Abstract
The adult human Vγ2Vδ2 T cell repertoire is a product of chronic selection in the periphery. Endogenous antigens drive the expansion of cells expressing the Vγ2Vδ2 TCR. Thus, we would expect the majority of circulating Vγ2Vδ2 T cells to be antigen experienced and to have memory phenotype, in contrast to the alpha/beta TCR+ subsets that include a substantial fraction of naive cells. We sought to characterize functional aspects of Vγ2Vδ2 T cells that might show whether circulating cells are memory or naive. For these studies, we focus on the expression of the CC chemokine regulated upon activation normal T cell expressed and secreted (RANTES). In naive αβ T cells, an initial stimulus triggers the onset of RANTES transcription followed later by protein expression. In memory CD8+ αβ T cells, RANTES mRNA is already present in unstimulated cells and protein expression is triggered immediately by TCR signaling; some cells may also contain RANTES protein in cytoplasmic stores. We show here that the vast majority of circulating human T cells contain RANTES protein in cytoplasmic stores and the chemokine is secreted rapidly after TCR signaling. Primary Vγ2Vδ2 T cell lines obtained after in vitro stimulation with phosphoantigens behaved similarly to circulating Vγ2Vδ2 T cells and contained both RANTES mRNA and protein, but only very low levels of mRNA or protein for macrophage inflammatory protein (MIP)-1α or MIP-1β. The presence of stored RANTES shows that circulating Vγ2Vδ2 T cells are mostly memory phenotype and capable of rapid chemokine responses to phosphoantigen stimulation. Considering that one of 40 circulating CD3+ lymphocytes is Vγ2Vδ2+, they comprise the largest circulating memory population against a single antigen, and phosphoantigen stimulation will trigger a rapid activation with immediate release of RANTES.
Keywords: chemokines, gamma/delta T cells, human, innate immunity, T cell memory, T cell receptors
Introduction
Human T cells express either the alpha/beta (αβ) or gamma/delta (γδ T cell) antigen receptor. The αβ T cells express lineage markers (CD4 or CD8) and recognize peptide antigens complexed with cell-surface major histocompatibility proteins. Alternatively, cells express the γδ TCR and recognize non-peptidic antigens and some tumor cell lines, in an MHC-unrestricted manner (1–3).
The naive αβ TCR repertoire is a product of thymic education (4, 5) and memory subsets arise after the initial antigen encounter. Repertoire selection and memory are controlled differently for γδ T cells. The γδ subset comprises 1–5% of circulating CD3+ lymphocytes in healthy adult human beings (6, 7) of which ~85% express the Vγ2Vδ2 TCR. Mature γδ T cells mostly do not express lineage markers. The αβ TCR repertoire is a product of thymic selection, and includes ~25 β chains families that combine with 100 α chain families to create ~105–106 unique TCRs present at any one time. The mature γδ repertoire is much different, being shaped mainly by peripheral selection and expansion starting with fewer γ and δ chain families. However, the repertoire in healthy adults shows that the Vγ2 chain is nearly always found with the Vδ2 chain.
Early in life, the more random γδ TCR repertoire seen in cord blood and infants is replaced by a repertoire that is biased in terms of receptor expression and antigen recognition (8). A dominant Vγ2Vδ2+ population emerges, is stably present at 3–10 times that of the next most abundant Vδ1-positive subset and manifests strong proliferative and cytokine responses to stimulation with low molecular weight, non-peptidic phosphoantigens including aminobisphosphonate compounds (9). Phosphoantigen recognition requires the Vγ2Vδ2 TCR (10, 11), and stimulated cells expand in a polyclonal or oligoclonal manner, with preferential use of the Vγ2-Jγ1.2 chain (12). Expanded cells are potently cytotoxic for several human tumor cell lines (13, 14).
Positive selection of Vγ2-Jγ1.2Vδ2 positive cells creates the mature repertoire and suggests that the circulating γδ T cell population is comprised mainly of antigen experienced, memory cells. However, phenotype studies of circulating γδ T cell produced conflicting results. Dieli et al. reported that up to 40% of circulating and 90% of lymph node Vγ2Vδ2 T cells have a naive phenotype. These results were contradicted by De Rosa et al. (15) who claimed that naive Vγ2Vδ2 T cells represent only a small population and most have a memory phenotype.
We decided to study other features of differentiated T cells, to show whether circulating Vγ2Vδ2 T cells were functionally similar to naive or memory cells. In particular, we examined the pattern of regulated upon activation normal T cell expressed and secreted (RANTES) expression. In αβ T cells, RANTES regulation differs in naive and memory subsets. Naive αβ T cells do not express late activation markers such as perforin, Granzymes A and B or granulysin, and they begin to express RANTES mRNA and protein only after 3–5 days of stimulation (16–19). Cell division and differentiation are required before initiating RANTES transcription, which appears as the rate-limiting step for expressing this chemokine (20, 21). Memory CD8+ T cells store preformed RANTES mRNA (22, 23) and cytoplasmic RANTES protein (24); a rapid translation or direct release from cytoplasmic stores of protein allows for rapid production of extracellular chemokine. Here, we show that circulating Vγ2Vδ2 T cells contain pre-existing RANTES protein and are functionally similar to CD8+ memory T cell subsets. There was little evidence for naive Vγ2Vδ2 T cells in circulation.
Methods
Cell culture
PBMCs were isolated from heparinized blood by Ficoll-Hypaque gradient centrifugation (Pharmacia Biotech, Piscataway, NJ, USA). PBMCs were cultured in RPMI 1640 supplemented with 10% FCS, 2 mmol l−1 L-glutamine, 100 U ml−1 penicillin and streptomycin (all from Invitrogen, Carlsbad, CA, USA) and 100 U ml−1 of recombinant IL-2. Isopentenyl pyrophosphate (IPP) (Sigma, St Louis, MO, USA) was used at 15 μM. Cells stimulated with IPP were incubated for 14 days at 37°C with 5% CO2 and replenished every 3 days with media containing 100 U ml−1 of IL-2 without IPP. Cells were then maintained at 10 units IL-2 for 8–10 additional days. Cells were fractionated using an NE-Per kit (Pierce, Rockford, IL, USA) according to the manufacturer's protocol.
