Abstract
Oocyte maturation in rodents is characterized by a dramatic reorganization of the endoplasmic reticulum (ER) and an increase in the ability of an oocyte to release Ca2+ in response to fertilization or inositol 1,4,5-trisphosphate (IP3). We examined if human oocytes undergo similar changes during cytoplasmic meiotic maturation both in vivo and in vitro. Immature, germinal vesicle (GV)-stage oocytes had a fine network of ER throughout the cortex and interior, whereas the ER in the in vivo-matured, metaphase II oocytes was organized in large (diameter, ∼2–3 μm) accumulations throughout the cortex and interior. Likewise, oocytes matured in vitro exhibited cortical and interior clusters with no apparent polarity in regard to the meiotic spindle. In vivo-matured oocytes contained approximately 1.5-fold the amount of IP3 receptor protein and released significantly more Ca2+ in response to IP3 compared with GV-stage oocytes; however, oocytes matured in vitro did not contain more IP3 receptor protein or release more Ca2+ in response to IP3 compared with GV-stage oocytes. These results show that at least one cytoplasmic change occurs during in vitro maturation of human oocytes that might be important for fertilization and subsequent embryonic development, but they suggest that a low developmental competence of in vitro-matured oocytes could be the result of deficiencies in the ability to release Ca2+ at fertilization.
Keywords: calcium, endoplasmic reticulum, human oocyte, IP3, oocyte maturation
Human oocytes undergo a reorganization of endoplasmic reticulum and become more sensitive to IP3 during meiotic maturation.
INTRODUCTION
A hallmark feature of fertilization is the release of Ca2+ from intracellular stores. Ca2+ is stored in the endoplasmic reticulum (ER) and is released into the cytoplasm following sperm-egg fusion by inositol 1,4,5-trisphosphate (IP3), which is produced from cleavage of the membrane lipid phosphatidylinositol 4,5-bisphosphate [1]. In mammals, an initial release of Ca2+ occurs soon after sperm-egg fusion, and this is followed by a series of repetitive Ca2+ oscillations that last until pronuclear formation [2]. Intracellular Ca2+ release is essential for polyspermy prevention, egg activation, and recruitment of maternal RNAs that initiate protein synthesis after fertilization [3, 4].
The ability to release Ca2+ at fertilization develops during oocyte maturation. Immature, prophase I oocytes (germinal vesicle [GV] stage) are less sensitive to IP3-induced Ca2+ release than mature, metaphase II (MII) oocytes [5–7]. An increase in the sensitivity to IP3 during oocyte maturation likely involves several modifications in the oocyte during maturation, including a reorganization of Ca2+ stores as well as increased numbers of IP3 receptors [8, 9]. Indeed, the ER undergoes a dramatic reorganization during maturation of a diverse array of species (from marine worms to starfish, frogs, rodents, and cows [10–16]) as well as an increase in the amount of IP3 receptor protein [8, 17]. In GV-stage mouse oocytes, the ER is continuous with the nuclear envelope and is present throughout the cytoplasm as well as in small accumulations throughout the oocyte interior. During maturation to MII, the ER changes such that clusters of ∼1–2 μm appear in the cortex opposite the meiotic spindle [9, 12]. These changes parallel a rearrangement of IP3 receptors, which also are generally absent from the cortex of immature oocytes but appear in clusters in the cortex of mature eggs [8, 18]. Both of these changes are thought to be involved in the increased sensitivity to IP3 in the mature egg as opposed to the immature oocyte.
Human oocytes undergo repetitive Ca2+ oscillations at fertilization [19], and the ability to release Ca2+ has been reported to develop, as it does in other species, during oocyte maturation [20]. The distribution of IP3 receptors has also been reported to change during oocyte maturation into an organization that is consistent with the structure of the ER in the cortex of the mouse oocyte [21]. However, it is not known if these changes are accompanied by a reorganization of the ER. In vitro-matured human oocytes have reportedly been unable to exhibit the same ability to release Ca2+ in response to the sulfhydryl agent thimerosal, which sensitizes the IP3 receptor as it does in vivo-matured oocytes, suggesting that these changes do not develop properly in oocytes matured in vitro [20]. In vitro-matured oocytes can be fertilized, but they have low developmental competence [22, 23]. Therefore, it is possible that Ca2+-releasing ability is deficient in oocytes matured in vitro. Because the ability to mature oocytes in vitro has important clinical applications, it is of interest to examine various components of normal cytoplasmic maturation to identify where oocytes matured in vitro might be deficient.
In the present study, we examined the ER distribution, the relative amount of IP3 receptor protein, and the ability to release Ca2+ in response to IP3 in GV-stage as well as in vivo- and in vitro-matured, MII oocytes. Our results show that in human oocytes, the ER undergoes a dramatic reorganization during maturation and that oocytes matured in vitro exhibit the same reorganization. Oocytes matured in vivo contain approximately 1.5-fold as much IP3 receptor protein as GV-stage oocytes; however, oocytes matured in vitro do not exhibit this increase. Likewise, the ability to release Ca2+ in response to IP3 increases during maturation in vivo but not in vitro.
