Abstract
Heterotopic hamster hearts transplanted to unmodified LEW rats underwent humoral rejection in 3 days. Survival was prolonged to a median of 4 days with 2 mg/kg/day FK506. As monotherapy, 15 mg/kg/day cyclophosphamide greatly prolonged graft survival—far more than could be accomplished with RS-61443, brequinar (BQR), mizoribine, methotrexate, or deoxyspergualin. However, when FK506 treatment, which was ineffective alone, was combined with a short induction course (14 or 30 days) of subtherapeutic BQR, RS-61443, or cyclophosphamide, routine survival of heart xenografts was possible for as long as the daily FK506 was continued. In addition, a single large dose of 80 mg/kg cyclophosphamide 10 days preoperatively allowed routine cardiac xenograft survival under FK506. The ability of these antimetabolites to unmask the therapeutic potential of FK506 correlated, although imperfectly, with the prevention of rises of preformed heterospecific cytotoxic antibodies immediately postoperatively. As an adjunct to FK506, azathioprine was of marginal value, whereas mizoribine, methotrexate, and deoxyspergualin (DSPG) were of intermediate efficacy.
After orthotopic hepatic xenotransplantation, the perioperative survival of the liver with its well-known resistance to antibodies was less dependent than the heart on the antimetabolite component of the combined drug therapy, but the unsatisfactory results with monotherapy of FK506, BQR, RS-61443, or cyclophosphamide were changed to routine success by combining continuous FK506 with a short course of any of the other drugs. Thus, by breaking down the antibody barrier to xenotransplantation with these so-called antiproliferative drugs, it has been possible with FK506 to transplant heart and liver xenografts with consistent long-term survival of healthy recipients.
FK506, which prevents T cell activation and cytokine secretion by inhibiting the transcription of early genes (1), has permitted improvements in the clinical transplantation of a variety of allografts (2, 3). However, the drug has a minimal effect on B cells and the antibody response, and does not prevent humoral allograft rejection in the presensitized recipient (4, 5) or the rejection of xenografts by heterospecific antibodies (6, 7).
In a hamster-to-rat xenograft model, we have combined FK506 with drugs that subvert the action of key enzymes of de novo purine and pyrimidine nucleotide synthesis, thereby inhibiting the DNA synthesis required for expansion of activated T and/or B cell clones. Brequinar (BQR),* an agent the immunosuppressive qualities of which were studied in rats by Cramer et al. (8) and RS-61443, a mycophenolic acid derivative showing considerable preclinical and clinical promise (9–12), where the new prototype drugs selected. However toward the end of the study, established anti-DNA drugs also were evaluated as adjuvants, including azathioprine, cyclophosphamide, and methotrexate; of these cyclophosphamide, was found to be spectacularly effective. Finally, FK506 was combined with deoxyspergualin (DSPG), a putative antimacrophage/monocyte drug that also has been said to suppress B cell activation and maturation (13).
MATERIALS AND METHODS
Animals
Inbred male Lewis rats (LEW, RT11) weighing 200–300 g were recipients, and Golden Syrian hamsters weighing 100–150 g were donors (Charles River Lab., Wilmington, MA).
Surgical procedure
The hamster-to-rat xenograft models were those characterized previously by Valdivia and Monden (14–17). Operations were under methoxyflurane anesthesia. Heterotopic heart transplantation was performed by anastomosing the donor aorta and pulmonary artery of the xenograft to the recipient infrarenal abdominal aorta and vena cava, respectively. The cardiac grafts were palpated daily for the first month and every other day thereafter. Rejection was diagnosed by the cessation of the heartbeat, and confirmed by direct inspection at reoperation and by histopathology. There was no discard rate of failed experiments.
Liver transplantation after graft cholecystectomy was performed with Kamada’s cuff technique for the portal and infrahepatic vena cava anastomoses, revascularizing the portal vein only (14–17). Rejection was diagnosed by the death of the recipients, followed by histopathological examination. Animals dying <3 days after surgery were excluded from the study (less than 5% of the total). This small rate of discard was from technical misadventures.
Immunosuppressive agents
FK506
FK506 (a gift of the Fujisawa Pharmaceutical Co., Osaka, Japan) was given i.m. after suspending it in normal saline. The dose for heart recipients was 2.0 mg/kg/day on days 0 to 5 followed by 1.0 mg/kg/day on days 6 to 30. For liver xenotransplantation, 1.0 mg/kg/day was used for the first 30 days. Both liver and heart recipients were given alternate-day injections of 0.5 mg/kg FK506 from days 31 to 100.
Antiproliferative drugs
All of these drugs were prepared daily and administered by gastric instillation. BQR (donated by Du Pont Medical Products, Wilmington, DE) was suspended in distilled water, and adjusted with NaOH to pH 9.0. RS-61443 (donated by Syntex Inc, Palo Alto, CA) was used in a special vehicle that contained 0.5% carboxymethyl-cellulose, 0.4% polysorbate 80, 0.9% benzyl alcohol, and 0.9% sodium chloride in distilled water. Mizoribine (Bredinin, donated by Asahi Chemical Industry Co. Ltd., Tokyo, Japan) was suspended in distilled water. Azathioprine, cyclophosphamide, and methotrexate were bought from commercial pharmacies and suspended in distilled water.
Deoxyspergualine
This gift from Nippon Kayaku Co. Ltd, Tokyo, (later Bristol-Myers Squibb) was suspended in sterile water and administered intramuscularly.
