Abstract
Introduction
Preoccupation with drug and drug-related items is a typical characteristic of cocaine addicted individuals. It has been shown in multiple accounts that prolonged drug use has a profound effect on the EEG recordings of drug addicts when compared to controls during cue reactivity tests. Cue reactivity refers to a phenomenon in which individuals with a history of drug abuse exhibit excessive psychophysiological responses to cues associated with their drug of choice. One of the aims of this pilot study was to determine the presence of an attentional bias to preferentially process drug-related cues using evoked and induced gamma reactivity measures in cocaine addicts before and after biobehavioral treatment based on neurofeedback. Another aim was to show that central SMR amplitude increase and frontal theta control is possible in an experimental outpatient drug users group over 12 neurofeedback sessions.
Method
Ten current cocaine abusers participated in this pilot research study using neurofeedback combined with Motivational Interviewing sessions. Eight of them completed all planned pre- and post –neurofeedback cue reactivity tests with event-related EEG recording and clinical evaluations. Cue reactivity test represented a visual oddball task with images from the International Affective Picture System and drug-related pictures. Evoked and induced gamma responses to target and non-target drug cues were analyzed using wavelet analysis.
Results
Outpatient subjects with cocaine addiction completed the biobehavioral intervention and successfully increased SMR while keeping theta practically unchanged in 12 sessions of neurofeedback training. The addition of Motivational Interviewing helped retain patients in the study. Clinical evaluations immediately after completion of the treatment showed decreased self-reports on depression and stress scores, and urine tests collaborated reports of decreased use of cocaine and marijuana. Effects of neurofeedback resulted in a lower EEG gamma reactivity to drug-related images in a post-neurofeedback cue reactivity test. In particular, evoked gamma showed decreases in power to non-target and to a lesser extent target drug-related cues at all topographies (left, right, frontal, parietal, medial, inferior); while induced gamma power decreased globally to both target and non-target drug cues. Our findings supported our hypothesis that gamma band cue reactivity measures are sufficiently sensitive functional outcomes of neurofeedback treatment. Both evoked and induced gamma measures were found capable to detect changes in responsiveness to both target and non-target drug cues.
Conclusion
Our study emphasizes the utility of cognitive neuroscience methods based on EEG gamma band measures for the assessment of the functional outcomes of neurofeedback-based biobehavioral interventions for cocaine use disorders. This approach may have significant potential for identifying both physiological and clinical markers of treatment progress. The results confirmed our prediction that EEG changes achieved with neurofeedback training will be accompanied by positive EEG outcomes in a cue reactivity and clinical improvements.
Keywords: Neurofeedback, substance use disorders, gamma band, EEG, cue reactivity
INTRODUCTION
Drug addiction is a psychoactive substance use disorder (SUD) which can be characterized by the physiological dependence of an afflicted individual upon a drug of choice. This dependence is coupled with the withdrawal syndrome upon discontinuation of drug use as well as physiological and psychological dependence with craving which motivates an addict to partake in drug-seeking behavior. Drug addiction is a chronic, relapsing mental disorder that results from the prolonged effects of drugs on the brain (Dackis & O’Brien, 2001; Leshner, 1997; Wexler et al., 2001). Addiction leads to behavioral, cognitive and socially adverse outcomes that incur substantial costs to society. In 2006, it was estimated by the Substance Abuse and Mental Health Service Administration (SAMHSA, 2007) that 19.9 million Americans used illicit drugs, computing to roughly 8 % of the United States population. In 2007, there were 2.1 million cocaine users, comprising 0.8 % of the population. The National Institute on Drug Abuse (NIDA) estimates that the total expenditure of drug-related complications is greater than 500 billion dollars when healthcare, legal procedures and job loss are considered.
Prolonged drug use can have profound effects upon normal brain activity which can be recorded and measured through the use of quantitative EEG (qEEG) techniques. One of the most difficult drug addictions to treat is that of cocaine as it is associated with a high rate of morbidity and mortality. Patients suffering cocaine addiction typically show low interest in drug treatment and hence treatment programs are often plagued with low retention rates. Some qEEG studies have highlighted signs of EEG activity that are significantly altered by cocaine abuse. It has been shown in several studies that cocaine abusers show increased beta as well as delta and alpha frequencies (Alper et al., 1990,1998; Costa & Bauer, 1997; Herning et al., 1985, 1994a,b; Noldy et al., 1994; Prichep et al., 1996, 1999, 2002). These changes are thought to be caused both by the neurotoxic side effects of cocaine use and as a result of the withdraw process (Alper, 1999). In light of these findings an effective and non-invasive method for treating the qEEG manifestations of addiction is needed. Neurofeedback is a technique employed to modify the electrical activity of the brain, including EEG, event-related potentials (ERP), slow cortical potentials (SCP), and other electrical activity of cortical origin. Detailed review of clinical efficacy of neurofeedback methods in SUD treatment and historic aspects of biofeedback-based behavioral intervention for drug addiction can be found in Sokhadze et al. (2008a) and Trudeau (2005).
Preoccupation with drug and drug-related items is a typical characteristic of cocaine addicted individuals. It has been shown in multiple accounts that prolonged drug use has a profound effect on the EEG recordings of drug addicts when compared to controls during cue reactivity tests. Cue reactivity refers to a phenomenon in which individuals with a history of drug abuse exhibit excessive verbal, physiological and behavioral responses to cues associated with their drug of choice (Carter & Tiffany, 1999; Franken et al., 1999) , suggesting a rearranging of neuronal networks in the brain of addicted individuals.
In cocaine addiction, items related to cocaine and drug paraphernalia are repeatedly selected by attention for conscious processing, and drug-related representations are disproportionately tagged as relevant. While studies with active cocaine users have indicated a strong physical reaction to drug-related stimuli (Carter & Tiffany, 1999, Childress et al., 1994, 1999; Grant et al., 1996, London et al., 2000), research examining cognitive aspects, for example attentional processes in cocaine addiction has been limited (Franken et al., 2000; Robbins et al., 1997). Several research studies provided support for the hypothesis that the process of alteration of attention takes place in addicts (Hester et al., 2006; Lyvers, 2000; Robinson & Berridge, 1993), so called “attentional bias” (Franken et al., 1999, 2000, 2003), and drug-related cues attain greater salience and motivational significance (Garavan et al., 2000; Koob, 1999; Koob & Le Moal, 2001; Robbins et al., 2000).
Cue reactivity expressed in physiological and behavioral responses to stimuli associated with the preferred substance of abuse (alcohol, nicotine, cocaine, heroin, etc.) is relatively well explored (Carter & Tiffany, 1999; Childress et al., 1999; Drummond et al., 1995; Ehrman et al., 1998; Lubman et al., 2000). One of the cognitive components of cue reactivity in substance abusers is the preferential allocation of attentional resources to items related to drugs (Lubman et al., 2000; Stormak et al., 2000). It has been proposed that conditioned sensitization in neural pathways associating incentives with stimulus items may be responsible for cue reactivity (Franken, 2003; Weiss et al., 2001). Several neuroimaging studies reported effects associated with drug cue-related responses and craving in cocaine addiction (Garavan et al., 2000; Hester & Garavan, 2004; Hester et al., 2006; Johnson et al., 1998; Kilts et al., 2004). Restructuring and reallocation of attentional resources suggests an over-attention to drug related cues and is believed to relate directly to the psychological symptoms of craving which leads to repeated drug use and relapse.
Several studies have been conducted to quantify the changes in qEEG values as a result of acute cocaine use as well as changes seen after prolonged abstinence and validated the findings that cocaine abusers typically elicit increased power in the beta, delta and alpha frequency patterns as compared to controls (Alper, 1999; Alper et al., 1990, 1998;Costa & Bauer, 1997; Herning et al., 1985, 1994b; Kilts et al., 2004; Noldy et al., 1994; Prichep et al., 2002). A more informative method of testing qEEG differences, as compared to resting, eyes closed EEG recordings, is through the use of both visual and auditory oddball tasks. SUD patients have been shown to illustrate a much higher response to emotionally salient stimuli. Hence in a visual oddball task involving neutral (e.g., household items and nature pictures) and drug related images drug addicts have shown a much higher response to drug-related cues (Sokhadze et al. 2008b).
Attentional bias toward the processing of salient stimuli is hypothesized to be an implicit cognitive process which is poorly controlled. Such automatic processing is similar to the orienting reflex to novel and significant signals. The automatic nature of addictive behaviors was outlined as well by other studies (Hester et al., 2006; Lubman et al., 2000). Drug abuse-related after-effects in the medial prefrontal cortex (PFC) could be accompanied by impairments in emotional regulation, and specifically in inhibition of all motivations and emotions other than craving (London et al., 2000; Volkow et al., 2003). Diminished PFC control of the fronto-striatal circuits allows more habitual responses mediated by the posterior and subcortical (e.g., basal ganglia, striatum) structures to take over regulation of behavior.
The gamma band (30–80 Hz), a high frequency rhythm of EEG activity, and more specifically gamma activity within 30–40 Hz range, is thought to represent the allocation of attentional resources and cognitive processes which take place in the brain (Karakaş et al., 2006; Muller et al., 2000; Tallon-Baudry et al., 1998, 2005). The gamma frequency oscillations have been speculated to play a role in several important cognitive functions. Widespread gamma band activity which can be seen in the EEG recordings may be connected to feature “binding” from separate parts of the brain in the attempt to make a coherent image from several perceived senses (Tallon-Baudry, 2003; Tallon-Baudry & Bertrand, 1999; Tallon-Baudry et al., 1998, 2005). More recent data involving new techniques such as magnetoencephalogram (MEG) and intra-cortical data collection have implicated the gamma band, especially frequencies around 40 Hz, in several higher level cognitive functions such as memory and learning through the synchronization of cortical cell networks (Gray & Singer, 1989; Muller et al., 2000) . Additional processes in which the gamma band has been highlighted as having a possible role are somato-sensory perception, visuo-motor coordination, music perception and conscious recollection (Herrmann & Mecklinger, 2000, 2001; Muller et al., 2000). These connections are thought to be reflected through the calculation of the power of the filtered gamma band at a given electrode of interest when presented with the appropriate stimulus.
The oscillatory response of gamma may be broken down into two main groups: evoked and induced responses. These two gamma responses may be discriminated on the basis of temporal localization and if they are time-locked to a stimulus. The early, or evoked, gamma responses occur in the 0–180 ms post-stimulus range. These early responses have been attributed to the early information processing linked to the sensation and perception of stimuli. These responses are also time locked to a specific stimulus. In contrast the late, or induced gamma response, manifests in the 280–480 ms post- stimulus time window or even later, depending on stimulus modality and complexity. These induced responses show a jitter in latency and are seen in task conditions which require pattern recognition or higher-order processes of the short-term memory (Tallon-Baudry & Bertrand, 1999). As such, these patterns have been linked to the possible indication of perceptual and cognitive processes. Based on these variable responses it is hypothesized that the gamma band is multifunctional and represents a broad based integration of attentional resources and cognitive patterns.