MATERIALS AND METHODS
Source and Culture of Oocytes
The present study was approved by the Institutional Review Board at the University of Connecticut Health Center (IRB 06–125). All patients gave informed consent to donate oocytes before participation in the study. Immature, GV-stage oocytes were retrieved from the ovaries of women aged 21–44 yr who were undergoing standard in vitro fertilization procedures using intracytoplasmic sperm injection. All patients underwent pituitary suppression using a gonadotropin-releasing hormone agonist or antagonist. Controlled ovarian stimulation was performed by injecting 150–450 IU of recombinant follicle-stimulating hormone (Gonal-F [EMD Serono] or Follistim [Schering Plough]) daily with or without 75–150 IU of human menopausal gonadotropin (Menopur or Repronex; Ferring Pharmaceuticals). Doses were adjusted based on follicular response as evidenced by serial transvaginal ultrasounds and serum estradiol levels. A subcutaneous injection of 3300–10 000 IU of human chorionic gonadotropin was administered when three or more follicles reached a mean diameter of 18 mm. In selected cases, final oocyte maturation was achieved with 20 IU of leuprolide acetate (Lupron; TAP Pharmaceuticals) [24]. Transvaginal ultrasound-guided oocyte retrieval was performed 35 h after human chorionic gonadotropin or Lupron injection.
Oocytes were aspirated from follicles with a diameter of ∼14–22 mm. Most oocytes retrieved from such follicles are at MII (mature eggs). However, a small percentage of oocytes are at prophase I; these oocytes are identified by the presence of a GV. Because these immature oocytes are not used clinically at our center, they are routinely discarded. In some cases, we obtained MII oocytes that were leftover from women who chose to expose only a few oocytes to sperm and that would have otherwise been discarded. Cumulus-oocyte complexes were aspirated into culture medium containing 5% human serum albumin (Global SL Behring) in Quinn Advantage Fertilization Medium (SAGE; Cooper Surgical) and incubated in an atmosphere of 5% CO2/95% at 37°C for 3–5 h. Cumulus cells were stripped enzymatically with hyaluronidase type VIII from bovine testes (catalog no. H-3757; Sigma-Aldrich) and mechanically by pipetting up and down through a small-bore pipette. Following cumulus removal, oocytes that had a GV were placed into blastocyst medium (SAGE) containing 10% serum protein substitute (Cooper Surgical) and 10 μM cilostamide (Calbiochem), a phosphodiesterase 3A-specific inhibitor that prevents spontaneous meiotic resumption. Oocytes retrieved from all patients on a given day were pooled.
We selected GV-stage oocytes that had a readily discernible GV following an overnight culture in medium containing 10 μM cilostamide except for some of the immunoblotting experiments, in which GV-stage oocytes were frozen on the day of retrieval. To obtain in vitro-matured, MII oocytes, we selected oocytes that were GV-intact after an overnight culture and transferred them to blastocyst medium that did not contain cilostamide. Oocytes were cultured in a humidified atmosphere of 5% CO2/95%, and oocytes that underwent GV breakdown and formed a first polar body within 24 h were selected for further experimentation.
Experiments using mice were performed in accordance with the Center for Laboratory Animal Care at the University of Connecticut Health Center. GV-stage mouse oocytes were retrieved from the ovaries of mice that had been injected 42–44 h previously with 10 IU of equine chorionic gonadotropin. Oocytes were retrieved in culture medium containing 10 μM milrinone to prevent spontaneous oocyte maturation and were transferred to medium that did not contain milrinone to initiate oocyte maturation. Zonae pellucidae were removed by pipetting the oocytes through a small-bore pipette in the presence of 10 μg/ml α-chymotrypsin.
Oocyte Microinjection, DiI, and Ca2+ Imaging
Oocytes were microinjected with DiI (1,1′-dihexadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate) as previously described [25]. Briefly, a saturated solution of DiI (Invitrogen) was prepared in soybean oil (Wesson Oil; ConAgra Foods, Inc.) and stored at 4°C. The DiI solution was front-loaded into a beveled, mercury-filled pipette connected to a micrometer syringe system filled with Fluorinert FC-70 (Sigma). The same pipette was used to inject several oocytes with ∼7 pl of the DiI solution, which formed an oil droplet inside the oocytes. The volume injected was calculated based on the diameter of the sphere that forms in the oocyte cytoplasm during microinjection. DiI-labeled oocytes were observed with a confocal microscope (Zeiss Pascal; Carl Zeiss Microimaging, Inc.) ∼1–2 h after microinjection. Fluorescence was excited with the 543-nm line of an HeNe laser and was detected using a 560-nm emission filter. Images were collected using a 40× NA 1.2 water-immersion objective (C-Apochromat; Carl Zeiss MicroImaging, Inc.).
Oocytes were microinjected with ∼30 pl of a 400 μM stock of calcium green 10-kDa dextran (final concentration in the oocyte, ∼13 μM; calculation based on an oocyte volume of 900 pl), and Ca2+ was imaged as previously described [26]. For IP3 injections, a baseline measurement was obtained from each oocyte, then the recording was interrupted while a pipette loaded with a solution of IP3 was inserted into the oocyte. After pipette insertion, the recording was started again. Following another baseline recording, 20 pl of a 5 μM solution of IP3 (final concentration in the oocyte, 100 nM) were injected while simultaneously measuring intracellular Ca2+. The amplitude of Ca2+ release was calculated based on the increase in the peak amplitude of the response to injection (F) subtracted by the baseline (F0) and then divided by the baseline signal [(F− F0)/F0]. Figures were made using Adobe Photoshop after scanning Ca2+ records into a computer.
FM 1–43 Labeling
To label plasma membranes, zonae pellucidae were removed from oocytes in serum-free blastocyst medium containing 0.5% pronase (Calbiochem). Zona-free oocytes were incubated in 2 μM FM 1–43 (Invitrogen) diluted in serum-free Hank balanced salt solution. Oocytes were examined with a confocal microscope after ∼1–2 h. Fluorescence was excited at 488 nm and was detected at 560 nm. Images were collected using a 40× NA 1.2 water-immersion objective.