Recipient humoral antibodies
Lymphocytotoxic antibodies
Complement-fixing lymphocytotoxic antibodies were measured in the recipient rat sera by Terasaki’s method (18), using target lymphocytes prepared from hamster cervical lymph nodes. After washing and isolation, the cells were resuspended at a concentration of 4×106/ml. Duplicate samples of 1 µl of various dilutions of serum samples and one µl of lymphocyte suspensions were placed into 72-well tissue-typing trays (Robbins Scientific, Sunnyvale, CA). After incubation for 60 min at room temperature, 5 µl of baby rabbit complement diluted 10 times (Cedarlane Laboratories Limited, Hornby, Ontario) was added to each well with reincubation for another 30 min at room temperature. Then 5 µl of 0.4% trypan blue and 15 µl of barbital buffer were added to each well for staining and fixation. The cell lysis ranged from 0 (undetectable) to 100%. The lymphocytotoxic antibody titer was defined as the highest serum dilution with more than 51% cell lysis. Normal hamster serum served as a negative control.
Indirect immunofluorescence
Snap-frozen normal hamster liver was the target tissue. It was cut into 2-µ sections, incubated with a protein blocking agent (Lipshaw, Pittsburgh, PA), and then incubated again with sera obtained from normal LEW rats (to detect preformed antibodies) or LEW recipients of hamster organs (to detect the antibody response). This was followed by goat antirat IgG or IgM to detect the localization, class, and intensity of the heterospecific antibodies. The location and intensity of the immunoglobulin deposits were determined without knowledge of the treatment regimen.
Statistical analyses
The graft survival was so predictable in unmodified rat recipients (3 days for hearts, 7 days for livers) that all survival extension beyond one day was statistically significant with the Mann-Whitney U test. Median survival figures were calculated but survival of individual animals was given in all experimental groups.
RESULTS
Heart xenograft survival
Single drug therapy
When used as a single agent in a dose of 2 mg/kg/day, FK506 increased graft survival by only one day, significantly less prolongation than could be accomplished with BQR, RS-61443, cyclophosphamide, or methotrexate (Table 1). The therapeutic window of BQR was narrow, the drug being almost ineffective at 3 mg/kg/day and clinically toxic if continued at 4.5 mg. The optimal dosing proved to be 4.0 mg/kg/day for 3 days and 3 mg/kg/day thereafter. The dose latitude with RS-61443 appeared to be somewhat greater (Table 1).
TABLE 1.
Hamster heart graft survival in LEW rat recipients treated with FK506, BQR, RS-61443, cyclophosphamide, methotrexate, mizoribine, and deoxyspergualin as single agentsa
| Group | Agent | Dose (mg/kg/ day) |
Duration (days) |
n | Survival (days) | Median survival (days) |
|---|---|---|---|---|---|---|
| 1 | — | — | — | 6 | 3, 3, 3, 3, 3, 3 | 3.0 |
| 2 | FK506 | 2.0 | 0 → | 6 | 4, 4, 4, 4, 5, 5 | 4.0 |
| 3 | BQR | 3.0 | −1 → | 4 | 5, 5, 6, 7 | 5.5 |
| 4 | BQR | 4.0 × 3 → 3.0 | −1 → | 6 | 5, 8, 8, 11, 12, >100 | 9.5 |
| 5 | BQR | 4.5 | −1 → | 4 | 7,b 9,b >75, >75 | >42.0 |
| 6 | RS-61443 | 20 | −1 → | 4 | 4, 5, 5, 6 | 5.0 |
| 7 | RS-61443 | 40 | −1 → | 4 | 6, 7, 7, 8 | 7.0 |
| 8 | RS-61443 | 60 × 3 → 40 | −1 → | 6 | 6, 8,b 9, 9, 20, 23 | 9.0 |
| 9 | RS-61443 | 60 | −1 → | 4 | 9, 10, 13, 15 | 11.5 |
| 10 | Cyclophosphamide | 7.5 | −1 → | 5 | 7, 8, 8, 8, 9 | 8.0 |
| 11 | Cyclophosphamide | 10.0 | −1 → | 5 | 14, >15, >15, >15, >15 | >15.0 |
| 12 | Cyclophosphamide | 15.0 | −1 → | 5 | >28, >28, >36, >36, >36 | >36.0 |
| 13 | Methotrexate | 0.5 | −1 → | 5 | 10, 11,b 13,b 14,b >16 | 13.0 |
| 14 | Mizoribine | 7.5 | −1 → | 5 | 4, 4, 4, 4, 5 | 4.0 |
| 15 | DSPG | 5.0 | −1 → | 3 | 4, 4, 4 | 4.0 |
BQR, RS-61443, Cyclophosphamide, Methotrexate, Mizoribine and Deoxyspergualin were administered from one day before transplantation and continued until graft rejection.
Animal died with functioning graft.
Cyclophosphamide was the only drug that permitted consistently successful heart transplantation. All of the animals given 10 or 15 mg/kg/day cyclophosphamide are alive. Although follow-up is short because the experiments were done last, the superiority of cyclophosphamide to any other single agent was evident (Table 1).
Combined therapy
Added to baseline therapy of 2 mg/kg/day FK506 that was ineffectual alone, BQR and RS-61443 both permitted a spectacular improvement in heart xenograft survival. As when it was used alone, the BQR had a narrow therapeutic window with a single safe dose. The RS-61443 could be used over a wide dose range in combination with FK506 with virtually 100% assurance of long survival (Table 2, groups 7–10), whether it was given for 14 or 30 days.
TABLE 2.
