SUMMARY
Histomonas meleagridis is a protozoan parasite of various galliform birds causing a type of enterohepatitis termed histomonosis or ‘blackhead disease’. Due to the ban of chemotherapeutic substances and an increase in free-range poultry production, histomonosis is currently a re-emerging disease. So far limited molecular knowledge is available. In the present work, mRNAs coding for antigenic proteins of H. meleagridis were identified. For this purpose, a cDNA expression library was constructed from a mono-eukaryotic culture of H. meleagridis. The library was screened with polyclonal rabbit serum raised against purified H. meleagridis trophozoites. Polyclonal rabbit serum specifically recognized the same major H. meleagridis antigens as chicken and turkey sera originating from animal trials, but displayed a significantly lower bacteria-dependent background signal. After 2 rounds of screening, a total of 95 positive clones were sequenced. Bioinformatics analyses were performed on nucleotide and deduced amino acid sequences, identifying 37 unique clones. Based on the homology to other protozoan parasites, mostly Trichomonas vaginalis, the clones were grouped according to functional aspects: structural proteins, possible surface proteins, oxygen reducing proteins, ribosomal proteins, protein kinases and various other intracellular proteins.
Keywords: Histomonas meleagridis, histomonosis, chicken, turkey, cDNA expression library, immuno-screening, amino acid sequence, Trichomonas vaginalis
INTRODUCTION
The flagellated protozoon, Histomonas meleagridis, is the aetioligical agent of an enterohepatitis termed histomonosis or ‘blackhead disease’ (Tyzzer, 1920). This parasitic disease is of economic importance in the poultry industry. Untreated, histomonosis causes a high rate mortality in turkeys, whereas clinical signs in chickens may vary considerably. For decades, the disease has been well controlled by the use of chemotherapeutics as preventative and curative drugs (McDougald, 2005). However, due to potential consumer health risks, effective drugs have been withdrawn from different markets, leading to the re-emergence of histomonosis in poultry.
Two different morphological forms of H. meleagridis have been described within its host, (i) a flagellated form with one anterior flagellum, residing in the caecal lumen, and (ii) an amoeboid form invading the intestinal mucosa and the liver (Bishop, 1938; Lund et al. 1967). A cyst stage, common to other related parasites as a survival form, has not been identified for H. meleagridis so far. Light and electron microscopy studies described the structure and division of flagellated forms of H. meleagridis in detail (Wenrich, 1943; Schuster, 1968; Honigberg and Bennett, 1971). Due to the amoeboid morphology under the light microscope, H. meleagridis was commonly placed among the Rhizopoda, which includes Entamoeba and its relatives. However, electron microscopy studies of H. meleagridis revealed morphological similarities with trichomonads, such as the presence of hydrogenosomes and numerous mastigont structures with 4 kinetosomes (Schuster, 1968; Rybicka et al. 1972). More recently, comparative analysis of small subunit rRNAs demonstrated a close phlyogenetic relationship with Dientamoeba fragilis (Gerbod et al. 2001), an atypical intestinal trichomonad that lacks flagella throughout its life cycle. Furthermore, the study linked Histomonas and Dientamoeba to the genus Trichomonas, suggesting that they might be representatives of a reductive evolution marked by the loss of several trichomonad cytoskeletal structures.
Until now, most of the work published on H. meleagridis characterizes its phylogenetic position, while detailed molecular studies, such as studies on proteins that cause an immune response in the host, are still missing. Most antigenic proteins of related protozoa like Tritrichomonas foetus, Trichomonas vaginalis and Entamoeba histolytica, are located on the cell surface or are secreted. These proteins are frequently involved in the colonization of mucosal surfaces as well as in the mechanisms of tissue damage and are therefore called virulence factors. Entamoeba histolytica produces an abundance of antigenic virulence factors that are involved in both host invasion and tissue destruction. These factors include a surface galactose-binding lectin (Gal/GalNAc lectin), amoebapores and a family of secreted cysteine proteinases (Chaudhry and Petri, 2005). In addition to surface antigens, a few antigenic proteins have been shown to be cytoplasmic proteins: elongation factor 1-alpha, enolase, 70 kDa heat-shock like protein, ribosomal protein L-23-a, cyclophillin and NADP+ dependant alcohol dehydrogenase (De Meester et al. 1991; Beanan and Bailey, 1995; Carrero et al. 2000; Sanuki et al. 2001). Major antigenic proteins, as well as the molecular mechanisms with which E. histolytica colonizes mucosal surfaces and causes tissue damage, are well defined. Characterizations of these proteins and mechanisms are not yet as developed for Trichomonas vaginalis and Tritrichomonas foetus. Nevertheless, several immunogenic virulence factors have been identified for T. vaginalis and include a variety of cysteine proteinases, alpha-actinin, P270 immunogen, alpha-enolase and tv44 (Alderete et al. 1991 a, b; Addis et al. 1999; Musatovova and Alderete, 1999; Mundodi et al. 2006, 2008).
In the present study, the aim was to identify and characterize antigenic proteins of H. meleagridis. For this purpose, a phage display method employing a cDNA expression library was used in combination with a polyclonal rabbit serum raised against purified H. meleagridis parasites. Screening of the cDNA expression library resulted in the identification of various surface and intracellular proteins.
MATERIALS AND METHODS
Culture of Histomonas meleagridis
A xenic mono-eukaryotic culture of Histomonas meleagridis was used for all experiments. The culture was established out of the caecal content and faecal material of a bronze turkey which died of histomonosis at the age of 20 weeks. In vitro isolation, establishment and propagation of the cloned parasite were performed as described recently (Hess et al. 2006b). Briefly, 1 g of caecal content, and some material scraped from the caecal wall were placed in 9 ml of Medium 199 containing Earl's salts, L-glutamine, 25 mM HEPES and L-amino acids (all Gibco™, Invitrogen GmbH, Lofer, Austria). Additionally, 15% foetal bovine serum (FBS) (Gibco™, Invitrogen GmbH, Lofer, Austria), antibiotics (200 IE penicillin and 200 μg streptomycin/ml of medium) (Sigma-Aldrich, Vienna, Austria), an anti-mycotic drug (2·5 μg amphotericin B/ml of medium) and 11 mg of rice starch (Sigma-Aldrich, Vienna, Austria) were added. This was the standard medium for in vitro cultivation of H. meleagridis. Cells were passaged every 2–3 days by transferring 1 ml of the old culture into a new sterile 50 ml tube (Sarstedt, Vienna, Austria) containing 9 ml fresh standard medium.
