Abstract
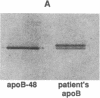
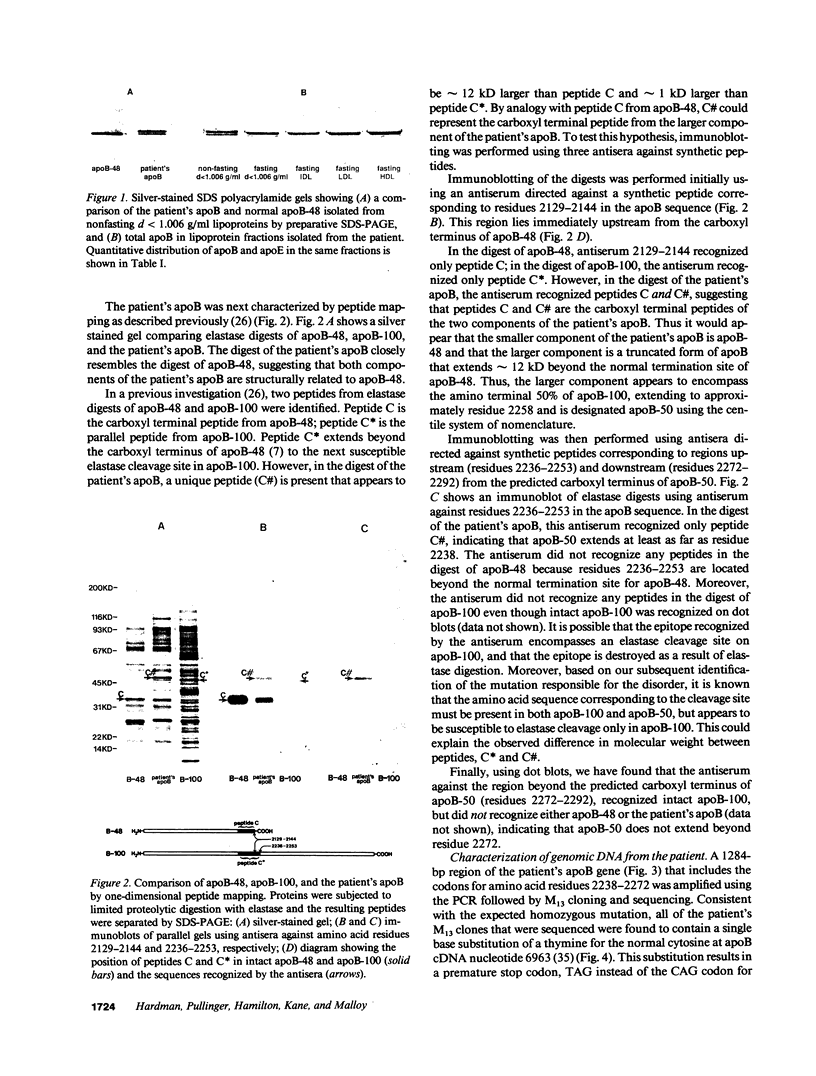
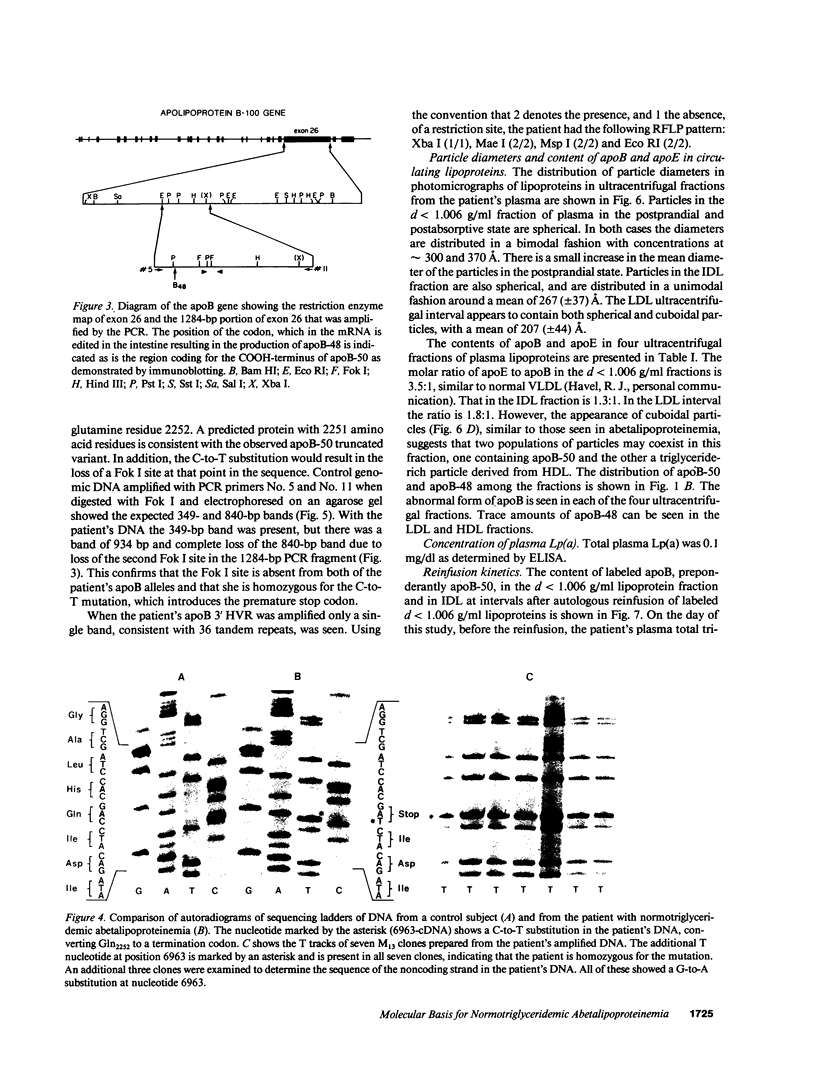
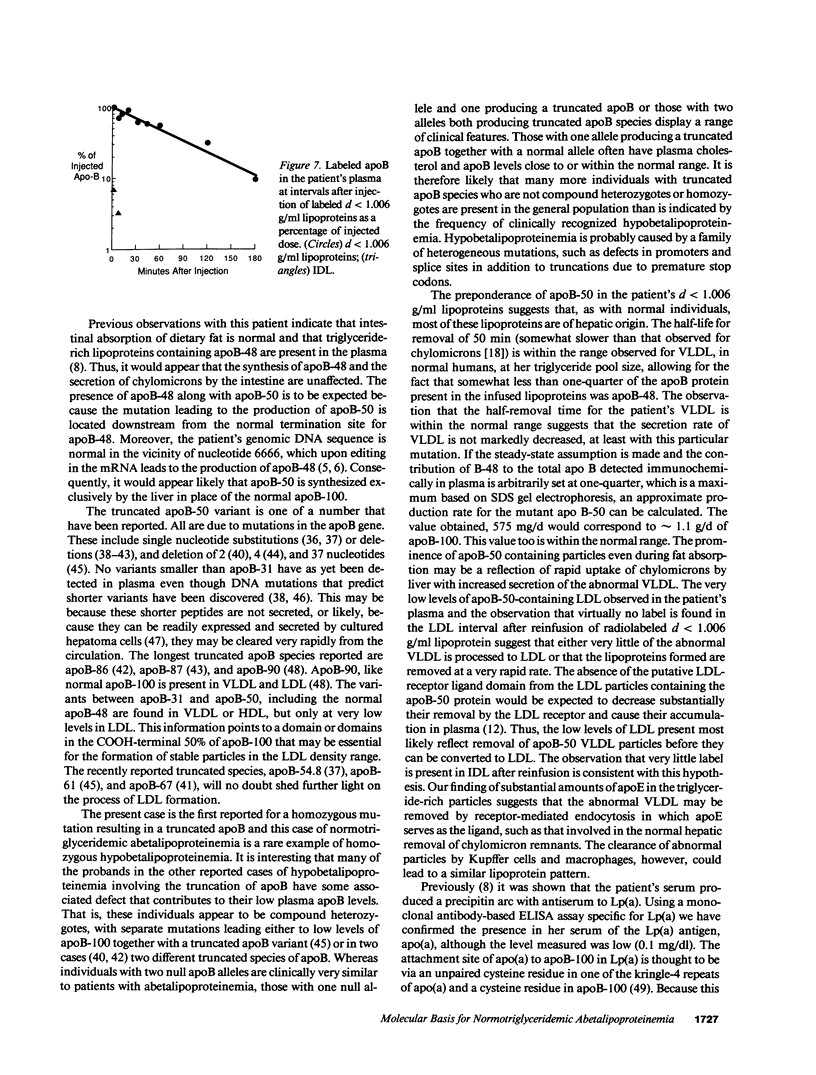
We have previously described a disorder, normotriglyceridemic abetalipoproteinemia, that is characterized by the virtual absence of plasma low density lipoproteins and complete absence of apoB-100, but with apparently normal secretion of triglyceride-rich lipoproteins containing apoB-48. The patient's plasma lipoproteins were shown on polyacrylamide gels and by antibody mapping to have a new truncated apoB variant, apoB-50, circulating along with her apoB-48. We have found this individual to be homozygous for a single C-to-T nucleotide substitution at apoB codon 2252, which produces a premature in-frame stop codon. Thus, this is a rare example of homozygous hypobetalipoproteinemia. Electron photomicrographs revealed that the diameters of particles in the d less than 1.006 g/ml lipoprotein fraction, in both the postprandial and postabsorptive state, are bimodally distributed. The molar ratio of apoE to apoB in these particles is 3.5:1, similar to normal VLDL. The plasma LDL interval contains both spherical and cuboidal particles. Autologous reinfusion of labeled d less than 1.006 g/ml lipoproteins showed exponential disappearance from plasma, with an apparent half-removal time of 50 min, somewhat slower than for normal chylomicrons but within the normal range for VLDL. The calculated production rate for apoB was within the normal range in this subject. A very small amount of label was found briefly in the IDL fraction, but none at any time in LDL or HDL. Therefore, because LDL particles that contain apoB-50 lack the putative ligand domain of the LDL receptor, we conclude that the very low level of LDL is due to the rapid removal of the abnormal VLDL particles before their conversion to LDL can take place.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg K., Powell L. M., Wallis S. C., Pease R., Knott T. J., Scott J. Genetic linkage between the antigenic group (Ag) variation and the apolipoprotein B gene: assignment of the Ag locus. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7367–7370. doi: 10.1073/pnas.83.19.7367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G. C., Kane J. P., Hamilton R. L. Thermal behavior of cores of human serum triglyceride-rich lipoproteins: a study of induced circular dichroism of beta-carotene. Biochemistry. 1984 Mar 13;23(6):1119–1124. doi: 10.1021/bi00301a013. [DOI] [PubMed] [Google Scholar]