Hamster heart graft survival in LEW recipients treated with combination treatment with FK506a
| Group | Drug combined with FK506 |
Dose (mg/kg/ day) |
Duration (days) |
n | Survival (days) | Median survival (days) |
|---|---|---|---|---|---|---|
| 1 | BQR | 3.0 | −1 → 30 | 6 | 4, 5, 5, 5, 7, >100 | 5.0 |
| 2 | BQR | 4.0 × 3 → 3.0 | −1 → 13 | 5 | >100, >100, >100, >100, >100 | >100 |
| 3 | BQR | 4.0 × 3 → 3.0 | −1 → 30 | 6 | 89, >100, >100, >100, >100, >100 | >100 |
| 4b | BQR | 4.0 × 3 → 3.0 | −1 → 30 | 6 | 5, 22, 24, >100, >100, >100 | >62.0 |
| 5 | BQR | 4.5 | −1 → 30 | 4 | 3,c 9,c >75, >75 | >42.0 |
| 6 | RS-61443 | 10 | −1 → 30 | 6 | 4, 5, 5, 5, >100, >100 | 5.0 |
| 7 | RS-61443 | 20 | −1 → 13 | 6 | 19, >78, >78, >78, >90, >90 | >78.0 |
| 8 | RS-61443 | 20 | −1 → 30 | 6 | >100, >100, >100, >100, >100, >100 | >100.0 |
| 9 | RS-61443 | 30 | −1 → 30 | 6 | >91, >91, >100, >100, >100, >100 | >100.0 |
| 10 | RS-61443 | 40 | −1 → 30 | 6 | >78, >92,d >92,d >92, >100, >100 | >92.0 |
| 11 | Cyclophosphamide | 5 | −1 → 30 | 5 | 5, 5, 5, 5, 6 | 5.0 |
| 12 | Cyclophosphamide | 7.5 | −1 → 7 | 5 | >35, >35, >35, >35, >35 | >35.0 |
| 13 | Cyclophosphamide | 7.5 | −1 → 13 | 5 | >49, >49, >49, >50, >50 | >49.0 |
| 14 | Cyclophosphamide | 7.5 | −1 → 30 | 5 | >64, >64, >64, >64, >64 | >64.0 |
| 15 | Cyclophosphamide | 10 | −1 → 13 | 6 | >50, >60, >63, >63, >64, >64 | >63.0 |
| 16 | Cyclophosphamide | 15 | −1 → 9 | 4 | 29, >43, >47, >47 | >45.0 |
| 17 | Methotrexate | 1.0 | −1 → 13 | 3 | 7,c 7,c 9c | 7.0 |
| 18 | Methotrexate | 1.0 | −1 → 5 | 3 | 9, c 10, c >40 | 10.0 |
| 19 | Methotrexate | 1.0 | −1 → 3 | 7 | 8, 10, 10, >19, >19, >49, >49 | >19.0 |
| 20 | Methotrexate | 1.0 | −1 → 1 | 2 | 8, 9 | 8.5 |
| 21 | Methotrexate | 0.5 | −1 → 9 | 4 | 10,c 13,c 43, >49 | 28.0 |
| 22 | Methotrexate | 0.25 | −1 → 13 | 3 | 4, 4, 6 | 4.0 |
| 23 | Mizoribine | 5.0 | −1 → 30 | 3 | 5, 5, 7 | 5.0 |
| 24 | Mizoribine | 7.5 × 3 → 5.0 | −1 → 30 | 6 | 3,c 5, 5, >77, >77, >100 | >41.0 |
| 25 | Azathioprine | 15 | −1 → 30 | 2 | 4, 5 | 4.5 |
| 26 | Azathioprine | 45 | −1 → 30 | 3 | 5, 5, 23c | 5.0 |
| 27 | Azathioprine | 60 | −1 → 30 | 4 | 5, 8,c 17,c 19c | 12.5 |
| 28 | DSPG | 2.5 | −1 → 30 | 4 | 4, 5, 5, 5 | 5.0 |
| 29 | DSPG | 5.0 × 3 → 2.5 | −1 → 30 | 6 | 23, 38, >70, >70, >70, >76 | >70.0 |
| 30 | DSPG | 5.0 | −1 → 30 | 4 | 9, 24,c 32,c >76 | 28.0 |
| 31 | Cyclophosphamide | 80.0 | −10 | 5 | >71, >71, >71, >79, >79 | >71.0 |
| (i.p.) |
FK506 was administered intramuscularly at a dose of 2.0 mg/kg/day for the first 6 days (days 0 to 5), then 1.0 mg/kg/day (days 6 to 30) and continued at 0.5 mg/kg/day every other day (days 31 to 100).
FK506 was used at 1.0 mg/kg/day for the first 30 days (0 to 30) and continued at 0.5 mg/kg/day every other day (days 31 to 100).
Animal died with functioning graft.
RS-61443 treatment was discontinued 25 days after transplantation because of the appearance of diarrhea and body weight loss.
Duration of follow-up with most of the other antimetabolite drugs has been shorter. However, the early success rate with cyclophosphamide induction 7 to 30 days with doses of 7.5, 10, or 15 mg/kg/day appeared to be at least equivalent to RS-61443 and BQR. Mizoribine was less effective and azathioprine had only a modest therapeutic effect when added in daily high doses to FK506, whereas mizoribine, methotrexate, and DSPG were of intermediate efficacy (Table 2). DSPG was the only drug tested as an adjuvant to FK506 that is not an antimetabolite. The animals given this drug developed alopecia and weight loss during its 30-day administration.
Cyclophosphamide pretreatment
The 5 rats given a single dose of 80 mg/kg cyclophosphamide 10 days preoperatively and then treated with the usual daily regimen of FK506 after transplantation have all accepted their cardiac xenografts, with follow-ups of 2 months (Table 2, group 31).