Stimulation of Vγ2Vδ2 T cells used 15 μM IPP, 10 ng ml−1 of phorbol myristate acetate (PMA) and 1 μg ml−1 of ionomycin (Sigma), plus plastic immobilized anti-CD3 antibody (Immunotech, Marseille, France) or 5 μg ml−1 PHA from Phaseolus spp. (Murex Diagnostics, UK). Cycloheximide and actinomycin D were used at 10 μg ml−1 (Sigma).
Purification of CD4, CD8 and γδ T cells
CD4 and CD8 lymphocytes were purified from peripheral blood of healthy volunteers using negative selection columns (R &D Systems, Minneapolis, MN, USA). Purity of the resulting preparations was assessed by flow cytometry using anti-CD4 and anti-CD8 antibodies (Beckton Dickinson, San Jose, CA, USA). The purity of recovered cells was >90% for CD4 and >85% for CD8 subsets, respectively. Since the majority of γδ T lymphocytes are CD4 and CD8 double negative, γδ T cells were enriched using a combination of RosetteSep protocols (StemCell Technologies, Vancouver, Canada). The combination of antibodies for negative selection of γδ T cells included anti-CD4, -CD8, -CD16, -CD19, -CD36 and -CD56 antibodies.
Quantitative PCR
RNA was isolated from a maximum of 1 × 107 cells using the RNeasy RNA extraction kit (Qiagen, Valencia, CA, USA). The lysate was homogenized with a Qiashredder (Qiagen). The RNA was eluted from the RNeasy column with two 40-μl washes with nuclease-free water. Quantitation of RNA was done by measuring absorbance at 260 nm. cDNA was produced by adding 1 μg of each RNA sample to the access reverse transcription (RT) kit (Promega, Madison, WI, USA). The cDNA was diluted to 100 μl and 5 μl were used in each replicate PCR assay.
Quantification of PCR products was done with the GeneAmp 5700 Sequence Detection System (Applied Biosystems, Foster City, CA, USA). The SYBR Green I PCR Core Reagent kit (Applied Biosystems) was used to produce fluorescent-labeled PCR products and we monitored increasing fluorescence during repetitive cycling of the amplification reaction. The only alteration of the manufacturer's protocol was to raise the annealing/extension temperature to 64°C. Primer sets for all amplicons were designed using the PrimerExpress 1.0 software system. The primer sequences are: RANTES: ACTCAAGAATGGGCGGAAAG and TGGCATGTTGCAGGCTCCT, MIP-1β: ACCCTCCCACCGCCTGCTGCTTTTCTTCAC and GTTGCAGGTCATACACGTACTCCTGGACCC, β-actin: GAAGCATTTGCGGTGGACGAT and TCCTGTGGCATCCACGAAACT and MIP-1α primers were from BioSource International (Camarillo, CA, USA).
Results from the RT-PCR assay were expressed as the threshold cycle (CT). The CT represents the number of reaction cycles at which the reporter fluorescence raises above a set baseline threshold, and indicates that the DNA amplicon is increasing exponentially. The ΔCT is the difference between the CT for a specific mRNA and the CT for a reference mRNA, β-actin. To determine relative mRNA levels, 2 was raised to the power of ΔΔCT (the difference between the ΔCT from treated cells and the ΔCT from untreated cells). This compares the relative levels of specific mRNA with β-actin mRNA in each individual sample, and then compares the level of the unknown mRNA from induced cells with that of control cells. Control cells were cultured in medium plus IL-2 and without IPP stimulation.
Flow cytometry
Cells were stained using a Cytofix/Cytoperm kit (PharMingen, San Diego, CA, USA) with a modified protocol. Briefly, cells were stained for 30 min at 4°C with either PE- or FITC-labeled mAb Vγ2 or appropriate isotype controls (PharMingen). Cells were then fixed for 10 min at room temperature and stained for 45 min at room temperature with FITC-labeled mAb RANTES (R&D Systems) or an appropriate isotype control. Cells were analyzed by flow cytometry using a FACSCalibur (Becton Dickinson, San Jose, CA, USA). Gated populations were analyzed using Flo-Jo software (Tree Star, San Carlos, CA, USA). Staining for intracellular Granzyme B used the mouse monoclonal clone GB12 against human Granzyme B, conjugated to PE (Caltag, Burlingame CA, USA).
Microscopy
For detecting the γδ TCR, we used a Cy5-labeled antibody. The FITC-labeled mAb RANTES (R &D Systems) was used for intracellular staining. Cells were spotted on poly-L-lysine-coated coverslips (Becton Dickinson), centrifuged for 5 min at 500 × g, then mounted in Vectashield (Vector Laboratories, Burlingame, CA, USA) on microscope slides, and examined using an Olympus BX51 microscope with a Magnafire camera and software (Optronics, Goleta, CA, USA). Changes in fluorescence were also recorded by a laser scanning confocal system (Zeiss LSM 510) using a Zeiss 63×/1.2 W corr objective. The system was operated in the frame-scan single or Z-stack modes. FITC and Cy5 were excited by light at 488 nm (30 mW argon laser). FITC fluorescence was measured using a bypass filter 505–530 nm, and Cy5 fluorescence was measured at wavelengths >650 nm. Images were acquired from optical slices 0.5–2 μm. Image processing and analysis were performed with Scion Image Beta 4.0.2 (NIH, Bethesda, MD, USA) software.
ELISA
Detection of MIP-1α, MIP-1β and RANTES in cytoplasmic fractions of cells or supernatant used chemokine ELISA kits (R &D Systems). Cell lysates were prepared using NE-PER kit (Pierce) according to manufacturer's instructions. Cytoplasmic lysates were diluted in the ELISA buffer (typically 1:20, corresponding to 1 000 000 lysed cells per 1 ml of assay buffer) and chemokine concentrations were measured in ELISA. Corresponding dilutions of the lysis buffer into the ELISA buffer did not affect chemokine detection (data not shown).
