Abstract
Kisspeptin, the product of the KISS1 gene, stimulates gonadotropin-releasing hormone (GnRH) secretion; gonadotropin inhibitory hormone (GnIH), encoded by the RF-amide-related peptide (RFRP) or NPVF gene, inhibits the reproductive axis. In sheep, kisspeptin neurons are found in the lateral preoptic area (POA) and the arcuate nucleus (ARC) and may be important for initiating the preovulatory GnRH/luteinizing hormone (LH) surge. GnIH cells are located in the ovine dorsomedial hypothalamic nucleus (DMN) and paraventricular nucleus (PVN), with similar distribution in the primate. KISS1 cells are found in the primate POA and ARC, but the function that kisspeptin and GnIH play in primates has not been elucidated. We examined KISS1 and NPVF mRNA throughout the menstrual cycle of a female primate, rhesus macaque (Macaca mulatta), using in situ hybridization. KISS1-expressing cells were found in the POA and ARC, and NPVF-expressing cells were located in the PVN/DMN. KISS1 expression in the caudal ARC and POA was higher in the late follicular phase of the cycle (just before the GnRH/LH surge) than in the luteal phase. NPVF expression was also higher in the late follicular phase. We ascertained whether kisspeptin and/or GnIH cells project to GnRH neurons in the primate. Close appositions of kisspeptin and GnIH fibers were found on GnRH neurons, with no change across the menstrual cycle. These data suggest a role for kisspeptin in the stimulation of GnRH cells before the preovulatory GnRH/LH surge in non-human primates. The role of GnIH is less clear, with paradoxical up-regulation of gene expression in the late follicular phase of the menstrual cycle.
Keywords: gonadotropin-releasing hormone, KISS1, kisspeptin, menstrual cycle, preovulatory surge, RFRP
The preovulatory LH surge in the non-human primate is associated with an increase in kisspeptin expression in neurons located in the preoptic area and arcuate nucleus of the brain.
INTRODUCTION
Reproduction depends upon secretion of the hypothalamic neuropeptide gonadotropin-releasing hormone (GnRH) from the brain, which stimulates the synthesis and release of the gonadotropins (luteinizing hormone [LH] and follicle-stimulating hormone) from the pituitary gland. The secretion of GnRH and gonadotropins is controlled by feedback effects of gonadal steroids. In females, tonic negative feedback effects of estrogen and progesterone prevail throughout most of the ovarian cycle. In the late follicular phase of the cycle, a neuroendocrine switch occurs, and a transient, estrogen-induced positive feedback effect causes the preovulatory surge in GnRH/LH [1]. The surge in LH secretion causes ovulation. Because GnRH neurons do not possess the requisite sex steroid receptors [2–4], feedback signals to these neurons rely on transmission through other steroid-receptive cells within the brain. Kisspeptin is an RF-amide peptide that appears to play a major role in transmission of steroid feedback signals to GnRH neurons [5, 6]. Other recent studies strongly suggest hypothalamic cells that produce gonadotropin inhibitory hormone (GnIH) may oppose the actions of kisspeptin and inhibit the reproductive system [7, 8].
The RF-amide neuropeptide family was first described in the bivalve mollusk by Price and Greenberg [9] in 1977 with the discovery of a cardioexcitatory peptide from the cerebral ganglia. Two recently discovered members of this peptide family, kisspeptin and GnIH, appear to be significant components of the neuroendocrine system that regulate GnRH secretion and reproductive function. Moreover, kisspeptin-producing neurons appear to be ideally placed to act as the conduit for sex steroid feedback control over GnRH neurons [5, 6]. Kisspeptins are the peptide product of the KISS1 gene; kisspeptins stimulate GnRH secretion [10–12] and appear to be critical for reproductive function [13, 14]. The stimulatory effect of kisspeptin on GnRH secretion appears to be fundamental to generation of the preovulatory LH surge in mice, rats, and sheep [15–17]. In sheep, KISS1 mRNA-expressing cells are located in the arcuate nucleus (ARC) and the dorsolateral region of the preoptic area (POA) [18, 19]. It appears that the former cell group is important for the negative feedback regulation of GnRH [19], and cells of both regions may be important for generation of the preovulatory LH surge [17]. Notably, direct input to GnRH neurons is from the kisspeptin cells in the POA, whereas kisspeptin cells in the ARC may regulate GnRH neurons via an interneuronal pathway [20].
In human and non-human primates, kisspeptin-immunoreactive (ir) and KISS1 mRNA-expressing cells are localized to the ARC [12, 21, 22], which is an area thought to be important for both positive and negative regulation to GnRH in these species [23, 24]. KISS1 expression in the rhesus monkey ARC appears to increase over pubertal development [12], and KISS1 mRNA expression increases in cynomolgus monkeys after ovariectomy [21], further substantiating a role for ARC kisspeptin cells in the negative feedback regulation of GnRH secretion. Evidence also exists, however, for the involvement of cells in the POA in generation of the preovulatory surge in primates [25], and KISS1 mRNA is expressed in this region [26]. Because earlier studies indicated a surge-generating mechanism may exist in the POA of the non-human primate [25], we sought to determine whether a population of kisspeptin cells is present in the primate POA and, if so, what functional role kisspeptin cells in this region play in the preovulatory GnRH/LH surge.
Gonadotropin inhibitory hormone was discovered in the brain of the Japanese quail [27], and similar peptides were subsequently identified in mammals [28–31]. The mammalian forms have been termed RF-amide-related peptides (RFRP) transcribed from the NPVF gene [32], but the original nomenclature can be applied to all species [8]. Despite mounting evidence for a role in the regulation of GnRH secretion, it is unclear if GnIH is an important regulator of mammalian reproduction. Mammalian GnIH (also termed RFRP-3) reduces plasma gonadotropin levels when administered intracerebroventricularly or peripherally to a range of species [28, 33–36] and, in sheep, appears to play a hypophysiotropic role, inhibiting gonadotropin synthesis and secretion at the level of the pituitary gland [29, 37]. GnIH inhibits the firing of a subset of GnRH neurons in mice [38, 39], and GnIH terminals appear to make close appositions to GnRH neurons in mice, rats, hamsters, and sheep [28, 33, 39, 40–42]. Using immunohistochemistry and in situ hybridization, GnIH cells have been found in the male rhesus monkey brain, located in the intermediate periventricular nucleus (IPe) [30]. This location may bear some homology to the dorsomedial hypothalamic nucleus (DMN)/paraventricular nucleus (PVN) population of GnIH cells seen in rodents and sheep [28, 29]. These data suggest GnIH may play a role in the regulation of GnRH secretion/action in primates, as it does in other vertebrates.