- Chen S. H., Habib G., Yang C. Y., Gu Z. W., Lee B. R., Weng S. A., Silberman S. R., Cai S. J., Deslypere J. P., Rosseneu M. Apolipoprotein B-48 is the product of a messenger RNA with an organ-specific in-frame stop codon. Science. 1987 Oct 16;238(4825):363–366. doi: 10.1126/science.3659919. [DOI] [PubMed] [Google Scholar]
- Collins D. R., Knott T. J., Pease R. J., Powell L. M., Wallis S. C., Robertson S., Pullinger C. R., Milne R. W., Marcel Y. L., Humphries S. E. Truncated variants of apolipoprotein B cause hypobetalipoproteinaemia. Nucleic Acids Res. 1988 Sep 12;16(17):8361–8375. doi: 10.1093/nar/16.17.8361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras M. A., Bale W. F., Spar I. L. Iodine monochloride (IC1) iodination techniques. Methods Enzymol. 1983;92:277–292. [PubMed] [Google Scholar]
- Edelstein C., Scanu A. M. Precautionary measures for collecting blood destined for lipoprotein isolation. Methods Enzymol. 1986;128:151–155. doi: 10.1016/0076-6879(86)28065-9. [DOI] [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harano Y., Kojima H., Nakano T., Harada M., Kashiwagi A., Nakajima Y., Hidaka T. H., Ohtsuki T., Suzuki T., Tamura A. Homozygous hypobetalipoproteinemia with spared chylomicron formation. Metabolism. 1989 Jan;38(1):1–7. doi: 10.1016/0026-0495(89)90172-8. [DOI] [PubMed] [Google Scholar]
- Hardman D. A., Protter A. A., Chen G. C., Schilling J. W., Sato K. Y., Lau K., Yamanaka M., Mikita T., Miller J., Crisp T. Structural comparison of human apolipoproteins B-48 and B-100. Biochemistry. 1987 Aug 25;26(17):5478–5486. doi: 10.1021/bi00391a040. [DOI] [PubMed] [Google Scholar]
- Hardman D. A., Protter A. A., Schilling J. W., Kane J. P. Carboxyl terminal analysis of human B-48 protein confirms the novel mechanism proposed for chain termination. Biochem Biophys Res Commun. 1987 Dec 31;149(3):1214–1219. doi: 10.1016/0006-291x(87)90537-7. [DOI] [PubMed] [Google Scholar]
