Abstract
Autoimmune chronic active liver disease (ACALD), a major indication for liver transplantation, is associated strongly with antigenic determinants HLA-B8 and DR3. A retrospective analysis of 43 patients who underwent OLTx for putative ACALD and who, as well as their tissue organ donors, were typed, was performed. Disease recurrence and graft rejection episodes were determined by chart review and histopathological review of all material available. Disease recurrence was histologically documented in 11 (25.6%) of these 43 cases. Graft rejection episodes occurred in 24 (55.8%). All recurrences were in recipients of HLA-DR3–negative grafts. Nine of the recurrences were in HLA-DR3–positive recipients (odds ratio: 6.14, P<0.03). Two of 11 cases of disease recurrence were in recipients who were HLA-DR3–negative. Nine of these 11 had received-HLA-DR3–negative grafts. Rejection occurred in 13 HLA-B8–positive recipients, 12 of whom received HLA-B8–negative grafts. Eleven HLA-B8–negative recipients experienced at least one rejection episode and 9 of these had received HLA-B8–negative grafts. Based upon these data we conclude: 1) that recurrence of putative ACALD is more likely to occur in HLA-DR3–positive recipients of HLA-DR3–negative grafts; (2) that recurrences were not seen in recipients of HLA-DR3–positive grafts; (3) that HLA-B8 status does not affect disease recurrence; and (4) that neither the HLA-B8 nor the DR3 status of the graft or recipient has an effect on the observed frequency of rejection.
Autoimmune chronic active liver disease (ACALD)* is known to be associated with certain HLA antigenic determinants, specifically the class I antigens HLA-A1 and B8 (1) and the class II antigen DR3 (2). These antigens are thought to reside near an abnormal immune response gene, found on chromosome 6, which is in genetic disequilibrium with these particular HLA antigens. It is the presence of the abnormal immune response gene that predisposes an individual to develop ACALD.
Currently, ACALD is one of the major specific disease indications for orthotopic liver transplantation. Disease recurrence following OLTx for this indication has occurred in at least one recipient (3). In this single report, the recipient was HLA-B8 and HLA-DR3–positive, while the liver donor was negative for both of these two antigens. In contradistinction to the situation with other organs, HLA typing has not been performed routinely in clinical OLTx, because several retrospective analyses have indicated that the HLA matching is not important in determining the long-term survival of either the graft or the patient following OLTx (4).
Because tissue typing of both the donor and the recipient has been standard practice at the University of Pittsburgh, it was possible to determine whether the presence or absence of HLA matching for HLA-B8 and DR3 affects the subsequent course in terms of disease recurrence and the frequency of rejection episodes. It was anticipated that a donor/recipient HLA match for DR3 and possibly for B8 would increase the frequency of disease recurrence while simultaneously decreasing the frequency of rejection episodes.
MATERIALS AND METHODS
The charts of all patients who were transplanted for putative autoimmune chronic active liver disease from November 1981 through July 1989 who had a typical disease course, including responsiveness to glucocorticoid treatment, absence of all viral markers, and typical serological markers for ACALD, were reviewed. The charts of all such liver transplant recipients with a minimum duration of posttransplantation follow-up of 10 months were evaluated in detail. A total of 114 such patients were identified. For 49 of these, the HLA typing data of both the donor and the recipient were available. Of these 49, 6 had died in the immediate posttransplant period and were excluded from the analysis. Two of these 6 died as a result of primary graft dysfunction, 2 died as a result of a vascular complication, and the remaining 2 died as a result of overwhelming sepsis.
All patients during this period were treated with standard immunosuppression consisting of cyclosporine and prednisone. For the purpose of this study, only the data relevant to the first organ graft, when more than one graft procedure was performed in a single recipient, were reviewed. All pre- and posttransplant liver biopsies were rereviewed. Each of the posttransplant liver biopsies was performed because of either a suspicion of graft rejection or an alteration in liver injury parameters and/or an increase in the serum bilirubin level. After rereview, those biopsies that were considered to be histologically consistent with a diagnosis of a recurrence of autoimmune chronic active liver disease were coded as cases of disease recurrence. Cases that were identified as showing either acute cellular rejection or chronic rejection were coded as cases of rejection. The histological diagnosis of rejection in each case were estimated using established using established criteria (5,6). Finally, the proportion of cases matched for HLA antigens B8 and DR3 in the donor and recipient was determined and compared with the proportion of cases with the same histologic findings but without such donor and recipient matching.
