identification of Histomonas meleagridis in tissues. J. Comp. Pathol. 2006;135:237–242. doi: 10.1016/j.jcpa.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Lund EE. Immunizing action of a nonpathogenic strain of Histomonas against blackhead in turkeys. J. Protozool. 1959;6:182–185. [Google Scholar]
- McDougald LR. Blackhead disease (histomoniasis) in poultry: a critical review. Avian Dis. 2005;49:462–476. doi: 10.1637/7420-081005R.1. [DOI] [PubMed] [Google Scholar]
- Singh A, Weissenbock H, Hess M. Histomonas meleagridis: immunohistochemical localization of parasitic cells in formalin-fixed, paraffin-embedded tissue sections of experimentally infected turkeys demonstrates the wide spread of the parasite in its host. Exp. Parasitol. 2008;118:505–513. doi: 10.1016/j.exppara.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Tyzzer EE. The flagellate character and reclassification of the parasite producing “blackhead” in turkeys-Histomonas (gen. nov.) meleagridis (Smith) J. Parasitol. 1920;6:124–131. [Google Scholar]