- Havel R. J., Kotite L., Vigne J. L., Kane J. P., Tun P., Phillips N., Chen G. C. Radioimmunoassay of human arginine-rich apolipoprotein, apoprotein E. Concentration in blood plasma and lipoproteins as affected by apoprotein E-3 deficiency. J Clin Invest. 1980 Dec;66(6):1351–1362. doi: 10.1172/JCI109988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L. S., Ripps M. E., Korman S. H., Deckelbaum R. J., Breslow J. L. Hypobetalipoproteinemia due to an apolipoprotein B gene exon 21 deletion derived by Alu-Alu recombination. J Biol Chem. 1989 Jul 5;264(19):11394–11400. [PubMed] [Google Scholar]
- Huang L. S., de Graaf J., Breslow J. L. ApoB gene MspI RFLP in exon 26 changes amino acid 3611 from Arg to Gln. J Lipid Res. 1988 Jan;29(1):63–67. [PubMed] [Google Scholar]
- Kane J. P. Characterization of apolipoprotein B-containing lipoproteins. Methods Enzymol. 1986;129:123–129. doi: 10.1016/0076-6879(86)29065-5. [DOI] [PubMed] [Google Scholar]
- Kane J. P., Hardman D. A., Paulus H. E. Heterogeneity of apolipoprotein B: isolation of a new species from human chylomicrons. Proc Natl Acad Sci U S A. 1980 May;77(5):2465–2469. doi: 10.1073/pnas.77.5.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott T. J., Pease R. J., Powell L. M., Wallis S. C., Rall S. C., Jr, Innerarity T. L., Blackhart B., Taylor W. H., Marcel Y., Milne R. Complete protein sequence and identification of structural domains of human apolipoprotein B. Nature. 1986 Oct 23;323(6090):734–738. doi: 10.1038/323734a0. [DOI] [PubMed] [Google Scholar]
- Knott T. J., Wallis S. C., Pease R. J., Powell L. M., Scott J. A hypervariable region 3' to the human apolipoprotein B gene. Nucleic Acids Res. 1986 Nov 25;14(22):9215–9216. doi: 10.1093/nar/14.22.9215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott T. J., Wallis S. C., Powell L. M., Pease R. J., Lusis A. J., Blackhart B., McCarthy B. J., Mahley R. W., Levy-Wilson B., Scott J. Complete cDNA and derived protein sequence of human apolipoprotein B-100. Nucleic Acids Res. 1986 Sep 25;14(18):7501–7503. doi: 10.1093/nar/14.18.7501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krul E. S., Kinoshita M., Talmud P., Humphries S. E., Turner S., Goldberg A. C., Cook K., Boerwinkle E., Schonfeld G. Two distinct truncated apolipoprotein B species in a kindred with hypobetalipoproteinemia. Arteriosclerosis. 1989 Nov-Dec;9(6):856–868. doi: 10.1161/01.atv.9.6.856. [DOI] [PubMed] [Google Scholar]