Purification of H. meleagridis trophozoites from mono-eukaryotic culture and RNA isolation
Since H. meleagridis grows in culture with ill-defined bacterial flora and rice starch, cells harvested directly from the in vitro culture are not suitable for the isolation of nucleic acids, proteins or antigens. Additional purification of H. meleagridis is required for the production of H. meleagridis-specific antibodies and the isolation of nucleic acids and proteins. For this purpose, a purification method including a Histopaque® 1077 (Sigma-Aldrich, Vienna, Austria) and different centrifugation steps was developed. Briefly, H. meleagridis cells were collected from 2-day-old cultures by centrifugation at 200 g for 5 min at room temperature. The supernatant containing bacteria was carefully discarded and the pellet containing Histomonas cells and rice starch was re-suspended in 1 ml of Medium 199 (Gibco™, Invitrogen, Lofer, Austria). This suspension was loaded onto a 3 ml Histopaque® 1077 (Sigma-Aldrich, Vienna, Austria) and centrifuged for 30 min at 650 g, room temperature. Then the supernatant containing Histomonas cells and the remaining bacteria was transferred to a fresh 15-ml tube (Sarstedt, Vienna, Austria) and centrifuged for 5 min at 200 g at room temperature. Following centrifugation, the supernatant containing bacteria was removed and the cell pellet was subsequently washed 3 times with 5 ml of Medium 199 (Gibco™, Invitrogen, Lofer, Austria) and centrifuged at 200 g for 5 min at room temperature. Finally, cells were re-suspended in 1 ml of phosphate-buffered saline (PBS) and 10 μl of this suspension was examined microscopically for the presence of bacteria and to quantify H. meleagridis cells. Purified cells were stored as a pellet at −80 °C.
Total RNA from purified H. meleagridis was prepared using TRIzol® Reagent (Invitrogen, Lofer, Austria) according to manufacturer's instructions.
Production of rabbit antisera against H. meleagridis trophozoites
Two rabbits were each immunized with 2 × 107 purified H. meleagridis cells suspended in 50% GERBU LQ (GERBU Biochemicals GmbH, Gaiberg, Germany). Rabbits were immunized subcutaneously and boosted 3 times at 6-week intervals. Serum to be used in the investigation was obtained 6 weeks after the final injection.
Production of chicken and turkey sera against H. meleagridis trophozoites
Chicken and turkey sera, used for immuno-detection of the Western blots, were collected during animal trials described recently. Briefly, chicken sera were gained from specified pathogen-free chickens cloacally infected at 14 days of age with 380 000 histomonads (Hess et al. 2006a). One serum sample taken from a non-infected control bird and 3 samples taken from infected birds (nos. 6, 10, 11) were used in the present study. As the birds did not die from histomonosis, they were killed 6 weeks post-infection. Serum samples were taken on the same day.
The 2 turkey sera were obtained from a vaccination experiment testing the efficacy of an inactivated vaccine (Hess et al. 2008). One serum sample was taken from a non-infected control bird (no. 849). The second sample was obtained from a turkey vaccinated at 7 days of age and boosted 2 weeks later (no. 828). This turkey was infected into the cloaca with 10 000 histomonads another 2 weeks later. The serum used was collected when the bird had to be euthanized due to its poor condition 19 days post-infection (p.i.), when histomonosis was diagnosed.
Purification of serum samples by pre-absorption
In order to decrease non-specific background reactions, pre-absorption of the sera was performed. For this purpose, serum samples were incubated with acetone powders made from the bacteria that cohabitate the mono-eukaryotic H. meleagridis culture. The purification was performed according to the protocol of Zhao and Siu (1995) including minor changes. Briefly, the bacterial supernatant harvested from the mono-eukaryotic culture of H. meleagridis, collected after the first step of purification, was centrifuged at 4000 g for 30 min at 4 °C. The bacterial pellet was washed in 1 ml of PBS and cells were collected by centrifugation at 16 000 g for 15 min at 4 °C. Cells were re-suspended in 300 μl of 0·9% NaCl and divided into 2 Eppendorf tubes. In one aliquot, cells were disrupted with 4 subsequent freeze-thaw cycles while the other aliquot was kept on ice. After freezing and thawing, both cell aliquots were pooled together to achieve a mixture of proteins and intact bacteria in a single sample. This sample was then mixed vigorously with 1·2 ml of cold acetone (−20 °C) by vortexing and incubated on ice for 30 min. The cell and protein pellet was collected by centrifugation at 10 000 g for 10 min at 4 °C. The pellet was re-suspended in acetone (−20 °C) and incubated on ice for 10 min. The cell and protein pellet was again collected by centrifugation, air-dried and ground with a sterile spatula to make a fine powder. The powder was stored in Eppendorf tubes at −20 °C until use. For pre-absorption, the final concentration of acetone powder was 1% (w/v). Sera were incubated by rocking for 1 h at room temperature or, alternatively, at 4 °C overnight. After incubation, sera were centrifuged at 10 000 g for 10 min at 4 °C and the supernatant was collected as a source for the primary antibody. Pre-absorption efficiency was examined by dot-blots, comparing pre-absorbed and non pre-absorbed sera.
SDS-PAGE, Western blotting and dot-blotting
For native protein preparations from purified H. meleagridis cells, pellets were re-suspended in PBS supplemented with 1% Nonidet P-40 and Complete protease inhibitors cocktail (Roche Diagnostics GmbH, Vienna, Austria). Cells were opened by 4 freeze-thaw cycles. Native protein samples of bacteria were prepared from the supernatant of the mono-eukaryotic H. meleagridis culture, collected after the first centrifugation step of H. meleagridis purification. The bacterial pellet was re-suspended in PBS supplemented with 1% Nonidet P-40 and Complete protease inhibitors cocktail (Roche Diagnostics GmbH, Vienna, Austria) and lysed by sonication (3 × 30 sec cycles with continuous power). Soluble proteins were separated from insoluble ones by centrifuging at 20 000 g for 10 min at 4 °C. Both fractions of Histomonas and bacterial proteins were mixed with SDS-PAGE loading buffer and analysed on 10% SDS-PAGE. After electrophoresis, proteins were transferred onto a polyvinyl difluoride (PVDF) membrane (Pall Corporation, VWR International GmbH, Vienna, Austria). For immuno-detection, membranes were saturated with 3% skimmed milk and incubated for 2 h with an adequate dilution of either pre-absorbed or non pre-absorbed immune sera: rabbit (1 : 50 000), chicken or turkey (each 1 : 500). After washing, membranes were incubated with horseradish conjugated donkey anti-rabbit IgG (1 : 100 000 dilution; Jackson ImmunoResearch Laboratories, Inc., Dianova, Hamburg, Germany), donkey anti-chicken IgY (IgG) (1 : 20 000 dilution; Jackson ImmunoResearch Laboratories, Inc., Dianova, Hamburg, Germany) or goat anti-turkey IgG (1 : 20 000 dilution; Southern Biotech, Biomedica, Vienna, Austria), respectively, and detected with SuperSignal® West Pico Chemiluminescent Substrate (Pierce, Thermo Fisher Scientific, Vienna, Austria).
Dot-blots were prepared on PVDF membranes and contained 2 spots of histomonad proteins and 2 spots of bacterial proteins (30 μg and 3 μg protein, respectively). The best serum concentration for the immunodetection of Western blots and the screening of the cDNA expression library, as well as the efficiency of the serum pre-absorption were determined using dot-blots.