Given the importance of these two RF-amide neuropeptide systems in the control of reproduction, we hypothesized that both systems play a role in regulation of the preovulatory LH surge in the non-human primate. Accordingly, the present study had two objectives: first, to determine whether mRNA expression of KISS1 and/or GnIH (NPVF) vary over the rhesus monkey menstrual cycle and, second, to determine whether neurons expressing these neuropeptides make close appositions to GnRH neurons.
MATERIALS AND METHODS
Animals
Spontaneously cycling female rhesus macaques (Macaca mulatta; age, 7–12 yr; body wt, 5.5–6.5 kg) were individually housed and maintained in quarters kept at temperatures between 21 and 25°C with a 12L:12D photoperiod in accordance with National Institutes of Health guidelines. Monkey chow (Ralston Purina) was provided twice daily, and fresh fruit was provided once daily. Fresh water was available ad libitum. Animals were killed humanely during three hormonally defined stages of the menstrual cycle: luteal phase (n = 3), early follicular phase (n = 3), and late follicular (periovulatory) phase (n = 4).
For brain fixation, animals were killed by intravenous injection with 25 mg/kg of sodium pentobarbital followed by exsanguination under deep anesthesia in accordance with the recommendation from the Panel on Euthanasia of the American Veterinary Medical Association. The brain was perfused through the aorta with 1 L of saline followed by 3 L of cold 4% paraformaldehyde in 0.1 M borate at pH 9.5. The cranial and upper cervical vertebrae bone structures were removed, and the brain was extracted. The hypothalamus was postfixed and cryoprotected in 4% paraformaldehyde containing 20% sucrose. The hypothalamus was subsequently sectioned at 20 μm and collected at a 1-in-10 series (i.e., sequential sections 200 μm apart). Sections were mounted onto poly-l-lysine-subbed slides (two or three sections per slide), dried in a vacuum overnight, and stored at −80°C in sealed slide boxes.
Menstrual Cycle Determination
Serum samples, collected three times a week from monkeys, were submitted to the Endocrine Services Laboratory at the Oregon National Primate Research Center for determination of serum estradiol and progesterone concentrations. Those monkeys showing a normal menstrual cycle were selected for daily steroid determination. On the day of brain perfusion (during the midluteal, midfollicular, or late follicular phase), a serum sample was examined in the early morning. Estradiol and progesterone results were obtained to confirm the stage of the menstrual cycle (i.e., estradiol levels of 60–120 pg/ml during the midfollicular phase and >200 pg/ml during the preovulatory phase, and progesterone levels of >3 ng/ml during the midluteal phase). At necropsy, a blood sample was collected again for estradiol or progesterone determination.
Serum concentrations of estradiol and progesterone were determined using a validated chemiluminescence-based automatic clinical platform (Roche Diagnostics Elecsys 2010) [43]. The assay sensitivity was 20 pg/ml for estradiol and 0.2 ng/ml for progesterone. The intra- and interassay variations are less than 10% for both assays. All quality-control samples and validations, provided by the company, were analyzed in each assay.
Radiolabeled cRNA Riboprobes
KISS1 riboprobe. A 311-base cDNA sequence of the human KISS1 gene (bases 209–519 of GenBank accession no. NM_002256) was cloned as previously described [12]. The antisense primate KISS1 riboprobe was transcribed from linearized plasmid containing the KISS1 insert with SP6 polymerase (Promega Corp.) and [35S]uridine 5-triphosphate (GE Healthcare Life Sciences) under a standard transcription protocol. The riboprobe was separated from unincorporated nucleotides on a Sephadex G-25 column.
NPVF riboprobe. A 460-base cDNA sequence of the ovine NPVF precursor (RFRP, bases 43–502 of GenBank accession no. NM_001127268) was cloned as previously described [29, 41]. We predicted an ovine riboprobe would hybridize to M. mulatta NPVF mRNA (GenBank accession no. NM_001033115) because of the 80% homology between ovine and M. mulatta cDNAs in the cloned region. The antisense NPVF riboprobe was transcribed from linearized plasmid with T7 polymerase and [35S]uridine 5-triphosphate as stated above.
KISS1 and NPVF In Situ Hybridization
In situ hybridization for KISS1 and NPVF mRNA was performed as previously described [19]. For KISS1 analysis, sections through the POA and ARC were chosen from each animal. For the POA, three or four sequential sections per animal were examined, and for the ARC, one or two sections per animal, representing the rostral, mid, and caudal regions of the ARC, were examined. For NPVF analysis, sections through the PVN/DMN region were chosen (n = 4–6 sequential sections per animal). The slides were prepared for in situ hybridization [19], and radiolabeled (35S) antisense KISS1 or NPVF riboprobe was denatured and then diluted in hybridization buffer at a concentration of 5 × 106 cpm/ml with tRNA (0.5 mg/ml). The hybridization solution was applied to slides (120 μl/slide) at 53°C for 16 h, after which the slides were treated with RNase A, washed in reducing concentrations of standard saline citrate, and dehydrated. The slides were then dipped in Ilford K5 photographic emulsion (Ilford Imaging), stored in the dark at 4°C, and developed 7 days later. No signal was observed after the application of radiolabeled sense probes (data not shown). Image analysis was carried out under dark-field illumination, and cells were counted when silver grain density was greater than fivefold the background level. The number of silver grains over each KISS1/NPVF cell (a semiquantitative index of mRNA content per cell) was determined using grain counting software (ImagePro Plus; Media Cybernetics, Inc.). For each animal, the number of KISS1 or NPVF mRNA cells per section and silver grains per cell was averaged to produce the mean ± SEM in each region.