It should be noted that the early time locked gamma response obtained remains less affected by changes in stimulus type and task descriptions and level of task complexity. As a result of these findings it has been suggested that early, time locked gamma is actually a sensory oriented process (Karakaş et al., 2006). In contrast to these findings, the late and non-phase locked gamma activity (occurring in the 200–500 ms window range) varies according to the levels of task complexity and stimulus (Herrmann & Mecklinger, 2000; Tallon-Baudry & Bertrand, 1999). These differences suggest that the late induced gamma response could be interpreted as perception process and higher cognitive function (Karakaş et al., 2006; Tallon-Baudry, 2003).
Another measurement variable to highlight differences in attentional resources altered during drug addiction is dense-array event-related potentials (ERP). The most commonly studied ERP is the P300 which looks at the window 250–600 ms post stimulus. It has been suggested the amplitude of this waveform may be attributed to the brain allocating attentional resources while the latency period has been correlated to stimulus classification processes. The P300 may be subdivided into amplitudes occurring over either the frontal regions or centro-parietal regions, and are named P3a and P3b respectively. When collected during the administration of an oddball task, as was done during this research, the P3a is correlated with an orientation of attention to a significant stimulus and processing of novelty, while P3b is thought to represent sustained attention upon a given stimulus (Katayama & Polich, 1998).
It has already been reported by the authors that significant changes occur in the ERP as a result of chronic cocaine use and are observable even after long periods of abstinence in recovering cocaine addicts (Sokhadze et al., 2008b). Changes reported include extended P300 latency. It was also shown that larger P3a and P3b amplitudes would be seen in addicts in response to drug cues as compared to controls. The results demonstrated clearly heightened ERP responses to drug-related cues in addicted individuals. It is reasonable to propose that excessive reactivity during exposure to drug cues in addicts can be detected not only in ERP but also in evoked and induced gamma responses. At some extent it is possible that evoked gamma responses might be even more sensitive than the P300 component of the ERP which is known to be a premorbid trait in SUD and many other psychopathologies such as schizophrenia, bipolar disorder. and affective disorders (Polich & Herbst, 2000). Early, exogenous ERPs occurring within 50–200 ms post-stimulus (e.g., P100, N100) are sufficiently well studied in the cue reactivity paradigm (Franken, van Strien, & Nijs, 2006). In our pilot study using pictorial drug-related cues several significant group differences were found in centro-parietal P100 and occipito-parietal N170 ERP components. In particular, the SUD group as compared to controls had more prolonged P100 latencies and higher amplitude to non-target drug cues in the right hemisphere, while P100 amplitude to target-drug cues was higher in cocaine addicts in both hemispheres (Sokhadze et al., 2008c).
It is thought that neurofeedback may be a non-invasive method of treatment which can lower drug-oriented attention and behavior, including craving. These changes may be measurable through the use of qEEG techniques such as gamma analysis and ERP calculation. Analysis of the qEEG results have shown that at baseline cocaine addicts exhibit decreased delta and theta activity, but increased alpha and frontal beta as compared to controls (Herning et al 1985, 1994a,b). It is thought therefore that drug addicts may benefit from a sensorimotor rhythm (SMR) neurofeedback protocol similar to that used in the treatment of attention deficit hyperactivity disorder (ADHD) patients. It is the hope of this research that through measuring pre- and post-treatment normalized power indices in the gamma band range we will be able to show reduced response to drug related items in post-neurofeedback cue reactivity tests in cocaine addicts. Both evoked and induced gamma power were analyzed at pre- and post-neurofeedback training time points and then compared for any statistical differences between topographic groupings of electrodes in the hope of highlighting topographic differences in the left and right hemispheres as well as in the anterior and posterior regions of the brain.
Our neurofeedback training protocol included up to 3 motivational interviewing (MI) sessions (Miller & Rollnick, 2002) as an integral part of biobehavioral intervention in outpatients, since we always emphasized that outpatient treatment programs are more effective in drug abusers when neurofeedback training is combined with any other cognitive-behavioral therapy treatment modalities (Sokhadze et al., 2008a). Several studies of brief MI with cocaine abusers (Stotts et al., 2001, 2006), including our own pilot study (Sokhadze et al., 2005), reported that cocaine dependent patients with lower initial motivation to change following brief MI intervention were more likely to achieve abstinence than those who did not receive MI intervention. Due to its brevity, MI is best suited to enhance compliance and facilitate treatment engagement (Burke et al., 2003; Miller & Rollnick, 2002; Treasure, 2004), which was one of the main rationales why we selected MI to engage patients in neurofeedback-based biobehavioral neurotherapy. Our hypotheses in this study were that outpatient cocaine users would show improvement in behavioral, EEG and clinical measures following 12 sessions of neurotherapy (SMR/theta neurofeedback and MI), and that repeated post-neurofeedback cue reactivity test would show decreased evoked and induced gamma frequency response to both target and non-target drug-related stimuli.
METHOD
Subjects: recruitment process
Patients with current cocaine use or cocaine dependence record were referred from the University of Louisville Hospital, drug abuse treatment outpatient services, such as Jefferson County Alcohol and Drug Abuse Center (JADAC), and other psychiatric ambulatory units. Dr. Stewart, a co-author in this study, is a Medical Director at JADAC and a clinical consultant at two residential addiction treatment centers located in Louisville metro area. He provided a substantial number of referrals through these programs and conducted Motivational Interviewing sessions. Participating subjects with SUD were provided with full information about the study including the purpose, requirements, responsibilities, reimbursement, risks, benefits, alternatives, and role of the local Institutional Review Board (IRB). The consent forms were reviewed and explained to all subjects who expressed interest to participate in cognitive tests with EEG recording, MI and neurofeedback sessions. If the individual agreed to participate, she/he signed and dated the consent form and received a copy countersigned by the investigator who obtained consent.
All procedures were conducted within the facilities of the Department of Psychiatry and Behavioral Science and the University of Louisville Hospital outpatient clinic. Initial contact with prospective participant was typically made via telephone screening. Responders were telephone screened to meet initial inclusion criteria. Following telephone screening, the subjects received a psychiatric assessment in the laboratory to verify screening results and rule out Axis I diagnoses using Structured Clinical Interview for DSM-IV (First et al., 2001). Since subjects participated in the research study they were reimbursed for their time and transportation costs. Payment methods followed the University of Louisville Health Science Center’s Committee for the Protection of Human Subjects’ guidelines concerning reimbursement for research time and parking. Participants were paid $20/hour for completing required research activities (e.g., EEG/ERP tests, providing urine sample, completing self-report forms, neurofeedback session, etc.) at each visit.
Psychiatric status questionnaires, drug use and psychosocial functioning screening
The Structured Clinical Interview for DSM-IV (SCID I) (First et al., 2001) was used for Axis I diagnoses. Posttraumatic Stress Disorder (PTSD) was assessed using The Post-traumatic Symptom Scale - Self Report (PSS-SR) (Foa et al., 1989, 1997) questionnaire. The Beck Depression Inventory (BDI-II, Beck et al., 1996) was used to measure symptoms of depression. PTSD and depression scores were assessed both before and after treatment. Handedness of patients was assessed using the Edinburgh inventory (Oldfield, 1971). Scores from the Addiction Severity Index (ASI) were used to measure problem severity in the areas of medical, employment, drug abuse, legal, family, social, and psychiatric difficulties (McLellan et al., 1980). Cocaine Negative Consequences Checklist (Michalec et al., 1996) was used to assess short-term and long-term adverse effects resulting from cocaine use. Psychosocial adjustment was assessed using the Social Adjustment Scale (SAS) (Weissman & Bothwell, 1976).
Qualitative urine toxicology screens (DrugCheck 4, NxStep, Amedica Biotech Inc., CA) were conducted in each subject to confirm cocaine abuse. In addition, qualitative urine toxicology screens for amphetamines, opiates and marijuana were performed to assess presence of additional abused substances (e.g., amphetamine, opiates, marijuana). Positive test for marijuana was not considered as exclusion criteria. Qualitative Saliva drug test (ALCO SCREEN, Chematics, Inc., IN) was used during each visit to rule out current alcohol use. Urine drug screens were conducted at the intake stage, and at post-neurofeedback assessment stage.
Subjects in the study
Ten cocaine abusing/dependent subjects (2 females, 8 males) mean age, 44.6 ±8.3, range 35–54 years, 70% Afro-Americans) participated in the study. Eight of them were current cocaine users and all subjects were without PTSD or any other comorbid mental conditions. Seven subjects tested positive for cocaine, and 7 of them tested positive for marijuana use as well. One tested positive for opiates and admitted use of heroin along with crack cocaine. Two subjects who did not test positive were recovering addicts enrolled in this study after the inpatient JADAC rehabilitation course with abstinence period of less than 30 days. Their use of cocaine within a month was confirmed by the hospital records. One of them tested inconclusive positive for cocaine at intake, but a repeated test on the following week did not confirm drug use. Therefore the majority of our outpatient population consisted of current cocaine users, with more than half of them using marijuana as a second drug of choice. The preferred form of drug administration was smoking crack cocaine. Only one cocaine addict subject in this study used cocaine intravenously. The majority of addicted subjects (80 %) reported regular use of nicotine/smoking. None of the subjects in the group were in any treatment program other than participating in Narcotics Anonymous (NA), Alcoholic Anonymous (AA) meetings or local church-based anti-drug counseling programs. Only one subject was left handed. Subjects enrolled in the study were fully informed about the nature of this research and signed informed consent form approved by the Institutional Review Board (IRB) of the University of Louisville. For the specimen collection (urine drug screens and alcohol saliva tests) subjects signed a separate consent form also approved by the IRB within the same study protocol.
Cue reactivity test: Stimulus presentation, EEG data acquisition and signal processing
All stimulus presentation, behavioral and subjective response collection was controlled by a computer running E-prime software (Psychology Software Tools [PST], PA). Visual stimuli were presented on a 15" flat-panel display. Behavioral responses (e.g., reaction time) were collected with a 5-button keypad (Serial Box, PST, PA). Subjects were instructed to press key number 1 when they saw a picture of target category, and to not press a key to non-target category images. In all experiments subjects were seated in a chair with their chin in a chinrest. The chinrest was placed so that subject's eyes were 50 cm from the center of the flat panel screen. Breaks were provided every 10 minutes. All EEG data were acquired with a 128 channel Electrical Geodesics system (Net Station 200, v. 4.0) (Electrical Geodesics Inc. [EGI], OR) running on a Macintosh G4 computer. EEG data are sampled at 500 Hz, 0.1 – 100 Hz analog filtered, referenced to the vertex (Cz). The Geodesic Sensor Net is a lightweight elastic thread structure containing silver/silver-chloride electrodes housed in a synthetic sponge on a pedestal. The sponges are soaked in a potassium chloride solution to render them conductive. Impedance of sensors was in maintained below the range recommended by the EGI manual (40 kOhm). Stimulus-locked EEG data are segmented off-line into 1000 ms epochs spanning 200 ms pre-stimulus to 800 ms post-stimulus around the critical stimulus events. For example in our cue reactivity task the events were: (1) neutral target of household category, (2) neutral non-target of household category, (3) neutral target of animal category, (4) neutral non-target of animal category; (5) drug target, (6) drug non-target, and (7) neutral non-target nature images (standards). Frequency of targets for each category (household, animal and drug) was 25%. There were always 50% of neutral pictorial (all non-drug, neutral other than household or animal category) standards in each block of trials. Data were first visually inspected and then digitally screened for artifacts (eye blinks, movement, etc.) and bad trials were removed using built-in EGI Net Station artifact rejection tools. The remaining data were sorted (segmented) by condition and exported for further analysis using MATLAB routines described below in the data analysis section. EEG sites presented in Figure 1 were selected for evoked and induced gamma response analysis.