Construction and screening of the cDNA expression library of H. meleagridis
Histomonas meleagridis cDNA library was constructed using the ZAP Express® cDNA Synthesis Kit and ZAP Express® cDNA Gigapack® II Gold Cloning Kit (both from Stratagene), according to manufacturer's instructions. Five micrograms poly (A)+ RNA were purified from total RNA using the Absolutely mRNA™ Purification Kit (Stratagene) and applied to cDNA synthesis. The library was immunologically screened with purified rabbit anti-H. meleagridis serum diluted 1 : 100 000 as described (Sambrook et al. 1989). After 2 rounds of screening and plaque purification, phagemids were excised with ExAssist interference-resistant Helper Phage according to manufacturer's instructions. Positive clones were sequenced using fluorescence-based sequencing with T3 and T7 primers or specific internal primers. Both DNA strands of each clone were sequenced.
Analysis of sequence data
Assembly and analyses of cDNA sequences as well as alignments of both nucleotide and amino acid sequences were performed with Accelrys Gene, version 2.5 (Accelrys, San Diego, CA) and Lasergene (DNASTAR Inc.) software packages. The cDNA sequences of unique positive clones were deposited in EMBL database and their accession numbers are listed in Table 2. GenBank™ database searches of obtained sequences were carried out with BlastN, BlastP and specialized BLASTs for conserved domains and conserved domain architecture with default settings.
Table 2.
List and detailed information of all unique clones isolated during immuno-screening of the cDNA expression library
| Category | Clone name |
Antigen homology | Signal intensity |
% identity to homologous parasites |
Accession number |
|---|---|---|---|---|---|
| Translation, ribosomal structure and biogenesis | 3/1 | T. vaginalis putative ribosomal protein S3 | Strong | 83% nsa | FM200059 |
| 91% aab | |||||
| 7/1 | T. vaginalis and E. histolytica glutaminyl tRNA synthetase family protein | Strong | 54% aa (T. vaginalis) | FM200060 | |
| 42% aa (E. histolytica) | |||||
| 29b/1 | T. vaginalis putative 60S acidic ribosomal protein P1 | Weak | 66% aa | FM200061 | |
| 32a/1 | T. vaginalis putative ribosomal protein L10 | Weak | 61% aa | FM200062 | |
| 79a/1 | T. vaginalis elongation factor 1-alpha (tef1) | Weak | 82–84% ns | FM200063 | |
| 83% aa | |||||
| 110a/1 | T. vaginalis putative ribosomal protein conserved domain L4/L1 family | Weak | 58% aa | FM200064 | |
| 177/2 | T. vaginalis putative ribosomal protein S27a | Weak | 57% aa | FM200065 | |
| 183c/2 | T. vaginalis putative ribosomal protein L18 | Weak | 64% aa | FM200066 | |
| 195a/2 | T. vaginalis ribosomal protein L8 | Strong | 68% aa | FM200067 | |
| Structural proteins | 9a/3 | T. vaginalis alpha-actinin | Strong | 46% aa | FM200068 |
| 48/3 | T. vaginalis putative fimbrin | Strong | 63% aa | FM200069 | |
| 73/2 | T. vaginalis actin | Strong | 78% ns | FM200070 | |
| 81% aa | |||||
| 163/1 | T. vaginalis alpha-actinin | Strong | 41% aa | FM200071 | |
| 192/1 | T. vaginalis alpha-actinin | Strong | 46% aa | FM200072 | |
| Energy construction and conversion | 11/2 | T. vaginalis putative hydrogenosomal oxygen reductase | Strong | 58% aab | FM200073 |
| 93a/2 | T. vaginalis hydrogenosomal malic enzyme subunit A (AP65-2) | Weak | 58% aa | FM200074 | |
| 117a/1 | T. vaginalis pyruvate : ferredoxin oxidoreductase E (PFOE) | Weak | 76% aa | FM200075 | |
| 177/1 | T. foetus cytosolic malate dehydrogenase 1 and 2 (mdh1, mdh2) | Weak | 70% aa | FM200076 | |
| Protein kinases | 25a/2 | T. vaginalis CAMK family protein kinase | Weak | 36–81% aa | FM200077 |
| 37b/1 | T. vaginalis C2 domian containing protein | Weak | 30% aa | FM200078 | |
| 141/2 | T. vaginalis CAMK family protein kinase | Weak | 40% aa | FM200079 | |
| Oxidative stress | 28/1 | T. vaginalis thioredoxin reductase (trxr) | Weak | 65% aa | FM200080 |
| 76b/1 | T. vaginalis DJ-1 family protein | Weak | 55% aa | FM200081 | |
| Outer membrane, cell envelope biogenesis | 93a/2 | T. vaginalis AP65-2 adhesin | Weak | 58% aa | FM200074 |
| 117a/1 | T. vaginalis pyruvate : ferredoxin oxidoreductase A (PFOA) | Weak | 69% aa | FM200075 | |
| 145/2 | T. vaginalis sugar isomerase domain containing protein | Strong | 60% aa | FM200082 | |
| Carbohydrate transport and metabolism | 58c/1 | T. vaginalis phosphoglucomutase/phosphomannomutase family protein | Weak | 74% aa | FM200083 |
| 110c/1 | T. vaginalis triosephosphat isomerase | Weak | 76% aa | FM200084 | |
| Signal transduction | 23b/2 | T. vaginalis putative guanine nucleotide-binding protein β subunit | Strong | 61% aab | FM200085 |
| Modification | 10a/3 | T. vaginalis ubiquitin family protein (UBA/TS-N domain containing protein) | Strong | 23% aa | FM200086 |
| Chromatin structure | 191b/1 | T. vaginalis putative histone 2A-IV | Weak | 86% nsa | FM200087 |
| 93% aa | |||||
| Lipid metabolism | 15c/1 | T. vaginalis AMP-binding enzyme protein | Weak | 48% aa | FM200088 |
| Metall-beta-lactamase superfamily III | 218/1 | T. vaginalis metallo-beta-lactamase superfamily III protein | Weak | 36% aa | FM200089 |
| Hypothetical proteins | 5a/3 | Hypothetical protein TVAG_151060 | Strong | 25% aa | FM200090 |
| 15a/2 | T. vaginalis conserved hypothetical protein EAY23136 | Weak | 40% aa (to both EAY23136 and EAY21702) | FM200091 | |
| T. vaginalis XYPPX repeat family protein EAY 21702 | |||||
| 97/1 | T. vaginalis hypothetical protein TVAG_533930 | Weak | 33% aa | FM200092 | |
| 152b/1 | T. vaginalis hypothetical protein TVAG_424960 | Weak | 68% aa | FM200093 | |
| 175/2 | T. vaginalis hypothetical protein TVAG_264950 | Weak | 35% aa | FM200094 | |
| 186a/1 | T. vaginalis hypothetical protein TVAG_161040 | Weak | 40% aa | FM200095 |
ns – nucleotide sequence.
aa – amino acid sequence.
