Abstract
We have previously reported a modest influence of moderate calorie restriction (CR) on testicular gene expression in young adult rhesus macaques (Macaca mulatta); however, it is unclear if these modifications correspond to subsequent changes in testicular function or sperm physiology. This study extends our earlier findings to examine potential physiological differences due to this differential gene expression. Animals were subjected to 30% CR (CR, n = 5) or were fed a standard control diet (CON, n = 5) starting during their peripubertal period. Circulating testosterone (T) levels were measured across a 24-h period after 7 yr of dietary treatment and were found to be similar in CR and CON males; however, maintenance of daily minimum T levels was significantly higher in the CR animals. Semen collection was performed on the same cohort of animals three times per male (CR, n = 4; CON, n = 4) after 8 yr of treatment, and samples were assessed by a variety of measures. Parameters, including semen quality and sperm cell viability and function, showed less variability in semen samples taken from CR males, but overall testicular function and sperm quality were comparable regardless of diet. There is mounting evidence that CR may promote health and longevity in a wide range of organisms, including nonhuman primates. Importantly, our data suggest that moderate CR has no obvious lasting detrimental effect on testicular function and sperm parameters in young adult primates and may in fact help maintain higher levels of circulating T.
Keywords: calorie restriction, rhesus macaque, semen collection, sperm, testis, testosterone
Moderate calorie restriction does not impact sperm parameters or semen characteristics in young adult rhesus macaques.
INTRODUCTION
Over the last 75 yr, calorie restriction (CR) has been established as the only proven nongenetic method of altering longevity and attenuating many of the biological changes associated with aging [1–3]. Moderate long-term CR is associated with extended life span and improved health measures such as reduced body mass and adiposity, lower body temperature, lower blood pressure, and reduction of glucose, fasting plasma insulin, and high-density lipoprotein levels, while increasing insulin sensitivity [3–7]. Remarkably, CR has consistent effects across taxa, including nematodes, spiders, flies, mollusks, birds, rodents, dogs, and nonhuman primates such as squirrel monkeys and cynomolgus and rhesus macaques [2, 8–11].
Its long history notwithstanding, the full impact of CR on healthy aging in nonhuman primates is still unknown. Although investigations in rhesus macaques (Macaca mulatta) have paralleled findings in other species [2, 8, 12–16], very little is known with regard to the exact mechanisms of action of CR on the hypothalamic-pituitary-gonadal axis, particularly the testicular portion of the axis. In male rhesus macaques, as in rats, the pubertal increase in circulating testosterone (T) concentrations is delayed by CR [4, 17]; consequently, there is concern that long-term CR could have detrimental effects on male reproduction and development.
We have previously demonstrated a limited impact of CR on testicular gene expression in young adult rhesus macaques based on mRNA expression and semiquantitative and quantitative real-time PCR data [18]. These findings suggest that CR can have general beneficial health effects without negative consequences for gonadal function. Calorie restriction may even benefit reproductive fitness through a reduction in body mass and adiposity, which has been shown to have significant effects on the reproductive axis. For example, it is known that obese men exhibit lower sperm concentrations and total sperm counts compared with men having a body mass index (BMI) below obese levels [19, 20], while an inverse relationship exists between BMI and total number of motile sperm cells and a positive relationship between BMI and DNA fragmentation [21]. Although our previous study [18] showed no deleterious effect of CR on testicular gene expression, research in rodents has shown significant effects on epididymal gene expression [22], which in turn could impact postproduction sperm maturation and function [23, 24].
The objective of the present study was to extend our previous findings and thoroughly ascertain the reproductive impact of moderate CR in young adult primates using semen analyses and assays of circulating T levels to assess circadian gonadal steroid production.
MATERIALS AND METHODS
Animals and Diet
A cohort of 10 male rhesus macaques (M. mulatta) was selected for study under protocols approved by the institutional animal care and use committees of the University of Maryland and the Oregon National Primate Research Center. The animals were housed in individual cages with auditory, visual, and olfactory interaction with male and female conspecifics in a temperature-controlled environment (24°C) under a fixed 12L:12D photoperiod (lights on from 700 h to 1900 h) with ad libitum access to drinking water. Individuals were cared for by the Oregon National Primate Research Center in accord with the National Research Council's Guide for the Care and Use of Laboratory Animals [25], which included daily health checks to ensure normal behavior, food consumption, and waste production. Additionally, routine physical examinations, hematological studies, fecal parasite checks, tuberculin testing, and dental cleaning were performed periodically.