Mass spectroscopy of RANTES isoforms present in culture supernatants
Mass spectral analysis was performed using the surface-enhanced laser desorption and ionization (SELDI) protein chip technology (Ciphergen Biosystems, Fremont, CA, USA). Three microliters of 50 nM sodium carbonate buffer pH 8.0 were added to each spot on a PS10 surface-enhanced protein chip, prior to the addition of antibody. Anti-RANTES antibody MAB678 (R&D Systems) or mouse IgG isotype control (Sigma) was added at 2 μg per spot overnight in a 4°C humid chamber and allowed to bind covalently to the pre-activated surface. Active sites were blocked using 1 M ethanolamine in PBS pH 8.0 for 1 h at room temperature. Up to 400 μl of culture supernatants from PHA-stimulated expanded Vγ2Vδ2 T cells (10 min at 37°C, 107 cells ml−1) were then incubated with the chip overnight at 4°C. After stringent washing, the samples were crystallized using saturated α-cyano-4-hydroxycinnamic acid (Sigma) in 50% acetonitrile in HPLC grade water mixed with bovine ubiquitin (Sigma) as the internal calibrant. The protein chips were activated by laser light and the masses of ionized and released products were measured as the time of flight from chip to detector. This system was optimized for studying RANTES protein (25) and discriminates intact and N-terminally truncated forms of RANTES.
Results
Peripheral blood Vγ2Vδ2 T cells contain intracellular RANTES
Intracellular RANTES was detected by flow cytometry in detergent-permeabilized cells from PBMC. Our protocol used double staining with PE-labeled anti-Vδ2 antibody to the cell-surface TCR chain and FITC-labeled RANTES antibody. RANTES-positive cells showed a reproducible increase in the mean fluorescence intensity compared with isotype controls. An increase of ~3-fold in fluorescence is consistent with data from other laboratories (13) and with the manufacturer's information for these antibodies. Isotype control antibodies labeled with FITC or PE were used to control for non-specific binding. We also demonstrated that an excess of unlabeled anti-RANTES prevented specific labeling of Vγ2Vδ2 T cells (data not shown).
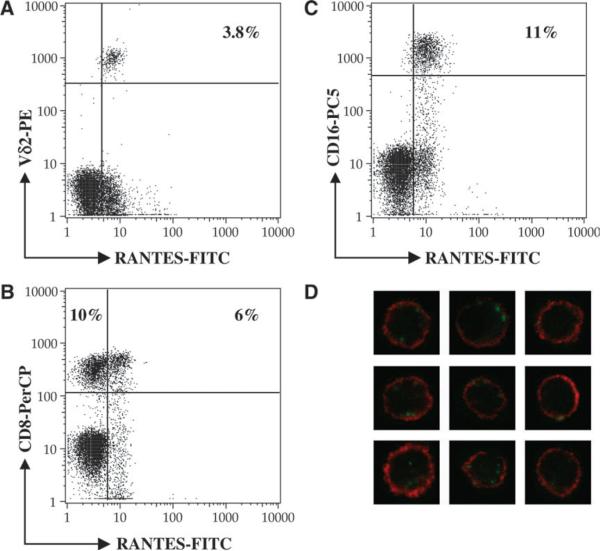
We routinely observed three main types of RANTES-positive cells in PBMC. They included Vγ2Vδ2 T cells, most of which were RANTES positive (Fig. 1A), approximately half of the CD8-positive T cells (Fig. 1B) likely representing the memory subset (23), and most of the CD16-positive NK cells (Fig. 1C). CD4-positive cells did not express RANTES (data not shown). Together, these populations accounted for the RANTES-positive cells in Ficoll-purified PBMC.
Fig. 1.
Peripheral Vδ2 T cells uniformly express cytoplasmic RANTES. Normal peripheral mononuclear cells were stained with antibodies to surface markers, then fixed and permeabilized by saponin treatment before staining with anti-RANTES FITC-labeled antibodies. Only the lymphocyte gate was analyzed in the panels A–C. RANTES-positive cells are present in three main subsets including Vδ2-positive T cells (A), some CD8-positive cells (B) and some CD16-positive cells (C). CD4-expressing cell were negative for RANTES (data not shown). For confocal microscopy (D), peripheral Vδ2 T cells were identified by anti-TCR Cy5-labeled antibody (red) and RANTES was detected in the cytoplasm using an FITC-labeled antibody (green).
We also studied the cytoplasmic distribution of RANTES using confocal microscopy. Peripheral blood Vγ2Vδ2 T cells were identified by anti-TCR Cy5-labeled antibody. RANTES was detected in the cytoplasm of permeabilized cells using an FITC-labeled antibody (Fig. 1D). The punctate staining pattern identified points of increased RANTES accumulation in the cytoplasm, similar to what was observed previously for RANTES in CD8+ T cells, where it was shown to occupy a novel secretory vesicle (24). We observed >100 Vγ2Vδ2-positive cells for each specimen and found that they were uniformly positive for RANTES staining.