Liver xenograft survival
Single drug therapy
FK506 alone at 1 mg/kg/day increased survival 5-fold to 34.5 days, with 3 of 10 animals surviving beyond 60 days and one beyond 100 days (Table 3); the results were similar to those reported earlier by Valdivia et al. (17). BQR alone doubled median survival but only one of 7 animals lived beyond 19 days (Table 3). Results also were poor with RS-61443 and with the lowest dose of 7.5 mg/kg/day cyclophosphamide.
TABLE 3.
Hamster liver graft survival in LEW rat recipients treated with FK506, BQR, RS-61443 and cyclophosphamide as single agents
| Group | Agent | Dose (mg/ kg/day) |
Duration (days) |
n | Survival (days) | Median survival (days) |
1-month survival rate (%) |
100-day survival rate (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | — | — | — | 8 | 6, 7, 7, 7, 7, 7, 7, 8 | 7.0 | 0 | 0 |
| 2 | FK506 | 1.0 | 0–30a | 10 | 7, 11, 18, 19, 21, 48, 58, 72, 94, >100 |
34.5 | 50 | 10 |
| 3 | BQR | 3.0 | 0-Death | 7 | 7, 8, 9, 12, 17, 19, 74 | 12.0 | (5/10) 14.3 |
(1/10) 0 |
| 4 | RS-61443 | 20.0 | 0-Death | 5 | 7, 7, 7, 7, 8 | 7.0 | (1/7) 0 |
0 |
| 5 | Cyclophosphamide | 7.5 | 0-Death | 5 | 7, 7, 9, 9, 9 | 9.0 | 0 | 0 |
FK506 was used at a dose of 1.0 mg/kg/day for the first 30 days (0 to 30) and continued at 0.5 mg/kg/day every other day (days 31 to 100).
Combined therapy
When maintenance FK506 was combined with a 14-day course of 3 mg/kg/day BQR, the survival achievable with either drug alone was remarkably enhanced (Table 4) with 4 of 7 animals living for 100 days (group 1). Extending the daily BQR to 30 days (group 2) or giving it every other day from day 14 until 100 days (groups 3) caused a high delayed mortality. The animals appeared to have toxic reactions.
TABLE 4.
Hamster liver graft survival in LEW rat recipients treated with combination treatment with FK506a
| Group | Agent | Dose (mg/ kg/day) |
Duration (days) |
n | Survival (days) | Median survival (days) |
1-month survival (%) |
|---|---|---|---|---|---|---|---|
| 1 | BQR | 3.0 | 0–13 | 7 | 21, 42, 81, >100, >100, >100, >100 | >100 | 85.7 |
| 2 | BQR | 3.0 | 0–30 | 7 | 13, 21, 23, 32, 36, 46, >100 | 32.0 | (6/7) 57.1 |
| 3 | BQR | 3.0 | 0–13b | 9 | 15, 15, 19, 30, 38, >100, >100, >100, >100 | 38.0 | (4/7) 66.7 |
| 4 | RS 61443 | 20 | 0–13 | 10 | 25, 83, >100, >100, >100, >100, >100, >100, >100, >100 | >100.0 | (6/9) 90.0 |
| 5 | RS 61443 | 20 | 0–30 | 9 | 12, 22, 30, 36, 54, >100, >100, >100, >100 | 54.0 | (9/10) 77.8 |
| 6 | RS 61443 | 30 | 0–13 | 10 | 15, 15, 39, 76, >82, >84, >96, >97, >97, >98 | >83.0 | (7/9) 80.0 |
| 7 | Splenectomy | — | — | 6 | 9, 11, 18, 61, >100, >100 | 39.5 | (8/10) 50.0 |
| 8 | Cyclophosphamide | 7.5 | 0→9 | 10 | 10, 31, >40, >45, >45, >45, >47, >48, >48, >51 | >45.0 | (3/6) 90.0 |
| 9 | Cyclophosphamide | 10.0 | 0→9 | 5 | >32, >32, >32, >39, >39 | >32.0 | (9/10) 100.0 |
| 10 | Cyclophosphamide | 80.0 | −10 | 12 | 12, 13, 20, >46, >47, >47, >47, >47, >65, >66, >66, >66 | >47.0 | (5/5) 75.0 |
| (i.p.) | (once) | (9/12) |
FK506 was used at 1.0 mg/kg/day for the first 30 days (0 to 30) and continued at 0.5 mg/kg/day every other day (days 31 to 100).
BQR was administered for the first 14 days and continued on alternate days from days 14 to 100 at a dose of 3.0 mg/kg/day.
RS-61443 with FK506 also greatly augmented survival when given for 14 days in the 2 relatively low doses of 20 and 30 mg/kg, but the success rate was reduced, apparently because of toxicity, when the RS-61443 was continued for 30 days (Table 4, group 5).
Although the follow-up periods are shorter, the best results were with cyclophosphamide plus FK506 (Table 4, groups 8 and 9). Virtually every animal had long survival—whether cyclophosphamide was given at the daily dose of 7.5 mg/kg, which was ineffective as monotherapy, or at the higher dose of 10 mg.
Splenectomy
The efficacy of FK506 also appeared to be improved with splenectomy (Table 4, group 7), a finding similar to that previously reported with hamster-to-rat heart xenotransplantation under monotherapy with FK506 (19) or with liver xenotransplantation under cyclosporine (16).
Cyclophosphamide pretreatment
Nine of the 12 rats pretreated 10 days preoperatively with a single dose of 80 mg/kg cyclophosphamide have had long survival posttransplantation under FK506.