Starting at ∼4 yr of age (i.e., the peripubertal period [26]), half of the animals were subjected to a continuous 30% CR diet (CR males) for ∼8 yr, as previously described [8, 14, 27]. The other half served as controls (CON males) and were fed ad libitum. This level of feeding was originally determined for each individual and was characterized by a few uneaten biscuits remaining in the animal's cage at the end of each day; CR males received 30% less food than age- and body weight-matched CON males. Each individual received a measured portion of specially formulated biscuits (Cargill, Minneapolis, MN) supplemented with daily fresh fruits or vegetables (10–40 cal) and was fed twice daily, at 0800 h and 1500 h. Biscuit composition was 15% protein, 5% fat, and 5% fiber, with a caloric content of ∼3.7 kcal/g, which included a vitamin/mineral mix that was 40% higher than the recommended allowance for rhesus macaques by the National Research Council [28]. This vitamin/mineral supplementation was designed to ensure sufficient availability of essential nutrients to both diet groups, but biscuits were otherwise similar to those used in many laboratory studies of rhesus macaques. Biochemical assays were performed periodically and with every new shipment to ensure diet content and quality [29, 30]. At the end of the study, the mean body masses of animals in the CR and CON groups were 9.19 and 10.72 kg, respectively. This difference in mean body mass (1.53 kg), while not significant due to the small sample sizes, was consistent with weight changes reported previously in CR studies [30, 31] involving larger cohorts of male rhesus macaques.
T Sampling and Analysis
Circulating levels of T vary widely throughout the day. Therefore, to more accurately assess the impact of CR on testicular T secretion, we collected serial blood samples across the day and night. At ∼11 yr of age, all animals were surgically fitted with an indwelling subclavian vein catheter connected to a swivel-tether remote blood sampling system [27, 32]. Before catheterization, the animals were allowed a minimum of 2 wk to become accustomed to wearing a protective nylon mesh jacket. Using this system, serial blood samples (1 ml) were collected remotely every 30 min over a 24-h period from an adjacent room, without need to disturb the animals. The samples were collected into edetic acid-coated glass tubes, and after centrifugation at 4°C, the plasma supernatant was stored at −20°C until assay for plasma T by radioimmunoassay [33, 34].
Penile Electrostimulation
At ∼12 yr of age, penile electrostimulation was conducted in the unanesthetized manually restrained subjects according to established methods [35–37]. After a 17-day habituation regimen and following methods described previously [38], penile electrostimulation was performed on three separate occasions for four animals in each treatment group, with one collection in the spring (March-April) and two collections in the fall (September-October). A decision was made following the habituation period to exclude one CON and one CR animal from the collection protocol for behavioral reasons.
Semen Collection and Processing
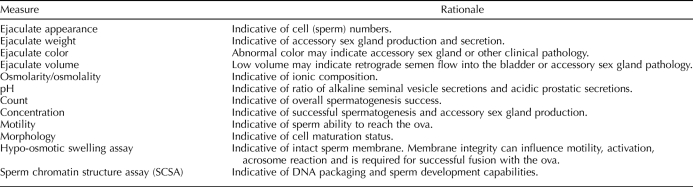
Semen samples were collected into sterile collection tubes and allowed to liquefy at room temperature for 30 min before evaluation. Ejaculate weight was obtained, and the liquid fraction of sample was transferred to a sterile centrifuge tube. Volume was recorded, and aliquots were removed for assessment of osmolarity, pH, and morphology. The remaining liquid fraction was resuspended in 15 ml of warm Tyrode albumin lactate pyruvate (TALP)-Hepes with bovine serum albumin (BSA [39]) and centrifuged at 130–150 × g for 10 min. The procedure was repeated twice, for a total of three washes. Following centrifugation, the sperm pellet was resuspended in 1 ml of warm TALP-BSA [39] and placed in 5% CO2/95% air at 37°C. Aliquots for viability, count, and concentration were taken, and sperm motility was measured. The remaining sample was then used for the sperm chromatin structure assay (SCSA) or frozen for future post-thaw analysis (Table 1). Cryopreservation was performed according to methods previously established for cynomolgus [40] and rhesus macaque [41] semen.
TABLE 1.
Summary of semen measurements.
Count, Concentration, and Motility Analysis
After washing and resuspension, count and concentration were measured on a Neubauer hemocytometer by phase-contrast microscopy. Percentage motility was determined for fresh washed and frozen-thawed samples with duplicate counts of 100 sperm on a phase-contrast microscope (200×). Motility was measured as total movement, not just forward progress, which was accounted for with a status rating.
Viability, Osmolarity, and pH Analysis
Sperm viability was determined in fresh semen samples using the hypo-osmotic swelling (HOS) assay [42]. Briefly, 5 μl of washed semen was incubated with 100 μl of HOS solution for 30 min in 5% CO2/95% air at 37°C. A minimum of 200 sperm were assessed for swelling by phase-contrast microscopy, and results were expressed as a percentage of the total count.
Osmolarity and pH were determined for fresh samples by osmometer (Vapro Vapor Pressure Osmometer; Wescor, Logan, UT) and pH strips (EMD colorpHast; Fisher Scientific, Hampton, NH).
Morphology Analysis
Sperm morphology was scored in fresh and frozen-thawed samples using one-step eosin-nigrosin staining (EN; IMV International Corp., Maple Grove, MN). Smears were made with equal volumes of semen and EN stain, air dried, coverslipped, and examined at 1000× under oil immersion (100× bright field). Two slides and 300 total sperm were examined for each collection and expressed as percentage normal or abnormal; abnormal sperm were further subdivided into head, midpiece, or tail abnormality.