RESULTS
Analysis of polyclonal rabbit, chicken and turkey sera raised against H. meleagridis
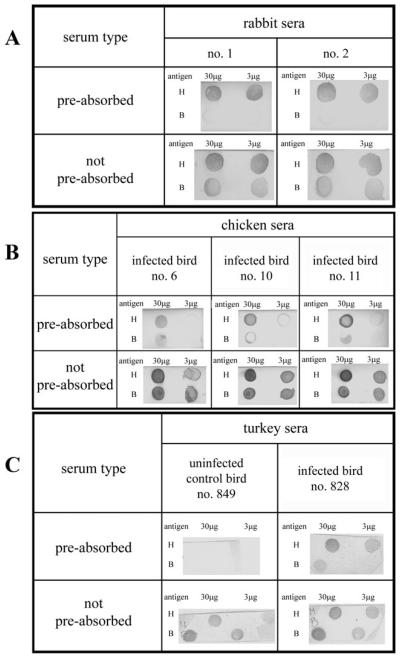
In order to determine which serum would be best for immuno-screening of the H. meleagridis cDNA expression library, both dot and Western blot analyses were performed with anti-H. meleagridis sera from rabbits, chickens and turkeys. Rabbit sera were included in the dot blot analysis to compare signals to poultry sera. As expected, the unspecific staining due to antibodies recognizing bacterial antigens was less prominent in rabbit sera (Fig. 1A). Since H. meleagridis is a poultry pathogen, sera from either infected chickens or turkeys would have been the obvious choice to identify H. meleagridis immuno-reactive antigens. But dot blot analyses of these sera demonstrated an extremely high bacteria-specific background, proving them inadequate for immuno-screening purposes (Fig. 1B and C). This result was not unexpected, since infections in these animal trials were performed with non-purified H. meleagridis cells. The sera from non-infected control chickens (data not shown) and a control turkey (Fig. 1C) were also included in dot-blot analyses and showed high bacterial background as well. This could be explained by the natural presence of these bacteria in the caeca of chickens and turkeys and in mono-eukaryotic cultures of H. meleagridis. In contrast to the infections induced in chickens and turkeys, rabbits were injected with purified and inactivated H. meleagridis trophozoites.
Fig. 1.
Immunodetection of dot-blots with pre-absorbed and not pre-absorbed sera. (A) Polyclonal rabbit anti-Histomonas meleagridis immune sera (1 : 50 000), (B) polyclonal chicken anti-H. meleagridis immune sera (1 : 500) and (C) polyclonal turkey anti-H. meleagridis immune serum (1 : 500). Dots H and B correspond to Histomonas meleagridis and bacteria, respectively.
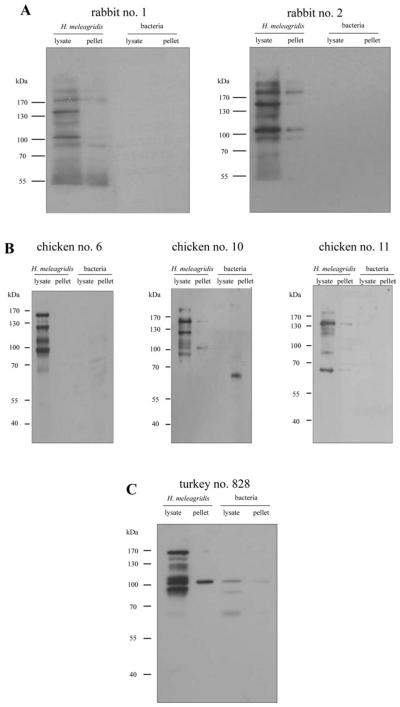
All positive sera were further analysed by Western blotting. For this purpose, H. meleagridis and bacterial proteins originating from the same preparation were separated on SDS-PAGE. Following electrophoresis, proteins were transferred to a PVDF membrane and incubated with an adequate dilution of rabbit, chicken or turkey immune sera (Fig. 2). The pattern of protein bands in Western blot analyses demonstrated major H. meleagridis antigens at 180 kDa, 150 kDa, 125 kDa, 105–110 kDa and 90 kDa (Table 1). All rabbit, chicken and turkey sera recognized these proteins with varying intensity. Since rabbit immune sera recognized the same major H. meleagridis antigens as chicken and turkey sera but displayed significantly lower bacteria-specific background, a rabbit serum was used for the immuno-screening of the H. meleagridis cDNA expression library.
Fig. 2.
Immuno-detection of Western blots with pre-absorbed sera. (A) Polyclonal rabbit anti-Histomonas meleagridis immune sera (1 : 50 000), (B) polyclonal chicken anti-H. meleagridis immune sera (1 : 500) and (C) polyclonal turkey anti-H. meleagridis immune serum (1 : 500). Lysate is the 1% Nonidet P40 soluble fraction of H. meleagridis and bacterial proteins. Pellet is the insoluble fraction of H. meleagridis and bacterial proteins. Molecular standards are in kDa.
Table 1.
Summary of major antigens recognized by rabbit, chicken and turkey immune sera
|
Histomonas meleagridis antigens |
Rabbit sera |
Chicken sera |
Turkey serum |
|||
|---|---|---|---|---|---|---|
| 1 | 2 | 6 | 10 | 11 | 828 | |
| >170 kDa | ++b | ++ | − | + | +/− | − |
| ca. 150 kDa | +++a | +++ | +++ | +++ | +++ | ++ |
| ca. 125 kDa | +c | + | +++ | +++ | + | ++ |
| ca. 115 kDa | − e | − | − | + | + | − |
| ca. 110 kDa | − | − | +++ | + | − | +++ |
| ca. 105 kDa | +++ | +++ | − | + | − | − |
| ca. 90 kDa | ++ | ++ | +++ | ++ | + | +++ |
| ca. 65 kDa | +/−d | +/− | +/− | − | ++ | − |
(+++) very strong band
(++) strong band
(+) weak band
(+/−) very weak band
(−) no band.
Screening of the cDNA expression library of H. meleagridis
In order to identify and characterize antigenic proteins of H. meleagridis, a cDNA expression library constructed from 6×107 purified H. meleagridis cells was immuno-screened with polyclonal rabbit serum raised against purified H. meleagridis. From the initial 612 000 cDNA library phages plated, 226 positive clones were obtained in the original screening. Upon a second round of screening, 95 clones were positive for coding for antigenic proteins. Before sequence analysis, all 95 clones were analysed for the presence and size of their cDNA insert by digesting the phagemid DNA with XhoI and PstI restriction endonucleases.
Analysis of positive clones
All 95 positive clones obtained after 2 rounds of immuno-screening were completely sequenced. In order to gain more information about the sequence of each clone, bioinformatics analyses such as BLAST search algorithm and conserved domain analysis were performed using nucleotide and deduced amino acid sequence. These analyses resulted in 37 H. meleagridis-specific sequences that were unique. Based on the homology to other protozoan parasites, mostly to Trichomonas vaginalis, positive clones were placed into several groups according to their functional aspects. Detailed information about all 37 unique clones is listed in Table 2. During the screening procedure, clones were additionally categorized according to the intensity of their reaction with polyclonal anti-H. meleagridis rabbit serum.
