FK506, a new and powerful immunosuppressant, has become the principal drug in the immunosuppressive regimens of organ transplant recipients at the University of Pittsburgh (1–3). It is well known that transplant patients often receive multiple-drug therapy, and that many of these co-administered agents can alter the kinetics of immunosuppressive drugs, such as cyclosporine and prednisone (4,5). In this communication we report for the first time a potential interaction between FK506 and the antifungal agent clotrimazole in a liver transplant recipient.
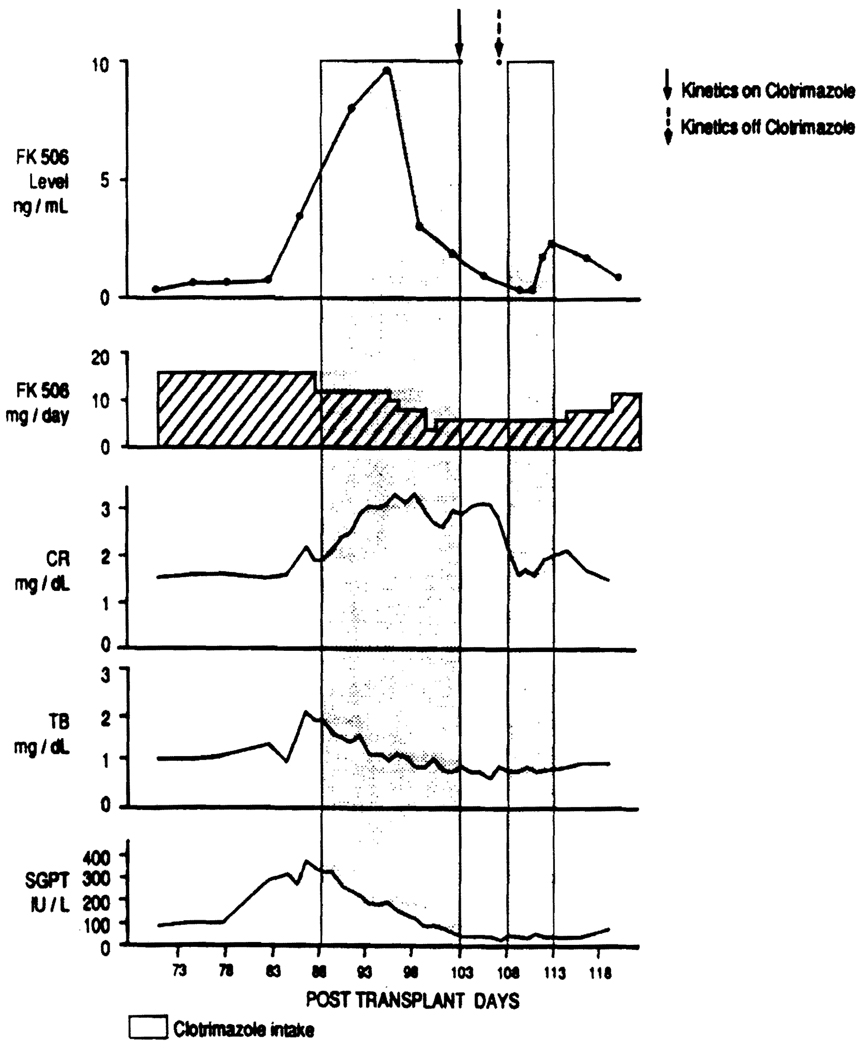
The recipient, a 55-year-old male received a liver transplant, on November 28, 1989, at the Veterans Affairs Medical Center of Pittsburgh, for chronic non-A–non-B hepatitis. His immunosuppressive regimen consisted of FK506 and prednisone, and after uneventful recovery he was discharged on the 15th postoperative day. On the 84th posttransplant day, he was readmitted with hyperglycemia, elevated liver function tests, mild elevation of the serum creatinine, and oropharyngeal moniliasis despite prophylactic nystatin therapy. The trough plasma FK506 concentration, 24 hr after admission, was 3.5 ng/ml. Since his liver biopsy disclosed mild lobular hepatitis, both his FK506 and steroid doses were decreased. The carbohydrate metabolism improved, but his serum creatinine and FK506 levels continued to rise in spite of a dose reduction (Fig. 1). Since the increase in plasma FK506 concentration could not be solely attributed to the mild hepatic dysfunction, a drug interaction with clotrimazole was suspected, since this drug, started on the 88th posttransplant day, was the only new medication added to the patient’s therapy.
FIGURE 1.
Patient’s serial biochemistries, trough FK506 plasma concentrations and FK506 dose, from posttransplant day 73 to posttransplant day 118. The shadowed areas correspond to the period of time during which the patient received Clotrimazole therapy, and the arrows indicate the dates during which the pharmacokinetics studies were performed.