Sperm Chromatin Structure Assay
The SCSA is a flow cytometric test that assesses the susceptibility of sperm nuclear DNA to acid-induced DNA denaturation in situ. Washed frozen-thawed sperm samples were sent to SCSA Diagnostics (Brookings, SD) for evaluation of chromatin structure.
Statistical Analysis
Data for semen parameters were averaged for the three collections from each male, with group treatment averages then determined. Data are expressed as group mean ± SEM (CON and CR, n = 4) for each parameter.
Group mean T values (CON and CR, n = 5) were determined by taking the overall mean of the individual hormone values spanning the entire 24-h sampling period. Group maximum T values were determined by first identifying the maximum value for an individual and then averaging it with two adjacent values on each side of the time point; the mean individual maximum values were then calculated. Similarly, the group minimum T values were determined by taking the mean of the minimum value for an individual and then averaging with two adjacent values on each side of the time point.
Statistical comparisons between CON and CR groups were performed by Student t-test using SPSS (SPSS Inc., Chicago, IL) or Excel (Microsoft, Redmond, WA). Power analysis and tests for heterogeneity of variance for semen parameters were conducted before parametric analysis using Statistical Analysis System (SAS Institute, Cary, NC). For all analyses, the minimum criterion for significance was P < 0.05.
RESULTS
Test for Seasonal Differences
Because distinct seasonal variations in testicular volume, semen quality, sperm number, sexual behavior, and frequency of birth rate have been observed in wild and captive rhesus macaque populations, even under constant light cycles [36, 43, 44], we tested for seasonal differences in animals before continuing other analyses.
No seasonal differences in the mean semen measurements (ejaculate weight, volume, count, concentration, motility, viability, osmolarity, and pH) were detected between spring and fall (paired Student t-test, data not shown). Additionally, no correlation was detected between individual animal weight and the proportion of morphological sperm abnormalities (head, midpiece, and tail; data not shown).
Semen Analyses
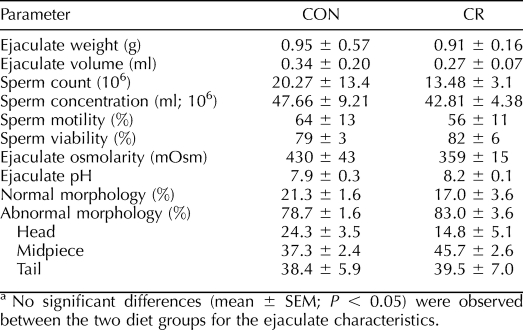
Ejaculate weight, liquid volume of ejaculate, sperm count, sperm concentration, sperm motility, sperm viability, ejaculate osmolarity, ejaculate pH, and morphology for freshly collected semen did not differ with diet (Table 2). Similarly, no significant treatment differences were detected in the percentage of head, midpiece, or tail abnormalities between CON and CR groups.
TABLE 2.
Fresh semen parameter values in young adult CON and CR rhesus macaques.a
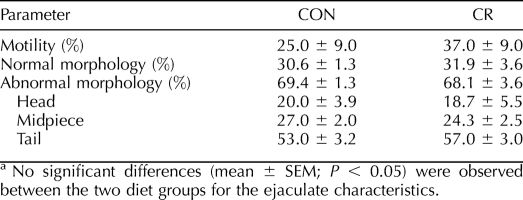
Two standard measures, motility and morphology, were also taken for frozen-thawed sperm. As with freshly processed samples, these end points showed no diet-induced differences in motility, the percentage of normal and abnormal sperm, or the three categories of abnormalities (Table 3).
TABLE 3.
Frozen-thawed sperm parameter values in young adult CON and CR rhesus macaques.a
Sperm Chromatin Structure Assay
Samples from CON and CR males (n = 4 per treatment) subjected to SCSA showed no statistically significant difference in sperm quality based on DNA fragmentation index (DFI) (CON, 8.5 ± 8.0; CR, 1.9 ± 1.1). Interestingly, one of the CON males had a 32% DFI, which was reflected in the large variability within the CON group.
T Concentrations
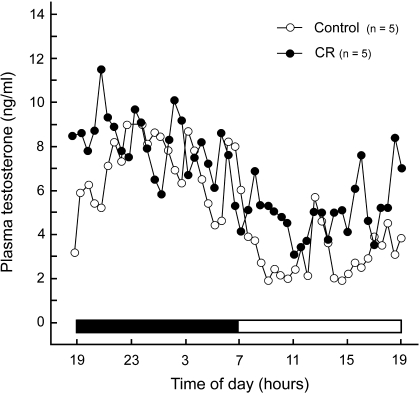
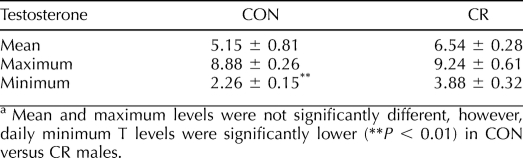
Daily circulating plasma T levels (ng/ml) had the same general pattern over the 24-h period in CON and CR males (Fig. 1). No significant differences were observed in the mean or maximum levels of circulating T; however, daily minimum T levels were significantly lower (P < 0.01) in CON versus CR males (Table 4).