- Malloy M. J., Kane J. P., Hardman D. A., Hamilton R. L., Dalal K. B. Normotriglyceridemic abetalipoproteinemia. absence of the B-100 apolipoprotein. J Clin Invest. 1981 May;67(5):1441–1450. doi: 10.1172/JCI110173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakane P. K., Kawaoi A. Peroxidase-labeled antibody. A new method of conjugation. J Histochem Cytochem. 1974 Dec;22(12):1084–1091. doi: 10.1177/22.12.1084. [DOI] [PubMed] [Google Scholar]
- Powell L. M., Wallis S. C., Pease R. J., Edwards Y. H., Knott T. J., Scott J. A novel form of tissue-specific RNA processing produces apolipoprotein-B48 in intestine. Cell. 1987 Sep 11;50(6):831–840. doi: 10.1016/0092-8674(87)90510-1. [DOI] [PubMed] [Google Scholar]
- Pullinger C. R., North J. D., Teng B. B., Rifici V. A., Ronhild de Brito A. E., Scott J. The apolipoprotein B gene is constitutively expressed in HepG2 cells: regulation of secretion by oleic acid, albumin, and insulin, and measurement of the mRNA half-life. J Lipid Res. 1989 Jul;30(7):1065–1077. [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider W. J., Beisiegel U., Goldstein J. L., Brown M. S. Purification of the low density lipoprotein receptor, an acidic glycoprotein of 164,000 molecular weight. J Biol Chem. 1982 Mar 10;257(5):2664–2673. [PubMed] [Google Scholar]
- Schuh J., Fairclough G. F., Jr, Haschemeyer R. H. Oxygen-mediated heterogeneity of apo-low-density lipoprotein. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3173–3177. doi: 10.1073/pnas.75.7.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalenhoef A. F., Malloy M. J., Kane J. P., Havel R. J. Metabolism of apolipoproteins B-48 and B-100 of triglyceride-rich lipoproteins in normal and lipoprotein lipase-deficient humans. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1839–1843. doi: 10.1073/pnas.81.6.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima Y., Kodama T., Iida H., Kawamura M., Aburatani H., Itakura H., Akanuma Y., Takaku F., Kawade M. Normotriglyceridemic abetalipoproteinemia in infancy: an isolated apolipoprotein B-100 deficiency. Pediatrics. 1985 Mar;75(3):541–546. [PubMed] [Google Scholar]
- Talmud P., King-Underwood L., Krul E., Schonfeld G., Humphries S. The molecular basis of truncated forms of apolipoprotein B in a kindred with compound heterozygous hypobetalipoproteinemia. J Lipid Res. 1989 Nov;30(11):1773–1779. [PubMed] [Google Scholar]