Immunoblotting
The GV-stage, in vivo- and in vitro-matured oocytes were washed with PBS containing 0.1% polyvinyl alcohol and then frozen in liquid nitrogen in a minimal amount of medium and stored at −80°C until use. Two to seven oocytes were solubilized in Laemmli sample buffer, and proteins were separated on 4–15% gradient gels. IP3 receptor protein was detected using an affinity-purified antibody kindly provided by Dr. Jim Watras (University of Connecticut Health Center, Farmington, CT) [27]. Blots were developed using enhanced chemiluminescence (ECLPlus; GE Healthcare). Densitometric analysis was carried out using ImageJ software (National Institutes of Health).
RESULTS
Distribution of ER in GV-Stage and MII Oocytes
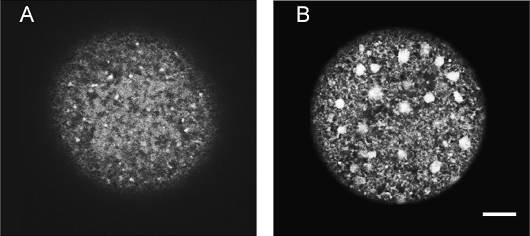
The cortical ER in GV-stage and MII mouse oocytes differs considerably, with the immature oocyte having little ER membrane in the cortex and the mature egg having cortical ER that is organized into large, distinct clusters [9, 12]. To examine the ER in human oocytes, we looked at the cortices of GV-stage and MII oocytes with confocal microscopy after injection of the lipophilic, fluorescent dye DiI [9, 25, 28]. DiI was microinjected into oocytes, where it contacts intracellular membranes; because the ER is a continuous network, it spreads throughout the ER in the entire cell [28]. We found that human, GV-stage oocytes contained little cortical ER that was not organized into cortical clusters (n = 21) (Fig. 1A), although in some cases, small, punctate spots of ER were observed in the cortex (not shown). In contrast, 69% of in vivo-matured, MII oocytes contained ER membrane throughout the cortex that was organized into large (diameter, ∼2–3 μm), distinct ER clusters (n = 16) (Fig. 1B). The cortical ER in human GV-stage and MII oocytes therefore undergoes changes during maturation similar to those in mouse oocytes [9, 12].
FIG. 1.
Cortical ER in GV-stage (A) and MII (B) oocytes. The ER was labeled with the lipophilic, fluorescent dye DiI and imaged with a confocal microscope. The cortical ER in GV-stage oocytes is largely devoid of ER, whereas the ER in MII oocytes is characterized by large ER accumulations. Bar = 10 μm.
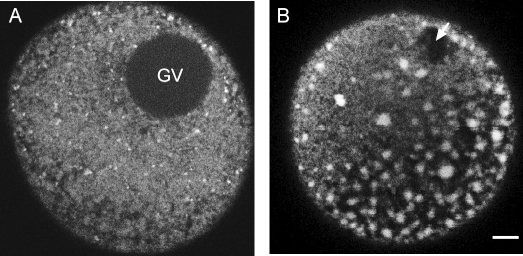
The ER in mature mouse oocytes is polarized such that the clusters of ER are localized in the cortex opposite the meiotic spindle, whereas immature oocytes contain smaller clusters of ER throughout the entire oocyte and surrounding the GV [9, 12]. Human oocytes differed in this regard. None of the GV-stage oocytes examined contained ER around the GV, and larger clusters of ER were absent from the oocyte interior (Fig. 2A). In MII oocytes, clusters of ER were present not only in the cortex but also throughout the entire oocyte (Fig. 2B). In addition, we generally observed no apparent polarity in relation to the meiotic spindle, although in one case, the clusters were concentrated in half of the oocyte at roughly a 45° angle to the spindle (Fig. 2B). This oocyte contained ER clusters throughout the rest of the oocyte as well.
FIG. 2.
Equatorial view of the ER in GV-stage (A) and MII (B) oocytes showing the lack of abundant ER in the immature oocyte and large ER accumulations throughout the cortex and inner cytoplasm of MII eggs. The arrow in B points to the meiotic spindle. Bar = 10 μm.
Distribution of ER in In Vitro-Matured Human Oocytes
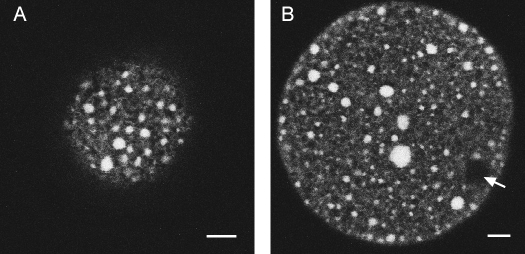
One issue with obtaining immature human oocytes and maturing them in vitro is that although these oocytes develop to MII, they have low developmental competence after fertilization [22, 23]. We sought to determine if oocytes matured in vitro were able to develop cortical ER accumulations following in vitro maturation to MII. We found that 79% of in vitro-matured oocytes exhibited large clusters of ER in the cortex (n = 14), similar to those in the in vivo-matured human oocytes (Fig. 3A). In addition, ER was distributed throughout the cytoplasm as well as in the cortex, with no polarity in regard to the meiotic spindle, as in the in vivo-matured oocytes (Fig. 3B). These results demonstrate that in vitro-matured oocytes are capable of undergoing the changes in ER reorganization that occur normally in vivo. Because Ca2+ is stored in the ER, this in turn suggests that in vitro-matured oocytes are able to mount a sufficient Ca2+ response at fertilization. Taken together, these results suggest that Ca2+-releasing ability in the in vitro-matured oocyte is not responsible for the developmental incompetence seen in the in vitro-matured human oocytes.