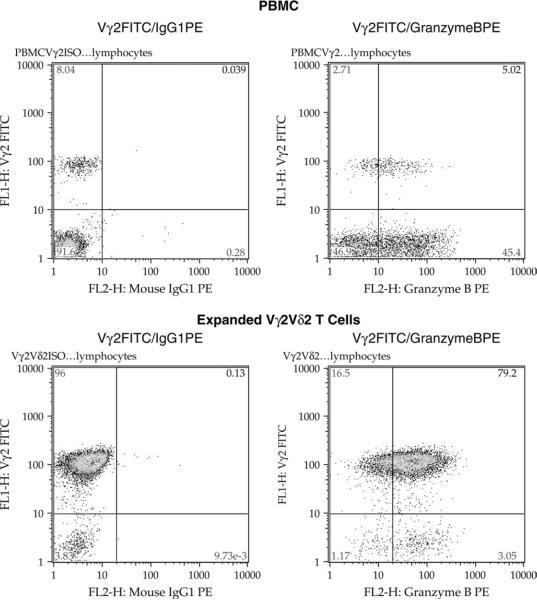
The peripheral blood Vγ2Vδ2 T cells were uniformly positive for RANTES in the cytoplasm, and the presence of RANTES did not require activation of these cells in vitro. We also know that Vγ2Vδ2+ T cells in peripheral blood are uniformly positive for expression of CD95 (Fas receptor) (15) and we confirmed these data in our laboratory (data not shown). Together, these data argue strongly that peripheral blood Vγ2Vδ2 T cells are all of the memory subset, a result that agrees with previous studies from other groups (26–28). We extended the characterization to include another cytoplasmic protein found in memory/effector T cells, which is Granzyme B. Staining of Vγ2Vδ2 T cells in peripheral blood or in expanded cell lines (described in the next section) showed a high degree of positive staining. In PBMC from one typical donor (Fig. 3), we found that 8% of CD3+ cells were in the Vγ2Vδ2 subset. Among these cells, ~5% were positive for cytoplasmic Granzyme B. Thus, the majority of Vγ2Vδ2 T cells in peripheral blood have the phenotype CD95+/cytoplasmic RANTES+/Granzyme B+. From other studies (29), we know that Vγ2Vδ2 are negative for surface expression of the lysosomal membrane protein CD107a, but that marker appears on the surface soon after antigen stimulation.
Fig. 3.
Primary and expanded Vγ2Vδ2 T cells contain cytoplasmic Granzyme B. PBMC (upper panels) or a Vγ2Vδ2 T cell line from the same donor (lower panels), were permeabilized and stained for cell-surface Vγ2 chain and for cytoplasmic Granzyme B. The left cytograms show the isotype control for each specimen, and the right panels show the frequency of cells positive for cell-surface Vγ2 and cytoplasmic Granzyme B. Approximately 65% of PBMC Vγ2Vδ2 T cells and 100% of expanded Vγ2Vδ2 T cells stained positively for Granzyme B expression.
Generating Vγ2Vδ2 T cell lines
Normal human PBMCs were stimulated with 15 μM IPP and cultured in the presence of 100 U ml−1 of recombinant IL-2. PBMCs cultured for 2 weeks after IPP stimulation were >85% positive for Vγ2Vδ2. During this time, the Vγ2Vδ2 T cell count increased ~500 times or by approximately nine cell divisions. Expanded Vγ2Vδ2 T cells were then rested for 8–10 days in medium with low (10 U ml−1) IL-2 to reduce proliferation and cytokine production to basal levels. The Vγ2Vδ2 T cells were not overgrown by other cell types during the rest period and remained >85% of the culture. Expanded and rested cells were sensitive to several stimuli, including IPP.
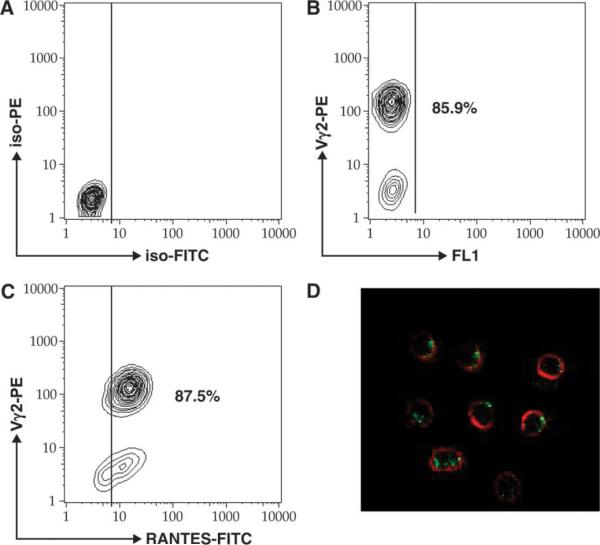
The Vγ2-positive cells were uniformly positive for RANTES by flow cytometry (Fig. 2C) or confocal microscopy (Fig. 2D). We noted that the content of RANTES was slightly higher in expanded T cells compared with peripheral cells. However, the pattern of staining and the morphology of expanded cells were similar to what we observed for primary Vγ2Vδ2 T cells. We also confirmed that expanded Vγ2Vδ2+ T cells were uniformly positive for cytoplasmic Granzyme B (Fig. 3). Overall, expanded Vγ2Vδ2 T cell lines had a phenotype that represented the majority of peripheral blood Vγ2Vδ2 T cells.
Fig. 2.
Expanded Vδ2 T cells uniformly express cytoplasmic RANTES. In vitro-expanded Vδ2 T cells were stained with PE-labeled antibodies to Vδ2 TCR chain, permeabilized, and then stained with anti-RANTES FITC-labeled antibodies. IgG isotype controls are presented a presented in panel A. More than 85% of cells were positive for Vδ2 (B) and double positive for Vδ2 and RANTES (C). A small population of RANTES single-positive cells were also detected (C). We confirmed the presence of cytoplasmic RANTES (green) in Vδ2-positive cells (red) by confocal microscopy.
Kinetics of RANTES release from Vγ2Vδ2 T cells after mitogenic stimulation
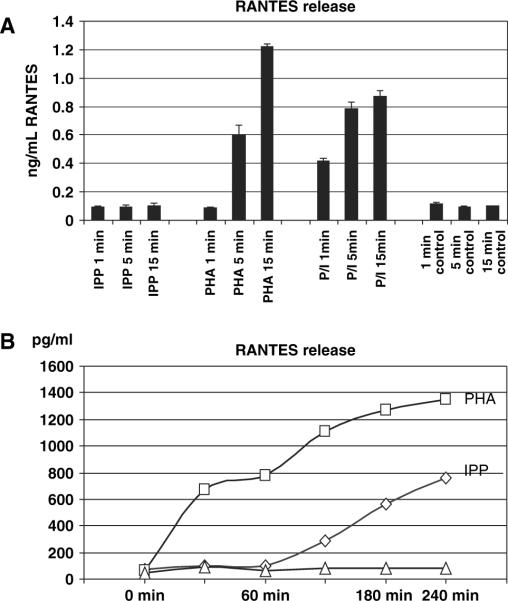
RANTES release after IPP stimulation was slow compared with other mitogenic stimuli. After 15 min of IPP treatment, there was only a low level of RANTES released to the medium, compared with very high levels with 5 min of PHA treatment or within 1 min of stimulation with PMA + ionomycin (Fig. 4A). RANTES release after IPP addition was considerably slower than in response to PHA, consistently demonstrating a 90- to 120-min gap between the time when IPP was added and the beginning of RANTES protein release (Fig. 4B). The rapid release (after PHA treatment) was >50% insensitive to cycloheximide treatment to block de novo RANTES synthesis, and was completely insensitive to actinomycin D that prevents mRNA accumulation (data not shown).