Antibody correlation
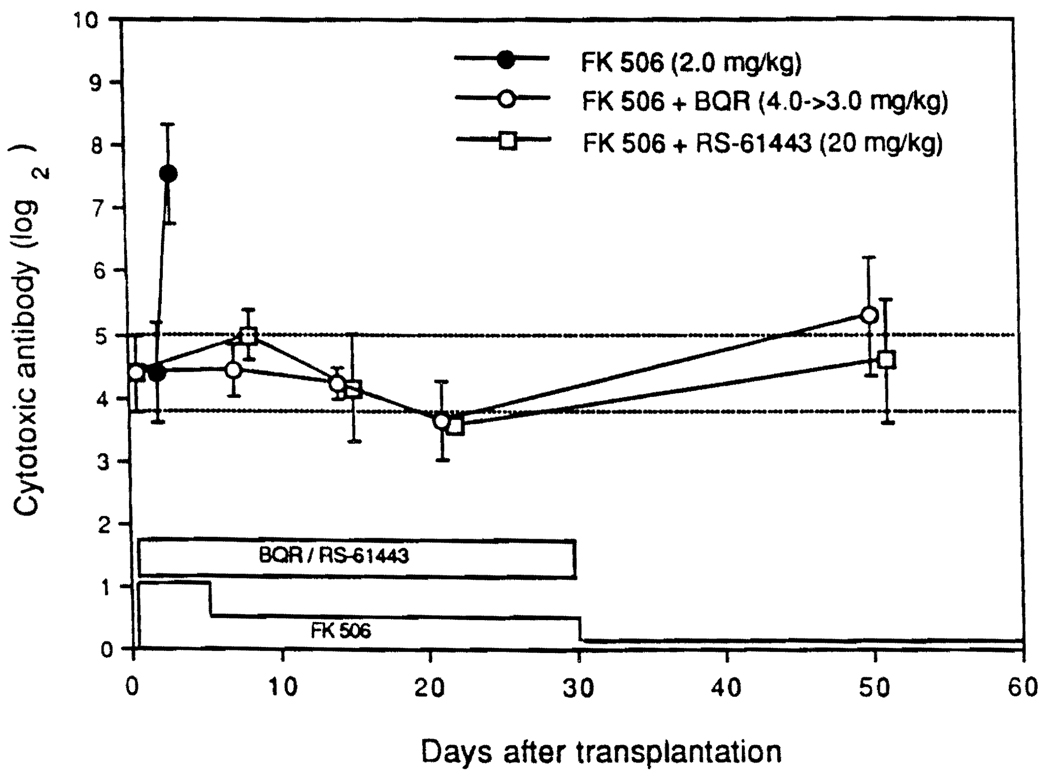
Under FK506 as single-drug treatment for cardiac xenotransplantation, the heterospecific lymphocytotoxic antibodies that were present pretransplantation had astronomic increases by 3 or 4 postoperative days, coinciding with the humoral rejection of the hearts. Thirty-day induction therapy with BQR and RS-61443 partially prevented this (Fig. 1). However, throughout the course until 100 days, low levels of xenospecific antibodies persisted without apparent harm (Fig. 1).
FIGURE 1.
Antihamster lymphocytotoxic antibody after heart transplantation.
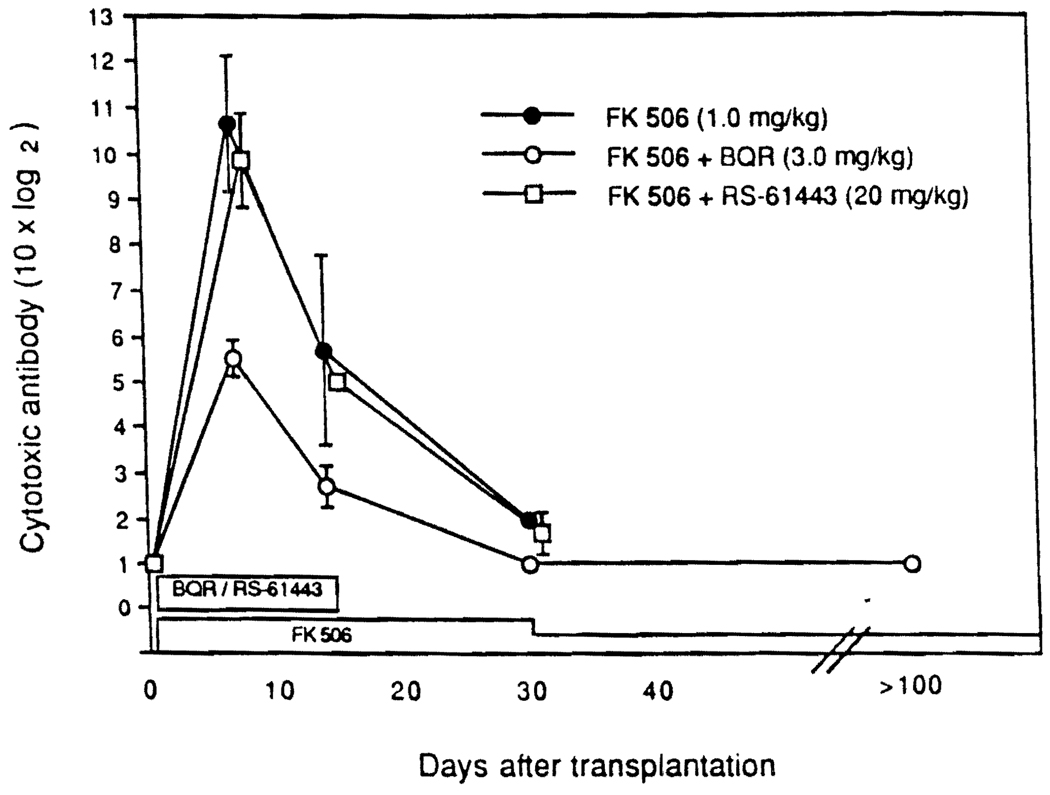
The antibody titers after liver xenotransplantation under FK506 alone rose 10 times higher than after heart transplantation alone, presumably because the livers survived long enough for the response to be complete (Fig. 2). The addition of BQR for a 14-day induction period blunted the antibody response more than RS-61443, but with both drugs the titer declined toward control levels after the 14-day course of the antiproliferative agents was stopped (Fig. 2).