Figure 1.
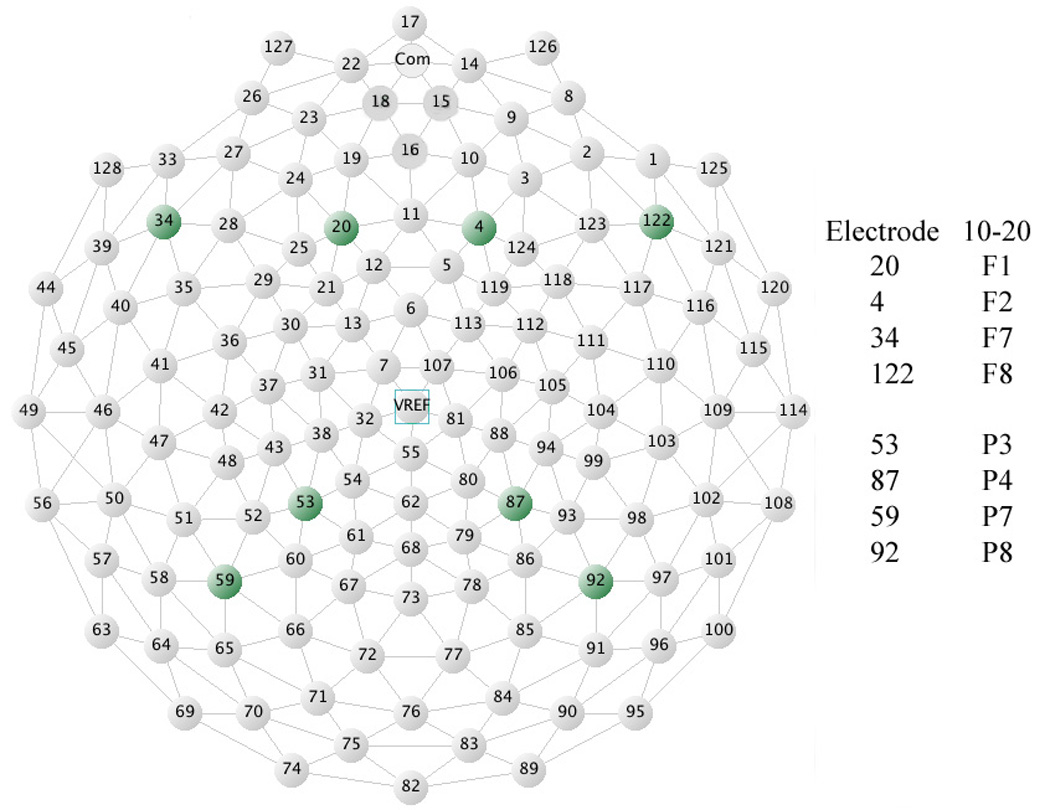
Graphical illustration of electrodes in the EGI layout format. Electrodes selected for this particular experiment included the EGI layout analogues of 10-5 system sites: F1, F2, F7, F8, P3, P4, P7, and P8 electrodes.
Pictorial stimuli
The pictorial materials were taken from the International Affective Picture System (IAPS, Lang et al., 2001). Numbers of each IAPS picture used in the study are available upon request. Cocaine images were selected and validated by a co-author (ES) during his post-doctoral fellowship at Rice University (Houston, TX). In that prior study (Potts, Martin, Stotts, George, & Sokhadze, unpublished report), 25 cocaine abusing patients rated 115 cocaine-related images on 5-point scale (5 being high) as to how evocative each drug image was. The mean rating for the entire set was 2.66, SD=0.48. There were selected 30 images that had the highest rating (all 30 with mean rating above 3.0) for use in this study. Valence, arousal, and dominance rates were matched within each set of images in neutral and traumatic stress categories using ratings from the IAPS database (Lang et al., 2001). The experiment used pictures from two neutral categories as targets: neutral (household items and animals), and one drug category (cocaine and drug paraphernalia). Subjects were instructed to respond to stimulus items from one of the categories, ignoring the others within each block (e.g., targets are household items in a “neutral” block, Figure 2). The order of blocks (with 240 trials per block) was counter-balanced. In the task a stimulus was presented on a screen for 200 ms, whereas recording of EEG data occurred for 1000 ms. Inter-trial interval varied in 1500~2000 ms range to avoid anticipation effects. Each of the three blocks of trials was followed by a short break. The task took approximately 30 minutes to complete. The cue reactivity test was followed by a 10–15 min debriefing to let cocaine cue-induced craving to fade out. Repeated cue reactivity was administered within a week after completion of 12 sessions of neurofeedback training.
Figure 2.
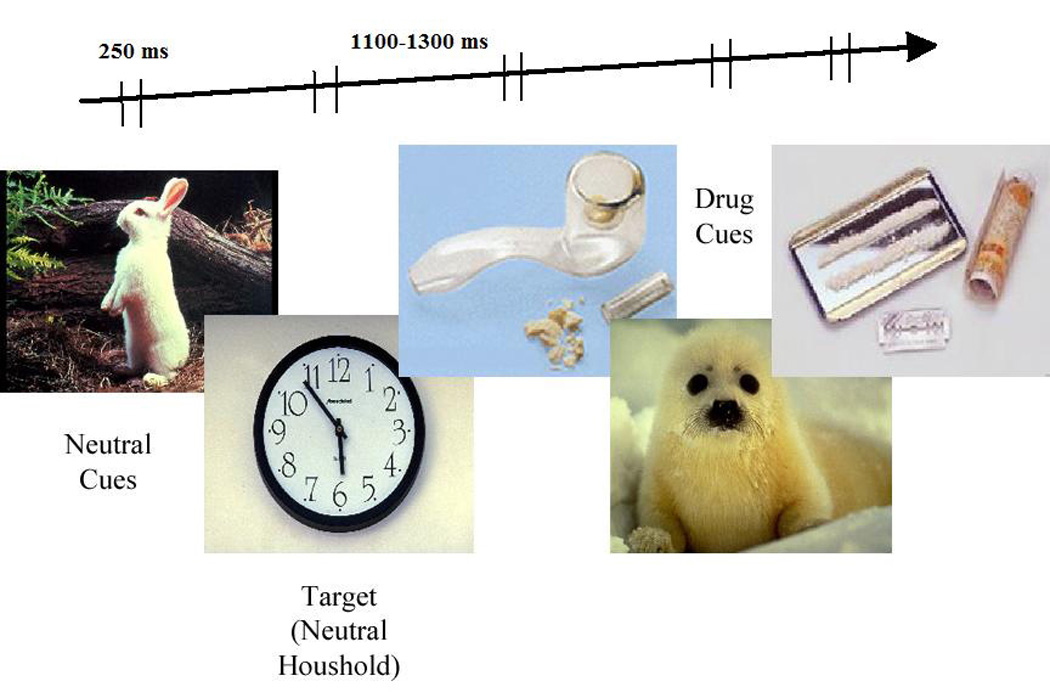
Examples of visual stimuli used during the cue reactivity test. In this experiment targets were household items (25%, target neutral cues). Drug cues were used as distracters (25%, non-target drug cues), while animals and other nature images were non-target neutral cues (50%, non-target standard cues).
Neurofeedback procedure
During neurofeedback treatment the subjects were trained to enhance amplitude of SMR within specified frequency band (12–15 Hz at C3 with a monopolar reference on the left mastoid) and/or decrease (suppress) amplitude of Theta frequency bands (4–7 Hz at F3 with monopolar reference to the left mastoid) over 12 sessions (2 sessions/week). Visual and auditory real time online feedback was provided using a C-2 J&J Engineering device with Physiodata software (J&J Engineering Inc, Poulsbo, WA). Each session in the SMR/Theta protocol was conducted using a standardized procedure lasting no more than 30 min.
Immediately after attachment of electrodes and impedance check (< 5 kOhms) and 4 min long baseline recording, subjects performed four 7-min long blocks of neurofeedback training (operant conditioning of specified EEG frequencies – suppression of Theta and enhancement of SMR). EEG was recorded with the sampling rate of 1024 Hz recorded from C3 with reference on the left mastoid and ground electrode placed on the right earlobe. The EEG biofeedback procedure was based on Lubar’s ADHD protocol in its late modifications (Lubar, 2003), and the first part of Scott & Kaiser’s modification of Peniston’s brainwave training protocol for alcohol/drug abuse treatment (Scott et al., 2005). During neurofeedback training, patients were trained to increase their SMR amplitude and decrease or keep on the same level their slow wave activity (e.g., theta). Out modified neurofeedback training protocol consisted of rewarding enhanced EEG amplitudes at the sensorimotor strip (C3) in the 12–15 Hz frequency range, while simultaneously inhibiting or at least trying to keep on the same level excessive low frequency (4–7 Hz) at the frontal F3 site. Self-adjusting thresholds were used for continuous visual and auditory feedback. SMR and theta amplitude changes (in percent vs. mean baseline level values) were calculated on a per minute basis. All four blocks of neurofeedback training included SMR enhancement, while two out of four had theta level control included along with the SMR enhancement task. One of these blocks included SMR/Theta ratio training.
As well as recording EEG, we also recorded electromyogram (EMG) from the left neck, skin conductance level and skin temperature for monitoring of associated peripheral physiological measures changes during neurofeedback session. Physiological data was stored for further analysis.
b. Motivational Interviewing procedure
Motivational Interviewing (MI) (Miller & Rollnick, 2002; Treasure, 2004) is a brief psychotherapeutic intervention for behavioral change aimed to bring about rapid commitment to change addictive behaviors. The MI (referred also as Motivation Enhancement Therapy [MET]) is designed to increase the compliance and probability of treatment entry and abstinence (Burke et al., 2003). This behavioral therapy is considered to be especially useful for the drug-dependent individuals who are ambivalent about changing their habits, since MI is specifically targeting ess motivated individuals. Two or three forty-five minute-long MI sessions were conducted by Dr. Stewart, specialist in addiction psychiatry trained in MET. Each subject received at least 2 sessions of MI, while 5 subjects from the group volunteered for a third (optional) MI session. There was at least 1 week waiting period between MI visits.
Data Analysis
Power and wavelet
Data were collected and stored using Net Station (EGI). Immediately following the cue reactivity test, the EEG data was tagged according to the appropriate triggers in Net Station and segmented into the appropriate response categories (e.g. drug-target, drug-non target, neutral-target and neutral-non target) and exported to MatLab for wavelet analysis. Waveleting was used to elucidate the frequency components of a signal as they vary in time. By plotting the resulting wavelet data it was possible to measure the precise timing and strength of the gamma response, both early evoked and late induced, in relation to a given cue. The data were subject to wavelet analysis using the continuous Morlet window and the continuous wavelet transform (Figure 3). The waveleted signal was further passed on for band pass filtering using a custom designed Harris 7 window (Figure 4). The flow chart of data processing is shown on Figure 5.
Figure 3.