Detailed analysis of clones 28-1 and 93a-2
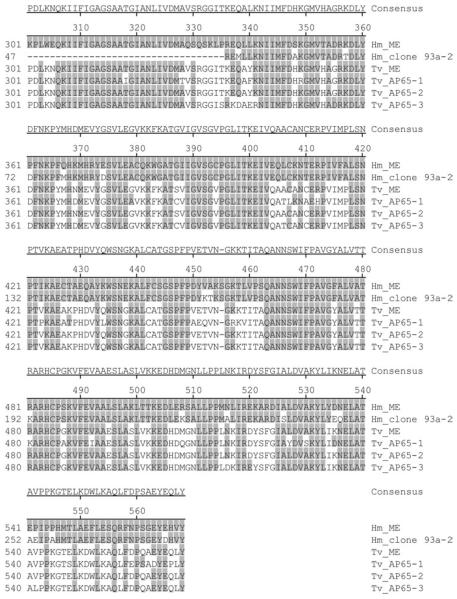
The BLAST search of the deduced amino acid sequence of clone 93a-2 demonstrated an homology to the T. vaginalis adhesin AP65-2/hydrogenosomal malic enzyme (Table 2). Since the existence of H. meleagridis hydrogenosomal malic enzyme was recently reported (Mazet et al. 2008), the identity of these 2 proteins was examined. Amino acid alignment of clone 93a-2, H. meleagridis malic enzyme (Hm_ME), T. vaginalis malic enzyme (Tv_ME) and the T. vaginalis adhesins AP65-1 (Tv_AP65-1), AP65-2 (Tv_AP65-2) and AP65-3 (Tv_AP65-3), was performed. The alignment of clone 93a-2 and Hm_ME in Fig. 3 shows that the 2 H. meleagridis homologues of adhesin AP65/hydrogenosomal malic enzyme are not identical, suggesting the existence of different AP65 adhesins in H. meleagridis.
Fig. 3.
Amino acid sequence alignment of the Histomonas meleagridis homologue of adhesin AP65-2 (Hm_clone 93a-2) with H. meleagridis malic enzyme (Hm_ME), Trichomonas vaginalis malic enzyme (Tv_ME) and the T. vaginalis adhesins AP65-1 (Tv_AP65-1), AP65-2 (Tv_AP65-2), AP65-3 (Tv_AP65-3). Amino acids are numbered on the left. The sequence of Hm_ME is shown at the top and only identities to this sequence are shaded grey. The deletions are indicated with dashes. The Hm_clone 93a-2 sequence originates from this study. GenBank™ Accession numbers for Hm_ME, Tv_ME, Tv_AP65-1, Tv_AP65-2, Tv_AP65-3 are FJ185157, AAA927141, AAA87406, AAA87407, and AAA91133, respectively.
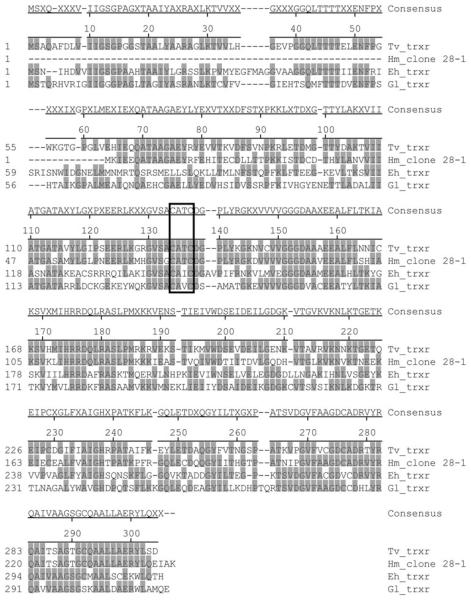
BLAST search of the deduced amino acid sequence of clone 28-1 demonstrated homology to T. vaginalis thioredoxin reductase (Table 2). In order to examine whether the key active site containing cysteine is conserved in the H. meleagridis homologue, the amino acid alignment of clone 28-1 and the thioredoxin reductases of T. vaginalis, E. histolytica and G. lamblia, was performed. The amino acid alignment in Fig. 4 shows that this active site in the protein is the only region well conserved among all 4 parasites.
Fig. 4.
Amino acid sequence alignment of the Histomonas meleagridis homologue of thioredoxin reductase (Hm_clone 28-1) with the thioredoxin reductases (TrxR) of Trichomonas vaginalis (Tv_trxr), Entamoeba histolytica (Eh_trxr) and Giardia lamblia (Gl_trxr). Amino acids are numbered on the left. The conserved active site, a redox centre is boxed. The sequence of Tv_trxr is shown at the top and only identities to this sequence are shaded grey. The deletions are indicated with dashes. The Hm_clone 28-1 sequence originates from this study. GenBank™ Accession numbers for Tv_trxr, Eh_trxr and Gl_trxr are XP_001316923, CAA56112 and AJ507833, respectively.
DISCUSSION
In recent years, histomonosis has re-emerged as the consequence of both the ban on prophylactic and therapeutic substances and the increased popularity of free-range housing. In contrast to this parasite's importance, its investigation on a molecular level is still rather poor. So far, merely the sequences of the 18S and 5.8S rRNA genes and those of the internal transcribed spacer regions 1 and 2 are available in databases. In a recent article (Mazet et al. 2008), information is given about protein-coding genes for malic enzyme, alpha-succinyl coenzyme A synthetase and iron-hydrogenase, which are all required for the hydrogenosomal carbon metabolism.
The present study attempts to broaden the molecular knowledge on H. meleagridis by identifying mRNAs that code for antigenic proteins. For this purpose, a cDNA library of H. meleagridis was constructed and immuno-screened with polyclonal rabbit serum raised against purified parasite cells. Extensive bioinformatics sequence analysis of positive clones revealed 37 unique clones of H. meleagridis-specific sequences. Most of these show a high level of homology to protein-coding genes of Trichomonas vaginalis. This finding supports the results of former investigations, indicating that H. meleagridis belongs to the class of Trichomonadea (Gerbod et al. 2001). Positive clones were grouped into several categories based on homologies to known proteins of other protozoan parasites: proteins for translation, ribosomal structure and biogenesis; structural proteins; proteins for energy construction and conversion; proteins for the biogenesis of the outer membrane and cell envelope; protein kinases; oxidative stress proteins and various other intracellular proteins.
Due to the broad spectrum of expression sequence tags (ESTs) identified in this study, only mRNAs coding for proteins involved in host cell adhesion and host tissue invasion are discussed in more detail. These proteins represent a first line of intrusion into the host by the parasite. Several clones described in this study show homology to such proteins. All 5 protein coding sequences from the category ‘structural proteins’ have homology to T. vaginalis proteins involved in the reorganization of actin fibres within the cell. It has been shown that redistribution of actin within T. vaginalis results in the transformation of the cell from a flagellated to an amoeboid form. This amoeboid form has been proven to be required for the parasite's cytopathogenicity (Fiori et al. 1999). In the obligatory amoebic parasite E. histolytica, the rearrangement of actin plays a central role in both movement and cellular interaction with the environment. This includes interaction with host cells (Guillen, 1996). Rearrangement of actin within T. vaginalis cells is accomplished by coordinated action of different actin-bundling proteins such as alpha-actinins (Bricheux et al. 1998; Addis et al. 1999). Since 2 morphological forms, flagellated and amoeboid, have been described for H. meleagridis (Bishop, 1938; Lund et al. 1967), a similar actin rearranging feature is likely. The flagellated form with one anterior flagellum, residing in the caecal lumen, can be considered a locomotive form, while the amoeboid parasite is found when invading the intestinal mucosa and the liver. In this study, homologous protein-coding sequences involved in cyto-skeletal rearrangements have been identified. This fact and the presence of 2 morphological forms of H. meleagridis suggest mechanisms of host tissue invasion similar to those of T. vaginalis. A sequence homologous to an additional actin-bundling protein, fimbrin, was identified as well. The presence and role of this protein for T. vaginalis has yet to be described, although its putative sequence has been detected in the completed genome of T. vaginalis (Carlton et al. 2007).