FIGURE 2.
Antihamster lymphocytotoxic antibody response after liver transplantation. Note that the log 2 scale is ×10, to accommodate the far greater rise in titer compared with that in heart recipients (see Fig. 1).
Humoral antibodies
Indirect immunofluorescence staining of heart and liver xenografts showed that the antibody was principally IgM with light IgG deposition (Table 5). Liver recipients treated with FK506 only had considerable antibody concentration in vessels, but when antiproliferative drug treatment was added the staining was lighter and less blood-vessel-directed.
TABLE 5.
Hepatic specificity of xenoantibodies in sera of liver recipients by indirect immunofluorescencea
| Treatment | 7–8 Days after transplantation | 30–40 Days after transplantation | ||
|---|---|---|---|---|
| Sinusoids | Large vessel endothelium | Sinuosids | Large vessel endothelium | |
| FK506 1.0 mg/kg | +++ | +++ | ± | ± |
| ++ | ++ | ± | ± | |
| FK506 1.0 mg/kg + RS-61443 20 mg/kg (0–13) | ++ | ++ | ± | ± |
| ++ | ++ | ± | ± | |
| FK506 1.0 mg/kg + BQR 3.0 mg/kg (0–13) | + | + | ± | ± |
| + | + | ± | ± | |
Immunofluorescent intensity was scored on a qualitative scale from 0 to 3+; values were shown as IgM deposits.
DISCUSSION
The moderately difficult hamster-to-rat model of xenotransplantation has been investigated extensively by Valdivia and Monden (14–17) and by others, including Knechtle et al. (20), who reported the mean survival of heterotopic hearts for >100 days with total-lymphoid irradiation plus cyclosporine. This accomplishment could not be duplicated by later workers (21, 22). Marchman et al. (23) reported that TLI plus DSPG increased heart xenograft survival to a mean of 26 days. By inhibiting complement fixation with snake venom in cyclosporine-treated rats, Van Den Bogaerde et al. (24) were able to increase heart graft survival to a mean of 50 days, with 2 of 10 animals living for 100 days. These last investigators emphasized the duality of the humoral and cellular mechanisms of xenograft rejection.
The special value of the hamster-to-rat xenograft models for the screening of immunosuppressive drug combinations was evident from our experiments. A hamster organ is confronted in the rat by a moderate titer (1:16–1:32) of preformed heterospecific cytotoxic antibodies, and subsequently by a rapidly gathering antibody storm that destroys cardiac grafts within 3 days in untreated recipients, before there is a trace histopathologically of immunocyte infiltration. The liver, which is more resistant to antibody-mediated injury (17, 25, 26), survives this initial onslaught but cellular rejection becomes increasingly evident beyond 3 days until death of the unmodified host after 7 days from combined humoral and cellular rejection. Thus the use of these 2 organs for testing allows at the outset a stratification in untreated animals of the 2 distinctive mechanisms of humoral and cellular graft rejection, and makes it clear that the mitigation or interdiction of either alone will not consistently permit long-term graft survival.
This generalization was verified with testing of all of the individual drugs that contributed to our most effective cocktails. Although FK506 and the antiproliferative drugs used most extensively (RS-61443 and BQR) significantly improved heart and liver xenograft survival when used alone, they did not permit consistent long-term survival. Survival became routinely possible when continuous FK506 was combined with a shout course of BQR, which inhibits de novo pyrimidine synthesis by blocking the enzyme dihydroorotate dehydrogenase (27), or of RS-61443, which (like mizoribine) inhibits de novo purine synthesis by blocking enzyme inosine monophosphate dehydrogenase (28).
Although less-extensive data were acquired with mizoribine and azathioprine, these antiproliferative agents appeared less capable of augmenting FK506 efficiency. In contrast, cyclophosphamide, another purine antimetabolite with considerable B cell specificity (29) was unexpectedly effective as monotherapy in prolonging heart xenograft survival and in allowing the routine acceptance of heart xenografts in FK506-treated recipients when the cyclophosphamide was given as a single large dose 10 days before transplantation or in a 9-day course postoperatively in smaller doses. The ability to effectively use cyclophosphamide for daily dosing in the xenograft model has direct clinical implications for clinical xenograft trials because this drug has been shown in extensive clinical trials of renal and liver transplantation to be as safe and effective as azathioprine when used as the baseline drug for chronic therapy (30–32). Azathioprine had low efficacy in the hamster-to-rat heart model, and methotrexate and mizoribine were in between.
DSPG, the synthetically hydroxylated fermentation product of Bacillus lactobacillus, the mechanism of action of which is not as well understood, appeared capable of filling the same handmaiden role to FK506 as the antimetabolite drugs, but the animals lost weight, developed alopecia, and appeared systemically toxic when effective doses were used. DSPG has been described as ameliorating preformed antibody states, including those in xenotransplantation (13, 23).
After orthotopic hepatic xenotransplantation, the perioperative survival of the liver with its well-known resistance to antibodies was less dependent than the heart on the antimetabolite component of the combined drug therapy—and, in contrast to the heart xenograft experiments, cyclophosphamide as monotherapy had little value for hamster-to-rat liver xenotransplantation—seemingly less than BQR or RS-61443. However, when any of these 3 agents was used in a brief induction course for continuous treatment with FK506, the results were converted from unpredictable and unacceptable to almost invariably successful.