- Thrift R. N., Forte T. M., Cahoon B. E., Shore V. G. Characterization of lipoproteins produced by the human liver cell line, Hep G2, under defined conditions. J Lipid Res. 1986 Mar;27(3):236–250. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trieu V. N., McConathy W. J. Lipoprotein(a) binding to other apolipoprotein B containing lipoproteins. Biochemistry. 1990 Jun 26;29(25):5919–5924. doi: 10.1021/bi00477a006. [DOI] [PubMed] [Google Scholar]
- Utermann G. The mysteries of lipoprotein(a). Science. 1989 Nov 17;246(4932):904–910. doi: 10.1126/science.2530631. [DOI] [PubMed] [Google Scholar]
- Wong W. L., Eaton D. L., Berloui A., Fendly B., Hass P. E. A monoclonal-antibody-based enzyme-linked immunosorbent assay of lipoprotein(a). Clin Chem. 1990 Feb;36(2):192–197. [PubMed] [Google Scholar]
- Wu M. J., Bütler E., Bütler R., Schumaker V. N. Identification of the base substitution responsible for the Ag(x/y) polymorphism of apolipoprotein B-100. Arterioscler Thromb. 1991 Mar-Apr;11(2):379–384. doi: 10.1161/01.atv.11.2.379. [DOI] [PubMed] [Google Scholar]
- Yang C. Y., Chen S. H., Gianturco S. H., Bradley W. A., Sparrow J. T., Tanimura M., Li W. H., Sparrow D. A., DeLoof H., Rosseneu M. Sequence, structure, receptor-binding domains and internal repeats of human apolipoprotein B-100. Nature. 1986 Oct 23;323(6090):738–742. doi: 10.1038/323738a0. [DOI] [PubMed] [Google Scholar]
- Young S. G., Hubl S. T., Chappell D. A., Smith R. S., Claiborne F., Snyder S. M., Terdiman J. F. Familial hypobetalipoproteinemia associated with a mutant species of apolipoprotein B (B-46). N Engl J Med. 1989 Jun 15;320(24):1604–1610. doi: 10.1056/NEJM198906153202407. [DOI] [PubMed] [Google Scholar]
- Young S. G., Hubl S. T., Smith R. S., Snyder S. M., Terdiman J. F. Familial hypobetalipoproteinemia caused by a mutation in the apolipoprotein B gene that results in a truncated species of apolipoprotein B (B-31). A unique mutation that helps to define the portion of the apolipoprotein B molecule required for the formation of buoyant, triglyceride-rich lipoproteins. J Clin Invest. 1990 Mar;85(3):933–942. doi: 10.1172/JCI114522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young S. G., Northey S. T., McCarthy B. J. Low plasma cholesterol levels caused by a short deletion in the apolipoprotein B gene. Science. 1988 Jul 29;241(4865):591–593. doi: 10.1126/science.3399894. [DOI] [PubMed] [Google Scholar]