Immunohistochemistry
Kisspeptin and GnRH double-label immunohistochemistry.
Three sequential sections through the POA and three through the mediobasal hypothalamus (MBH) were chosen from each animal for analysis. Antigen retrieval was performed using 1 M citrate buffer (pH 6) in a microwave oven at 1000 W (twice for 5 min each time). A blocking solution containing 10% normal goat serum and 0.3% Triton X-100 in 0.1 M Tris-buffered saline (TBS) was applied (2 h at room temperature). The sections were then incubated for 72 h at 4°C with a sheep polyclonal antibody against synthetic human kisspeptin-54 (GQ2; kindly supplied by Dr. Stephen Bloom, Imperial College London, U.K.) previously used in monkeys [22] and a rabbit polyclonal antibody against GnRH (LR1; kindly supplied by Dr. Robert Benoit, Montreal General Hospital, Canada); both antibodies were at a dilution of 1:2000. The sections were washed in TBS, incubated with donkey anti-sheep Alexa 488 and goat anti-rabbit Alexa 546 (diluted 1:400; Molecular Probes, Inc.) for 2 h at room temperature, rinsed in TBS, and then counterstained with 0.3% Sudan Black B to minimize autofluorescence. Following rinses in TBS and then 0.1 M phosphate buffer, coverslips were applied using antifade mounting solution (DAKO).
GnIH and GnRH double-label immunohistochemistry.
Three sequential sections through the POA and three through the MBH from each animal were chosen for analysis. Immunohistochemistry was performed as described above with the following exceptions: GnIH cells were visualized with a guinea pig polyclonal antibody against human GnIH (diluted 1:1000; human RFRP-3, VPNLPQRF-NH2; Antibodies Australia), which was previously shown to be specific in ovine tissue [42]. Goat anti-guinea pig Alexa 488 (diluted 1:400; Molecular Probes, Inc.) was used as a second antibody.
Image Analysis
The GnRH-ir cells were identified under fluorescent illumination, with a single observer counting the total number of GnRH cells and the number of GnRH cells with kisspeptin- or GnIH-ir terminal appositions. Putative contacts of kisspeptin and GnIH fibers on GnRH neurons were examined with a Zeiss Apotome microscope (Carl Zeiss, Inc.). Z-stacks of optical sections (1 μm, ×126 magnification) were captured through GnRH-ir neurons. Putative contacts were defined as apposition of terminals with soma or proximal dendrites when no pixelation occurred between the two objects. Using the Apotome system, Z-stacks were rotated to confirm the lack of pixelation between the objects when viewed in different planes. This method has been reported previously [42, 44]. For each animal, the percentage of “contacted” GnRH-ir cells in each region was averaged to produce the mean ± SEM.
Statistical Analysis
Data are presented as the mean ± SEM, and one-way ANOVA was used to determine the effect of menstrual cycle stage on NPVF mRNA expression as well as on estradiol and progesterone data. Two-way ANOVA was used to determine the effect of menstrual cycle stage on KISS1 mRNA in the POA and rostral, mid, and caudal divisions of the ARC. Variation in the percentage of GnRH neurons with kisspeptin- or GnIH-ir terminal appositions was assessed by two-way ANOVA on arc-sine-transformed data, appropriate for the analysis of percentages. When significance was reached (P < 0.05), post hoc analysis of differences between means was performed by use of the least-significant-difference test.
RESULTS
Regulation of KISS1 and NPVF mRNA During the Menstrual Cycle
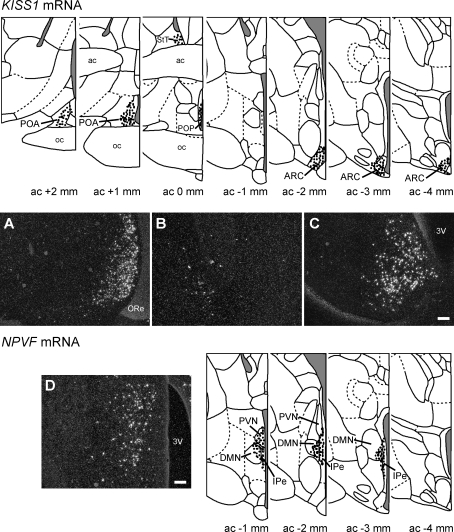
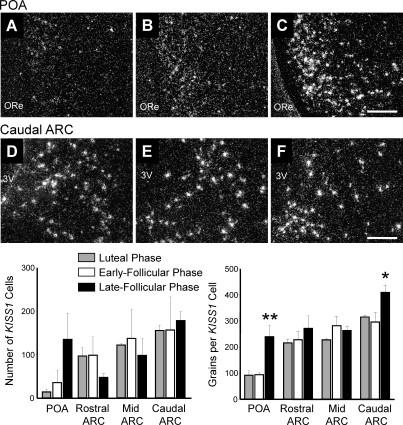
KISS1 mRNA-expressing cells were located in the POA and continued caudally to the preoptic periventricular nucleus (POP) (Fig. 1) [45]. KISS1 cells were also abundant in the ARC (Fig. 1). A very small population of KISS1-expressing cells was also seen in the bed nucleus of the stria terminalis (Fig. 1), although no effect of menstrual cycle stage was found in this region (data not shown). The number of KISS1-expressing cells in the POA and the ARC did not vary across the menstrual cycle (Fig. 2), although a nonsignificant trend for an increase in KISS1 cells was noted in the POA. In the POA, the KISS1 mRNA expression per cell was 2.5-fold higher (P < 0.01) during the late follicular phase of the menstrual cycle (239 ± 45 grains/cell) than in the luteal phase (92 ± 17 grains/cell) and early follicular phase (94 ± 9 grains/cell) (Fig. 2). In the caudal ARC, KISS1 mRNA expression per cell was higher (P < 0.05) during the late follicular phase of the menstrual cycle (410 ± 26 grains/cell), being 38% higher than in the early follicular phase (296 ± 35 grains/cell) and 30% higher than in the luteal phase (315 ± 7 grains/cell; both P < 0.05) (Fig. 2). No change in KISS1 mRNA expression per cell was observed in the rostral or mid ARC (Fig. 2).