Thus, by breaking down the antibody barrier to xenotransplantation with several of these so-called antiproliferative drugs as well as with DSPG, it has been possible with FK506 to transplant hearts and livers in the moderately difficult hamster-to-rat xenotransplantation model as easily as in most allogeneic strain combinations and more easily than in some. The therapeutic benefit of the adjuvant agents correlated with the ability of the adjuvant drug to inhibit the antihamster antibody response perioperatively, although this correlation was imprecise later in the course, the reappearance of the antibodies was not predictably harmful. Once the first 2 weeks were past, treatment with antiproliferative agents was no longer necessary—and, in fact, its continuation may have been a liability in that some of the rats appeared ill unless these agents were stopped. The minor benefit of splenectomy in one of our liver subgroups was thought to be by abridging the humoral immune response. The imperfect correlation between the suppression of generic heterospecific antibodies by these drugs and the clinical results, particularly with cyclophosphamide, has been explained by further studies (manuscript in preparation) showing that xenograft rejection is dependent on an IgM-producing subpopulation of splenic B cells and NK cells.
Thus, we are reporting here the conclusion that interdiction of the B cell proliferative response holds the key to the critical first step of xenotransplantation. This concept should be clinically relevant, provided that the humoral antibody reaction is not so instantaneous that it causes hyperacute rejection in a matter of a few minutes or hours. It is known from experience that baboon-to-human xenotransplantation fulfils this condition (33, 34). The prime candidate drug to prevent the heterospecific humoral rejection response is cyclophosphamide, although the experimental drugs RS-61443 and BQR are promising.
Once the antibody barrier is breached, the need for the antiproliferative drugs apparently diminishes, as was shown by the ability to routinely stop cyclophosphamide, RS-61443, and BQR in 2 weeks or less without subsequent humoral rejection in spite of the continued presence or in some animals a delayed rise of the antihamster antibodies. In the hamster-to-rat model, monotherapy with FK506 was all that was required from 2 weeks onward. It was shown previously that with stoppage of FK506 at 100 days, the hamster heart or liver xenograft is rejected, primarily by cellular mechanisms, after several weeks to months (19).
Supplementary Material
Footnotes
Presented at the 18th Annual Meeting of the American Society of Transplant Surgeons, May 27–29, 1992, Chicago, IL.
This work was supported by Project Grant DK 29961 from the National Institutes of Health, Bethesda, MD.
Abbreviations: BQR, brequinar; DSPG, deoxyspergualin.
REFERENCES
- 1.Tocci MJ, Matkovich DA, Collier KA, et al. The immunosuppressant FK506 selectively inhibits expression of early T cell activation genes. J Immunol. 1989;143:718. [PubMed] [Google Scholar]
- 2.Starzl TE, Todo S, Fung J, Demetris AJ, Venkataramanan R, Jain A. FK506 for human liver, kidney and pancreas transplantation. Lancet. 1989;2:1000. doi: 10.1016/s0140-6736(89)91014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Todo S, Fung JJ, Starzl TE, et al. Liver, kidney, and thoracic organ transplantation under FK506. Ann Surg. 1990;212:295. doi: 10.1097/00000658-199009000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Makowka L, Chapman F, Qian S, et al. The effect of FK506 on hyperacute rejection in presensitized rats. Transplant Proc. 1987;19 suppl 6:79. [PMC free article] [PubMed] [Google Scholar]
- 5.Starzl TE, Fung J, Jordan M, et al. Kidney transplantation under FK506. JAMA. 1990;264:63. [PMC free article] [PubMed] [Google Scholar]
- 6.Nakajima K, Sakamoto K, Ochiai T, Nagato M, Asano T, Isono K. Prolongation of cardiac xenograft survival in rats treated with 15-deoxyspergualin alone and in combination with FK506. Transplantation. 1988;45:1146. doi: 10.1097/00007890-198806000-00033. [DOI] [PubMed] [Google Scholar]
- 7.Gudas VM, Carmichael PG, Morris RE. Comparison of the immunosuppressive and toxic effects of FK506 and cyclosporine in xenograft recipients. Transplant Proc. 1989;21:1072. [PubMed] [Google Scholar]
- 8.Cramer DV, Chapman FA, Jaffee BD, et al. The effect of a new immunosuppressive drug, brequinar sodium, on heart, liver, and kidney allograft in the rat. Transplantation. 1992;53:303. doi: 10.1097/00007890-199202010-00009. [DOI] [PubMed] [Google Scholar]
- 9.Sollinger H, editor. Transplant Proc. Vol. 23. 1991. Immunosuppressive drugs: targets and mechanisms of action; p. 1. [Google Scholar]
- 10.Eugui EM, Almquist SJ, Muller CD, Allison AC. Lymphocyte-selective cytostatic and immunosuppressive effects of mycophenolic acid in vitro: role of deoxyguanosine nucleotide depletion. Scand J Immunol. 1991;33:161. doi: 10.1111/j.1365-3083.1991.tb03746.x. [DOI] [PubMed] [Google Scholar]
- 11.Morris RE, Wang J, Blum JR, et al. Immunosuppressive effects of the morpholinoethyl ester of mycophenolic acid (RS-61443) in rat and nonhuman primate recipients of heart allografts. Transplant Proc. 1991;23 suppl 2:2. 19. [PubMed] [Google Scholar]