Fig. 4.
Rapid release of RANTES from Vγ2Vδ2 T cells. PHA or PMA/ionomycin stimulation induced rapid release of RANTES from expanded Vδ2 T cells (A). IPP treatment produced no soluble RANTES by 15 min (B), and the release was detected within 60–90 min after stimulation (B).
Regulation of RANTES protein release and mRNA synthesis in Vγ2Vδ2 T cells
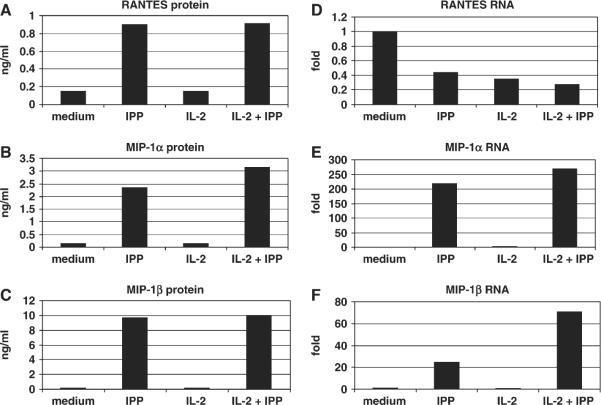
Previous studies using repertoire analysis (12) or functional assays showed that expanded cells behave similarly to primary Vγ2Vδ2 T cells and serve as a good model for biochemical studies. Within 4 h after re-stimulation with IPP, the rested Vγ2Vδ2 T cells released RANTES, MIP-1β (Fig. 5A and B) and MIP-1α (data not shown) into the culture medium. RANTES mRNA was detected in cell lysates from rested Vγ2Vδ2 T cell lines and further accumulation of RANTES mRNA was not induced by IPP stimulation (Fig. 5C). In contrast, these same resting cells contained barely detectable levels of MIP-1β and MIP-1α (data not shown) mRNAs and required de novo RNA synthesis before the initial chemokine release (Fig. 5D).
Fig. 5.
Regulation of RANTES mRNA and protein in Vγ2Vδ2 T cells. A Vγ2/Vδ2 T cell line was rested in medium supplemented with 10 U ml−1 IL-2 for 8–10 days, then washed and re-suspended in the same medium. Control cells were cultured in medium plus 10 U ml−1 IL-2. Secreted RANTES and MIP-1β levels were measured after 4 h of IPP treatment (A and B). The mRNA for RANTES was present at highest amounts prior to IPP addition and was reduced after stimulation (C). In contrast, MIP-1β mRNA was not present before IPP addition and required IPP for maximal expression (D). Cell stimulation decreased the levels of cytoplasmic RANTES and the addition of cycloheximide had little effect (E), compared with the increase in cytoplasmic MIP-1β that was sensitive to cycloheximide inhibition (F). The experiment was repeated three times using expanded Vγ2Vδ2 T cells from three different donors. The pattern of responses was identical for each Vγ2Vδ2 T cell line, but the absolute levels of chemokines varied for each donor. Accordingly, a representative experiment is shown here.
RANTES was already present in cells prior to IPP re-stimulation and was released mostly independently of de novo translation (Fig. 5E). In contrast, MIP-1β accumulated in the cells after stimulation and this accumulation was abrogated by cycloheximide (Fig. 5F). As expected, the translation inhibitor actinomycin D did not affect RANTES secretion by Vγ2Vδ2 T cells, but did block MIP-1α and MIP-1β production (data not shown).
Overall, cytoplasmic RANTES protein levels declined during the first few hours after re-stimulation as might be expected for products that were stored in resting cells (Fig. 5E). Activation of Vγ2Vδ2 T cells by PMA/ionomycin, PHA or anti-CD3 antibodies resulted in a similar pattern of RANTES release. Cycloheximide partially inhibited RANTES release, and actinomycin D was without effect, showing that RANTES secretion is due mostly to the release of the preformed protein and partly to translation of pre-existing mRNA.
Cytoplasmic RANTES is intact in Vγ2Vδ2 T cells
Vγ2Vδ2 T cells were shown to express high levels of CCR5, a receptor for RANTES. We wanted to rule out the possibility that cytoplasmic RANTES accumulation might be the result of receptor-mediated internalization of RANTES protein released previously into the medium during cell activation and expansion. Knowing that RANTES protein is processed rapidly after exposure to serum proteases and that this exposure leads to loss of N-terminal protein sequences (30, 31), we used SELDI technology to characterize RANTES in Vγ2Vδ2 T cells. We detected only the full-length form of RANTES with an intact amino terminus (Fig. 6). It is unlikely that RANTES present in Vγ2Vδ2 T cells was taken up from the medium. This result argues strongly that cytoplasmic RANTES was synthesized and stored in Vγ2Vδ2 T cells and was ready to be released when cells were re-stimulated.
Fig. 6.