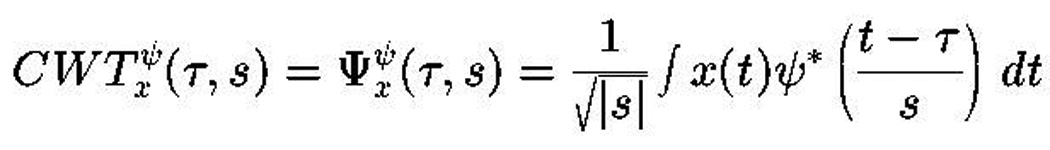
Continuous Wavelet Transform:
S (Scale) = 1/frequency
Tau (τ) = time shift
Psi (ψ) = mother wavelet (in our case the Morlet window)
Figure 4.
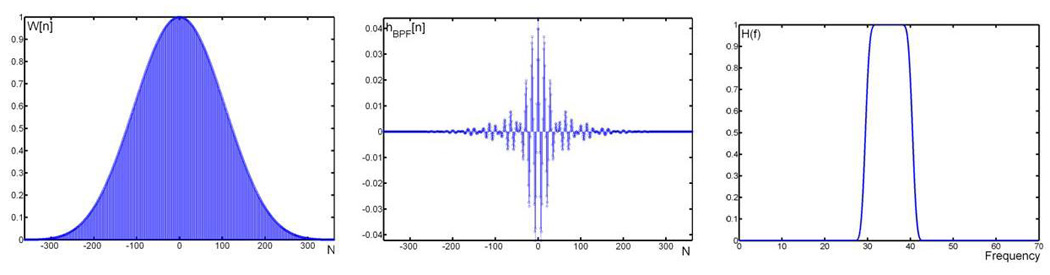
Left: Harris 7 window with a band pass filter centered at 35 Hz is created (using 725 samples). Middle: Impulse response of the designed Harris Window. Right: Pass band frequencies of the constructed band pass filter.
Figure 5.

Flow-chart outlining the power analysis process.
The Harris window used a number of samples (n) of 725 and was designed to allow complete passage of signals from the 30–40 Hz range. An attenuation band of one Hz was present in the system. The resulting signals now consisted of only the gamma band frequency components and could be summated to yield the relative power of the gamma band.
Statistical analysis
Statistical analysis was performed on the subject-averaged data with the subject averages being the observations. The primary analysis model implemented was the repeated measures ANOVA, with physiological dependent variables being those described above. Each single gamma oscillations trial was analyzed for pre-selected frontal and parietal EEG sites and time window (0–180, 280–480 ms post-stimulus). Data for each dependent gamma EEG variable was analyzed using repeated-measures ANOVA. Factors included Stimulus (target or non target), Cue (drug or neutral), Hemisphere (right or left) and Topographic location (anterior or posterior). Using SPSS (v. 18) analysis package a model was created to test for significant interactions between electrodes in both lateral (i.e., inferior that include F7, F8, P7 and P8 EEG sites) and medial (F1, F2, P3, and P4) locations pre and post neurofeedback training. Analysis was carried out for both early and late gamma windows. A-priori hypotheses were tested with two-tailed Student’s t-tests for 2 groups with unequal variance. In all ANOVAs, Greenhouse-Geisser corrected p-values were employed where appropriate.
RESULTS
SMR and Theta changes in neurofeedback sessions
All subjects successfully completed twelve 25–30 min long sessions of SMR-up/theta-down sessions and at least 2 Motivational Interviewing sessions (conducted by Dr. Stewart and his associate, addiction psychiatry fellow Dr. Husk). The mean increase of the SMR amplitude as compared to daily baseline level across all neurofeedback sessions was 17.06 %, SD=15.04 (t=3.20, p=0.007), but mean change of theta amplitude was not significant . Regression analysis showed that increase of SMR vs. baseline along with neurofeedback session numbers was not linear. Considering that out of 10 participants only 8 were available for the post-neurofeedback (within a week after completion) clinical assessments and cue reactivity test, all results are reported for 8 subjects (i.e., hereafter all statistical calculations used N=8/group).
Effects of neurofeedback on RT and EEG gamma power in cue reactivity test (post-treatment)
Behavioral responses
There were no significant differences in reaction time (RT, Mean 603.6 ± Standard Deviation 120.6 ms pre- vs. 576.9 ± 122.4 ms for drug targets post-neurofeedback, n.s.) and accuracy (percentage of commission and omission errors) in the cue reactivity test following neurofeedback treatment.
Effects of neurofeedback on evoked (early) EEG gamma responses in cue reactivity test
Neurofeedback affected predominantly evoked early gamma responses to non-target drug stimuli bilaterally at the frontal and parietal sites (all p<0.05). The power of gamma oscillations to non-target drug cues significantly decreased post-treatment with decreases ranging from −23.6 % (P8) up to −44.94 % (P3), mean − 35.84 % with SD across the EEG channels 7.43 %. Gamma response to target drug cues was less pronounced (−9.65 ± 7.21 %) and was significant only at F2, F8, P3, and P7 sites. Changes of gamma power in response to target and non-target drug cues at each EEG recording site are presented in Table 1.
Table 1.
Pre- and post-neurofeedback values of relative gamma (30–40 Hz) power and changes in percent (pre-minus-post) for evoked and induced gamma responses to target and non-target drug cues at different topographies.
| EEG Channel/condition |
Early Evoked Gamma Power | Late Induced Gamma Power | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | % Change F(1,14) | Sig. | Pre | Post | % Change F(1,14) | Sig. | |||
| AFz-Non-target drug | 0.399 | 0.347 | −15.0 | 18.8 | 0.001 ** | 0.436 | 0.297 | −46.8 | 182.4 | 0.000*** |
| AFz-Target drug | 0.520 | 0.499 | −4.2 | 0.4 | 0.524 | 0.563 | 0.472 | −19.3 | 28.2 | 0.000*** |
| F1-Non-target drug | 0.392 | 0.321 | −22.1 | 8.5 | 0.014 * | 0.429 | 0.355 | −20.8 | 8.1 | 0.014* |
| F1-Target drug | 0.495 | 0.467 | −6.0 | 1.0 | 0.332 | 0.552 | 0.436 | −26.6 | 15.0 | 0.003** |
| F2-Non-target drug | 0.400 | 0.319 | −25.4 | 18.1 | 0.001*** | 0.427 | 0.360 | −18.6 | 7.3 | 0.018* |
| F2-Target drug | 0.523 | 0.478 | −9.4 | 7.2 | 0.023* | 0.563 | 0.445 | −26.5 | 17.4 | 0.002** |
| F7-Non-target drug | 0.382 | 0.318 | −20.1 | 11.4 | 0.006** | 0.426 | 0.309 | −37.9 | 59.1 | 0.000*** |
| F7-Target drug | 0.46 | 0.485 | 5.2 | 0.9 | 0.376 | 0.546 | 0.475 | −14.9 | 6.5 | 0.029* |
| F8- Non-target drug | 0.388 | 0.299 | −29.8 | 77 | 0.000*** | 0.436 | 0.314 | −38.9 | 37 | 0.000*** |
| F8-Target drug | 0.499 | 0.469 | −6.4 | 2.6 | 0.139 | 0.476 | 0.467 | −1.9 | 0.0 | 0.924 |
| P3- Non-target drug | 0.387 | 0.276 | −40.2 | 22.5 | 0.001** | 0.419 | 0.285 | −47.0 | 55.0 | 0.000*** |
| P3-Target drug | 0.513 | 0.442 | −16.1 | 4.1 | 0.071 | 0.432 | 0.438 | 1.4 | 0.0 | 0.958 |
| P4- Non-target drug | 0.399 | 0.325 | −22.8 | 5.5 | 0.039* | 0.436 | 0.322 | −35.4 | 20.5 | 0.001** |
| P4- Target drug | 0.465 | 0.453 | −2.6 | 0.2 | 0.641 | 0.536 | 0.457 | −17.3 | 12.3 | 0.006** |
| P7- Non-target drug | 0.386 | 0.281 | −37.4 | 23.7 | 0.000*** | 0.417 | 0.279 | −49.5 | 120.8 | 0.000*** |
| P7-Target drug | 0.496 | 0.433 | −14.5 | 3.7 | 0.082 | 0.554 | 0.433 | −27.9 | 24.3 | 0.001** |
| P8- Non-target drug | 0.379 | 0.326 | −16.3 | 5.0 | 0.048* | 0.438 | 0.313 | −39.9 | 38.7 | 0.000*** |
| P8-Target drug | 0.456 | 0.465 | 1.9 | 0.1 | 0.71 | 0.560 | 0.465 | −20.4 | 26.1 | 0.001** |
Cue (drug, neutral) had main effects both at medial (i.e., F1, F2, P3, P4) and lateral (i.e., inferior, F7, F8, P7, P8) EEG channels with more at medial (F=9.43, p=0.001) compared to lateral (F=5.05, p=0.044). The Stimulus (non-target, target) main effect was highly significant both medially and laterally (medial, F=268.05, p<0.0001; lateral F=196.75, p<0.0001).
Effects of neurofeedback on induced (late) EEG gamma responses
Neurofeedback affected induced gamma responses both to target and non-target drug stimuli bilaterally at most frontal and parietal sites, except responses to targets at P3. The power of gamma oscillations to non-target drug cues significantly decreased post-treatment (across all channels, mean −47.17 ± 9.88 %), while decreases to target drug cues was also significant but slightly less expressed (−21.58 ± 5.09 %).
Cue (drug, neutral) had main effects both at medial and lateral EEG channel groups (F=34.28, p<0.001, and F=27.20, p<0.001 respectively). The Stimulus (non-target, target) main effect was also significant medially and laterally (medial sites – across F1, F2, P3, and P4, F=80.52, p<0.0001; lateral sites- F7, F8, P7, and P8, F=1173.16, p<0.0001).
Topographic differences and interaction effects
Evoked gamma
Early gamma responses showed a Stimulus (non-target, target) × Treatment (pre-, post-NFB) interaction both at medial (F=34.82, p<0.001) and lateral (F=29.82, p<0.001) channels with more of a pronounced decrease in gamma activity to non-target compared to target cues. A three-way Stimulus × Cue (drug, neutral) × Treatment interaction was significant only at the medial channel group (F=7.99, p=0.015) and can be described as more of a significant decrease to non-target rather than target drug cues following neurofeedback training. There was a tendency for a Hemisphere (left, right) × Topography (anterior, posterior) × Treatment interaction, but the effect did not reach significance (F=4.56, p=0.056, n.s.).
Induced gamma
Induced gamma responses showed a Stimulus (non-target, target) × Treatment (pre-, post-NFB) interaction only at lateral EEG channels (F=60.78, p<0.001). Again, the effect manifested as a clearer global decrease in gamma power to non-target cues (Figure 6). A Stimulus × Cue × Treatment interaction was significant both at the medial (F=6.29, p=0.022) and lateral (inferior) channels (F=4.72, p=0.049) and was characterized by more significant decreases in gamma induced by non-target compared to target drug cues post- neurofeedback. Figure 6 and Figure 7 show a relatively more visible decrease of evoked and induced gamma responses to non-target and as compared to target drug cues after neurofeedback based therapy.
Figure 6.