FIG. 1.
Schematic drawings of coronal sections through the rhesus macaque brain showing the location of KISS1 mRNA and NPVF mRNA (GnIH)-expressing cells (dots). Representative sections (modified from [45]) are 1 mm apart. Section serial numbers indicate the number of millimeters anterior (+) or posterior (−) from the complete anterior commissure (ac). A–C) Representative dark-field photomicrographs showing the distribution of KISS1 mRNA-expressing cells in the POA (A), bed nucleus of the stria terminalis (B), and ARC (C). D) Representative dark-field photomicrographs showing the distribution of NPVF mRNA-expressing cells in the PVN/DMN. 3V, third ventricle; oc, optic chiasm; ORe, optic recess; POP, preoptic periventricular nucleus; StT, bed nucleus of the stria terminalis. Bars = 200 μm.
FIG. 2.
Representative dark-field photomicrographs showing KISS1 mRNA-expressing cells (as reflected by the presence of white clusters of silver grains) in the POA and ARC from rhesus macaques at the luteal phase (A and D), early follicular phase (B and E), and late follicular phase (C and F). Quantification of KISS1 mRNA in the rostral, mid, and caudal ARC and the POA is shown. The number of KISS1 mRNA-positive cells per section was unchanged over the cycle, but the number of grains per KISS1 cell in the caudal ARC and POA was significantly greater in animals at the late follicular phase Values are presented as the mean ± SEM (n = 3–4 per group). 3V, third ventricle; ME, median eminence; ORe, optic recess. Bars = 200 μm. *P < 0.05, **P < 0.01.
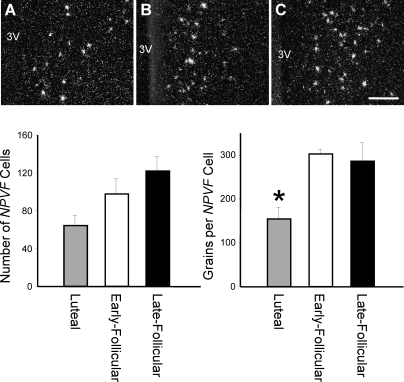
NPVF mRNA-expressing cells were located in the PVN and DMN and extended medially into the IPe (Fig. 1). The number of NPVF mRNA-expressing cells did not differ during the menstrual cycle (Fig. 3). NPVF mRNA expression per cell was significantly reduced during the luteal phase of the menstrual cycle (155 ± 27 grains/cell) compared to the early follicular phase (49% reduced, 303 ± 11 grains/cell) and late follicular phase (46% reduced, 286 ± 42 grains/cell) (Fig. 3).
FIG. 3.
Representative dark-field photomicrographs showing NPVF mRNA-expressing cells (GnIH; as reflected by the presence of white clusters of silver grains) in the PVN/DMN region from rhesus macaques at the luteal phase (A), early follicular phase (B), and late follicular phase (C). Quantification of NPVF mRNA shows the number of NPVF mRNA-positive cells per section was unchanged over the cycle, but the number of grains per NPVF cell was significantly reduced in luteal-phase animals. Values are presented as the mean ± SEM (n = 3–4 per group). 3V, third ventricle. Bars = 200 μm. *P < 0.05.
Kisspeptin- and GnIH-ir Terminal Appositions to GnRH Neurons
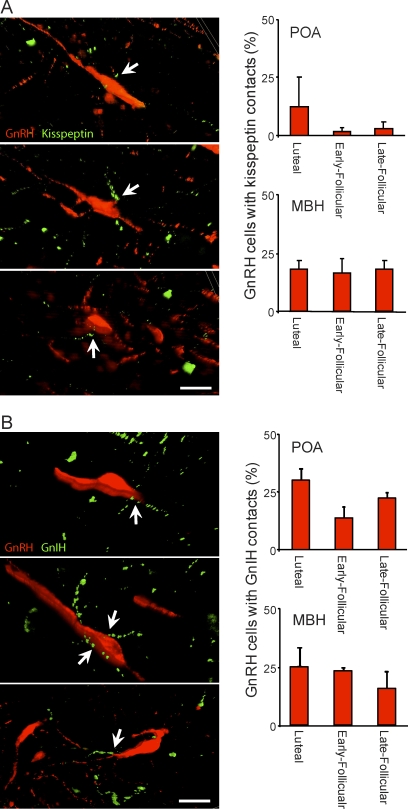
Kisspeptin-ir fibers in close apposition to GnRH neurons were located in the POA and MBH (Fig. 4A). The percentage of GnRH neurons displaying kisspeptin terminal appositions was greater in the MBH than in the POA irrespective of menstrual cycle stage (P < 0.05). Across the menstrual cycle, the percentage of GnRH neurons with kisspeptin appositions was similar in both the POA and the MBH (Fig. 4A).
FIG. 4.
Z-stack apotome analysis showing putative input to GnRH neurons from kisspeptin and GnIH terminals. The images show examples of GnRH neurons (red) that have kisspeptin fibers (A) or GnIH fibers (B; green) in close apposition. The percentage of GnRH neurons with kisspeptin or GnIH appositions was similar over the menstrual cycle in the POA and MBH. Values are presented as the mean ± SEM (n = 3–4 per group). Bars = 20 μm.
We observed GnIH-ir fibers in close apposition to GnRH neurons in cells of the POA and MBH (Fig. 4B). No difference was observed in the percentage of GnRH neurons with GnIH terminal appositions between the POA and MBH or during the menstrual cycle.