Although cytomegalovirus was never isolated from the liver, it was found in the patient’s buffy coat, stomach mucosa, and urine. He received gancyclovir from the 99th to the 112th posttransplant day and was discharged on the 114th posttransplant day, with normal fasting blood sugar, a serum creatinine of 2.0 mg/dl, and normal liver biochemistry.
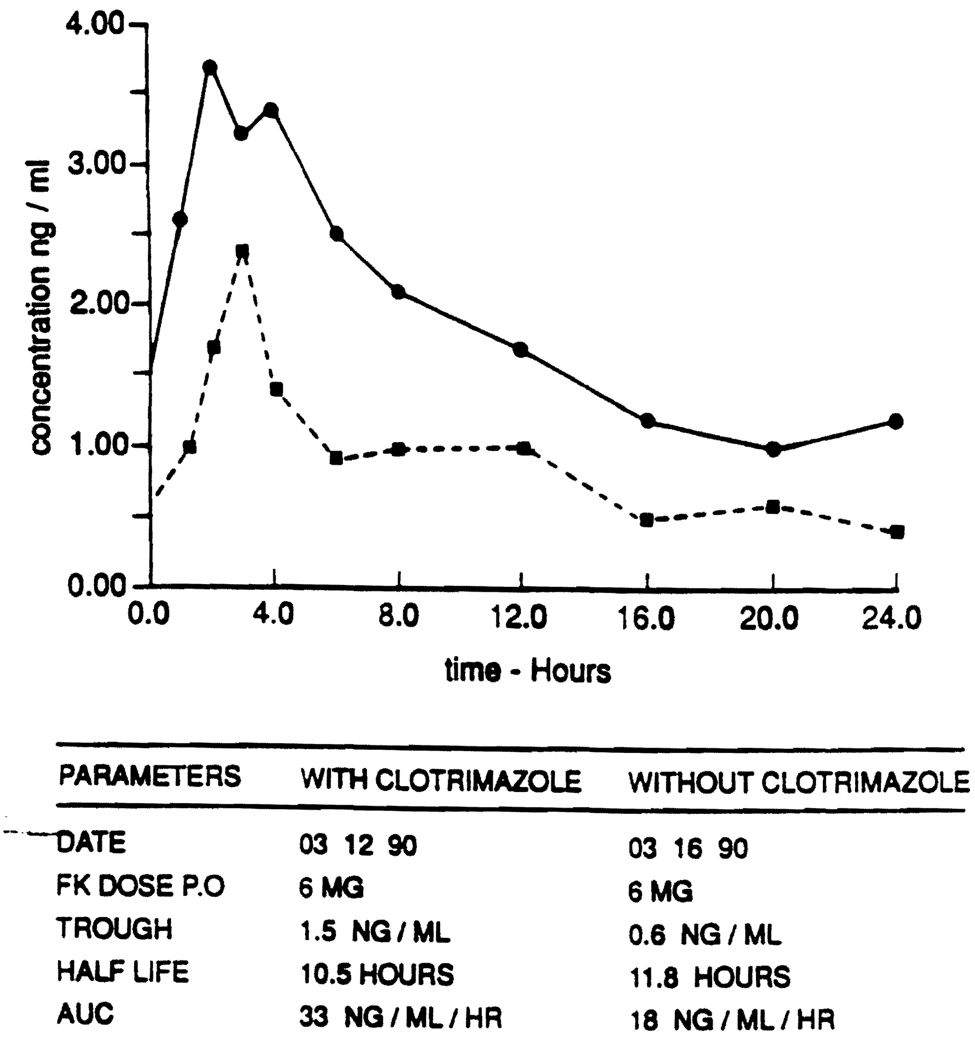
Two pharmacokinetic studies were performed after the patient had been placed on a fixed dose of FK506 (6 mg once a day). The first study was carried out on the 103rd posttransplant day, 15 days after starting clotrimazole therapy (10 mg 4 times a day). The second study was performed on the 107th posttransplant day, 4 days after discontinuation of clotrimazole therapy (Fig. 1). In order to reconfirm the drug interaction, clotrimazole was restarted the day after the second kinetic study for 5 days, and daily FK506 concentrations were monitored (Fig. 1). On the study days, multiple blood samples (11) were collected just prior to the oral FK506 dose and at various time periods for the next 24 hr. Blood samples were incubated at 37°C for 30 min and centrifuged at 37°C to separate the plasma. Plasma samples were frozen at −70°C and analyzed for FK506, within a week, using an ELISA assay (6).
As seen in Figure 1, prior to adding the clotrimazole, the trough plasma FK506 concentrations increased from 0.7 ng/ml to 3.5 ng/ml. This was probably due to mild hepatic dysfunction. The addition of clotrimazole further increased the trough levels to 5.6 ng/ml in a day and to greater than 9.0 ng/ml within 8 days.