FIG. 3.
ER distribution in an in vitro-matured oocyte at the MII stage. A) ER in the cortex just under the plasma membrane. B) Equatorial view showing cortical and cytoplasmic ER accumulations. Arrow in B points to the meiotic spindle. Bar = 10 μm.
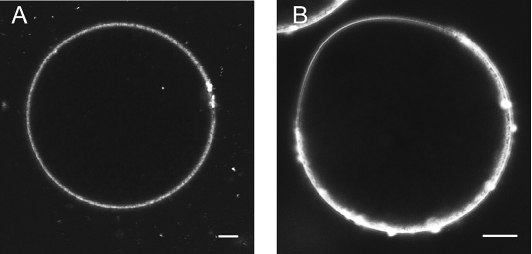
It has been reported that human oocytes contain no polarity with regard to the meiotic spindle and that sperm are able to fuse anywhere on the surface of the oocyte [29], whereas in mouse oocytes, a microvilli-free domain is present above the meiotic spindle that prevents the sperm from binding in this region [30, 31]. This might explain the lack of ER polarity with regard to the meiotic spindle in human oocytes, because Ca2+ stores would be expected to be present even if the sperm fuses in the region of the oocyte containing the meiotic spindle. To confirm the lack of polarity in the mature human oocyte, we used FM 1–43, a dye that is fluorescent when it contacts membranes. We found that in the in vitro-matured oocytes, the fluorescence intensity was the same around the entire surface of the oocyte, with no apparent polarity (Fig. 4A). To illustrate that this dye can, indeed, show polarity in an oocyte, we used mouse oocytes. Figure 4B shows the presence in a mouse oocyte of a microvilli-free domain in the region of the meiotic spindle.
FIG. 4.
The membrane of the mature, MII human oocyte does not exhibit polarity with regard to the meiotic spindle. A) Plasma membrane of an in vitro-matured human oocyte labeled with the fluorescent membrane dye FM 1–43. B) Polarization with regard to the meiotic spindle in an MII mouse oocyte. Bar = 10 μm.
Human Oocytes Synthesize IP3 Receptor Protein During In Vivo, but Not In Vitro, Oocyte Maturation
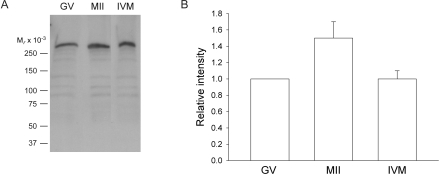
In addition to changes in reorganization of ER during maturation in mouse oocytes, the amount of IP3 receptor protein approximately doubles [8]. IP3 receptors are present on the ER [18] and allow the oocyte to release Ca2+ at fertilization in response to sperm-egg fusion. Using Western blot analysis, we examined if human oocytes undergo such an increase in IP3 receptor protein during maturation. We examined the type 1 IP3 receptor, both because it has been shown previously to be present in human oocytes [21] and because it is the receptor responsible for Ca2+ release after fertilization or injection of IP3 in rodent as well as in human oocytes [32–34]. The IP3 receptor was abundant in the oocyte, because we were able to detect protein in a single oocyte. Compared with the amount of IP3 receptor protein in GV-stage oocytes, the amount of receptor protein in the in vivo-matured, MII oocytes increased by approximately 50% (Fig. 5). In contrast, oocytes matured in vitro did not synthesize IP3 receptor protein to the same extent as oocytes matured in vivo (Fig. 5). Although the amount of protein in the in vivo- versus in vitro-matured oocytes did not quite reach statistical significance (P = 0.05), these results suggest that oocytes are deficient in their ability to synthesize protein during in vitro maturation.
FIG. 5.
The amount of IP3 receptor protein increases during oocyte maturation in vivo, but not in vitro. A) Western blot showing IP3 receptor protein in lysates from seven GV-stage, in vivo-matured (MII), and in vitro-matured (IVM) oocytes per lane. B) Densitometric analysis of IP3 receptor protein amounts in in vivo-matured and in vitro-matured oocytes compared to GV-stage oocytes. Densitometric values were obtained from four separate experiments using in vivo-matured oocytes and from eight separate experiments using in vitro-matured oocytes each. Values are reported as the intensity of MII oocytes relative to that of GV-stage oocytes that were run on the same blot. Overall, the relative amount of IP3 receptor protein increased by 1.5-fold in oocytes matured in vivo but showed no increase in oocytes matured in vitro. Data are presented as the mean ± SEM are shown. The amount of protein in in vivo-matured oocytes versus in vitro-matured oocytes did not quite reach statistical significance (P = 0.05).