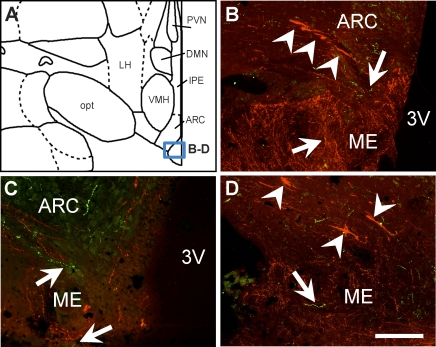
Kisspeptin and GnIH fibers were distributed throughout the MBH (data not shown), as previously described in this species [22, 30]. One exception to these previous reports was that GnIH fibers were scarce in the median eminence at all stages of the menstrual cycle (Fig. 5).
FIG. 5.
Localization of GnIH-ir terminals in the median eminence of the rhesus macaque. Fluorescence photomicrographs show few GnIH fibers (green) located in the median eminence, where an abundance of GnRH fibers is noted (red). Arrows indicate GnIH fibers; arrowheads indicate GnRH neurons in the MBH. Box in A demonstrates the approximate position of photomicrographs B–D. 3V, third ventricle; ARC, arcuate nucleus; DMN, dorsomedial nucleus of the hypothalamus; LH, lateral hypothalamus; ME, median eminence; opt, optic tract; VMH, ventromedial hypothalamus. Bars = 100 μm.
Plasma Estradiol and Progesterone Concentrations
Estradiol levels were below the detectable limit of the assay in luteal-phase animals (14 ± 9 pg/ml). During the late follicular phase, estradiol levels (258 ± 46 pg/ml) were higher (P < 0.01) than during the early follicular phase (65 ± 11 pg/ml). During the luteal phase, progesterone levels (2.8 ± 0.9 ng/ml) were higher (P < 0.05) than in the early follicular phase (0.2 ± 0.1 ng/ml) and the late follicular phase (0.6 ± 0.05 ng/ml).
DISCUSSION
We describe the expression of KISS1 and NPVF mRNA in the female non-human primate brain during the menstrual cycle. Our data show KISS1 gene expression is greatest in the ARC and POA during the late follicular phase of the menstrual cycle, immediately preceding the preovulatory LH surge. NPVF mRNA expression was lowest during the luteal phase of the cycle (higher during the follicular phase). Both kisspeptin- and GnIH-ir terminals were seen to come into close contact with GnRH neurons. Together, these observations suggest kisspeptin neurons are involved as central processors for the preovulatory GnRH/LH surge in the non-human primate. The role GnIH neurons play in this species is yet to be fully determined.
In a number of species, kisspeptin cells are ideally placed to act as the interneuronal link connecting levels of sex steroids to GnRH feedback regulation [5, 6]. Moreover, the acute positive feedback effects of estradiol to induce the preovulatory GnRH/LH surge appear to be transmitted (at least in part) by kisspeptin cells. In mice and rats, Kiss1 mRNA expression is increased in the anteroventral periventricular nucleus (AVPV) by estradiol treatment, and this area of the brain is thought to be important for generation of the LH surge in these species [46–49]. Therefore, it is not surprising that Kiss1 expression and activation of kisspeptin neurons (as indicated by the induction of FOS) increases in the AVPV at the time of the LH surge [15, 50]. Interestingly, in this species, KISS1 expression in the ARC appears to decrease or remain constant during this time [15, 50]. Estradiol acts via estrogen receptor α in the AVPV to regulate Kiss1 in mice [51], and recent data show this to be via a classical estrogen-response element-dependent pathway [52]. Importantly, kisspeptin/GPR54 signaling, presumably in the AVPV, is essential for the LH surge in mice [16]. In sheep, a clear species difference is apparent: The MBH region of the brain, and not the AVPV, is critical for the acute positive feedback effects of estradiol on GnRH secretion [53, 54]. Consistent with this, KISS1 mRNA expression in the caudal region of the ARC is greatest in the late follicular phase of the estrous cycle (immediately before the LH surge) [17, 18]. Moreover, kisspeptin neurons in the mid and caudal ARC become transcriptionally activated in response to an estrogen stimulus that induces an LH surge [17]. The kisspeptin cells in this region also appear to mediate the negative feedback effects of gonadal steroids, being up-regulated with ovariectomy and down-regulated with chronic estrogen replacement [19]. Interestingly, our recent data further suggest that, in addition to the KISS1 gene expression in the ARC, KISS1 gene expression in the ovine POA is up-regulated immediately before the LH surge [17], which may indicate similarities to the rodent species. Thus, we have proposed that both the ARC and the POA kisspeptin cells are involved in generation of the preovulatory LH surge in the ovine species.
The present results indicate the distribution of kisspeptin cells and changes in KISS1 mRNA expression across the menstrual cycle in the female rhesus monkey are similar to that in the sheep. Moreover, we describe the distribution of kisspeptin cells in the POA of the non-human primate brain, which to our knowledge has not been described previously. It is possible a similar population of preoptic kisspeptin cells exists in the human brain [21], but this has not been scrutinized. KISS1 expression in cells of the caudal ARC and POA was increased during the late follicular phase of the menstrual cycle. This indicates the likely involvement of both these populations of kisspeptin cells in generation of the estrogen positive feedback preovulatory LH surge in the primate. These findings in the non-human primate POA may be synonymous with those reported in the rodent AVPV, but definition of a precise role for these rostral kisspeptin cells may be problematic. Classic studies in rhesus monkeys where the MBH was surgically isolated from the rest of the brain show no interference in estrogen negative or positive feedback [23, 24, 55]. Such data potentially eliminate a critical role for the POA population of kisspeptin cells in generation of the preovulatory LH surge, but an active role cannot be ruled out. Thus, bilateral lesions to the POA in monkeys compromised the LH surge and ovulation [25]. Similar results were also noted after separation of the anterior hypothalamus and MBH [56], although blockade of LH surges and anovulation were only apparent in the short term (LH surges spontaneously resumed 120–210 days postoperatively). It is possible the anterior hypothalamic nuclei (potentially kisspeptin cells in the POA) do play a role in controlling the menstrual cycle of non-human primates but may not be essential.