The pharmacokinetic studies (Fig. 2) revealed a twofold increase in the area under the plasma concentration versus time curve (AUC) of FK506 in the presence of clotrimazole, as compared with the AUC in the absence of clotrimazole. The half-life of FK506 did not appear to be significantly different in the presence (10.5 hr) and in the absence of clotrimazole (11.8 hr). Reinitiation of clotrimazole therapy for 5 days after the kinetic study disclosed again an increase in FK506 concentration in the presence of clotrimazole.
FIGURE 2.
Patient’s FK506 pharmacokinetic profile with clotrimazole (continuous line) and without clotrimazole (interrupted line). AUC: area under the curve.
Clotrimazole (Mycelex troches), an imidazole antifungal, is occasionally used for the treatment and/or prophylaxis of mucocutaneous candidiasis. In spite of being poorly and erratically absorbed, it is suspected that clotrimazole can interact with other medications that are metabolized through the same P-450 enzymatic system of the liver, leading to an increased half-life of drugs such as cyclosporine (5). However, the pharmacokinetics studies performed in this patient disclosed an increased plasma concentration of the FK506 in the presence clotrimazole, without any change in the half-life. The exact mechanism of this interaction is not completely clear at the present time. One possible explanation is that the intestinal metabolism of FK506 may be reduced in the presence of clotrimazole. Recent evidence has suggested: (1) that there is abundant cytochrome P-450 in the enterocytes of the human jejunal mucosa (7); (2) that there is metabolism of FK506 (8) and of cyclosporine (9), in the intestinal microsomes; and (3) that drug interactions in the intestine can lead to altered kinetics of drugs, as is the case of cyclosporine in the presence of erythromycin (10). Therefore, it is conceivable, that since most of the clotrimazole from the troche eventually reach the intestine, it can compete with FK506 for the binding sites of P-450 enzymatic system, thereby decreasing the amount of FK506 metabolized by the intestinal mucosa and increasing the amount of drug available for absorption. On the other hand, clotrimazole to a certain extent could also decrease the hepatic P-450 enzymes responsible for FK506 metabolism, without significantly affecting the half-life of the drug. Independent of the mechanism involved, it is clear that coadministration of clotrimazole with FK506 causes an increase in the FK506 concentrations, and that the dose of the latter has to be reduced in such cases, to avoid toxicity. Finally, as already noted with cyclosporine and diltiazem, this interaction could be useful in reducing the dose of FK506 required to achieve a desired FK506 therapeutic concentration.
Footnotes
This work was supported by research grants from the Veterans Administration and Project Grant No. DK 29961 from the National Institutes of Health, Bethesda, Maryland.
REFERENCES
- 1.Starzl TE, Todo S, Fung J, Demetris AJ, Venkataramanan R, Jain A. FK 506 for human liver, kidney and pancreas transplantation. Lancet. 1989;2:1000. doi: 10.1016/s0140-6736(89)91014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Todo S, Fung JJ, Starzl TE, et al. Liver, kidney, and thoracic organ transplantation under FK 506. Ann Surg. 1990;212:295. doi: 10.1097/00000658-199009000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fung JJ, Todo S, Tzakis A, et al. Conversion of liver allograft recipients from cyclosporine to FK 506 based immunosuppression: benefits and pitfalls. Transplant Proc. 1991;23:1421. [PMC free article] [PubMed] [Google Scholar]
- 4.Venkataramanan R, Habucky K, Burckart GJ, Ptachcinski RJ. Clinical pharmacokinetics in organ transplant patients. Clin Pharmacokinetic. 16:134. doi: 10.2165/00003088-198916030-00002. [DOI] [PubMed] [Google Scholar]
- 5.Ptachcinski RJ, Venkataramanan R, Burckart GJ. Clinical pharmacokinetics of cyclosporin. Clin Pharmacokinet. 1986;11:107. doi: 10.2165/00003088-198611020-00002. [DOI] [PubMed] [Google Scholar]
- 6.Cadoff EM, Venkataramanan R, Krajack A, et al. Assay of FK 506 in plasma. Transplant Proc. 1990;22:50. [PMC free article] [PubMed] [Google Scholar]
- 7.Watkins PB, Wrighton SA, Schuetz EG, Molowa DT, Guzelian PS. Identification of glucocorticoid-inducible cytochromes P-450 in the intestinal mucosa of rats and man. J Clin Invest. 1987;80:1029. doi: 10.1172/JCI113156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christians U, Kruse C, Kownatzki R, et al. Measurement of FK 506 by HPLC and isolation and characterization of its metabolites. Transplant Proc. 1991;23:940. [PubMed] [Google Scholar]
- 9.Kolars JC, Duell EA, Benedict PE, et al. P-450 III metabolizes cyclosporine in intestinal mucosa: observations in a novel rat model. Clin Res. 1989;37:933A. [Abstract]. [Google Scholar]
- 10.Gupta SK, Bakran A, Johnson RW, Rowland M. Cyclosporinerythromycin interaction in renal transplant patients. Br J Clin Pharmacol. 1989;27:475. doi: 10.1111/j.1365-2125.1989.tb05396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]