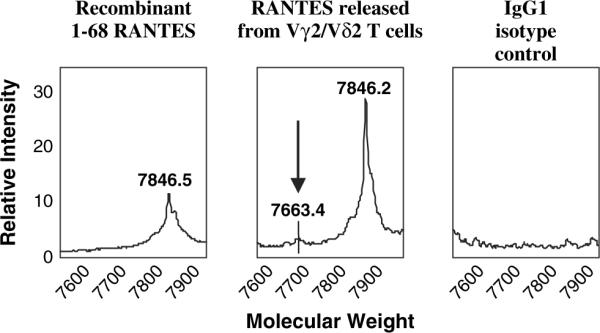
Secreted RANTES is a full-length protein. An anti-RANTES antibody was used to capture a synthetic, full-length RANTES (A) or chemokine released from Vγ2Vδ2 T cells after PHA stimulation (B). The protein masses were nearly identical, and were clearly distinct from the mass (7663.4) expected for truncated RANTES (indicated in B). A control antibody (C) did not capture protein in this mass range from the Vγ2Vδ2 T cell supernatant.
Discussion
Primary, peripheral blood Vγ2Vδ2 T cells were uniformly positive for cytoplasmic RANTES protein and contained RANTES mRNA. Circulating Vc2Vd2 T cells can be divided into a very small naive subset, a T effector memory CD45RA+ subset (IFN-γ negative), a T effector memory h (high for IFN-γ) and a T central memory (26). Of these, the T effector memory h are CD45RA− and CD27−. Expanded Vγ2Vδ2 T cell lines are uniformly CD45RA− and CD27−, thus they resemble the T effector memory h population as described (26). The expanded Vγ2Vδ2 T cell lines do not represent all subsets of circulating cells, but include the major phenotype that is responsive to phosphoantigen stimulation. We observed positive staining for cytoplasmic RANTES in virtually all Vγ2Vδ2 T cells in peripheral blood indicating that chemokine accumulation was a feature of all memory subsets.
RANTES release from expanded cells occurred independently of de novo transcription or translation and was distinct from the regulation of MIP-1α or MIP-1β expression. Cytoplasmic RANTES was not the result of absorbing RANTES from the culture medium, as there was no evidence for amino-terminus proteolysis of RANTES that would be expected after even a brief exposure to serum (25). The regulation of RANTES secretion was most similar to what had been observed previously for memory CD8+ T cells (22–24). Despite the identification of small numbers of naive cells using CD45RA and CD27 cell-surface markers (27), the population of Vγ2Vδ2 T cells in blood are mainly memory cells (15, 26, 27), and the majority express cytoplasmic RANTES.
The mature repertoire of Vγ2Vδ2 T cells is formed by peripheral selection and expansion of the Vγ2-Jγ1.2Vδ2 positive Tcell subset (12). Selection for this TCR also produces a population poised for responding to phosphoantigens (32). Vγ2Vδ2 T cells seem to recognize antigen directly, in an Ig-like manner (10, 33), but there are no co-crystallization studies of Vγ2Vδ2 TCR and phosphoantigen and the details of antigen recognition remain unclear. We observed a significant delay in the Vγ2Vδ2 Tcell response to IPP compared with other stimuli. This observation suggests that an intermediate event such as IPP forming a complex with other macromolecules, or the induction of new presenting antigens, may be required for effective IPP stimulation. It is also possible that model phosphoantigens used in vitro do not adequately represent the endogenous antigens used for selecting the mature repertoire even if they stimulate proliferation or cytokine release.
The in vitro-expanded Vγ2Vδ2 T cells provide a model for cells that expand in vivo after antigen stimulation, but have not yet declined back to their original population. In the examples of Brucella abortus infection (34, 35) or plague (36), >30% of circulating T cells will have the Vγ2Vδ2 phenotype, especially during convalescence. This is a substantial expansion for a subset primed to respond to phosphoantigens, and represents one of the largest antigen-specific memory subsets ever documented. With so many T cells capable of responding to a single stimulus, the Vγ2Vδ2 T cells will exert significant effects on regulation of immune responses and RANTES may be an important mediator of these functions. In human beings, RANTES promotes type 1 immune responses by preferentially attracting CCR5-positive Th1 cells (37, 38), acts as an antigen-independent activator of T cells (39, 40) and stimulates the cytolytic function of CD8 T cells (41). RANTES is a powerful chemotactic agent for professional antigen-presenting cells such as dendritic cells (42, 43), and γδ T cells may promote their recruitment and maturation partly through cytokine secretion.
The Vγ2Vδ2 T cell subset is often described as a population linking innate and adaptive immunity. These cells are found uniquely among human and non-human primates (2, 44) and the lack of small animal models has impeded their study. The normal repertoire is highly skewed in healthy adults, by selective amplification of the Vγ2-Jγ2Vδ2 rearrangement (4), and this creates a population where most cells are primed for rapid responses to a limited number of antigens. The capacity for rapid and uniform responses, especially considering that Vγ2-Jγ2Vδ2 cells recognize antigen in the absence of MHC restriction, accounts for including these cells under the broad category of innate immunity. However, they are indeed the product of a lineage with rearranging TCRs and antigen selection drives repertoire maturation; these are characteristics of cells in the acquired arm of immunity. However we view the Vγ2-Jγ2Vδ2 T cell subset, it is important to remember that cells carrying just this TCR rearrangement comprise 1–5% of circulating CD3+ T cells in healthy adult human beings, and because of their uniform responses to a limited number of antigens, provide for very rapid and potent responses at the site of bacterial or viral infections. By secreting chemokine including RANTES, these rapid responding cells will promote the recruitment of other immune cell types to accelerate and shape the subsequent response to infection.
Acknowledgements
This work was supported by PHS grant AI51212 and CA113261 (C.D.P.)