Recent data in humans and non-human primates suggest KISS1 expression in the ARC plays a role in estrogen negative feedback [21]. We now show these cells may participate in the positive feedback phenomenon. Thus, as in the ovine species [17–19, 41], kisspeptin cells located in the ARC of the non-human primate appear to be involved in estrogen positive feedback regulation of GnRH, yet also play a role in chronic estrogen negative feedback regulation. Discrete regions of the ARC may be able to distinguish different estrogen stimuli and transmit negative or positive regulation of GnRH secretion. Alternatively, the same kisspeptin cells could respond to both negative and positive feedback stimuli, possibly involving classical and nonclassical ER pathways, as recently reported in the mouse [52]. In this regard, it is pertinent to note that acute (surge-inducing) estradiol treatment induced Fos expression in the vast majority of caudal ARC kisspeptin cells in sheep, whereas after ovariectomy, approximately half of the same kisspeptin cells had induced Fos expression [17]. Thus, it is probable some kisspeptin cells are responding to both positive and negative feedback signals.
Mammalian GnIH (RFRP) was first identified in the human, bovine, rat, and mouse [32] and was later characterized as the mammalian homolog to the avian GnIH [28]. GnIH inhibits gonadotropin secretion in hamsters [28], rats [33], and sheep [29, 37]. In the female rhesus monkey, we observed NPVF mRNA-expressing cells in the DMN and PVN, and this population appeared to extend medially into the IPe. In a recent study, GnIH (NPVF)-expressing cells were observed only in the IPe of the male rhesus monkey brain [30]. We believe this population of GnIH cells in males to be homologous to the one we describe in females. It is possible that disparities may relate to sex differences in GnIH expression in the rhesus monkey. In females, we show lower NPVF mRNA expression during the luteal phase than in the follicular phase of the menstrual cycle. Given the proposed role of GnIH in mammalian reproduction, especially in relation to the LH surge (in hamsters) [57], this result was unexpected. Functional studies are warranted to decipher the precise role of GnIH for various reasons. In rats, gonadal steroids do not appear to affect GnIH expression [58]. In sheep, similar results are apparent, because no change in NPVF mRNA expression occurs between breeding seasons or with ovariectomy and estradiol replacement [41]. However, changes in GnIH protein levels have been noted [41]. This inconsistency may relate to the nature of the NPVF mRNA, which may be rapidly degraded, or the NPVF riboprobe, which spans the 460-nucleotide ovine NPVF precursor. It is possible that posttranslational proteolytic cleavage of the GnIH preprotein may be regulated, leading to specific control of mature GnIH peptides.
In mice, GnIH directly inhibits the activity of GnRH neurons [38, 39], and kisspeptin stimulates their activity [59, 60]. Supporting this, GnIH- and kisspeptin-ir varicose fibers make close appositions to GnRH neurons in the mouse [39, 61], rat [33, 62], hamster [28], sheep [41, 42], and male rhesus monkey [22, 30]. We confirm these findings in female rhesus monkeys. Our previous data show GnIH and kisspeptin appositions to GnRH neurons vary with season in sheep, with GnIH appositions being fewer and kisspeptin appositions greater during the breeding season than during the nonbreeding anestrous season [41]. In the female rhesus monkey, both GnIH and kisspeptin appositions to GnRH neurons were unchanged over the menstrual cycle, indicating that involvement of these neuropeptides in the preovulatory LH surge is unlikely to occur at this specific level. In the non-human primate, GnIH-ir projections were also seen in the bed nucleus of the stria terminalis, habenular nucleus, and the PVN of the hypothalamus [30]. In the hypothalamus, GnIH fibers were mostly isolated to midline structures, such as the POA, PVN, ARC, and the DMN [30], and our data showed a similar distribution (data not shown). In the ovine brain, GnIH cells also project to appetite-regulating cells in the hypothalamus, particularly those producing neuropeptide Y and melanocortins in the ARC, those producing orexin and melanin-concentrating hormone in the lateral hypothalamic area, orexin cells in the DMN, and corticotrophin-releasing hormone and oxytocin cells in the PVN [42]. This is consistent with the proposed role for GnIH in the regulation of food intake [33, 35, 63]. Whether the same is true for the non-human primate remains to be determined.
The GnIH-ir terminals have also been visualized in the neurosecretory zone of the median eminence in hamsters [28] and sheep [29, 64], but not in rats [65]. Thus, a hypophysiotropic role has been proposed for some species but argued for others. In male rhesus monkeys, GnIH-ir terminals are seen in the median eminence [30], but we observed far fewer fibers in the present study. This difference may again relate to sex differences in the non-human primate but may also relate to the different antibodies used for immunodetection of GnIH. Kisspeptin-ir terminals have recently been detailed in the male rhesus monkey. In that study, abundant kisspeptin fibers were observed in the median eminence, and kisspeptin fibers also made infrequent contacts to GnRH neurons in the MBH [22]. Our data, obtained in the female rhesus monkey, are consistent with these observations. Alternatively, in the male rhesus monkey, kisspeptin-positive perikarya were only observed in the ARC [22], whereas we observed a population of KISS1 mRNA-positive cells in the POA. Two reasons are possible for this discrepancy. First, the initial study was performed in castrated males. Castration has been shown to reduce Kiss1 mRNA expression in AVPV of rodents [51, 66], and it may reduce expression in the primate POA. Second, sex differences may be apparent in the more rostral populations of kisspeptin neurons (females > males), as also shown in rats [67], mice [68], and sheep [69]. The anatomical origin of kisspeptin inputs to GnRH neurons is yet to be fully determined in any species. In mice, data indicate the AVPV population of kisspeptin neurons as being the population that projects to GnRH neuronal cell bodies [61]. Consistent with this, estrogen-sensitive neurons located in the AVPV provide one of the largest direct inputs to GnRH neurons [48]. In sheep, close contacts of kisspeptin fibers on GnRH neurons may also come from the more rostral kisspeptin neurons. Credence for this is gained by the observation that the kisspeptin neurons of the ovine ARC do not appear to project to GnRH neurons, but those of the POA do [20]. Potentially, the POA kisspeptin population may form part of an interneuronal pathway connecting kisspeptin cells in the ARC to GnRH neurons.