FIG. 1.
Mean daily circulating plasma T levels for young adult CON and CR rhesus macaques. The line plot shows a similar pattern of daily circulating T in both CON and CR animals measured during a 24-h period.
TABLE 4.
Daily mean, maximum, and minimum circulating plasma testosterone levels for adult CON and CR rhesus macaques.a
DISCUSSION
We have previously reported that moderate CR modestly influenced pituitary and testicular gene expression in young adult rhesus macaques, without any apparent deleterious effect on the reproductive axis [18]. The present study extends these findings to demonstrate no detrimental impact of CR on sperm parameters and testicular function in these same study subjects. The nine components of the classical spermiogram investigated in our study give an indication of the probable success a male would have in siring offspring following mating. Our findings indicated no significant differences (P < 0.05) between CON and CR groups in any of these ejaculate characteristics. Furthermore, the means for each group appear to fall within the normal ranges for macaque semen [36, 45].
Although sperm count and concentration were not different between treatment groups, variability was much less in the CR-treated animals. Sperm motility in both treatment groups was generally good, with no significant differences observed. It is important to remember, however, that although good sperm are necessarily motile, motile sperm are not necessarily fertile, thus the need for a complete battery of tests in determining reproductive potential. From a purely observational standpoint, it seems that sperm from CR animals may have tolerated freezing slightly better, as motility dropped only from 56% to 37% following post-thaw analysis compared with the CON group, which dropped from 64% to 25%. Again, the differences were not statistically significant due to biological variation, and post-thaw motility was generally lower in both groups compared with values reported in the literature [46]. Evaluation of sperm morphology in both fresh and frozen-thawed samples showed no diet-induced differences between treatments. There were significant differences between fresh and frozen-thawed samples, but these differences are simply an artifact of the cryopreservation and/or thawing process and are not attributable to diet.
Membrane viability is important not only for sperm metabolism but also for the critically timed changes in membrane properties required for successful sperm activation, acrosome reaction, and oocyte binding. We chose to use the HOS assay to evaluate the functional integrity of the sperm membrane rather than the “live-dead” EN stain, which only measures whether the membrane is morphologically intact [47]. The percentage of viable sperm was not significantly different between CON and CR animals in the present study.
Ejaculate osmolarity and pH are dependent on seminal vesicle and prostate secretions and can be influenced by the nutritional status of the individual [48–52]. Our data showed no significant differences between diet groups for either of these parameters; thus, the nutritional status evoked by CR relative to seminal plasma characteristics was not evident in macaques.
It should be noted that the animals in our study were part of a long-term CR aging study under the auspices of the National Institute of Health's National Institute on Aging [8]. Due to the nature of the ongoing study, we were only able to collect ejaculates from the animals on three separate occasions; no collections were made during the habituation phase. As a result, small group size and few ejaculates collected per animal may have made it more difficult to detect significant differences, especially if true biological differences are subtle. Power analysis based on current sample size and observed variability for each semen characteristic showed, for example, that a 137-mOsm difference in ejaculate osmolarity or a 1.05 difference in pH would have been necessary to detect differences between our treatments with 80% power. Alternatively, to detect significance for our observed apparent differences in these end points would have required sample sizes of 15 and 40 animals, respectively. Even more extreme was ejaculate weight, which was almost identical between treatment groups and would have required a difference of 2.4 g to detect differences with 80% power, which is not biologically possible.
Morphology (and to a lesser extent motility) measures also varied widely from reports in the literature [38]. It may be that the epididymides were not cleared out on a regular basis, which can result in old and degraded sperm accumulating in the seminiferous tubules and vas deferens. For this reason, sperm from macaques involved in semen studies are often collected weekly throughout the year regardless of whether or not the ejaculate is to be analyzed. As such, our values may not be directly comparable to those of animals whose semen is regularly collected, but more importantly our animals were directly comparable to each other, with no detectable differences between treatment groups.
While the semen parameters analyzed were similar between experimental groups, it is worth noting that in every instance, with the exception of sperm viability, biological variation in the CR-treated animals was the same or less than that measured in their CON counterparts. In fact, CR variability was found to be significantly less for sperm count (P < 0.04) and tended toward significance for three other ejaculate parameters, namely, volume, weight, and osmolarity (range, P < 0.06 to P < 0.11).
Taken together, these data demonstrate that moderate CR had limited impact on semen quality in young adult rhesus macaques. Despite these similarities, it is still possible that sperm competency could be affected at some point following fertilization. Accumulating human and animal data suggest that alterations in genomic organization of the sperm nuclei are negatively correlated with the fertility potential of sperm [53, 54] and subsequent embryo survival [55]. The SCSA has proven to be highly effective in predicting fertility outcome both in vivo and in vitro [56]. In humans, an SCSA index finding above 30% is associated with reduced fertilizing capability of the sperm [55, 56]. Similarly, a large meta-analysis [57] conducted in the Georgetown Male Factor Infertility Study showed that the SCSA infertility test is significantly predictive of reduced pregnancy success.