Statistical analysis
All results are reported as absolute numbers. The true odds ratio was computed. The 95% confidence interval for the true odds ratio was calculated by the exact maximum likelihood method. Fisher’s exact test was used to examine relationships of disease status with HLA markers. A P value less than 0.05 was considered to represent a significant difference.
RESULTS
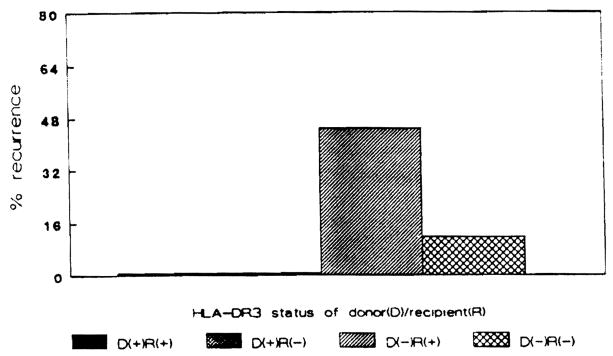
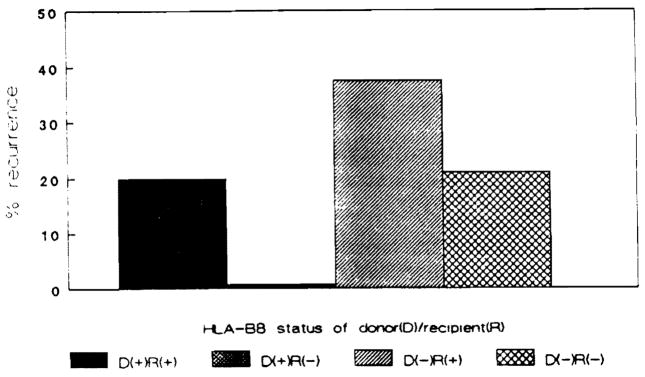
Eleven cases of disease recurrence were noted in these 43 patients. All 11 cases of disease recurrence were in recipients who received HLA-DR3–negative donor organs (Fig. 1). The clinical characteristics of these 11 cases are shown in Table 1. All were positive for one or more autoantibodies and had hypergammaglobulinemia before and after OLTx at the time of disease recurrence defined histologically. Moreover, as all posttransplant liver biopsies were obtained only as indicated for clinical reasons, all had abnormal liver enzymes consistent with a diagnosis of recurrent chronic active hepatitis. Six recipients received HLA-DR3–positive grafts, and no histologic evidence for disease recurrence was noted in any. Nine of 20 HLA-DR3–positive recipients experienced histologic recurrence of their chronic active hepatitis. In contrast, only two of 17 HLA-DR3–negative recipients experienced evidence for disease recurrence defined histologically (odds ratio: 6.14, P<0.03). All but one of the cases of disease recurrence were in recipients of HLA-B8–negative donor organs (Fig. 2). Six of these recipients were HLA-B8–positive. Eight patients received HLA-B8–positive grafts. Only one of these found to experience histologic recurrence. Seven of 21 HLA-B8–positive recipients experienced disease recurrence. Four of 22 HLA-B8–negative recipients experienced histologic disease recurrence. All had received HLA-B8–negative grafts.
Figure 1.
Percentage of recipients experiencing disease recurrence based upon the HLA-DR3 status of the donor-recipient pairing.
Table 1.
Clinical characteristics of the 11 subjects with recurrent ACALD
| A. General Characteristics | ||
|---|---|---|
| Characteristic | No. | |
| Gender | All women | |
| Age | 49±3 years | |
| Time posttransplant when histology positive for ACALD | 18±4 months | |
| B. Autoantibody status | ||
| Autoantibodies positive | Pre-OLTx | Post-OLTx |
| ANA | 6/11 | 5/11 |
| Antimicrosomal | 11/11 | 11/11 |
| Antithyroglobulin | 10/11 | 9/11 |
| Antismooth muscle | 8/11 | 8/11 |
| Symptomatica | 11/11 | 11/11 |
| Fivefold elevation of transaminases | 5/11 | 8/11 |
| Twofold increase in gamma-globulin levels | 11/11 | 11/11 |
Symptoms including arthritis, fatigue, and abdominal (hepatic) discomfort.
Figure 2.
Percentage of recipients experiencing disease recurrence based upon the HLA-B8 status of the donor-recipient pairing.
Rejection episodes occurred in two of the six recipients that received HLA-DR3–positive donor grafts and in 22 of 37 who received HLA-DR3–negative grafts (Fig. 3). Thirteen of 24 HLA-DR3–positive recipients experienced at least one episode of rejection. All of these had received HLA-DR3–negative donor organs. Four of the recipients had HLA-DR3–positive grafts and none of them rejected. Eleven of 19 HLA-DR3–negative recipients experienced at least one episode of rejection; 9 of these 11 received HLA-DR3–negative grafts. Only two HLA-DR3–negative recipients received HLA-DR3–positive grafts, and both of these experienced at least one episode of rejection.