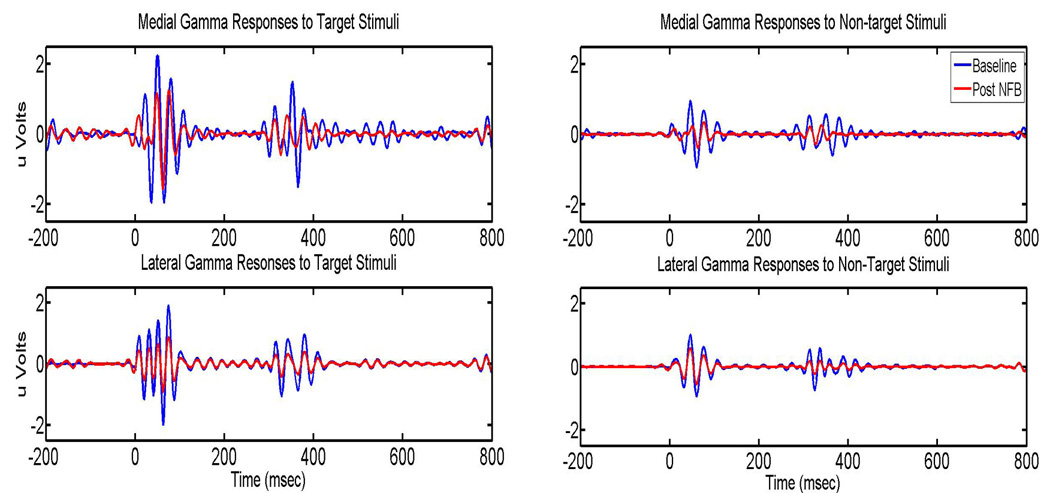
Baseline and post neurofeedback gamma responses (both early and late) for both target and non-target stimuli. Top pane depicts grand-average waveforms for the medial region (F1, F2, P3 and P4) while the bottom pane depicts the grand-average waveforms for the lateral region (F7, F8, P7, and P8).
Figure 7.
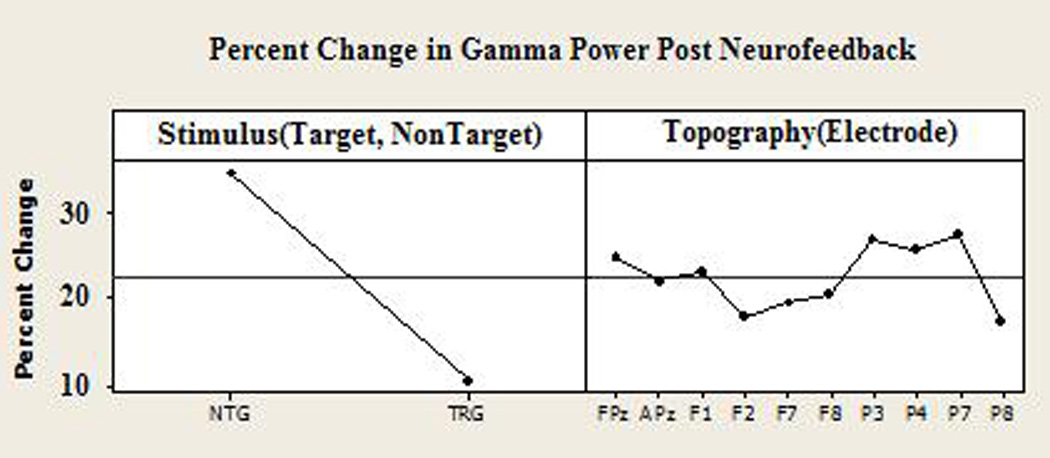
Analysis of gamma response change (in percent) across electrodes. Results show a higher percent change to non-target rather than target stimuli (left), and all electrodes changing in a consistent pattern (i.e. Topography was not a significant factor in percent change of gamma power).
Clinical evaluations and drug tests after neurofeedback and Motivational Interviewing
Results of the clinical evaluations showed decreased perceived depression and stress. Following neurofeedback sessions, subjects reported to have reduced depression scores (from 22.2 ± 6.9 at pre- to 13.6 ± 8.7 at post-NFB, two-tailed Student’s t-test, t=3.30, p=0.004) as measured by the BDI-II (Beck et al., 1996); additionally there was a reduced stress score (from 29.9 ± 8.6 to 20.1 ± 13.9, t=1.95, p=0.041) as measured by the PSS-SR (Foa et al., 1989, 1997). Post-neurofeedback urine drug screens showed a marginal decrease in positive cocaine tests (t=1.96, p=0.04) and a significant decrease in positive tests for marijuana use (t=2.44, p=0.018). Most of the subjects reported a decrease in the amount of cocaine and marijuana used and improvements in social status (i.e., resuming study at school, employment, housing, financial security, problems with law, etc.); however, in this study we did not have any independent sources (e.g., family members, neighbors or social workers reports) to confirm self-reported data collected from our subjects. Considering that from ten participants originally enrolled in this neurofeedback study all planned clinical, behavioral, and EEG data were collected from 8, a retention rate of 80% was maintained for the outpatient subjects in the study.
DISCUSSION
Outpatient subjects with cocaine addiction completed the biobehavioral intervention and successfully increased SMR while keeping theta practically unchanged in 12 sessions of neurofeedback training. The addition of Motivational Interviewing helped retain patients in the study. Two subjects were not available for post-neurofeedback cue reactivity EEG testing. Even though both of them were tested at a 3-month follow-up stage we report here data on 8 patients only. Clinical evaluations immediately after completion of the treatment showed decreased self-reports on depression and stress scores, and urine tests collaborated reports of decreased use of cocaine and marijuana.
Effects of neurofeedback resulted in a lower EEG reactivity to drug-related images in a post-neurofeedback cue reactivity test. In particular, evoked gamma showed decreases in power to non-target and to a lesser extent target drug-related cues at all topographies (left, right, frontal, parietal, medial, inferior); while induced gamma power decreased globally to both target and non-target drug cues. Our findings supported our hypothesis that gamma band cue reactivity measures are sufficiently sensitive functional outcomes of neurofeedback treatment. Both evoked and induced gamma measures were found capable to detect changes in responsiveness to both target and non-target drug cues.
Application of wavelet transformation analysis and custom-made MATLAB routines provided more advanced and refined outcomes for neurotherapy and functional diagnostics research; these methods should be incorporated in future studies when EEG measures are used for the assessment of attentional biases to drug-related cues in addicts enrolled in biobehavioral therapy. Our findings support the notion that neurofeedback combined with cognitive-behavioral therapy (Motivational Interviewing) is a potentially effective approach to the treatment of outpatient substance abusers. Our data are in accord with a previous report that even brief motivational interviewing may reduce cocaine use and engage drug abusers in treatment (Bernstein et al., 2005; Burke, Arkowitz, & Menchola, 2003).
According to Lenz et al. (2008) even in the new era of functional magnetic resonance imaging (fMRI), the EEG still represents an important tool for psychiatry research. The EEG reflects the electrical activity of large populations of synchronized neurons, mostly cortical pyramidal neurons. Therefore, some mental diseases can be more easily identified with EEG than with functional imaging, especially when the disease manifests in a form of altered electrical brain activity such as in ADHD (Barry et al., 2003; Clarke et al., 1998, 2001). Cocaine addiction is also accompanied by alterations in EEG responses (Alper et al., 1990, Costa & Bauer, 1997; Herning et al., 1985, 1994b). New trends in cognitive neuroscience make it possible to study the neural network dynamics of mental disorder, and they have strongly contributed to the study of predisposition and brain dysfunction in psychiatric populations--specifically in substance use disorders (Banaschewski et al., 2003, Herrmann & Demiralp, 2005, Prichep & John, 1997; Prichep et al., 1996, 1999; Sokhadze et al., 2007; van der Stelt et al., 2001).
Models of brain function in psychopathologies presented with executive deficit symptoms emphasize frontal/parietal interactions in deficits of attention (Silberstein et al., 1998, Shaw et al., 2006) and anterior cingulate/lateral pre-frontal cortex interactions in behavioral disinhibition (Barkley, 1997, Williams, 2006). Mechanisms of large-scale coordination between cortical areas can be explored via measures of specific frequency waves in the EEG. The present research focuses on theta (4–7 Hz) and sensorimotor (12–15 Hz) rhythm training with neurofeedback, and gamma (30–40 Hz) wave oscillatory activity during a cue reactivity test in SUD.
Previous EEG, ERP, and fMRI research has contributed to the understanding of impairments in attention, executive functions, and memory in cocaine addiction (Volkow et al., 2003). However, there is a lack of studies investigating substance abuse-related differences in the gamma range of EEG although gamma oscillations are directly associated with executive prefrontal processes (e.g., cortical inhibition, attention, error monitoring, working memory, etc.), which are thought to be impaired in addicted patients. Deviations of gamma responses indicate that early mechanisms of sensory stimulus processing are altered in addiction, resulting in disproportional significance and pre-attentive orienting to stimuli associated with drugs (Carter & Tiffany, 2007; Franken et al., 2004,2006; Garavan et al., 2000; Sokhadze, Stewart, & Hollifield, 2007). Sensitization to drug-related cues is a well known phenomenon in addiction research (Franken 2003; Robinson & Berridge, 1993), even though the neurobiological mechanisms of this over-reactivity and its behavioral consequences are not yet clearly defined. We believe that post-treatment assessments following intervention in substance-dependent individuals should incorporate physiological drug reactivity measures and cannot be limited to self-reported outcomes or clinical evaluations only.
Historically evoked and event-related potentials have been the primary electrophysiological indices of cognitive processes and have provided important insights into human brain functions. There is now substantial research suggesting that some ERP features arise from oscillatory changes due to sensory and cognitive processes, and these changes influence the dynamics of EEG in different frequency bands (Rangaswamy & Porjesz, 2008; Herrmann & Demiralp, 2005; Yordanova et al., 2001). The study of the functional correlates of evoked and event-related EEG oscillations, including those in portions of the high frequency gamma band, has recently become an important branch of cognitive neuroscience. The current review by Başar & Güntekin (2008) includes analysis of EEG oscillations in ADHD, alcoholism, substance abuse and those with genetic disorders.
The early, time-locked gamma response is mainly related to the earlier operations of information processing that culminate in sensation and perception (Karakaş et al., 2006). The gamma response that occurs as non-phase-locked activity in the late time window (i.e., induced) varies as a function of task demands and represents perceptual/cognitive processes. The non-phase-locked activity of the late gamma response is thus basically a phenomenon of pattern recognition or short-term memory, and it fulfills perceptual and cognitive functions (Karakaş et al., 2006; Muller et al, 2000; Tallon-Baudry, 2003). According to Tallon-Baudry & Bertrand (1999) induced gamma activity could reflect the activation of an object representation, both in the visual and auditory modality. According to some studies, the area-specific 40 Hz centered gamma activity response represents “perception of coherent visual patterns” and is related to “perception of meaningful visual elements” and thus to higher cognitive processes (Lutzenberger et al., 1995). Our study showed that gamma responses to motivationally salient visual stimuli (i.e., drug images to substance addicted subjects) were not significantly dependent on topography; this was probably due to the coherent simultaneous activation of gamma activity at functionally connected brain areas.
EEG coherence analysis is a technique that investigates the pair-wise correlations of power spectra obtained from different electrodes. It measures the functional interaction between cortical areas in different frequency bands. A high level of coherence between two EEG signals indicates co-activation of neuronal populations and provides information on functional coupling between these areas (Franken et al., 2004). EEG coherence abnormalities were reported in patients with cocaine (Roemer et al., 1995), heroin (Fingelkurts et al., 2006a,b), and marijuana dependence (Struve et al., 1989, 1999, 2003). In the next stage of our research we plan to conduct coherence analysis of oscillations in the gamma band both at early (80–180 ms) and late (280–480 ms) post-stimulus phases of oscillatory responses to drug cues.