Our study did not detect any significant difference between treatment groups in sperm quality based on DNA fragmentation index (CON, 8.5 ± 8.0; CR, 1.9 ± 1.1), confirming that DNA integrity was very high in all eight of the study animals. The one exception was a CON animal for which there was only one available sample for assay. In this instance, the subject demonstrated a DFI of 32.5%. If the 30% threshold observed in human studies was applied, it would appear that this animal could be infertile. Such judgment is speculative without further samples, however, and while a 30% threshold is highly indicative of infertility, it is not the only relevant measure, as there are many other factors that can affect whether sperm can initiate and sustain embryo development.
Finally, there have been sporadic studies of T measurement in rhesus macaques, and they are often contradictory. Some reports claim nonsignificant declines in testicular mass, serum T levels, and pulsatile T release in aged animals [58, 59], while others show no evidence of different T levels with age [14, 60]. This can perhaps be attributed to poor sampling and/or the fact that T levels vary widely among individuals, throughout the day, and even from day to day.
In our study, treatment groups had a similar pattern of daily circulating plasma T levels. Likewise, no differences were detected between CON and CR animals for the mean or maximum circulating T levels. Daily minimum levels, however, were significantly lower (P < 0.01) in CON subjects than in their CR counterparts. This differs from our previously published luteinizing hormone (LH) data [18], which detected no significant differences between the same animals with regard to daytime, nighttime, or overall mean plasma LH levels (P > 0.05). The biological relevance of this increase in daily minimum T levels in CR animals is uncertain, but it may be an indication of physiological efficiency. Alternatively, elevated basal plasma T levels in the CR animals may reflect enhanced conversion of the adrenal steroid dehydroepiandrosterone to T in organs such as the liver. By decreasing the daily swing between maximum and minimum levels and maintaining tighter control over T release, CR animals may be able to divert energy toward more critical functions of life maintenance [59]. This may in turn account for the decreased variability observed in the majority of our spermiogram parameters.
Our findings may also be an indication that CR animals can maintain T levels, which would be of great benefit because age-related T decline can cause weakened muscle function, lower bone density, and loss of cognitive function [15, 61–63]. Investigations in Brown Norway rats have shown that CR initiated at 4 mo of age and applied continuously for 30 mo results in significantly higher concentrations of serum T compared with control animals, suggesting that long-term CR can transiently suppress the reductions in steroidogenesis that are characteristic of aging [64]. Should CR be shown to elicit the same biological response in a nonhuman primate model, it could potentially be implemented as a counterbalancing force in human aging by exerting positive overall effects on metabolic health and maintenance of other physiological systems. This could have far-reaching social consequences for aging populations through improving quality of life, resistance to disease, maintained bone and muscle health, retention of libido, and cognition.
The present study is among the first to address the potential impact of moderate CR on sperm parameters and circulating T levels in young adult rhesus macaques. As such, it represents a unique and valuable opportunity to contribute to the growing body of literature regarding the effects of this dietary paradigm and its impact on biological function. Overall, the data suggest that moderate CR has no obvious lasting negative impact on semen quality and may in fact have a beneficial effect on maintaining daily minimum T levels, perhaps even during the age-related decline that occurs with aging. Whether CR impacts these same parameters in aging male macaques remains to be determined. Thus, the advantageous health benefits of CR may potentially be achieved without interfering with male reproductive potential.
Acknowledgments
The authors would like to thank the following members of the Oregon National Primate Research Center: Maralee Lawson, Diana Takahashi, Michelle Sparman, Carrie Thomas, Audrey Trupp, Kaleb Grund, Marci Szlavich, Norma Myers, and the late Dr. John Fanton. We would also like to thank Tia Dixon for her assistance with morphology scoring and Dr. Cathi VandeVoort of the California National Primate Research Center for her editorial comments.
Footnotes
Supported by National Institutes of Health grants AG-19914, AG-21380, AG-21382, AG-29612, HD-18185, HD-29186, and RR-00163. Additional support was provided by the Department of Animal and Avian Sciences at the University of Maryland and the Intramural Research Program of the National Institute on Aging.
These authors contributed equally as senior authors.