Figure 3.
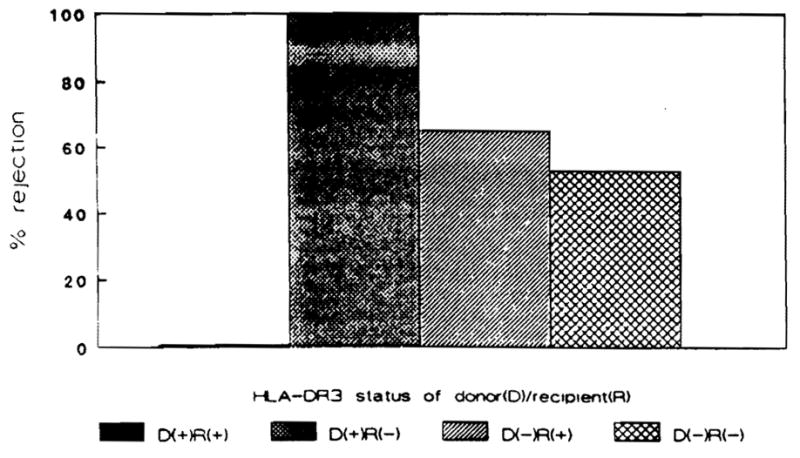
Percentage of recipients experiencing graft rejection based upon the HLA-DR3 status of the donor-recipient pairing.
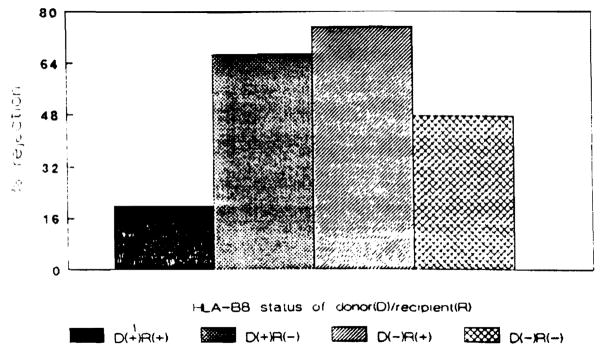
Episodes of rejection occurred in three of eight patients who received HLA-8–positive grafts (Fig. 4). Two of these patients were negative for HLA-B8. Twenty-one of 35 recipients of HLA-B8–negative grafts experienced rejection. Nine of these were HLA-B8–negative. Thirteen of 21 HLA-B8–positive recipients and 11 of 22 HLA-B8–negative recipients experienced at least one episode of rejection.
Figure 4.
Percentage of recipients experiencing graft rejection based upon the HLA-B8 status of the donor-recipient pairing.
DISCUSSION
In this study, an attempt was made to elucidate the influence of donor/recipient matching or mismatching for HLA-B8 and DR3 antigenic determinants on subsequent episodes of rejection and disease recurrence in patients transplanted for ACALD. These antigens were chosen for study because they are known to be associated with autoimmune chronic active liver disease (2). All recipients transplanted for ACALD who survived for 10 months or more were studied. Their records were examined for episodes of graft rejection and histologic evidence of disease recurrence. The data available indicate that recipients who are HLA-DR3–positive and receive HLA-DR3–negative donor organs are at increased risk to experience histologic recurrence of ACALD. Although a similar trend was evident for recipients having the HLA-B8 antigen, the finding failed to achieve the level of statistical significance.
It has been shown previously that the B8/DR3 phenotype is associated with a exaggerated humoral immune response (7), increased mixed leukocyte reactions (8), increased in vitro responses to wheat antigens (9), and decreased concanavalin A–induced suppression of immunoglobulin synthesis, along with an increased spontaneous in vitro Ig synthesis (10). Nouri-Aria et a1. (11) have shown an association between the presence of HLA antigens A1/B8/DR3 in families of patients with autoimmune chronic active hepatitis and a defect in nonantigen-specific suppressor T-cell control of immunoglobulin production. Such pathogenetic mechanism(s) might playa role in the pathogenesis of ACALD and possibly disease recurrence should it occur.