- 12.Sollinger HW, Belzer FO, Deierhoi MH, et al. RS-61443: a multi-center study for refractory kidney transplant rejection. Ann Surg. 1992;216:513. doi: 10.1097/00000658-199210000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morris RE. 15-deoxyspergualin: a mystery wrapped within an enigma. Clin Transplant. 1991;5:530. [Google Scholar]
- 14.Valdivia LA, Monden M, Gotoh M, et al. Hepatic xenografts from hamster to rat. Transplant Proc. 1987;19:1158. [PubMed] [Google Scholar]
- 15.Monden M, Valdivia LA, Gotoh M, et al. Hamster-to-rat orthotopic liver xenografts. Transplantation. 1987;43:745. [PubMed] [Google Scholar]
- 16.Valdivia LA, Monden M, Gotoh M, et al. Prolonged survival of hamster-to-rat liver xenografts using splenectomy and cyclosporine administration. Transplantation. 1987;44:759. doi: 10.1097/00007890-198712000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Valdivia LA, Fung JJ, Demetris AJ, Starzl TE. Differential survival of hamster-to-rat liver and cardiac xenografts under FK-506 immunosuppression. Transplant Proc. 1991;23:3269. [PMC free article] [PubMed] [Google Scholar]
- 18.Terasaki PI, Bernoco D, Park MS, Dzturk GO, Iwaki Y. Micro-droplet testing for HLA-A, -B, -C, and -D antigens. Am J Clin Pathol. 1978;69:103. doi: 10.1093/ajcp/69.2.103. [DOI] [PubMed] [Google Scholar]
- 19.Valdivia LA, Demetris AJ, Langer AM, Celli S, Fung JJ, Starzl TE. Dendritic cell replacement in the long-surviving liver and cardiac xenografts. Transplantation. doi: 10.1097/00007890-199308000-00048. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knechtle SJ, Halperin EC, Bollinger RR. Xenograft survival in two species combinations using total-lymphoid irradiation and cyclosporine. Transplantation. 1987;43:173. doi: 10.1097/00007890-198702000-00002. [DOI] [PubMed] [Google Scholar]
- 21.DeMasi T, Alqaisi M, Araneda D, et al. Reevaluation of total-lymphoid irradiation and cyclosporine therapy in the Syrian hamster–to–Lewis rat cardiac xenograft model. Transplantation. 1990;49:639. [PubMed] [Google Scholar]
- 22.Steinbruchel DA, Madsen HH, Nielsen HH, Kemp E, Larsen S, Koch C. Graft survival in a hamster-to-rat heart transplantation model after treatment with total lymphoid irradiation, cyclosporine A, and a monoclonal anti–T cell antibody. Transplant Proc. 1990;22:1088. [PubMed] [Google Scholar]
- 23.Marchman W, Araneda D, DeMasi R, et al. Prolongation of xenograft survival after combination therapy with 15-deoxyspergualin and total-lymphoid irradiation in the hamster-to-rat cardiac xenograft model. Transplantation. 1992;53:30. doi: 10.1097/00007890-199201000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Van Den Bogaerde J, Aspinall R, Wang MW, et al. Induction of long-term survival of hamster heart xenografts in rats. Transplantation. 1991;52:15. doi: 10.1097/00007890-199107000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Starzl TE, Putnam CW, Ishikawa M, et al. Progress in and deterrents to orthotopic liver transplantation, with special reference to survival, resistance to hyperacute rejection, and biliary duct reconstruction. Transplant Proc. 1974;6:129. [PMC free article] [PubMed] [Google Scholar]
- 26.Kamada N, Davies HFFS, Roser B. Reversal of transplantation immunity by liver grafting. Nature. 1981;292:840. doi: 10.1038/292840a0. [DOI] [PubMed] [Google Scholar]
- 27.Chen SF, Ruben RL, Dexter DL. Mechanism of action of the novel anticancer agent 6-fluoro-2-(2′-fluoro-1,1′-biphenyl-4-yl)-3-methyl-4-quinolinecarboxylic acid sodium salt (NSC 368390): inhibition of de novo pyrimidine nucleotide biosynthesis. Cancer Res. 1986;46:5014. [PubMed] [Google Scholar]
- 28.Nelson PH, Eugui E, Wang CC, Allison AC. Synthesis and immunosuppressive activity of some side-chain variants of mycophenolic acid. J Med Chem. 1990;33:833. doi: 10.1021/jm00164a057. [DOI] [PubMed] [Google Scholar]
- 29.Putnam CW, Halgrimson CG, Groth CG, Kashiwagi N, Porter KA, Starzl TE. Immunosuppression with cyclophosphamide in the dog. Clin Exp Immunol. 1975;22:323. [PMC free article] [PubMed] [Google Scholar]
- 30.Starzl TE, Halgrimson CG, Penn I, et al. Cyclophosphamide and human organ transplantation. Lancet. 1971;2:70. doi: 10.1016/s0140-6736(71)92046-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Starzl TE, Putnam CW, Halgrimson CG, et al. Cyclophosphamide and whole organ transplantation in human beings. Surg Gynecol Obstet. 1971;133:981. [PMC free article] [PubMed] [Google Scholar]
- 32.Starzl TE, Groth CG, Putnam CW, et al. Cyclophosphamide for clinical renal and hepatic transplantation. Transplant Proc. 1973;5:511. [PMC free article] [PubMed] [Google Scholar]
- 33.Starzl TE, Marchioro TL, Pteres GN, et al. Renal homotransplantation from baboon to man: experience with 6 cases. Transplantation. 1964;2:752. doi: 10.1097/00007890-196411000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bailey L, Nelson-Cannarella S, Concepcion W, Jolley WB. Baboon-to-human cardiac xenotransplantation in a neonate. JAMA. 1985;254:3321. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.