Ability to Release Ca2+ Increases in Response to IP3 During In Vivo, but Not In Vitro, Oocyte Maturation
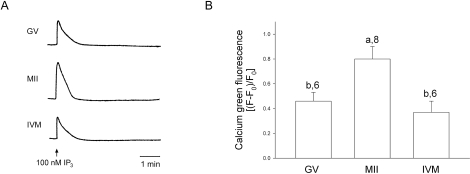
It has been reported previously that human oocytes develop the ability to release Ca2+ in response to the sulfhydryl reagent thimerosal, which sensitizes the IP3 receptor to low levels of IP3 [35], during maturation in vivo [20]. This previous study [20] indicated that oocytes matured in vitro, however, were deficient in their ability to release Ca2+ following in vitro maturation. The oocytes used in that study were obtained from ovaries of unstimulated patients, so it differed from our study, which used oocytes following hormonal stimulation. Here, we chose to measure Ca2+ release in response to the more physiological Ca2+-releasing agent IP3. We found that oocytes matured in vivo exhibited a single Ca2+ transient in response to injection of 100 nM IP3 (Fig. 6). This concentration was chosen because it is not saturating in mouse oocytes [7]. GV-stage oocytes also released a single Ca2+ transient in response to IP3, but the amplitude of the Ca2+ transient was significantly lower than that in the in vivo-matured oocytes (Fig. 6). However, oocytes matured in vitro showed a single transient that did not differ in amplitude from that of GV-stage oocytes (Fig. 6). In all cases, the duration of the transient was the same. These results demonstrate that immature oocytes develop an increased ability to release Ca2+ in response to a physiological stimulus in vivo, but that the ability to release Ca2+ in response to IP3 does not increase during in vitro maturation under our culture conditions.
FIG. 6.
Human oocytes increase their ability to release Ca2+ in response to injection of IP3 during in vivo, but not in vitro, oocyte maturation. A) Pattern of Ca2+ release following injection of 100 nM IP3. Oocytes were injected with the Ca2+ indicator dye calcium green dextran, and Ca2+ release was monitored during injection of IP3. B) Quantification of the amplitude of Ca2+ release in response to IP3 injection. Results shown are the average increase in calcium green fluorescence after subtracting the baseline fluorescence (F0) by the peak amplitude (F) and then dividing by the baseline ([F − F0)/F0]). Average amplitude and SEM are shown. Different letters represent significant differences in amplitude (P < 0.001), and numbers are the number of oocytes tested.
DISCUSSION
The present study demonstrates that human oocytes undergo a dramatic reorganization of ER during meiotic maturation. GV-stage oocytes contain a fine ER network throughout the cortex and interior, whereas mature, MII oocytes contain striking, ∼2- to 3-μm accumulations in both the cortex and throughout the cytoplasm, with no apparent polarity in regard to the meiotic spindle. The ER in oocytes matured in vitro is indistinguishable from the ER of in vivo-matured oocytes, with membrane accumulations of the same size located both in the cortex and throughout the cytoplasm. In addition, during in vivo maturation, the amount of IP3 receptor protein increases by approximately 1.5-fold, and oocytes develop an increased ability to release Ca2+ in response to IP3. However, oocytes matured in vitro are deficient in their ability to synthesize IP3 receptor protein and fail to develop an increased sensitivity to release Ca2+ in response to IP3.
The ER is the major Ca2+ storage organelle in the oocyte [36]. As such, it plays an important role in Ca2+ release that occurs at fertilization in all species that have been studied [37, 38]. Ca2+ release is necessary for polyspermy prevention, egg activation, and recruitment of maternal RNAs that initiate protein synthesis following fertilization [3, 4]. In mature mouse oocytes, the ER is polarized such that clusters of ER are present in the cortex opposite the meiotic spindle [9]. This region of the oocyte corresponds to the region that contains both microvilli and cortical granules [30, 39, 40], is the region where sperm-egg fusion occurs [30, 31], and is the site for the generation of repetitive Ca2+ waves following fertilization [18]. We did not observe polarity of the ER in the human oocyte. Because the human oocyte does not contain a microvilli-free area over the meiotic spindle and, thus, the sperm is able to fuse anywhere over the oocyte surface [29], this is not unexpected. A previous study indicated that the IP3 receptor is polarized in the human oocyte with regard to the meiotic spindle [21], as in mouse oocytes. Although we did not label the IP3 receptor, it is likely that IP3 receptors are located on the ER even in the region of the meiotic spindle, because IP3 receptors have been shown previously to be localized to the ER in mouse oocytes [18].
A rearrangement of ER during meiotic maturation, including the development of cortical clusters, has been reported in other species, including the marine worm Cerebratulus lacteus [10] and the frog Xenopus laevis [16]. Among the mammals, mouse oocytes develop cortical clusters during maturation [9, 12], whereas hamster and bovine oocytes exhibit a change from cortical localization to the formation of loosely organized clusters of ER throughout the cytoplasm [14, 15]. Some of these species have been found to develop the ability to release Ca2+ during maturation [6, 7, 28], and the rearrangement of Ca2+ stores is thought to play a role in this ability. The arrangement of ER could also play a role in the ability of a mature oocyte to generate Ca2+ waves at fertilization, because immature C. lacteus and hamster oocytes do not generate Ca2+ waves at fertilization [14], nor do mouse oocytes generate Ca2+ waves in response to PLCζ [41], a sperm-specific phospholipase C thought to initiate Ca2+ oscillations at fertilization [42]. In the present study, we found that in vitro-matured human oocytes undergo a reorganization of ER but do not develop the ability to release Ca2+ in response to IP3, suggesting there is more to increasing IP3-induced sensitivity than simply a rearrangement of ER structure.