A major issue in this study was how certainly could we distinguish histologic disease recurrence in a liver graft as opposed to new-onset viral hepatitis. Immunoperoxidase stains for CMV and EBV were negative in all of the cases with putative disease recurrence. Similarly, all 11 cases were negative for hepatitis A, B, and D markers. The possibility of a non A, non B hepatitis (HCV) was ruled out also, with the use of the recently available HCV antibody assay developed by Abbott Laboratories. All 11 cases of histologic disease recurrence of ACALD were negative for HCV, despite the reports of false positive HCV-Ab responses in individuals with ACALD (12).
The results of this study indicate an increased susceptibility to “recurrent autoimmune chronic hepatitis” characterized by piecemeal necrosis and a dense portal inflammatory infiltrate consisting of mature plasma cells and lymphocytes as opposed to immunoblasts in recipients who are HLA-DR3–positive. Moreover, the presence of any biliary tract lesion responsible for the histologic findings was excluded by cholangiography in each case at the time of the disease recurrence defined histologically. Moreover, these histologic findings suggestive of recurrent ACALD occurred in the absence of any evidence for viral hepatitis either before or after OLTx.
In contrast to these data suggesting disease recurrence, no difference in the rejection episode rate was evident among the various subgroups of transplant recipients assessed based upon the presence or absence of any HLA match or mismatch of the antigens studied. This latter finding confirms the earlier finding that HLA matching does not influence graft rejection rates in liver transplantation (4).
Footnotes
Abbreviations: ACALD, autoimmune chronic active liver disease.
References
- 1.Mackay RR, Morris PJ. Association of autoimmune chronic active hepatitis with HLA-A1, B8. Lancet. 1972;2:793. doi: 10.1016/s0140-6736(72)92149-6. [DOI] [PubMed] [Google Scholar]
- 2.MacKay IR, Tait BD. HLA associations with autoimmune-type chronic active hepatitis: identification of B8-DRw3 haplotype by family studies. Gastroenterology. 1980;79:95. [PubMed] [Google Scholar]
- 3.Neuberger J, Portmann B, Calne R, Williams R. Recurrence of autoimmune chronic active hepatitis following orthotopic liver grafting. Transplantation. 1984;37:363. doi: 10.1097/00007890-198404000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Gordon RD, Fung JJ, Markus B, et al. The antibody crossmatch in liver transplantation. Surgery. 1986;100:705. [PMC free article] [PubMed] [Google Scholar]
- 5.Snover DC, Sibley RK, Freese DK, et al. Orthotopic liver transplantation: a pathological study of 63 serial liver biopsies from 17 patients with specific reference to the diagnostic features and natural history of rejection. Hepatology. 1984;4:1212. doi: 10.1002/hep.1840040620. [DOI] [PubMed] [Google Scholar]
- 6.Adams DH, Neuberger JM. Patterns of graft rejection following liver transplantation. J Hepatol. 1990;10:113. doi: 10.1016/0168-8278(90)90081-2. [DOI] [PubMed] [Google Scholar]
- 7.Kallenberg CGM, Klaassen RJL, Beelen JM, Haw The T. HLA-B8/DR3 phenotype and the primary immune response. Clin Immunol Immunopathol. 1985;34:135. doi: 10.1016/0090-1229(85)90017-0. [DOI] [PubMed] [Google Scholar]
- 8.Osoba D, Falk J. HLA-B8 phenotype associated with an increased mixed leukocyte reaction. Immunogenetics. 1978;6:425. [Google Scholar]
- 9.Cunningham-Rundles S, Cunningham-Rundles C, Pollack MS, Good RA, Dupont B. Response to wheat antigen in vitro lymphocyte transformation among HLA-B8 positive normal donors. Transplant Proc. 1978;10:977. [PubMed] [Google Scholar]
- 10.Ambinder JM, Chiorazzi N, Gibofsky A, Fotino M, Kundel HG. Special characteristics of cellular immune function in normal individuals of the HLA-DR3 type. Clin Immunol Immunopathol. 1982;23:269. doi: 10.1016/0090-1229(82)90113-1. [DOI] [PubMed] [Google Scholar]
- 11.Nouri-Aria KT, Donaldson PT, Hegarty JE, Eddleston AL, Williams R. HLA A1-B8-DR3 and suppressor cell function in first-degree relatives of patients with autoimmune chronic active hepatitis. J Hepatol. 1985;1:235. doi: 10.1016/s0168-8278(85)80051-9. [DOI] [PubMed] [Google Scholar]
- 12.McFarlane IG, Smith HM, Johnson PJ, Bray GP, Vergani D, Williams R. Hepatitis C virus antibodies in chronic active hepatitis: pathogenetic factor or false-positive result? Lancet. 1990;335:754. doi: 10.1016/0140-6736(90)90870-b. [DOI] [PubMed] [Google Scholar]