Another group of proteins that play a role in the invasion of host tissue are adhesins. In this study, 2 clones, termed 93a-2 and 117a-1, were detected to be homologous to T. vaginalis adhesins AP65 and AP120, respectively. Both adhesins have been described as having additional functions as metabolic enzymes within the T. vaginalis hydrogenosomes. The hydrogenosome is a double membrane-bound organelle involved in the fermentative oxidation of pyruvate derived from glycolysis (Muller, 1997). The AP65 adhesin of T. vaginalis is the decarboxylating malic enzyme (ME), while the AP120 adhesin of T. vaginalis functions as pyruvate : ferredoxin oxidoreductase A (PFOA) (Engbring et al. 1996; Alderete et al. 2001; Moreno-Brito et al. 2005). Due to possessing 2 unrelated functions, that of a metabolic enzyme and an adhesion function, these proteins represent moonlighting proteins of T. vaginalis (Jeffery, 1999; Hirt et al. 2007). Since they are both members of multi-gene families in T. vaginalis, it is still an open question whether only some or all genes code for both enzymatic and adhesive functions. The presence of a H. meleagridis homologue of malic enzyme (Hm_ME) together with another T. vaginalis moonlighting protein, an alpha-subunit of a succinyl coenzyme A synthetase (Hm_aSCS), was recently reported (Mazet et al. 2008). Yet the present study is the first to report the existence of another H. meleagridis homologue of a T. vaginalis moonlighting protein, namely AP120/pyruvate : ferredoxin oxido-reductase A (PFOA).
Sequence divergences shown in the protein sequence alignment of clone 93a-2, H. meleagridis malic enzyme (Hm_ME), T. vaginalis malic enzyme (Tv_ME) and T. vaginalis adhesins AP65-1, (Tv_ AP65-1), AP65-2 (Tv_AP65-2), AP65-3 (Tv_AP65-3) suggest that the hydrogenosomal malic enzyme in H. meleagridis, as in T. vaginalis, is most likely a member of a multi-gene family. Another clone isolated during this study, termed clone 177-1, showed homology to cytosolic malic enzyme. This enzyme is a member of another family of malic enzymes usually found in prokaryotes. The differences between the two families of malic enzymes are obvious in several aspects: (i) localization: the cytosolic enzyme is localized within the cytoplasm, while the hydrogenosomal one is related to the enzyme found in mitochondria and plastids, (ii) size: the cytosolic enzyme is smaller (42 kDa subunit) and forms a dimer, while the hydogenosomal enzyme is bigger (60 kDa subunit) and forms a tetramer and (iii) coenzyme specificity: the cytosolic enzyme has a strict specificity to nicotinamide adenine dinucleotide phosphate (NADP+), while the hydrogenosomal one preferentially uses nicotinamide adenine dinucleotide (NAD+). The existence of 2 different families of malic enzymes in different compartments of a single eukaryotic cell appears to be unique in nature and was described in detail for T. vaginalis (Dolezal et al. 2004).
The fact that homologous protein-coding sequences of adhesins/hydrogenosomal enzymes and of a cytosolic malic enzyme were identified in H. meleagridis is very interesting on several points. Firstly, it suggests that the mechanisms of both host-cell adhesion and energy production and conservation are very similar between H. meleagridis and T. vaginalis. Secondly, it emphasizes the close phylogenetic relationship between these two parasites. However, it still remains to be investigated whether the same phenomenon of moonlighting is true for H. meleagridis proteins.
Another interesting clone detected during this study, clone 28-1, shows strong homology (65%) to thioredoxin reductase (TrxR) of T. vaginalis, an enzyme involved in the response to oxidative stress. As a microaerophilic parasitic protozoon, T. vaginalis depends on an anaerobic metabolism. When exposed to oxygen, T. vaginalis must be able to cope with the resultant oxidative stress. Thioredoxin reductase functions together with thioredoxin and thioredoxin peroxidase to detoxify potentially damaging oxidants (Coombs et al. 2004). This system does not use a glutathione as the reductant and is thus of particular importance to amitochondriate eukaryotes, such as Trichomonas, Entamoeba and Giardia, which lack glutathione (Ellis et al. 1994; Flohe et al. 1999; Muller et al. 2003). The protein alignment of the H. meleagridis clone 28-1 with thioredoxin reductases of other protozoan parasites demonstrates its strong homology to the T. vaginalis protein, as compared to the E. histolytica and G. lamblia homologues. In this protein, the only well-conserved part among all 4 parasites is the key active site containing cysteine residues (boxed in Fig. 4). H. meleagridis is regarded as an anaerobic parasite, but strict anaerobic incubation is not obligatory for the parasite to multiply in vitro (Hess et al. 2006b). The presence of the EST sequence in H. meleagridis with a high homology to thioredoxin reductase indicates that Histomonas is capable of combating oxidative stress. Furthermore, the mechanism to detoxify damaging oxidants seems to be very similar to that of other homologous parasites. In T. vaginalis, thioredoxin reductase is one of the proteins also found to be secreted in response to the parasite's contact with host cells (Kucknoor et al. 2007). It is most likely involved in modifying disulphide bonds of host proteins, thereby altering the microenvironment to one more suitable for the parasite's survival. Although the invasion of caecal epithelium by H. meleagridis seems to resemble the invasion of vaginal mucosa surfaces by T. vaginalis in many ways, it still remains elusive whether thioredoxin reductase is secreted by Histomonas, thereby actively contributing to the virulence of this parasite.
During the present study, a broad spectrum of protein-coding sequences showing homology to both intracellular and surface proteins was identified. Since the original intention of the study was the identification of H. meleagridis antigens, one would expect these to be located on the cell surface. However, in addition to surface antigens, numerous intracellular proteins were detected. It is evident from the literature that some intracellular proteins of E. histolytica were also found to be antigenic (De Meester et al. 1991; Beanan and Bailey, 1995; Carrero et al. 2000; Sanuki et al. 2001). It is possible, though, that some of the intracellular proteins identified in this study, especially weak signal translation machinery proteins, might not represent true H. meleagridis immunogenic proteins. Reporting sequences of such proteins, nevertheless, has its scientific importance, since until now only sequences of 3 H. meleagridis proteins are available in the database. The detection of intracellular proteins is obviously a consequence of the rabbit serum used for screening the cDNA library, because the inoculum administered to rabbits contained both destroyed and intact parasite cells. The detection of intracellular proteins during screening would have also been expected using chicken or turkey sera, which were gained from the natural host of the parasite after lethal infection. Prior to screening the H. meleagridis cDNA expression library, we used dot blot analysis to compare the polyclonal sera of the 3 different species. Polyclonal rabbit serum had the advantage of displaying a much weaker bacteria-specific background as compared to chicken and turkey sera. This background would obscure the screening procedure. Therefore it was decided to use polyclonal rabbit serum in subsequent experiments. Furthermore, the comparative analyses of the sera by Western blot analysis from all 3 animal species showed that the same major H. meleagridis antigens were detected by all polyclonal sera. It is nevertheless possible that polyclonal chicken and turkey sera would detect additional proteins. This, however, is the scope of further investigations.