The amount of IP3 receptor protein increased by approximately 50% during meiotic maturation in vivo. These mature oocytes also released significantly more Ca2+ in response to IP3 injection compared with GV-stage oocytes. However, we found that oocytes matured in vitro possessed similar amounts of IP3 receptor protein and released Ca2+ in response to IP3 injection to the same extent as immature oocytes, demonstrating that they did not increase their sensitivity to IP3 during maturation. These results are in agreement with those of a previous study showing that the human oocyte develops an increased Ca2+-releasing ability during oocyte maturation and that oocytes matured in vitro do not have the same ability to release Ca2+ in response to the sulfhydryl reagent thimerosal compared with oocytes matured in vivo [20]. The finding that GV-stage and in vitro-matured oocytes had a completely different ER arrangement yet released comparable amounts of Ca2+ in response to IP3 suggests that the amount of IP3 receptor protein or other factors, such as an increase in Ca2+ stores, is perhaps as important as the presence of ER clusters.
The finding that oocytes matured in vitro do not release as much Ca2+ as those matured in vivo in response to IP3 suggests this could contribute to the lower developmental competence seen after in vitro maturation. Other factors could also contribute to this lower developmental competence. For example, microtubules or microfilaments could be disrupted during maturation in vitro. The cytoskeleton has been shown to be necessary for reorganization of the ER during both GV breakdown and development of clusters afterward [12]. Our results showing that ER clusters form during in vitro maturation suggest that microtubules and microfilaments function normally, at least during this process, but they could potentially affect other aspects of cytoplasmic maturation. Another contributor to low developmental competence could be the particular culture medium used, because this has been shown to be important for proper ER reorganization during in vitro maturation of mouse oocytes [25]. Our results demonstrate that the culture medium used in the present study supports ER reorganization. It would be interesting to test a variety of maturation media to see if they have positive or negative effects on the ability of IP3-induced Ca2+ release to increase during maturation.
Despite our inability to detect a differential development of Ca2+ signals during in vitro maturation, our study nonetheless shows that at least one important event during cytoplasmic maturation occurs during oocyte maturation in vitro. Other cytoplasmic changes, such as changes in IP3 receptor phosphorylation during maturation, also likely are involved. For example, the type I IP3 receptor becomes phosphorylated early after the onset of maturation by Polo-like kinase 1 (PLK1) and mitogen-activated protein kinase in mouse and pig oocytes [43–45], and this phosphorylation is associated with the ability of the oocyte to form cortical IP3 clusters during maturation and to mount a full Ca2+ response following IP3 stimulation or in response to other agonists known to cause Ca2+ release [43, 46]. Further studies will be needed to determine if cell-cycle regulatory proteins likewise contribute to the increased sensitivity that has reportedly been seen during maturation of human oocytes [20]. Indeed, mitogen-activated protein kinase activity has been shown to be lower in human oocytes matured in vitro than in those matured in vivo [47]. Because we did not observe a noticeable increase in the ability of in vitro-matured oocytes to release Ca2+ in response to IP3, such oocytes possibly are deficient in other, as-yet-unidentified factors that change during cytoplasmic maturation.
In summary, we have shown that human oocytes undergo a dramatic reorganization of the ER during progression from the GV stage to MII and develop an increased sensitivity to IP3-induced Ca2+ release during maturation. Whereas oocytes matured in vitro retain their ability to reorganize the ER, they do not release as much Ca2+ in response to IP3 as oocytes matured in vivo, and this likely results, at least in part, from their inability to synthesize IP3 receptor protein during in vitro maturation. The ability of an oocyte to release Ca2+ could potentially be used as an endpoint to examine proper events of cytoplasmic maturation in oocytes matured in vitro using different culture conditions.
Acknowledgments
We thank the attending physicians and reproductive endocrinology fellows at the University of Connecticut Health Center for supporting this work. We also thank Victoria Scranton and research nurses for obtaining patient consents, as well as all of the embryologists at the Center for Advanced Reproductive Services at the University of Connecticut Health Center for their excellent cooperation and willingness to prepare oocytes for this study. We would also like to thank Dr. Laurinda Jaffe for helpful comments on the manuscript.
Footnotes
Supported by NIH grant HD056366 to L.M.M.