For a neurofeedback protocol we used EEG recordings over the sensorimotor cortex (e.g., C3, C4) which shows a very distinctive oscillatory pattern in the 12–15 Hz frequency range and is termed the ‘sensorimotor rhythm’ (Hoedlmoser et al., 2008; Howe & Sterman, 1972; Sterman Wyrwicka, 1967) or the ‘Rolandic mu rhythm.’ The SMR usually appears as the dominant EEG activity during quiet and relaxed but alert wakefulness, and becomes desynchronized during planning, execution, or the imagination of movements and motor acts (Pfurtscheller et al., 2006; Neuper et al., 2006; Howe & Sterman, 1972). There is a growing body of evidence suggesting the feasibility of self-regulation training of the SMR for the correction of altered EEG patterns associated with specific disorders (e.g., ADHD, epilepsy, insomnia, etc.) (reviewed in Arns et al., 2009; Sterman & Egner, 2006; Monastra, 2003; Rossiter & La Vaque, 1995). Prior studies of SMR neurofeedback had methodological shortcomings, such as missing clearly defined treatment targets, neglecting pre- post SMR neurofeedback assessments of EEG, etc. (Hoeldmoser et al., 2008). The SMR is known to be associated with inhibition of motor activity (Chase & Harper, 1972) and can be considered as the dominant ‘standby’ frequency of the integrated thalamo-cortical, somato-sensory and somato-motor pathways (Sterman & Egner, 2006). Similarly, synchronization of the SMR has been suggested to be a correlate of an ‘idling’ state (Mullholand & Human,1995, Hummel et al., 2002). According to a theoretical perspective (Hoeldmoser et al., 2008), the utility of SMR relates to the numerous findings that define this EEG rhythm as a thalamo-cortical consequence of decreased motor excitability (Pfurtscheller et al., 1996) arising both intentionally and passively from the imposition of motor inhibition from functional reorganization within striatal (basal ganglia) circuits (Sterman & Egner, 2006).
It is possible to propose that a treatment approach that uses neuromodulation techniques to activate and strengthen circuits involved in inhibitory control, including self-regulation training directed at the normalization of frontal and central cortical activity, may increase ability to successfully maintain abstinence from drug-seeking and drug-taking behaviors typical of addicted individuals.
This pilot study has several limitations that need to be addressed. The size of the sample was relatively small and the majority of patients were males with a relatively long history of crack cocaine use, though only half of them had a documented cocaine dependence diagnosis. The study can be considered an extended case series since we did not have a control group. Motivational Interviewing sessions were combined with neurofeedback making it impossible to differentiate between effects of these two arms. The study was limited to paarticipants without a comorbid mental condition, though in our previous studies using similar pictorial and verbal cue reactivity tests we demonstrated important behavioral and ERP group differences in patients with and without co-occurring psychiatric conditions--specifically in dually diagnosed patients with cocaine abuse comorbid with post-traumatic stress disorder (PTSD) (Sokhadze et al., 2007, 2008b). Despite the above listed limitations it should be taken into account that the study had a clearly defined methodological emphasis and the main claims are related to improvements of cue reactivity tests in addiction using advanced methods of high frequency EEG band response measures. We admit in the development of the notion that this case series is limited in that no far going conclusions can be drawn from it, but we believe that a suggested methodological approach lays the methodological and rationale groundwork for further studies in the future. We plan to use gamma responses along with the more standard qEEG measures (e.g., resting eyes-open and eyes closed EEG conditions using dense-array EEG recording) of neurofeedback-based treatment outcomes.
Future studies have to address questions of whether neurotherapy integrated with specific forms of cognitive-behavioral therapy (CBT, which should not be limited to Motivational Interviewing) might be successfully applied to dually-diagnosed patients with different combinations of SUD and mental disorders, and whether observed changes are stable in the long term. The crucial point concerning this type of neurotherapy approach is that it directly acts on the brain oscillations which are altered in SUD and comorbid conditions (e.g., excess of theta in ADHD). Future studies should continue to focus on the clinical, electrophysiological (e.g., qEEG) and cognitive effects of neurofeedback integrated with CBT on more extended sets of neurocognitive tasks and also address the possible clinical significance of the integrated biobehavioral training as a treatment arm for dual diagnosis.
CONCLUSIONS
Our study emphasizes the utility of cognitive neuroscience methods based on qEEG measures for the assessment of the functional outcomes of EEG neurofeedback-based biobehavioral interventions for addictive disorders. This approach may have significant potential for identifying both physiological and clinical markers of treatment progress. These markers may provide useful information for planning interventions in substance use disorders. The specific aim of this pilot study was to determine the presence of an attentional bias to preferentially process drug-related cues (using evoked and induced gamma reactivity measures) in cocaine addicts before and after 12 sessions of behavioral treatments based on neurofeedback. Our hypothesis was that central SMR amplitude increase is possible in an experimental outpatient SUD group over 12 operant conditioning sessions. The results confirmed out prediction that EEG changes achieved with neurofeedback training will be accompanied by positive EEG outcomes in cue reactivity and clinical improvements.
Acknowledgements
The project was funded by NIDA R03 DA021821, ISNR Research Committee grant to Estate Sokhadze, and AAPB Foundation for Education and Research in Biofeedback research grant for graduate students awarded to Tim Horrell.
REFERENCES
- Alper KR. The EEG and cocaine sensitization. Journal of Neuropsychiatry and Clinical Neuroscience. 1999;11:209–221. doi: 10.1176/jnp.11.2.209. [DOI] [PubMed] [Google Scholar]
- Alper KR, Chabot RJ, Kim AH, Prichep LS, John ER. Quantitative EEG correlates of crack cocaine dependence. Psychiatry Research. 1990;35:95–106. doi: 10.1016/0925-4927(90)90013-v. [DOI] [PubMed] [Google Scholar]
- Alper KR, Prichep LS, Kowalik S, Rosenthal MS, John ER. Persistent QEEG abnormality in crack cocaine users at 6 months of drug abstinence. Neuropsychopharmacology. 1998;19:1–9. doi: 10.1016/S0893-133X(97)00211-X. [DOI] [PubMed] [Google Scholar]
- Arns M, de Ridder S, Strehl U, Breteler M, Coenen A. Efficacy of neurofeedback treatment in ADHD: The effects on inattention, impulsivity and hyperactivity: A meta-analysis. Clinical EEG Neurosciences. 2009;40(3):180–189. doi: 10.1177/155005940904000311. [DOI] [PubMed] [Google Scholar]
- Banaschewski T, Brandeis D, Heinrich H, Albrecht B, Brunner E, Rothenberger A. Association of ADHD and conduct disorder—brain electrical evidence for the existence of a distinct subtype. Journal of Child Psychology and Psychiatry. 2003;44:356–376. doi: 10.1111/1469-7610.00127. [DOI] [PubMed] [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychological Bulletin. 1997;121(1):65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Barry RJ, Clarke AR, Johnstone SJ. A review of electrophysiology in attention-deficit/hyperactivity disorder: I. Qualitative and quantitative electroencephalography. Clinical Neurophysiology. 2003;114:171–183. doi: 10.1016/s1388-2457(02)00362-0. [DOI] [PubMed] [Google Scholar]
- Başar E, Güntekin B. A review of brain oscillations in cognitive disorders and the role of neurotransmitters. Brain Research. 2008;1235:172–193. doi: 10.1016/j.brainres.2008.06.103. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Beck Depression Inventory II. San Antonio: The Psychological Corporation; 1996. p. 38. [Google Scholar]
- Bernstein J, Bernstein E, Tassiopoulos K, Heeren T, Levenson S, Hingson R. Brief motivational intervention at a clinic visit reduces cocaine and heroin use. Drug Alcohol Dependence. 2005;77(1):49–59. doi: 10.1016/j.drugalcdep.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Burke BL, Arkowitz H, Menchola M. The efficacy of motivational interviewing. Journal of Consulting and Clinical Psychology. 2003;71:843–861. doi: 10.1037/0022-006X.71.5.843. [DOI] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327–340. [PubMed] [Google Scholar]
- Chase M, Harper RM. Somatomotor and visceromoior correlates of operantly condiltoned 12–14 C-SEC sensoriirioior cortical activity. Electroencephalography and Clinical Neurophysiololgy. 1971;31:85–92. doi: 10.1016/0013-4694(71)90292-6. [DOI] [PubMed] [Google Scholar]
- Childress AR, Ehrman R, McLellan A, MacRae J, O'Brien CP. Can induced moods trigger drug-related responses in opiate abuse patients? Journal of Substance Abuse Treatment. 1994;11:17–23. doi: 10.1016/0740-5472(94)90060-4. [DOI] [PubMed] [Google Scholar]
- Childress AR, Mozley D, McElgin W, Fitzgerald J, Reivich M, et al. Limbic activation during cue-induced cocaine craving. American Journal of Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke AR, Barry RJ, McCarthy R, Selikowitz M. EEG analysis in attention-deficit/hyperactivity disorder: a comparative study of two subtypes. Psychiatry Research. 1998;81:19–29. doi: 10.1016/s0165-1781(98)00072-9. [DOI] [PubMed] [Google Scholar]
- Clarke A, Barry RJ, McCarthy R, Selikowitz M. EEG-defined subtypes of children with attention-deficit/hyperactivity disorder. Clinical Neurophysiology. 2001;112:2098–2105. doi: 10.1016/s1388-2457(01)00668-x. [DOI] [PubMed] [Google Scholar]
- Costa L, Bauer L. Quantitative electroencephalographic differences associated with alcohol, cocaine, heroin and dual-substance dependence. Drug & Alcohol Dependence. 1997;46:87–93. doi: 10.1016/s0376-8716(97)00058-6. [DOI] [PubMed] [Google Scholar]
- Dackis CA, O’Brien CP. Cocaine dependence: a disease of the brain’s reward centers. Journal of Substance Abuse Treatment. 2001;21:111–117. doi: 10.1016/s0740-5472(01)00192-1. [DOI] [PubMed] [Google Scholar]
- Drummond DC, Tiffany ST, Glautier S, Remington B. Addictive Behaviour: Cue Exposure Theory and Practice. Chichester: Wiley; 1995. [Google Scholar]