Finally, this study represents a major breakthrough for the molecular investigation of H. meleagridis, since it is the first to report 37 H. meleagridis-specific protein-coding sequences. Various ESTs that might have an impact on host-cell invasion were detected and have been commented on in more detail. Discussing all 37 H. meleagridis-specific EST sequences identified during the present study would definitely reach beyond the capacity of this investigation. Nevertheless, the remaining clones listed in Table 2 should not be ignored. Since little molecular data are available on H. meleagridis, these cDNA sequences represent a foundation for further molecular studies on this parasite.
Acknowledgments
This investigation was supported by the Austrian Science Fund (FWF), grant no. P19315.
REFERENCES
- Addis MF, Rappelli P, Pinto De Andrade AM, Rita FM, Colombo MM, Cappuccinelli P, Fiori PL. Identification of Trichomonas vaginalis alpha-actinin as the most common immunogen recognized by sera of women exposed to the parasite. The Journal of Infectious Diseases. 1999;180:1727–1730. doi: 10.1086/315095. [DOI] [PubMed] [Google Scholar]
- Alderete JF, Millsap KW, Lehker MW, Benchimol M. Enzymes on microbial pathogens and Trichomonas vaginalis: molecular mimicry and functional diversity. Cellular Microbiology. 2001;3:359–370. doi: 10.1046/j.1462-5822.2001.00126.x. [DOI] [PubMed] [Google Scholar]
- Alderete JF, Newton E, Dennis C, Neale KA. Antibody in sera of patients infected with Trichomonas vaginalis is to trichomonad proteinases. Genitourinary Medicine. 1991a;67:331–334. doi: 10.1136/sti.67.4.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete JF, Newton E, Dennis C, Neale KA. The vagina of women infected with Trichomonas vaginalis has numerous proteinases and antibody to trichomonad proteinases. Genitourinary Medicine. 1991b;67:469–474. doi: 10.1136/sti.67.6.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beanan MJ, Bailey GB. The primary structure of an Entamoeba histolytica enolase. Molecular and Biochemical Parasitology. 1995;69:119–121. doi: 10.1016/0166-6851(94)00201-w. [DOI] [PubMed] [Google Scholar]
- Bishop A. Histomonas meleagridis in domestic fowls (Gallus gallus). Cultivation and experimental infection. Parasitology. 1938;30:181–194. [Google Scholar]
- Bricheux G, Coffe G, Pradel N, Brugerolle G. Evidence for an uncommon alpha-actinin protein in Trichomonas vaginalis. Molecular and Biochemical Parasitology. 1998;95:241–249. doi: 10.1016/s0166-6851(98)00105-4. [DOI] [PubMed] [Google Scholar]
- Carlton JM, Hirt RP, Silva JC, Delcher AL, Schatz M, Zhao Q, Wortman JR, Bidwell SL, Alsmark UC, Besteiro S, Sicheritz-Ponten T, Noel CJ, Dacks JB, Foster PG, Simillion C, Van de PY, Miranda-Saavedra D, Barton GJ, Westrop GD, Muller S, Dessi D, Fiori PL, Ren Q, Paulsen I, Zhang H, Bastida-Corcuera FD, Simoes-Barbosa A, Brown MT, Hayes RD, Mukherjee M, Okumura CY, Schneider R, Smith AJ, Vanacova S, Villalvazo M, Haas BJ, Pertea M, Feldblyum TV, Utterback TR, Shu CL, Osoegawa K, de Jong PJ, Hrdy I, Horvathova L, Zubacova Z, Dolezal P, Malik SB, Logsdon JM, Jr., Henze K, Gupta A, Wang CC, Dunne RL, Upcroft JA, Upcroft P, White O, Salzberg SL, Tang P, Chiu CH, Lee YS, Embley TM, Coombs GH, Mottram JC, Tachezy J, Fraser-Liggett CM, Johnson PJ. Draft genome sequence of the sexually transmitted pathogen Trichomonas vaginalis. Science. 2007;315:207–212. doi: 10.1126/science.1132894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrero JC, Petrossian P, Acosta E, Sanchez-Zerpa M, Ortiz-Ortiz L, Laclette JP. Cloning and characterization of Entamoeba histolytica antigens recognized by human secretory IgA antibodies. Parasitology Research. 2000;86:330–334. doi: 10.1007/s004360050052. [DOI] [PubMed] [Google Scholar]
- Chaudhry OA, Petri WA., Jr. Vaccine prospects for amebiasis. Expert Review of Vaccines. 2005;4:657–668. doi: 10.1586/14760584.4.5.657. [DOI] [PubMed] [Google Scholar]
- Coombs GH, Westrop GD, Suchan P, Puzova G, Hirt RP, Embley TM, Mottram JC, Muller S. The amitochondriate eukaryote Trichomonas vaginalis contains a divergent thioredoxin-linked peroxiredoxin antioxidant system. Journal of Biological Chemistry. 2004;279:5249–5256. doi: 10.1074/jbc.M304359200. [DOI] [PubMed] [Google Scholar]
- De Meester F, Bracha R, Huber M, Keren Z, Rozenblatt S, Mirelman D. Cloning and characterization of an unusual elongation factor-1 alpha cDNA from Entamoeba histolytica. Molecular and Biochemical Parasitology. 1991;44:23–32. doi: 10.1016/0166-6851(91)90217-t. [DOI] [PubMed] [Google Scholar]
- Dolezal P, Vanacova S, Tachezy J, Hrdy I. Malic enzymes of Trichomonas vaginalis: two enzyme families, two distinct origins. Gene. 2004;329:81–92. doi: 10.1016/j.gene.2003.12.022. [DOI] [PubMed] [Google Scholar]
- Ellis JE, Yarlett N, Cole D, Humphreys MJ, Lloyd D. Antioxidant defences in the microaerophilic protozoan Trichomonas vaginalis: comparison of metronidazole-resistant and sensitive strains. Microbiology. 1994;140:2489–2494. doi: 10.1099/13500872-140-9-2489. [DOI] [PubMed] [Google Scholar]
- Engbring JA, O'Brien JL, Alderete JF. Trichomonas vaginalis adhesin proteins display molecular mimicry to metabolic enzymes. Advances in Experimental Medicine and Biology. 1996;408:207–223. doi: 10.1007/978-1-4613-0415-9_25. [DOI] [PubMed] [Google Scholar]
- Fiori PL, Rappelli P, Addis MF. The flagellated parasite Trichomonas vaginalis: new insights into cytopathogenicity mechanisms. Microbes and Infection. 1999;1:149–156. doi: 10.1016/s1286-4579(99)80006-9. [DOI] [PubMed] [Google Scholar]
- Flohe L, Hecht HJ, Steinert P. Glutathione and trypanothione in parasitic hydroperoxide metabolism. Free Radical Biology and Medicine. 1999;27:966–984. doi: 10.1016/s0891-5849(99)00172-0. [DOI] [PubMed] [Google Scholar]