REFERENCES
- Runft LL, Jaffe LA, Mehlmann LM.Egg activation at fertilization: where it all begins. Dev Biol 2002; 245: 237–254. [DOI] [PubMed] [Google Scholar]
- Jones KT, Carroll J, Merriman JA, Whittingham DG, Kono T.Repetitive sperm-induced Ca2+ transients in mouse oocytes are cell cycle dependent. Development 1995; 121: 3259–3266. [DOI] [PubMed] [Google Scholar]
- Ducibella T, Huneau D, Angelichio E, Xu Z, Schultz RM, Kopf GS, Fissore R, Madoux S, Ozil JP.Egg-to-embryo transition is driven by differential responses to Ca2+ oscillation number. Dev Biol 2002; 250: 280–291. [PubMed] [Google Scholar]
- Ducibella T, Fissore R.The roles of Ca2+, downstream protein kinases, and oscillatory signaling in regulating fertilization and the activation of development. Dev Biol 2008; 315: 257–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba K, Kado RT, Jaffe LA.Development of calcium release mechanisms during starfish oocyte maturation. Dev Biol 1990; 140: 300–306. [DOI] [PubMed] [Google Scholar]
- Fujiwara T, Nakada K, Shirakawa H, Miyazaki S.Development of inositol trisphosphate-induced calcium release mechanism during maturation of hamster oocytes. Dev Biol 1993; 156: 69–79. [DOI] [PubMed] [Google Scholar]
- Mehlmann LM, Kline D.Regulation of intracellular calcium in the mouse egg: calcium release in response to sperm or inositol trisphosphate is enhanced after meiotic maturation. Biol Reprod 1994; 51: 1088–1098. [DOI] [PubMed] [Google Scholar]
- Mehlmann LM, Mikoshiba K, Kline D.Redistribution and increase in cortical inositol 1,4,5-trisphosphate receptors after meiotic maturation of the mouse oocyte. Dev Biol 1996; 180: 489–498. [DOI] [PubMed] [Google Scholar]
- Mehlmann LM, Terasaki M, Jaffe LA, Kline D.Reorganization of the endoplasmic reticulum during meiotic maturation of the mouse oocyte. Dev Biol 1995; 170: 607–615. [DOI] [PubMed] [Google Scholar]
- Stricker SA, Silva R, Smythe T.Calcium and endoplasmic reticulum dynamics during oocyte maturation and fertilization in the marine worm Cerebratulus lacteus. Dev Biol 1998; 203: 305–322. [DOI] [PubMed] [Google Scholar]
- Ajduk A, Malagocki A, Maleszewski M.Cytoplasmic maturation of mammalian oocytes: development of a mechanism responsible for sperm-induced Ca2+ oscillations. Reprod Biol 2008; 8: 3–22. [DOI] [PubMed] [Google Scholar]
- FitzHarris G, Marangos P, Carroll J.Changes in endoplasmic reticulum structure during mouse oocyte maturation are controlled by the cytoskeleton and cytoplasmic dynein. Dev Biol 2007; 305: 133–144. [DOI] [PubMed] [Google Scholar]
- Jaffe LA, Terasaki M.Structural changes in the endoplasmic reticulum of starfish oocytes during meiotic maturation and fertilization. Dev Biol 1994; 164: 579–587. [DOI] [PubMed] [Google Scholar]
- Payne C, Schatten G.Golgi dynamics during meiosis are distinct from mitosis and are coupled to endoplasmic reticulum dynamics until fertilization. Dev Biol 2003; 264: 50–63. [DOI] [PubMed] [Google Scholar]
- Shiraishi K, Okada A, Shirakawa H, Nakanishi S, Mikoshiba K, Miyazaki S.Developmental changes in the distribution of the endoplasmic reticulum and inositol 1,4,5-trisphosphate receptors and the spatial pattern of Ca2+ release during maturation of hamster oocytes. Dev Biol 1995; 170: 594–606. [DOI] [PubMed] [Google Scholar]
- Terasaki M, Runft LL, Hand AR.Changes in organization of the endoplasmic reticulum during Xenopus oocyte maturation and activation. Mol Biol Cell 2001; 12: 1103–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, White KL, Reed WA, Campbell KD.Dynamic changes to the inositol 1,4,5-trisphosphate and ryanodine receptors during maturation of bovine oocytes. Cloning Stem Cells 2005; 7: 306–320. [DOI] [PubMed] [Google Scholar]
- Kline D, Mehlmann L, Fox C, Terasaki M.The cortical endoplasmic reticulum (ER) of the mouse egg: localization of ER clusters in relation to the generation of repetitive calcium waves. Dev Biol 1999; 215: 431–442. [DOI] [PubMed] [Google Scholar]
- Taylor CT, Lawrence YM, Kingsland CR, Biljan MM, Cuthbertson KS.Oscillations in intracellular free calcium induced by spermatozoa in human oocytes at fertilization. Hum Reprod 1993; 8: 2174–2179. [DOI] [PubMed] [Google Scholar]
- Herbert M, Gillespie JI, Murdoch AP.Development of calcium signaling mechanisms during maturation of human oocytes. Mol Hum Reprod 1997; 3: 965–973. [DOI] [PubMed] [Google Scholar]
- Goud PT, Goud AP, Van Oostveldt P, Dhont M.Presence and dynamic redistribution of type I inositol 1,4,5-trisphosphate receptors in human oocytes and embryos during in-vitro maturation, fertilization and early cleavage divisions. Mol Hum Reprod 1999; 5: 441–451. [DOI] [PubMed] [Google Scholar]
- Chian RC, Lim JH, Tan SL.State of the art in in-vitro oocyte maturation. Curr Opin Obstet Gynecol 2004; 16: 211–219. [DOI] [PubMed] [Google Scholar]
- Jurema MW, Nogueira D.In vitro maturation of human oocytes for assisted reproduction. Fertil Steril 2006; 86: 1277–1291. [DOI] [PubMed] [Google Scholar]
- Engmann L, DiLuigi A, Schmidt D, Nulsen J, Maier D, Benadiva C.The use of gonadotropin-releasing hormone (GnRH) agonist to induce oocyte maturation after cotreatment with GnRH antagonist in high-risk patients undergoing in vitro fertilization prevents the risk of ovarian hyperstimulation syndrome: a prospective randomized controlled study. Fertil Steril 2008; 89: 84–91. [DOI] [PubMed] [Google Scholar]