REFERENCES
- McCay CM, Crowell MF, Maynard LA.The effect of retarded growth upon the length of the life span and upon the ultimate body size. J Nutr 1935; 10: 63–70. [PubMed] [Google Scholar]
- Lane MA, Tilmont EM, De Angelis H, Handy A, Ingram DK, Kemnitz JW, Roth GS.Short-term calorie restriction improves disease-related markers in older male rhesus monkeys (Macaca mulatta). Mech Ageing Dev 1999; 112: 185–196. [DOI] [PubMed] [Google Scholar]
- Koubova J, Guarente L.How does calorie restriction work? Genes Dev 2003; 17: 313–321. [DOI] [PubMed] [Google Scholar]
- Lane MA, Ingram DK, Roth GS.Beyond the rodent model: calorie restriction in rhesus monkeys. Age 1997; 20: 45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresl TA, Colman RJ, Roecker EB, Havighurst TC, Huang Z, Allison DB, Bergman RN, Kemnitz JW.Dietary restriction and glucose regulation in aging rhesus monkeys: a follow-up report at 8.5 yr. Am J Physiol Endocrinol Metab 2001; 281: E757–E765. [DOI] [PubMed] [Google Scholar]
- Heilbronn LK, Ravussin E.Calorie restriction and aging: review of the literature and implications for studies in humans. Am J Clin Nutr 2003; 78: 361–369. [DOI] [PubMed] [Google Scholar]
- Gredilla R, Barja G.Minireview: the role of oxidative stress in relation to caloric restriction and longevity. Endocrinology 2005; 146: 3713–3717. [DOI] [PubMed] [Google Scholar]
- Ingram DK, Cutler RG, Weindruch R, Renquist DM, Knapka JJ, April M, Belcher CT, Clark MA, Hatcherson CD, Marriott BM, Roth GS.Dietary restriction and aging: the initiation of a primate study. J Gerontol A Biol Sci Med Sci 1990; 45: B148–B163. [DOI] [PubMed] [Google Scholar]
- Ottinger MA, Mobarak M, Abdelnabi MA, Roth GS, Proudman JA, Ingram DK.Effects of caloric restriction on reproductive and adrenal systems in Japanese quail: are responses similar to mammals, particularly primates? Mech Ageing Dev 2005; 126: 967–975. [DOI] [PubMed] [Google Scholar]
- Roth GS, Ingram DK, Lane MA.Calorie restriction in primates: will it work and how will we know? J Am Geriatr Soc 1999; 47: 896–903. [DOI] [PubMed] [Google Scholar]
- Weindruch R, Walford RL.The Retardation of Aging and Disease by Dietary Restriction. Springfield, IL: Charles C. Thomas;1988. [Google Scholar]
- Roth GS, Ingram DK, Black A, Lane MA.Effects of reduced energy intake on the biology of aging: the primate model. Eur J Clin Nutr 2000; 54: S15–S20. [DOI] [PubMed] [Google Scholar]
- Roth GS, Lane MA, Ingram DK, Mattison JA, Elahi D, Tobin JD, Muller D, Metter EJ.Biomarkers of caloric restriction may predict longevity in humans. Science 2002; 297: 811 [DOI] [PubMed] [Google Scholar]
- Mattison JA, Lane MA, Roth GS, Ingram DK.Calorie restriction in rhesus monkeys. Exp Gerontol 2003; 38: 35–46. [DOI] [PubMed] [Google Scholar]
- Sitzmann BD, Urbanski HF, Ottinger MA.Aging in male primates: reproductive decline, effects of calorie restriction and future research potential. Age 2008; 30: 157–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, Weindruch R.Caloric restriction delays disease onset and mortality in rhesus monkeys. Science 2009; 325: 201–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth GS, Blackman MR, Ingram DK, Lane MA, Ball SS, Cutler RG.Age related changes in androgen levels of rhesus monkeys subjected to diet restriction. Endocr J 1993; 1: 227–234. [Google Scholar]
- Sitzmann BD, Mattison JA, Ingram DK, Roth GS, Ottinger MA, Urbanski HF.Impact of moderate calorie restriction on the reproductive neuroendocrine axis of male rhesus macaques. Open Longevity Sci 2009; 3: 38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursting SD, Lavigne JA, Berrigan D, Perkins SN, Barrett JC.Calorie restriction, aging, and cancer prevention: mechanisms of action and applicability to humans. Ann Rev Med 2003; 54: 131–152. [DOI] [PubMed] [Google Scholar]
- Jensen TK, Andersson AM, Jorgensen N, Andersen AG, Carlsen E, Petersen JH, Skakkebaek NE.Body mass index in relation to semen quality and reproductive hormones among 1,558 Danish men. Fertil Steril 2004; 82: 863–870. [DOI] [PubMed] [Google Scholar]
- Kort HI, Massey JB, Elsner CW, Mitchell-Leef D, Shapiro DB, Witt MA, Roudebush WE.Impact of body mass index values on sperm quantity and quality. J Androl 2006; 27: 450–452. [DOI] [PubMed] [Google Scholar]
- Jervis KM, Robaire B.Effects of caloric restriction on gene expression along the epididymis of the Brown Norway rat during aging. Exp Gerontol 2003; 38: 549–560. [DOI] [PubMed] [Google Scholar]