Abbreviations
- IPP
isopentenyl pyrophosphate
- MIP
macrophage inflammatory protein
- PMA
phorbol myristate acetate
- RANTES
regulated upon activation normal T cell expressed and secreted
- RT
reverse transcription
- SELDI
surface-enhanced laser desorption and ionization
References
- 1.Tanaka Y, Sano S, Nieves E, et al. Nonpeptide ligands for human gamma delta T cells. Proc. Natl Acad. Sci. USA. 1994;91:8175. doi: 10.1073/pnas.91.17.8175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tanaka Y, Morita CT, Nieves E, Brenner MB, Bloom BR. Natural and synthetic non-peptide antigens recognized by human gamma delta T cells. Nature. 1995;375:155. doi: 10.1038/375155a0. [DOI] [PubMed] [Google Scholar]
- 3.Bukowski JF, Morita CT, Brenner MB. Human gamma delta T cells recognize alkylamines derived from microbes, edible plants, and tea: implications for innate immunity. Immunity. 1999;11:57. doi: 10.1016/s1074-7613(00)80081-3. [DOI] [PubMed] [Google Scholar]
- 4.Spits H, Lanier LL, Phillips JH. Development of human T and natural killer cells. Blood. 1995;85:2654. [PubMed] [Google Scholar]
- 5.Davis MM, Bjorkman PJ. T-cell antigen receptor genes and T-cell recognition. Nature. 1988;334:395. doi: 10.1038/334395a0. [DOI] [PubMed] [Google Scholar]
- 6.Carding SR, Egan PJ. Gammadelta Tcells: functional plasticity and heterogeneity. Nat. Rev. Immunol. 2002;2:336. doi: 10.1038/nri797. [DOI] [PubMed] [Google Scholar]
- 7.Groh V, Porcelli S, Fabbi M, et al. Human lymphocytes bearing T cell receptor gamma/delta are phenotypically diverse and evenly distributed throughout the lymphoid system. J. Exp. Med. 1989;169:1277. doi: 10.1084/jem.169.4.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parker CM, Groh V, Band H, et al. Evidence for extrathymic changes in the T cell receptor gamma/delta repertoire. J. Exp. Med. 1990;171:1597. doi: 10.1084/jem.171.5.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kunzmann V, Bauer E, Feurle J, Weissinger F, Tony HP, Wilhelm M. Stimulation of gammadelta T cells by aminobisphosphonates and induction of antiplasma cell activity in multiple myeloma. Blood. 2000;96:384. [PubMed] [Google Scholar]
- 10.Allison TJ, Garboczi DN. Structure of gammadelta T cell receptors and their recognition of non-peptide antigens. Mol. Immunol. 2002;38:1051. doi: 10.1016/s0161-5890(02)00034-2. [DOI] [PubMed] [Google Scholar]
- 11.Allison TJ, Winter CC, Fournie JJ, Bonneville M, Garboczi DN. Structure of a human gammadelta T-cell antigen receptor. Nature. 2001;411:820. doi: 10.1038/35081115. [DOI] [PubMed] [Google Scholar]
- 12.Evans PS, Enders PJ, Yin C, Ruckwardt TJ, Malkovsky M, Pauza CD. In vitro stimulation with a non-peptidic alkylphosphate expands cells expressing Vgamma2-Jgamma1.2/ Vdelta2 T-cell receptors. Immunology. 2001;104:19. doi: 10.1046/j.0019-2805.2001.01282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Groh V, Rhinehart R, Secrist H, Bauer S, Grabstein KH, Spies T. Broad tumor-associated expression and recognition by tumor-derived gamma delta T cells of MICA and MICB. Proc. Natl Acad. Sci. USA. 1999;96:6879. doi: 10.1073/pnas.96.12.6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moris A, Rothenfusser S, Meuer E, Hangretinger R, Fisch P. Role of gammadelta T cells in tumor immunity and their control by NK receptors. Microbes Infect. 1999;1:227. doi: 10.1016/s1286-4579(99)80038-0. [DOI] [PubMed] [Google Scholar]
- 15.De Rosa SC, Andrus JP, Perfetto SP, Mantovani JJ, Herzenberg LA, Roederer M. Ontogeny of gamma delta T cells in humans. J. Immunol. 2004;172:1637. doi: 10.4049/jimmunol.172.3.1637. [DOI] [PubMed] [Google Scholar]
- 16.Pena SV, Hanson DA, Carr BA, Goralski TJ, Krensky AM. Processing, subcellular localization, and function of 519 (granulysin), a human late T cell activation molecule with homology to small, lytic, granule proteins. J. Immunol. 1997;158:2680. [PubMed] [Google Scholar]
- 17.Podack ER, Kupfer A. T-cell effector functions: mechanisms for delivery of cytotoxicity and help. Annu. Rev. Cell Biol. 1991;7:479. doi: 10.1146/annurev.cb.07.110191.002403. [DOI] [PubMed] [Google Scholar]
- 18.Ortiz BD, Nelson PJ, Krensky AM. Switching gears during T-cell maturation: RANTES and late transcription. Immunol. Today. 1997;18:468. doi: 10.1016/s0167-5699(97)01128-6. [DOI] [PubMed] [Google Scholar]
- 19.Song A, Nikolcheva T, Krensky AM. Transcriptional regulation of RANTES expression in T lymphocytes. Immunol. Rev. 2000;177:236. doi: 10.1034/j.1600-065x.2000.17610.x. [DOI] [PubMed] [Google Scholar]
- 20.Song A, Chen YF, Thamatrakoln K, Storm TA, Krensky AM. RFLAT-1: a new zinc finger transcription factor that activates RANTES gene expression in T lymphocytes. Immunity. 1999;10:93. doi: 10.1016/s1074-7613(00)80010-2. [DOI] [PubMed] [Google Scholar]
- 21.Nikolcheva T, Pyronnet S, Chou SY, et al. A translational rheostat for RFLAT-1 regulates RANTES expression in T lymphocytes. J. Clin. Invest. 2002;110:119. doi: 10.1172/JCI15336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Swanson BJ, Murakami M, Mitchell TC, Kappler J, Marrack P. RANTES production by memory phenotype T cells is controlled by a posttranscriptional, TCR-dependent process. Immunity. 2002;17:605. doi: 10.1016/s1074-7613(02)00456-9. [DOI] [PubMed] [Google Scholar]