- Gerbod D, Edgcomb VP, Noel C, Zenner L, Wintjens R, Delgado-Viscogliosi P, Holder ME, Sogin ML, Viscogliosi E. Phylogenetic position of the trichomonad parasite of turkeys, Histomonas meleagridis (Smith) Tyzzer, inferred from small subunit rRNA sequence. Journal of Eukaryotic Microbiology. 2001;48:498–504. doi: 10.1111/j.1550-7408.2001.tb00185.x. [DOI] [PubMed] [Google Scholar]
- Guillen N. Role of signalling and cytoskeletal rearrangements in the pathogenesis of Entamoeba histolytica. Trends in Microbiology. 1996;4:191–197. doi: 10.1016/0966-842x(96)10033-0. [DOI] [PubMed] [Google Scholar]
- Hess M, Grabensteiner E, Liebhart D. Rapid transmission of the protozoan parasite Histomonas meleagridis in turkeys and specific pathogen free chickens following cloacal infection with a mono-eukaryotic culture. Avian Pathology. 2006a;35:280–285. doi: 10.1080/03079450600815507. [DOI] [PubMed] [Google Scholar]
- Hess M, Kolbe T, Grabensteiner E, Prosl H. Clonal cultures of Histomonas meleagridis, Tetratrichomonas gallinarum and a Blastocystis sp. established through micromanipulation. Parasitology. 2006b;133:547–554. doi: 10.1017/S0031182006000758. [DOI] [PubMed] [Google Scholar]
- Hess M, Liebhart D, Grabensteiner E, Singh A. Cloned Histomonas meleagridis passaged in vitro resulted in reduced pathogenicity and is capable of protecting turkeys from histomonosis. Vaccine. 2008;26:4187–4199. doi: 10.1016/j.vaccine.2008.05.071. [DOI] [PubMed] [Google Scholar]
- Hirt RP, Noel CJ, Sicheritz-Ponten T, Tachezy J, Fiori PL. Trichomonas vaginalis surface proteins: a view from the genome. Trends in Parasitology. 2007;23:540–547. doi: 10.1016/j.pt.2007.08.020. [DOI] [PubMed] [Google Scholar]
- Honigberg BM, Bennett CJ. Lightmicroscopic observations on structure and division of Histomonas meleagridis (Smith) Journal of Protozoology. 1971;18:687–700. doi: 10.1111/j.1550-7408.1971.tb03398.x. [DOI] [PubMed] [Google Scholar]
- Jeffery CJ. Moonlighting proteins. Trends in Biochemical Sciences. 1999;24:8–11. doi: 10.1016/s0968-0004(98)01335-8. [DOI] [PubMed] [Google Scholar]
- Kucknoor AS, Mundodi V, Alderete JF. The proteins secreted by Trichomonas vaginalis and vaginal epithelial cell response to secreted and episomally expressed AP65. Cellular Microbiology. 2007;9:2586–2597. doi: 10.1111/j.1462-5822.2007.00979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund EE, Augustine PC, Chute AM. Histomonas meleagridis after one thousand in vitro passages. Journal of Protozoology. 1967;14:349–351. doi: 10.1111/j.1550-7408.1967.tb02007.x. [DOI] [PubMed] [Google Scholar]
- Mazet M, Diogon M, Alderete JF, Vivares CP, Delbac F. First molecular characterisation of hydrogenosomes in the protozoan parasite Histomonas meleagridis. International Journal for Parasitology. 2008;38:177–190. doi: 10.1016/j.ijpara.2007.06.006. [DOI] [PubMed] [Google Scholar]
- McDougald LR. Blackhead disease (histomoniasis) in poultry: a critical review. Avian Diseases. 2005;49:462–476. doi: 10.1637/7420-081005R.1. [DOI] [PubMed] [Google Scholar]
- Moreno-Brito V, Yanez-Gomez C, Meza-Cervantez P, Avila-Gonzalez L, Rodriguez MA, Ortega-Lopez J, Gonzalez-Robles A, Arroyo R. A Trichomonas vaginalis 120 kDa protein with identity to hydrogenosome pyruvate:ferredoxin oxidoreductase is a surface adhesin induced by iron. Cellular Microbiology. 2005;7:245–258. doi: 10.1111/j.1462-5822.2004.00455.x. [DOI] [PubMed] [Google Scholar]
- Muller M. Evolutionary origins of trichomonad hydrogenosomes. Parasitology Today. 1997;13:166–167. doi: 10.1016/s0169-4758(97)01036-3. [DOI] [PubMed] [Google Scholar]
- Muller S, Liebau E, Walter RD, Krauth-Siegel RL. Thiol-based redox metabolism of protozoan parasites. Trends in Parasitology. 2003;19:320–328. doi: 10.1016/s1471-4922(03)00141-7. [DOI] [PubMed] [Google Scholar]
- Mundodi V, Kucknoor AS, Alderete JF. Immunogenic and plasminogen-binding surface-associated alpha-enolase of Trichomonas vaginalis. Infection and Immunity. 2008;76:523–531. doi: 10.1128/IAI.01352-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundodi V, Kucknoor AS, Chang TH, Alderete JF. A novel surface protein of Trichomonas vaginalis is regulated independently by low iron and contact with vaginal epithelial cells. BMC Microbiology. 2006;6:6. doi: 10.1186/1471-2180-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musatovova O, Alderete JF. The Trichomonas vaginalis phenotypically varying P270 immunogen is highly conserved except for numbers of repeated elements. Microbial Pathogenesis. 1999;27:93–104. doi: 10.1006/mpat.1999.0281. [DOI] [PubMed] [Google Scholar]
- Rybicka K, Honigberg BM, Holt SC. Fine structure of the mastigont system in culture forms of Histomonas meleagridis (Smith) Protistologica. 1972;8:107–120. [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: a Laboratory Manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY, USA: 1989. [Google Scholar]
- Sanuki J, Nakano K, Tokoro M, Nozaki T, Okuzawa E, Kobayashi S, Asai T. Purification and identification of major soluble 40-kDa antigenic protein from Entamoeba histolytica: its application for serodiagnosis of asymptomatic amebiasis. Parasitology International. 2001;50:73–80. doi: 10.1016/s1383-5769(01)00062-9. [DOI] [PubMed] [Google Scholar]
- Schuster FL. Ultrastructure of Histomonas meleagridis (Smith) Tyzzer, a parasitic amebo-flagelate. The Journal of Parasitology. 1968;54:725–737. [Google Scholar]
- Tyzzer EE. The flagellate character and reclassification of the parasite producing ‘Blackhead’ in turkeys- Histomonas (gen. nov.) meleagridis (Smith) The Journal of Parasitology. 1920;6:124–131. [Google Scholar]
- Wenrich DH. Observations on the morphology of Histomonas (Protozoa, Mastigophora) from phesants and chickens. Journal of Morphology. 1943;72:279–303. [Google Scholar]
- Zhao X, Siu CH. Colocalization of the homophilic binding site and the neuritogenic activity of the cell adhesion molecule L1 to its second Ig-like domain. Journal of Biological Chemistry. 1995;270:29413–29421. doi: 10.1074/jbc.270.49.29413. [DOI] [PubMed] [Google Scholar]