- Harrison RM, Lewis RW.The male reproductive tract and its fluids. Dukelow WR, Erwin J.Comparative Primate Biology: Reproduction and Development, vol. 3. New York:Alan R. Liss, Inc.;1986: 101–148. [Google Scholar]
- Elder K, Dale B.In Vitro Fertilization. Cambridge, UK:Cambridge University Press;2000. [Google Scholar]
- National Research Council. Guide for the Care and Use of Laboratory Animals. Washington, DC:National Academies Press;1996. [Google Scholar]
- Urbanski HF, Pau FKY.A biphasic developmental pattern of circulating leptin in the male rhesus macaque (Macaca mulatta). Endocrinology 1998; 139: 2284–2286. [DOI] [PubMed] [Google Scholar]
- Downs JL, Mattison JA, Ingram DK, Urbanski HF.Effect of age and caloric restriction on circadian adrenal steroid rhythms in rhesus macaques. Neurobiol Aging 2008; 29: 1412–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council. Nutrient Requirements of Nonhuman Primates, 2nd rev. ed. Washington, DC:National Academies Press;2003. [Google Scholar]
- Black A, Allison DB, Shapses SA, Tilmont EM, Handy AM, Ingram DK, Roth GS, Lane MA.Calorie restriction and skeletal mass in rhesus monkeys (Macaca mulatta): evidence for an effect mediated through changes in body size. J Gerontol A Biol Sci Med Sci 2001; 56: B98–B107. [DOI] [PubMed] [Google Scholar]
- Mattison JA, Black A, Huck J, Moscrip T, Handy A, Tilmont E, Roth GS, Lane MA, Ingram DK.Age-related decline in caloric intake and motivation for food in rhesus monkeys. Neurobiol Aging 2005; 26: 1117–1127. [DOI] [PubMed] [Google Scholar]
- Mattison JA, Croft MA, Dahl DB, Roth GS, Lane MA, Ingram DK, Kaufman PL.Accommodative function in rhesus monkeys: effects of aging and calorie restriction. J Am Aging Assoc 2005; 27: 59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanski HF, Garyfallou VT, Kohama SG, Hess DL.α-Adrenergic receptor antagonism and N-methyl-D-aspartate (NMDA) induced luteinizing hormone release in female rhesus macaques. Brain Res 1997; 744: 96–104. [DOI] [PubMed] [Google Scholar]
- Resko JA, Malley A, Begley D, Hess DL.Radioimmunoassay of testosterone during fetal development of the rhesus monkey. Endocrinology 1973; 93: 156–161. [DOI] [PubMed] [Google Scholar]
- Urbanski HF, Downs JL, Garyfallou VT, Mattison JA, Lane MA, Roth GS, Ingram DK.Effect of caloric restriction on the 24-h plasma DHEAS and cortisol profiles of young and old male rhesus macaques. Ann N Y Acad Sci 2004; 1019: 443–447. [DOI] [PubMed] [Google Scholar]
- Mastroianni L, Jr, Manson WA., JrCollection of monkey semen by electroejaculation. Proc Soc Exp Biol Med 1963; 112: 1025–1027. [DOI] [PubMed] [Google Scholar]
- Gould KG, Mann DR.Comparison of electrostimulation methods for semen recovery in the rhesus monkey (Macaca mulatta). J Med Primatol 1988; 17: 95–103. [PubMed] [Google Scholar]
- Kholkute SD, Gopalkrishnan K, Puri CP.Variations in seminal parameters over a 12-month period in captive bonnet monkeys. Primates 2000; 41: 393–405. [DOI] [PubMed] [Google Scholar]
- Sarason RL, Vandevoort CA, Mader DR, Overstreet JW.The use of nonmetal electrodes in electroejaculation of restrained but unanesthetized macaques. J Med Primatol 1991; 20: 122–125. [PubMed] [Google Scholar]
- Lanzendorf SE, Zelinski-Wooten MB, Stouffer RL, Wolf DP.Maturity at collection and the developmental potential of rhesus monkey oocytes. Biol Reprod 1990; 42: 703–711. [DOI] [PubMed] [Google Scholar]
- Tollner TL, VandeVoort CA, Overstreet JW, Drobnis EZ.Cryopreservation of spermatozoa from cynomolgus monkeys (Macaca fascicularis). J Reprod Fertil 1990; 90: 347–352. [DOI] [PubMed] [Google Scholar]
- Leibo SP, Kubisch HM, Schramm RD, Harrison RM, VandeVoort CA.Male-to-male differences in post-thaw motility of rhesus spermatozoa after cryopreservation of replicate ejaculates. J Med Primatol 2007; 36: 151–163. [DOI] [PubMed] [Google Scholar]
- Jeyendran RS.Protocols for Semen Analysis in Clinical Diagnosis. New York:Parthenon Publishing Group;2003. [Google Scholar]
- Gupta G, Maikhuri J, Setty B, Dhar JD.Seasonal variations in daily sperm production rate of rhesus and bonnet monkeys. J Med Primatol 2000; 29: 411–414. [DOI] [PubMed] [Google Scholar]
- Muehlenbein MP, Campbell BC, Murchison MA, Phillippi KM.Morphological and hormonal parameters in two species of macaques: impact of seasonal breeding. Am J Phys Anthropol 2002; 117: 218–227. [DOI] [PubMed] [Google Scholar]