- Ehrman RN, Robbins SJ, Childress AR, Goehl L, Hole AV, et al. Laboratory exposure to cocaine cues does not increase cocaine use by outpatient subjects. Journal of Substance Abuse Treatment. 1998;15(5):431–435. doi: 10.1016/s0740-5472(97)00290-0. [DOI] [PubMed] [Google Scholar]
- Fingelkurts AA, Fingelkurts AA, Kivisaari R, Autti T, Borisov S, Puuskari V, Jokela O, Kähkönen S. Reorganization of the composition of brain oscillations and their temporal characteristics in opioid dependent patients. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2006a;30(8):1453–1465. doi: 10.1016/j.pnpbp.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Fingelkurts AA, Fingelkurts AA, Kivisaari R, Autti T, Borisov S, Puuskari V, Jokela O, Kähkönen S. Increased local and decreased remote functional connectivity at EEG alpha and beta frequency bands in opioid-dependent patients. Psychopharmacology. 2006b;188(1):42–52. doi: 10.1007/s00213-006-0474-4. [DOI] [PubMed] [Google Scholar]
- First MD, Spitzer RI, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV-TR axis I disorders-patient edition (SCID-I/P) New York: NY State Psychiatric Institute; 2001. [Google Scholar]
- Foa EB, Cashman L, Jaycox L, Perry K. The validation of a self-report measure of posttraumatic stress disorder The Posttraumatic Diagnostic Scale. Psychological Assessment. 1997;9:445–451. [Google Scholar]
- Foa EB, Steketee G, Rothbaum BO. Behavioral/cognitive conceptualisations of post-traumatic stress disorder. Behavior Therapy. 1989;20:155–176. [Google Scholar]
- Franken IH, de Haan HA, van der Meer CW, Haffmans PM, Hendriks VM. Cue reactivity and effects of cue exposure in abstinent posttreatment drug users. Journal of Substance Abuse Treatment. 1999;16:81–85. doi: 10.1016/s0740-5472(98)00004-x. [DOI] [PubMed] [Google Scholar]
- Franken IHA, Kroon LY, Hendriks VM. Influence of individual differences in craving and obsessive cocaine thoughts on attentional processes in cocaine abuse patients. Addictive Behaviours. 2000;25:99–102. doi: 10.1016/s0306-4603(98)00112-9. [DOI] [PubMed] [Google Scholar]
- Franken IHA. Drug craving and addiction: integrating psychological and psychopharmacological approaches. Progress Neuro-Pharmacology Biological Psychiatry. 2003;27:563–579. doi: 10.1016/S0278-5846(03)00081-2. [DOI] [PubMed] [Google Scholar]
- Franken IHA, Stam CJ, Hendriks VM, van den Brink W. Electroencephalographic power and coherence analysis suggest altered brain function in abstinent male heroin-dependent patients. Neuropsychobiology. 2004;49:105–110. doi: 10.1159/000076419. [DOI] [PubMed] [Google Scholar]
- Franken IHA, Van Strien JW, Nijs IM. Effect of hedonic tone on event-related potential measures of cognitive processing. Psychiatry Research. 2006;142:233–239. doi: 10.1016/j.psychres.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Garavan H, Pankiewicz J, Bloom A, Cho J-K, Sperry L, et al. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry. 2000;157:1789–1798. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C, Phillips RL, Kimes AS, Margolin A. Activation of memory circuits during cue-elicited cocaine craving. Proceedings National Academy of Sciences USA. 1996;93:12040–12045. doi: 10.1073/pnas.93.21.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray CM, Singer W. Stimulus-specific neuronal oscillations in orientation columns of cat visual cortex. Proceedings National Academy of Sciences USA. 1989;86:1698–1702. doi: 10.1073/pnas.86.5.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herning RI, Glover BJ, Guo X. Effects of cocaine on P3B in cocaine abusers. Neuropsychobiology. 1994a;30:132–142. doi: 10.1159/000119148. [DOI] [PubMed] [Google Scholar]
- Herning RI, Glover BJ, Koeppl B, Phillips RL, London ED. Cocaine induced increases in EEG alpha and beta activity: evidence for reduced cortical processing. Neuropsychopharmacology. 1994b;11:1–9. doi: 10.1038/npp.1994.30. [DOI] [PubMed] [Google Scholar]
- Herning RI, Jones RT, Hooker WD, Mendelson J, Blackwell L. Cocaine increases EEG beta: a replication and extension of Hans Berger's historic experiments. Electroencephalography and Clinical Neurophysiology. 1985;60(6):470–477. doi: 10.1016/0013-4694(85)91106-x. [DOI] [PubMed] [Google Scholar]
- Hester R, Garavan H. Executive dysfunction in cocaine addiction: evidence for discordant frontal, cingulate, and cerebellar activity. The Journal of Neuroscience. 2004;24:11017–11022. doi: 10.1523/JNEUROSCI.3321-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann CS, Mecklinger A. Magnetoencephalographic responses to illusory figures: early evoked gamma is affected by processing of stimulus features. International Journal of Psychophysiology. 2000;38:265–281. doi: 10.1016/s0167-8760(00)00170-7. [DOI] [PubMed] [Google Scholar]
- Herrmann CS, Mecklinger A. Gamma activity in human EEG is related to highspeed memory comparisons during object selective attention. Visual Cognition. 2001;8(3–5):593–608. [Google Scholar]
- Herrmann CS, Demiralp T. Human EEG gamma oscillations in neuropsychiatric disorders. Clinical Neurophysiology. 2005;116:2719–2733. doi: 10.1016/j.clinph.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Hester R, Dixon V, Garavan H. A consistent attentional bias for drug-related material in active cocaine users across word and picture versions of the emotional Stroop task. Drug and Alcohol Dependence. 2006;81:251–257. doi: 10.1016/j.drugalcdep.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Hester R, Garavan H. Executive dysfunction in cocaine addiction: evidence for discordant frontal, cingulate, and cerebellar activity. The Journal of Neuroscience. 2004;24:11017–11022. doi: 10.1523/JNEUROSCI.3321-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoedlmoser K, Pecherstorfer T, Gruber G, Anderer P, Doppelmayr M, Klimesch W, Schabus M. Instrumental conditioning of human sensorimotor rhythm (12–15 Hz) and its impact on sleep as well as declarative learning. Sleep. 2008;31(10):1401–1408. [PMC free article] [PubMed] [Google Scholar]
- Howe RC, Sterman MB. Cortical-subcortical EEG correlats of suppressed motor behavior during sleep and waking in the cat. Electroencephalography and Clinical Neurophysiology. 1972;32:681–695. doi: 10.1016/0013-4694(72)90104-6. [DOI] [PubMed] [Google Scholar]
- Hummel F, Andres F, Altenmuller F, Dichgans J, Gerloff C. Inhibitory control of acquired motor programmes in the human brain. Brain. 2002;125:404–420. doi: 10.1093/brain/awf030. [DOI] [PubMed] [Google Scholar]
- Karakaş S, Tüfekçi I, Bekçi B, Çakmak E, Doğutepe E, Erzengin OU, Özkan A, Arıkan O. Early time-locked gamma response and gender specificity. International Journal of Psychophysiology. 2006;60:225–239. doi: 10.1016/j.ijpsycho.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Katayama J, Polich J. Stimulus context determines P3a and P3b. Psychophysiology. 1998;35:23–33. [PubMed] [Google Scholar]
- Kilts CD, Gross RE, Ely TD, Drexler KPG. The neural correlates of cue-induced craving in cocaine-dependent women. American Journal of Psychiatry. 2004;161:233–241. doi: 10.1176/appi.ajp.161.2.233. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Chen YR, Schmitz J, Bordnic P, Shafer A. Cue reactivivty in cocaine-dependent subjects: effects of cue type and cue modality. Addictive Behavior. 1998;23:7–15. doi: 10.1016/s0306-4603(97)00014-2. [DOI] [PubMed] [Google Scholar]
- Koob GF. Stress, corticotropin-releasing factor, and drug addiction. Annals of New York Academy of Sciences. 1999;897:27–45. doi: 10.1111/j.1749-6632.1999.tb07876.x. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS): Instruction manual and affective ratings Tech. report A-5. CRP: University Florida; 2001. [Google Scholar]
- Lenz D, Krauel K, Schadow J, Baving L, Duzel E, Herrmann CS. Enhanced gamma-band activity in ADHD patients lacks correlation with memory performance found in healthy children. Brain Research. 2008;1235:117–132. doi: 10.1016/j.brainres.2008.06.023. [DOI] [PubMed] [Google Scholar]
- Leshner AI. Addiction is a brain disease, and it matters. Science. 1997;278:45–47. doi: 10.1126/science.278.5335.45. [DOI] [PubMed] [Google Scholar]
- London ED, Ernst M, Grant S, Bonson K, Weinstein A. Orbitofrontal cortex and human drug abuse: functional imaging. Cerebral Cortex. 2000;10:334–342. doi: 10.1093/cercor/10.3.334. [DOI] [PubMed] [Google Scholar]
- Lubar JF. Neurofeedback for the management of attention deficit disorders. In: Schwartz MS, Andrasik F, editors. Biofeedback: A practitioner’s guide. 3rd edition. New York: Guilford Press; 2003. pp. 409–437. [Google Scholar]
- Lubman D, Peters L, Mogg K, Bradley B, Deakin J. Attentional bias for drug cues in opiate dependence. Psychological Medicine. 2000;30:169–175. doi: 10.1017/s0033291799001269. [DOI] [PubMed] [Google Scholar]
- Lutzenberger W, Pulvermüller F, Elbert F, Birbaumer N. Visual stimulation alters 40 Hz response in humans: an EEG study. Neuroscience Letters. 1995;183:1–4. doi: 10.1016/0304-3940(94)11109-v. [DOI] [PubMed] [Google Scholar]
- Lyvers M. “Loss of Control" in alcoholism and drug addiction: a neuroscientific interpretation. Experimental and Clinical Psychopharmacology. 2000;8:225–249. doi: 10.1037//1064-1297.8.2.225. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Woody GE, O’Brien CP. An improved diagnostic evaluation instrument for substance abuse patients: The Addiction Severity Index. Journal Nervous and Mental Diseases. 1980;168:26–33. doi: 10.1097/00005053-198001000-00006. [DOI] [PubMed] [Google Scholar]
- Michalec EM, Rohsenow DJ, Monti PM, Varney SM, Martin RA, Dey AN, Myers MG, Sirota AD. A Cocaine Negative Consequences Checklist: development and validation. Journal of Substance Abuse. 1996;8(2):181–193. doi: 10.1016/s0899-3289(96)90238-2. [DOI] [PubMed] [Google Scholar]
- Miller W, Rollnick S. Motivational Interviewing. NY, Guilford: 2002. [Google Scholar]
- Monastra VJ. Clinical applications of electroencephalographic biofeedback. In: Schwartz MS, Andrasik F, editors. Biofeedback: A practitioner’s guide. 3rd edition. New York: Guilford Press; 2003. pp. 438–463. [Google Scholar]
- Mulholland T, Human E. Behavioral stillness and biofeedback. International Journal of Psychophiology. 1995;19:26–27. doi: 10.1016/0167-8760(95)00019-o. [DOI] [PubMed] [Google Scholar]
- Muller MM, Gruber T, Keil A. Modulation of induced gamma band activity in the human EEG by attention and visual information processing. International Journal of Psychophysiology. 2000;38:283–299. doi: 10.1016/s0167-8760(00)00171-9. [DOI] [PubMed] [Google Scholar]
- Noldy NE, Santos CV, Politzer N, Blair RD, Carlen PL. Quantitative EEG changes in cocaine withdrawal: evidence for long-term CNS effects. Neuropsychobiology. 1994;30:189–196. doi: 10.1159/000119160. [DOI] [PubMed] [Google Scholar]