Overall, our data describe the distribution of KISS1 mRNA- and NPVF mRNA-expressing cells in the brain of the female rhesus monkey over the menstrual cycle. We show that levels of KISS1 mRNA expression in the POA and caudal ARC increase during the late follicular phase immediately before the preovulatory LH surge. In addition, kisspeptin-ir fibers were seen to make close appositions to GnRH neurons (although this did not change across the menstrual cycle). These data suggest that both POA and ARC kisspeptin neurons are involved in the preovulatory GnRH/LH surge of the non-human primate. NPVF mRNA expression in the DMN/PVN region appeared to decrease during the luteal phase of the menstrual cycle, and GnIH-ir terminals made close appositions to GnRH neurons. The precise role GnIH cells play in the primate is yet to be determined.
Acknowledgments
The authors thank Dr. Tony Plant, Dr. Stephen Bloom, and Dr. Robert Benoit for reagents.
Footnotes
Supported by the National Health and Medical Research Council of Australia (NHMRC) grants 384124, 384362, 545919, and 606538. Preparation of monkey brain sections were supported by National Institutes of Health (NIH) grants HD-16631 and RR-00163. J.T.S. is supported by an Australian Research Council Future Fellowship (FT0990986).
REFERENCES
- Pau KY, Berria M, Hess DL, Spies HG.Preovulatory gonadotropin-releasing hormone surge in ovarian-intact rhesus macaques. Endocrinology 1993; 133: 1650–1656. [DOI] [PubMed] [Google Scholar]
- Shivers BD, Harlan RE, Morrell JI, Pfaff DW.Absence of estradiol concentration in cell nuclei of LHRH-immunoreactive neurones. Nature 1983; 304: 345–347. [DOI] [PubMed] [Google Scholar]
- Herbison AE, Theodosis DT.Localization of estrogen receptors in preoptic neurons containing neurotensin but not tyrosine hydroxylase, cholecystokinin or luteinizing hormone-releasing hormone in the male and female rat. Neuroscience 1992; 50: 283–298. [DOI] [PubMed] [Google Scholar]
- Skinner DC, Caraty A, Allingham R.Unmasking the progesterone receptor in the preoptic area and hypothalamus of the ewe: no colocalization with gonadotropin-releasing neurons. Endocrinology 2001; 142: 573–579. [DOI] [PubMed] [Google Scholar]
- Smith JT.Kisspeptin signaling in the brain: Steroid regulation in the rodent and ewe. Brain Res Rev 2007; 57: 288–298. [DOI] [PubMed] [Google Scholar]
- Smith JT.Sex steroid control of hypothalamic Kiss1 expression in sheep and rodents: comparative aspects. Peptides 2009; 30: 94–102. [DOI] [PubMed] [Google Scholar]
- Clarke IJ, Qi Y, Puspita Sari I, Smith JT.Evidence that RF-amide-related peptides are inhibitors of reproduction in mammals. Front Neuroendocrinol 2009; 30: 371–378. [DOI] [PubMed] [Google Scholar]
- Smith JT, Clarke IJ.Gonadotropin inhibitory hormone function in mammals. Trends Endocrinol Metab 2010; 21: 255–260. [DOI] [PubMed] [Google Scholar]
- Price DA, Greenberg MJ.Purification and characterization of a cardioexcitatory neuropeptide from the central ganglia of a bivalve mollusk. Prep Biochem 1977; 7: 261–281. [DOI] [PubMed] [Google Scholar]
- Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA.A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology 2004; 145: 4073–4077. [DOI] [PubMed] [Google Scholar]
- Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, Thresher RR, Malinge I, Lomet D, Carlton MB, Colledge WH, Caraty A, et al. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci U S A 2005; 102: 1761–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahab M, Mastronardi C, Seminara SB, Crowley WF, Ojeda SR, Plant TM.Increased hypothalamic GPR54 signaling: a potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci U S A 2005; 102: 2129–2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E.Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci U S A 2003; 100: 10972–10976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, et al. The GPR54 gene as a regulator of puberty. N Engl J Med 2003; 349: 1614–1627. [DOI] [PubMed] [Google Scholar]
- Smith JT, Popa SM, Clifton DK, Hoffman GE, Steiner RA.Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. J Neurosci 2006; 26: 6687–6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson J, d'Anglemont de Tassigny X, Moreno AS, Colledge WH, Herbison AE.Kisspeptin-GPR54 signaling is essential for preovulatory gonadotropin-releasing hormone neuron activation and the luteinizing hormone surge. J Neurosci 2008; 28: 8691–8697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JT, Shahab M, Pau KY, Clarke IJ.Kisspeptin neurons in the arcuate nucleus and preoptic area are central regulators for the preovulatory luteinizing hormone surge in the sheep and monkey. Program No. 665.13. 2009 Neuroscience Meeting Planner. Chicago, IL: Society for Neuroscience, 2009. Online. [Google Scholar]
- Estrada KM, Clay CM, Pompolo S, Smith JT, Clarke IJ.Elevated KiSS-1 expression in the arcuate nucleus prior to the cyclic preovulatory gonadotrophin-releasing hormone/luteinizing hormone surge in the ewe suggests a stimulatory role for kisspeptin in estrogen-positive feedback. J Neuroendocrinol 2006; 18: 806–809. [DOI] [PubMed] [Google Scholar]