- Lanzendorf SE, Gliessman PM, Archibong AE, Alexander M, Wolf DP.Collection and quality of rhesus monkey semen. Mol Reprod Dev 1990; 25: 61–66. [DOI] [PubMed] [Google Scholar]
- Nichols SM, Bavister BD.Comparison of protocols for cryopreservation of rhesus monkey spermatozoa by post-thaw motility recovery and hyperactivation. Reprod Fertil Dev 2006; 18: 777–780. [DOI] [PubMed] [Google Scholar]
- Jeyendran RS, Vanderven HH, Perezpelaez M, Crabo BG, Zaneveld LJD.Development of an assay to assess the functional integrity of the human-sperm membrane and its relationship to other semen characteristics. J Reprod Fertil 1984; 70: 219–228. [DOI] [PubMed] [Google Scholar]
- Wong WY, Thomas CMG, Merkus JMWM, Zielhuis GA, Steegers-Theunissen RPM.Male factor subfertility: possible causes and the impact of nutritional factors. Fertil Steril 2000; 73: 435–442. [DOI] [PubMed] [Google Scholar]
- Yousef MI, Abdallah GA, Kamel KI.Effect of ascorbic acid and vitamin E supplementation on semen quality and biochemical parameters of male rabbits. Anim Reprod Sci 2003; 76: 99–111. [DOI] [PubMed] [Google Scholar]
- Hammoud AO, Gibson M, Peterson CM, Meikle AW, Carrell DT.Impact of male obesity on infertility: a critical review of the current literature. Fertil Steril 2008; 90: 897–904. [DOI] [PubMed] [Google Scholar]
- Biswas A, Mohan J, Sastry KVH.Effect of higher dietary vitamin E concentrations on physical and biochemical characteristics of semen in Kadaknath cockerels. Br Poult Sci 2009; 50: 733–738. [DOI] [PubMed] [Google Scholar]
- Mendiola J, Torres-Cantero AM, Moreno-Grau JM, Ten J, Roca M, Moreno-Grau S, Bernabeu R.Food intake and its relationship with semen quality: a case-control study. Fert Steril 2009; 91: 812–818. [DOI] [PubMed] [Google Scholar]
- Penfold LM, Jost L, Evenson DP, Wildt DE.Normospermic versus teratospermic domestic cat sperm chromatin integrity evaluated by flow cytometry and intracytoplasmic sperm injection. Biol Reprod 2003; 69: 1730–1735. [DOI] [PubMed] [Google Scholar]
- Ostermeier GC, Sargeant GA, Yandell BS, Evenson DP, Parrish JJ.Relationship of bull fertility to sperm nuclear shape. J Androl 2001; 22: 595–603. [PubMed] [Google Scholar]
- Larson-Cook KL, Brannian JD, Hansen KA, Kasperson KM, Aamold ET, Evenson DP.Relationship between the outcomes of assisted reproductive techniques and sperm DNA fragmentation as measured by the sperm chromatin structure assay. Fertil Steril 2003; 80: 895–902. [DOI] [PubMed] [Google Scholar]
- Bungum M, Humaidan P, Spano M, Jepson K, Bungum L, Giwercman A.The predictive value of sperm chromatin structure assay (SCSA) parameters for the outcome of intrauterine insemination, IVF, and ICSI. Hum Reprod 2004; 19: 1401–1408. [DOI] [PubMed] [Google Scholar]
- Evenson DP, Wixon RL.Use of the sperm chromatin structure assay (SCSA) as a diagnostic tool in the human infertility clinic. Fertil Magazine 2006; 4: 15–18. [Google Scholar]
- Black A, Lane MA.Nonhuman primate models of skeletal and reproductive aging. Gerontology 2002; 48: 72–80. [DOI] [PubMed] [Google Scholar]
- Roth GS, Mattison JA, Ottinger MA, Chachich ME, Lane MA, Ingram DK.Aging in rhesus monkeys: relevance to human health interventions. Science 2004; 305: 1423–1426. [DOI] [PubMed] [Google Scholar]
- Mattison JA, Roth GS, Ingram DK, Lane MA.Endocrine effects of dietary restriction and aging: the National Institute on Aging study. J Anti-Aging Med 2001; 4: 215–223. [Google Scholar]
- Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR.Longitudinal effects of aging on serum total and free testosterone levels in healthy men. J Clin Endocrinol Metab 2001; 86: 724–731. [DOI] [PubMed] [Google Scholar]
- Moffat SD, Zonderman AB, Metter EJ, Blackman MR, Harman SM, Resnick SM.Longitudinal assessment of serum free testosterone concentration predicts memory performance and cognitive status in elderly men. J Clin Endocrinol Metab 2002; 87: 5001–5007. [DOI] [PubMed] [Google Scholar]
- Schlatt S, Pohl CR, Ehmcke J, Ramaswamy S.Age-related changes in diurnal rhythms and levels of gonadotropins, testosterone, and inhibin B in male rhesus monkeys (Macaca mulatta). Biol Reprod 2008; 79: 93–99. [DOI] [PubMed] [Google Scholar]
- Chen HL, Luo LD, Liu J, Brown T, Zirkin BR.Aging and caloric restriction: effects on Leydig cell steroidogenesis. Exp Gerontol 2005; 40: 498–505. [DOI] [PubMed] [Google Scholar]