- Neuper C, Wortz M, Pfurtscheller G. ERD/ERS patterns reflecting sensorimotor activation and deactivation. Progress in Brain Research. 2006;159:211–222. doi: 10.1016/S0079-6123(06)59014-4. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Brunner C, Schlogl A, Lopes da Silva F. Mu rhythm (de)synchronization and EEG single-trial classification of different motor imagery tasks. NeuroImage. 2006;31:153–159. doi: 10.1016/j.neuroimage.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Staneak A, Neuper C. Event-related synchronization (ERS) in the alpha related brain potentials in an auditory selective-attention task. European Archive Psychiatry Clinical Neuroscience. 1996;250(2):101–110. doi: 10.1007/s004060070042. [DOI] [PubMed] [Google Scholar]
- Polich J, Herbst KL. P300 as a clinical assay: Rationale, evaluation and findings. International Journal of Psychophysiology. 2000;38(1):3–19. doi: 10.1016/s0167-8760(00)00127-6. [DOI] [PubMed] [Google Scholar]
- Prichep LS, Alper KA, Kowalik SC, Rosenthal M. Neurometric QEEG studies of crack cocaine dependence and treatment outcome. Journal of Addictive Disorders. 1996;15(4):39–53. doi: 10.1300/J069v15n04_03. [DOI] [PubMed] [Google Scholar]
- Prichep LS, Alper KR, Kowalik SC, Vaysblat LS, Merkin HA, et al. Prediction of treatment outcome in cocaine dependent males using quantitative EEG. Drug and Alcohol Dependence. 1999;54:35–43. doi: 10.1016/s0376-8716(98)00147-1. [DOI] [PubMed] [Google Scholar]
- Prichep LS, John ER. Quantitative EEG (QEEG) and psychiatric classification. Biological Psychiatry. 1997;42:64S. [Google Scholar]
- Prichep LS, Alper KA, Sverdlov L, Kowalik SC, John ER, Merkin H, Tom ML, Rosenthal M. Outcome related electrophysiological subtypes of cocaine dependence. Clinical Electroencephalography. 2002;33(1):8–20. doi: 10.1177/155005940203300104. [DOI] [PubMed] [Google Scholar]
- Rangaswamy M, Porjesz B. Uncovering genes for cognitive (dys)function and predisposition for alcoholism spectrum disorders: A review of human brain oscillations as effective endophenotypes. Brain Research. 2008;1235:153–171. doi: 10.1016/j.brainres.2008.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Research Reviews. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robbins SJ, Ehrman RN, Childress AR, Cornish JW, O’Brien CP. Mood state and recent cocaine use are not associated with levels of cocaine cue reactivity. Drug and Alcohol Dependence. 2000;59:33–42. doi: 10.1016/s0376-8716(99)00103-9. [DOI] [PubMed] [Google Scholar]
- Robbins SJ, Ehrman RN, Childress AR, O’Brien CP. Relationships among physiological and self-report responses produced by cocaine-related cues. Addictive Behaviours. 1997;22(2):157–167. doi: 10.1016/s0306-4603(96)00007-x. [DOI] [PubMed] [Google Scholar]
- Roemer RA, Cornwell A, Dewart D, Jackson P, Ercegovac DV. Quantitative electroencephalographic analysis in cocaine-preferring polysubstance abusers during abstinence. Psychiatry Research. 1995;58:247–257. doi: 10.1016/0165-1781(95)02474-b. [DOI] [PubMed] [Google Scholar]
- Rossiter TR, LaVaque TJ. A comparison of EEG biofeedback and psychostimulants in treating attention deficit/hyperactivity disorders. Journal of Neurotherapy. 1995;1:48–59. [Google Scholar]
- Scott WC, Kaiser D, Othmer S, Sideroff SI. Effects of an EEG biofeedback protocol on a mixed substance abusing population. American Journal of Drug Alcohol Abuse. 2005;31(3):455–469. doi: 10.1081/ada-200056807. [DOI] [PubMed] [Google Scholar]
- Shaw P, Lerch J, Greenstein D, Sharp W, Clasen L, et al. Longitudinal mapping of cortical thickness and clinical outcome in children and adolescents with attention-deficit/hyperactivity disorder. Archives of General Psychiatry. 2006;63:540–549. doi: 10.1001/archpsyc.63.5.540. [DOI] [PubMed] [Google Scholar]
- Silberstein RB, Farrow M, Levy F, Pipingas A, Hay DA, Jarman FC. Functional brain electrical activity mapping in boys with attention-deficit/hyperactivity disorder. Archives of General Psychiatry. 1998;55:1105–1112. doi: 10.1001/archpsyc.55.12.1105. [DOI] [PubMed] [Google Scholar]
- Sokhadze EM, Potts GF, Martin LE, Stotts AL. Brief psychotherapeutic intervention enhances cognitive ERPs in a selective attention task in cocaine addiction. Association Applied Psychophysiology & Biofeedback Meeting; April 1–4; Austin, TX. 2005. [Google Scholar]
- Sokhadze E, Stewart C, Hollifield M. Integrating cognitive neuroscience methods with neurofeedback therapy in treatment of substance use disorder comorbid with PTSD. Journal of Neurotherapy. 2007;11(2):13–44. [Google Scholar]
- Sokhadze TM, Cannon R, Trudeau DL. EEG biofeedback as a treatment for substance use disorders: Review, rating of efficacy and recommendations for future research. Applied Psychophysiology and Biofeedback. 2008a;33:1–28. doi: 10.1007/s10484-007-9047-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokhadze E, Stewart C, Hollifield M, El-Baz A, Singh S, Tasman A. Attentional bias to stress-related pictorial cues in cocaine addiction comorbid with PTSD. Journal of Neurotherapy. 2008b;12:205–225. doi: 10.1080/10874200802502185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokhadze E, Singh S, Stewart C, Hollifield M, Tasman A. Psychophysiological reactivity to drug and trauma cues in cocaine addiction co-occurring with PTSD. Applied Psychophysiology and Biofeedback. 2008c;33:176. [Google Scholar]
- Sterman MB, Wyrwicka W. EEG correlates of sleep: evidence for separate forebrain substrates. Brain Research. 1967;1:143–163. doi: 10.1016/0006-8993(67)90186-2. [DOI] [PubMed] [Google Scholar]
- Sterman MB, Egner T. Foundation and practice of neurofeedback for the treatment of epilepsy. Applied Psychophysiology and Biofeedback. 2006;31:21–35. doi: 10.1007/s10484-006-9002-x. [DOI] [PubMed] [Google Scholar]
- Stormark KM, Laberg JC, Nordby H, Hugdahl K. Alcoholics' selective attention to alcohol stimuli: Automated processing? Journal of Studies on Alcohol. 2000;61:18–23. doi: 10.15288/jsa.2000.61.18. [DOI] [PubMed] [Google Scholar]
- Stotts AL, Schmitz JM, Rhoades HM, Grabowski J. Motivational interviewing with cocaine-dependent subjects: a pilot study. Journal of Consulting and Clinical Psychology. 2001;69:858–862. doi: 10.1037//0022-006x.69.5.858. [DOI] [PubMed] [Google Scholar]
- Stotts AL, Potts GF, Ingersoll G, George MR, Martin LE. Preliminary feasibility and efficacy of a brief motivational intervention with psychophysiological feedback for cocaine abuse. Substance Abuse. 2006;27(4):9–20. doi: 10.1300/j465v27n04_02. [DOI] [PubMed] [Google Scholar]
- Struve FA, Straumanis JJ, Patrick G, Price L. Topographic mapping of quantitative EEG variables in chronic heavy marihuana users: empirical findings with psychiatric patients. Clinical Electroencephalography. 1989;20(1):6–23. doi: 10.1177/155005948902000106. [DOI] [PubMed] [Google Scholar]
- Struve FA, Straumanis JJ, Patrick G, Leavitt J, Manno J, Manno BR. Topographic quantitative EEG sequelae of chronic marihuana use: a replication using medically and psychiatrically screened normal subjects. Drug and Alcohol Dependence. 1999;56:167–179. doi: 10.1016/s0376-8716(99)00029-0. [DOI] [PubMed] [Google Scholar]
- Struve FA, Manno BR, Kemp P, Patrick G, Manno J. Acute marihuana (THC) exposure produces a “transient” topographic quantitative EEG profile identical to the “persistent” profile seen in chronic heavy users. Clinical Electroencephalography. 2003;34:75–83. doi: 10.1177/155005940303400206. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2006 National Survey on Drug Use and health: National Findings (Office of Applied Studies, NSDUH Series H-32, DHHS Publication SMA 07-4293) Rockville, MD: 2007. [Google Scholar]
- Tallon-Baudry C. Oscillatory synchrony and human visual cognition. Journal of Physiology-Paris. 2003;97:355–363. doi: 10.1016/j.jphysparis.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O. Oscillatory gamma activity in humans and its role in object representation. Trends in Cognitive Sciences. 1999;3(4):151–162. doi: 10.1016/s1364-6613(99)01299-1. [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O, Henaff M-A, Isnard J, Fischer C. Attention modulates gamma-band oscillations differently in the human lateral occipital cortex and fusiform gyrus. Cerebral Cortex. 2005;15:654–662. doi: 10.1093/cercor/bhh167. [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O, Peronnet F, Pernier J. Induced gamma-Band activity during the delay of a visual short-term memory task in humans. The Journal of Neuroscience. 1998;18(11):4244–4254. doi: 10.1523/JNEUROSCI.18-11-04244.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treasure J. Motivational interviewing. Advances in Psychiatric Treatment. 2004;10:331–337. [Google Scholar]
- Trudeau DL. EEG Biofeedback for addictive disorders - The state of the art in 2004. Journal of Adult Development. 2005;12:139–146. [Google Scholar]
- Van der Stelt, van der Molen M, Gunning WB, Kok A. Neuroelectrical signs of selective attention to color in boys with attention-deficit hyperactivity disorder. Cognitive Brain Research. 2001;12:245–264. doi: 10.1016/s0926-6410(01)00055-6. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. The addicted human brain: insights from imaging studies. Journal of Clinical Investigations. 2003;111:1444–1451. doi: 10.1172/JCI18533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wexler BE, Gottschalk CH, Fulbright RK, Prohovnik I, Lacadie CM, Rounsaville BJ, Gore JC. Functional Magnetic Resonance Imaging of cocaine craving. American Journal of Psychiatry. 2001;158:86–95. doi: 10.1176/appi.ajp.158.1.86. [DOI] [PubMed] [Google Scholar]
- Weiss F, Ciccocioppo R, Parsons LH, Katner S, Liu X, Zorrilla EP, et al. Compulsive drug-seeking behavior and relapse. Neuroadaptation, stress, and conditioning factors. Annals of the New York Academy of Sciences. 2001;937:1–26. doi: 10.1111/j.1749-6632.2001.tb03556.x. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Bothwell S. Assessment of social adjustment by patient self-report. Archives of General Psychiatry. 1976;33(9):1111–1115. doi: 10.1001/archpsyc.1976.01770090101010. [DOI] [PubMed] [Google Scholar]
- Williams LM. An integrative neuroscience model of “significance” processing. Journal or Integrative Neuroscience. 2006;5:1–47. doi: 10.1142/s0219635206001082. [DOI] [PubMed] [Google Scholar]
- Yordanova J, Banaschewski T, Kolev V, Woerner W, Rothenberger A. Abnormal early stages of task stimulus processing in children with attention-deficit hyperactivity disorder--evidence from event-related gamma oscillations. Clinical Neurophysiology. 2001;112(6):1096–1108. doi: 10.1016/s1388-2457(01)00524-7. [DOI] [PubMed] [Google Scholar]