- Lowther KM, Weitzman VN, Maier D, Mehlmann LM.Maturation, fertilization, and the structure and function of the endoplasmic reticulum in cryopreserved mouse oocytes. Biol Reprod 2009; 81: 147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlmann LM, Carpenter G, Rhee SG, Jaffe LA.SH2 domain-mediated activation of phospholipase Cγ is not required to initiate Ca2+ release at fertilization of mouse eggs. Dev Biol 1998; 203: 221–232. [DOI] [PubMed] [Google Scholar]
- Runft LL, Watras J, Jaffe LA.Calcium release at fertilization of Xenopus eggs requires type I IP3 receptors, but not SH2 domain-mediated activation of PLCγ or Gq-mediated activation of PLCβ. Dev Biol 1999; 214: 399–411. [DOI] [PubMed] [Google Scholar]
- Terasaki M, Jaffe LA.Imaging endoplasmic reticulum in living sea urchin eggs. Methods Cell Biol 1993; 38: 211–220. [DOI] [PubMed] [Google Scholar]
- Santella L, Alikani M, Talansky BE, Cohen J, Dale B.Is the human oocyte plasma membrane polarized? Hum Reprod 1992; 7: 999–1003. [DOI] [PubMed] [Google Scholar]
- Nicosia SV, Wolf DP, Inoue M.Cortical granule distribution and cell surface characteristics in mouse eggs. Dev Biol 1977; 57: 56–74. [DOI] [PubMed] [Google Scholar]
- Talansky BE, Malter HE, Cohen J.A preferential site for sperm-egg fusion in mammals. Mol Reprod Dev 1991; 28: 183–188. [DOI] [PubMed] [Google Scholar]
- Goud PT, Goud AP, Leybaert L, Van Oostveldt P, Mikoshiba K, Diamond MP, Dhont M.Inositol 1,4,5-trisphosphate receptor function in human oocytes: calcium responses and oocyte activation-related phenomena induced by photolytic release of InsP(3) are blocked by a specific antibody to the type I receptor. Mol Hum Reprod 2002; 8: 912–918. [DOI] [PubMed] [Google Scholar]
- Miyazaki S, Yuzaki M, Nakada K, Shirakawa H, Nakanishi S, Nakade S, Mikoshiba K.Block of Ca2+ wave and Ca2+ oscillation by antibody to the inositol 1,4,5-trisphosphate receptor in fertilized hamster eggs. Science 1992; 257: 251–255. [DOI] [PubMed] [Google Scholar]
- Xu Z, Kopf GS, Schultz RM.Involvement of inositol 1,4,5-trisphosphate-mediated Ca2+ release in early and late events of mouse egg activation. Development 1994; 120: 1851–1859. [DOI] [PubMed] [Google Scholar]
- Missiaen L, Taylor CW, Berridge MJ.Luminal Ca2+ promoting spontaneous Ca2+ release from inositol trisphosphate-sensitive stores in rat hepatocytes. J Physiol 1992; 455: 623–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JK, Nuccitelli R.Inositol 1,4,5-trisphosphate-induced calcium release in the organelle layers of the stratified, intact egg of Xenopus laevis. J Cell Biol 1990; 110: 1103–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki S.Thirty years of calcium signals at fertilization. Semin Cell Dev Biol 2006; 17: 233–243. [DOI] [PubMed] [Google Scholar]
- Stricker SA.Comparative biology of calcium signaling during fertilization and egg activation in animals. Dev Biol 1999; 211: 157–176. [DOI] [PubMed] [Google Scholar]
- Ducibella T, Anderson E, Albertini DF, Aalberg J, Rangarajan S.Quantitative studies of changes in cortical granule number and distribution in the mouse oocyte during meiotic maturation. Dev Biol 1988; 130: 184–197. [DOI] [PubMed] [Google Scholar]
- Longo FJ, Chen DY.Development of cortical polarity in mouse eggs: involvement of the meiotic apparatus. Dev Biol 1985; 107: 382–394. [DOI] [PubMed] [Google Scholar]
- Carroll J, Swann K, Whittingham D, Whitaker M.Spatiotemporal dynamics of intracellular [Ca2+]i oscillations during the growth and meiotic maturation of mouse oocytes. Development 1994; 120: 3507–3517. [DOI] [PubMed] [Google Scholar]
- Swann K, Saunders CM, Rogers NT, Lai FA.PLCζ(zeta): a sperm protein that triggers Ca2+ oscillations and egg activation in mammals. Semin Cell Dev Biol 2006; 17: 264–273. [DOI] [PubMed] [Google Scholar]
- Ito J, Yoon SY, Lee B, Vanderheyden V, Vermassen E, Wojcikiewicz R, Alfandari D, De Smedt H, Parys JB, Fissore RA.Inositol 1,4,5-trisphosphate receptor 1, a widespread Ca2+ channel, is a novel substrate of polo-like kinase 1 in eggs. Dev Biol 2008; 320: 402–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito J, Yoshida T, Kasai Y, Wakai T, Parys JB, Fissore RA, Kashiwazaki N.Phosphorylation of inositol 1,4,5-trisphosphate receptor 1 during in vitro maturation of porcine oocytes. Anim Sci J 2010; 81: 34–41. [DOI] [PubMed] [Google Scholar]
- Lee B, Vermassen E, Yoon SY, Vanderheyden V, Ito J, Alfandari D, De Smedt H, Parys JB, Fissore RA.Phosphorylation of IP3R1 and the regulation of [Ca2+]i responses at fertilization: a role for the MAP kinase pathway. Development 2006; 133: 4355–4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderheyden V, Wakai T, Bultynck G, De Smedt H, Parys JB, Fissore RA.Regulation of inositol 1,4,5-trisphosphate receptor type 1 function during oocyte maturation by MPM-2 phosphorylation. Cell Calcium 2009; 46: 56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combelles CM, Fissore RA, Albertini DF, Racowsky C.In vitro maturation of human oocytes and cumulus cells using a coculture three-dimensional collagen gel system. Hum Reprod 2005; 20: 1349–1358. [DOI] [PubMed] [Google Scholar]