- 23.Walzer T, Marcais A, Saltel F, Bella C, Jurdic P, Marvel J. Cutting edge: immediate RANTES secretion by resting memory CD8 T cells following antigenic stimulation. J. Immunol. 2003;170:1615. doi: 10.4049/jimmunol.170.4.1615. [DOI] [PubMed] [Google Scholar]
- 24.Catalfamo M, Karpova T, McNally J, et al. Human CD8+ T cells store RANTES in a unique secretory compartment and release it rapidly after TcR stimulation. Immunity. 2004;20:219. doi: 10.1016/s1074-7613(04)00027-5. [DOI] [PubMed] [Google Scholar]
- 25.Lim JK, Burns JM, Lu W, DeVico AL. Multiple pathways of amino terminal processing produce two truncated variants of RANTES/CCL5. J. Leukoc. Biol. 2005;78:442. doi: 10.1189/jlb.0305161. [DOI] [PubMed] [Google Scholar]
- 26.Angelini DF, Borsellino G, Poupot M, et al. FcgammaRIII-discriminates between 2 subsets of Vgamma9Vdelta2 effector cells with different responses and activation pathways. Blood. 2004;104:1801. doi: 10.1182/blood-2004-01-0331. [DOI] [PubMed] [Google Scholar]
- 27.Dieli F, Poccia F, Lipp M, et al. Differentiation of effector/ memory Vdelta2 T cells and migratory routes in lymph nodes or inflammatory sites. J. Exp. Med. 2003;198:391. doi: 10.1084/jem.20030235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Rosa SC, Mitra DK, Watanabe N, Herzenberg LA, Roederer M. Vdelta1 and Vdelta2 gammadelta T cells express distinct surface markers and might be developmentally distinct lineages. J. Leukoc. Biol. 2001;70:518. [PubMed] [Google Scholar]
- 29.Deetz CO, Hebbeler AM, Propp NA, Cairo C, Tikhonov I, Pauza CD. Interferon-gamma secretion by human Vγ2Vδ2 T cells after stimulation with antibody against the T cell receptor plus the Toll-like receptor 2 agonist, Pam3Cys. Infect. Immun. 2006 doi: 10.1128/IAI.00088-06. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boykins RA, Oravecz T, Unsworth E, Syin C. Chemical synthesis and characterization of chemokine RANTES and its analogues. Cytokine. 1999;11:8. doi: 10.1006/cyto.1998.0391. [DOI] [PubMed] [Google Scholar]
- 31.Proost P, Menten P, Struyf S, Schutyser E, De Meester I, Van Damme J. Cleavage by CD26/dipeptidyl peptidase IV converts the chemokine LD78beta into a most efficient monocyte attractant and CCR1 agonist. Blood. 2000;96:1674. [PubMed] [Google Scholar]
- 32.Miyagawa F, Tanaka Y, Yamashita S, et al. Essential contribution of germline-encoded lysine residues in Jgamma1.2 segment to the recognition of nonpeptide antigens by human gammadelta T cells. J. Immunol. 2001;167:6773. doi: 10.4049/jimmunol.167.12.6773. [DOI] [PubMed] [Google Scholar]
- 33.Bukowski JF, Morita CT, Band H, Brenner MB. Crucial role of TCR gamma chain junctional region in prenyl pyrophosphate antigen recognition by gamma delta T cells. J. Immunol. 1998;161:286. [PubMed] [Google Scholar]
- 34.Bertotto A, Gerli R, Spinozzi F, et al. Lymphocytes bearing the gamma delta T cell receptor in acute Brucella melitensis infection. Eur. J. Immunol. 1993;23 doi: 10.1002/eji.1830230531. [DOI] [PubMed] [Google Scholar]
- 35.Ottones F, Dornand J, Naroeni A, Liautard JP, Favero J. V gamma 9V delta 2 T cells impair intracellular multiplication of Brucella suis in autologous monocytes through soluble factor release and contact-dependent cytotoxic effect. J. Immunol. 2000;165:7133. doi: 10.4049/jimmunol.165.12.7133. [DOI] [PubMed] [Google Scholar]
- 36.Gossman W, Oldfield E. Quantitative structure–activity relations for gammadelta T cell activation by phosphoantigens. J. Med. Chem. 2002;45:4868. doi: 10.1021/jm020224n. [DOI] [PubMed] [Google Scholar]
- 37.Loetscher P, Uguccioni M, Bordoli L, et al. CCR5 is characteristic of Th1 lymphocytes. Nature. 1998;391:344. doi: 10.1038/34814. [DOI] [PubMed] [Google Scholar]
- 38.Bonecchi R, Bianchi G, Bordignon PP, et al. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J. Exp. Med. 1998;187:129. doi: 10.1084/jem.187.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bacon KB, Szabo MC, Yssel H, Bolen JB, Schall TJ. RANTES induces tyrosine kinase activity of stably complexed p125FAK and ZAP-70 in human T cells. J. Exp. Med. 1996;184:873. doi: 10.1084/jem.184.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bacon KB, Premack BA, Gardner P, Schall TJ. Activation of dual T cell signaling pathways by the chemokine RANTES. Science. 1995;269:1727. doi: 10.1126/science.7569902. [DOI] [PubMed] [Google Scholar]
- 41.Appay V, Rowland-Jones SL. RANTES: a versatile and controversial chemokine. Trends Immunol. 2001;22:83. doi: 10.1016/s1471-4906(00)01812-3. [DOI] [PubMed] [Google Scholar]
- 42.Caux C, Ait-Yahia S, Chemin K, et al. Dendritic cell biology and regulation of dendritic cell trafficking by chemokines. Springer Semin. Immunopathol. 2000;22:345. doi: 10.1007/s002810000053. [DOI] [PubMed] [Google Scholar]
- 43.Sozzani S, Allavena P, Vecchi A, Mantovani A. Chemokines and dendritic cell traffic. J. Clin. Immunol. 2000;20:151. doi: 10.1023/a:1006659211340. [DOI] [PubMed] [Google Scholar]
- 44.Cairo C, Propp N, Hebbeler AM, Colizzi V, Pauza CD. The Vgamma2/Vdelta2 T-cell repertoire in Macaca fascicularis: functional responses to phosphoantigen stimulation by the Vgamma2/Jgamma1.2 subset. Immunology. 2005;115:197. doi: 10.1111/j.1365-2567.2005.02153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]