- Smith JT, Clay CM, Caraty A, Clarke IJ.KiSS-1 messenger ribonucleic acid expression in the hypothalamus of the ewe is regulated by sex steroids and season. Endocrinology 2007; 148: 1150–1157. [DOI] [PubMed] [Google Scholar]
- Backholer K, Smith JT, Clarke IJ.Melanocortins may stimulate reproduction by activating orexin neurons in the dorsomedial hypothalamus and kisspeptin neurons in the preoptic area of the ewe. Endocrinology 2009; 150: 5488–5497. [DOI] [PubMed] [Google Scholar]
- Rometo AM, Krajewski SJ, Voytko ML, Rance NE.Hypertrophy and increased kisspeptin gene expression in the hypothalamic infundibular nucleus of postmenopausal women and ovariectomized monkeys. J Clin Endocrinol Metab 2007; 92: 2744–2750. [DOI] [PubMed] [Google Scholar]
- Ramaswamy S, Guerriero KA, Gibbs RB, Plant TM.Structural interactions between kisspeptin and GnRH neurons in the mediobasal hypothalamus of the male rhesus monkey (Macaca mulatta) as revealed by double immunofluorescence and confocal microscopy. Endocrinology 2008; 149: 4387–4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krey LC, Butler WR, Knobil E.Surgical disconnection of the medial basal hypothalamus and pituitary function in the rhesus monkey. I. Gonadotropin secretion. Endocrinology 1975; 96: 1073–1087. [DOI] [PubMed] [Google Scholar]
- Knobil E, Plant TM, Wildt L, Belchetz PE, Marshall G.Control of the rhesus monkey menstrual cycle: permissive role of hypothalamic gonadotropin-releasing hormone. Science 1980; 207: 1371–1373. [DOI] [PubMed] [Google Scholar]
- Norman RL, Resko JA, Spies HG.The anterior hypothalamus: how it affects gonadotropin secretion in the rhesus monkey. Endocrinology 1976; 99: 59–71. [DOI] [PubMed] [Google Scholar]
- Kim W, Jessen HM, Auger AP, Terasawa E.Postmenopausal increase in KiSS-1, GPR54, and luteinizing hormone releasing hormone (LHRH-1) mRNA in the basal hypothalamus of female rhesus monkeys. Peptides 2009; 30: 103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsui K, Saigoh E, Ukena K, Teranishi H, Fujisawa Y, Kikuchi M, Ishii S, Sharp PJ.A novel avian hypothalamic peptide inhibiting gonadotropin release. Biochem Biophys Res Commun 2000; 275: 661–667. [DOI] [PubMed] [Google Scholar]
- Kriegsfeld LJ, Mei DF, Bentley GE, Ubuka T, Mason AO, Inoue K, Ukena K, Tsutsui K, Silver R.Identification and characterization of a gonadotropin-inhibitory system in the brains of mammals. Proc Natl Acad Sci U S A 2006; 103: 2410–2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke IJ, Sari IP, Qi Y, Smith JT, Parkington HC, Ubuka T, Iqbal J, Li Q, Tilbrook A, Morgan K, Pawson AJ, Tsutsui K, et al. Potent action of RF-amide-related peptide-3 on pituitary gonadotropes indicative of a hypophysiotropic role in the negative regulation of gonadotropin secretion. Endocrinology 2008; 149: 5811–5821. [DOI] [PubMed] [Google Scholar]
- Ubuka T, Lai H, Kitani M, Suzuuchi A, Pham V, Cadigan PA, Wang A, Chowdhury VS, Tsutsui K, Bentley GE.Gonadotropin-inhibitory hormone identification, cDNA cloning, and distribution in rhesus macaque brain. J Comp Neurol 2009; 517: 841–855. [DOI] [PubMed] [Google Scholar]
- Ubuka T, Morgan K, Pawson AJ, Osugi T, Chowdhury VS, Minakata H, Tsutsui K, Millar RP, Bentley GE.Identification of human GnIH homologs, RFRP-1 and RFRP-3, and the cognate receptor, GPR147 in the human hypothalamic pituitary axis. PLoS One 2009; 4: e8400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinuma S, Shintani Y, Fukusumi S, Iijima N, Matsumoto Y, Hosoya M, Fujii R, Watanabe T, Kikuchi K, Terao Y, et al. New neuropeptides containing carboxy-terminal RF-amide and their receptor in mammals. Nat Cell Biol 2000; 2: 703–708. [DOI] [PubMed] [Google Scholar]
- Johnson MA, Tsutsui K, Fraley GS.Rat RF-amide-related peptide-3 stimulates GH secretion, inhibits LH secretion, and has variable effects on sex behavior in the adult male rat. Horm Behav 2007; 51: 171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MA, Fraley GS.Rat RFRP-3 alters hypothalamic GHRH expression and growth hormone secretion but does not affect KiSS-1 gene expression or the onset of puberty in male rats. Neuroendocrinology 2008; 88: 305–315. [DOI] [PubMed] [Google Scholar]
- Murakami M, Matsuzaki T, Iwasa T, Yasui T, Irahara M, Osugi T, Tsutsui K.Hypophysiotropic role of RF-amide-related peptide-3 in the inhibition of LH secretion in female rats. J Endocrinol 2008; 199: 105–112. [DOI] [PubMed] [Google Scholar]
- Kadokawa H, Shibata M, Tanaka Y, Kojima T, Matsumoto K, Oshima K, Yamamoto N.Bovine C-terminal octapeptide of RF-amide-related peptide-3 suppresses luteinizing hormone (LH) secretion from the pituitary as well as pulsatile LH secretion in bovines. Domest Anim Endocrinol 2009; 36: 219–224. [DOI] [PubMed] [Google Scholar]
- Sari IP, Rao A, Smith JT, Tilbrook AJ, Clarke IJ.Effect of RF-amide-related peptide-3 on luteinizing hormone and follicle-stimulating hormone synthesis and secretion in ovine pituitary gonadotropes. Endocrinology 2009; 150: 5549–5556. [DOI] [PubMed] [Google Scholar]
- Ducret E, Anderson GM, Herbison AE.RF-amide-related peptide-3, a mammalian gonadotropin-inhibitory hormone ortholog, regulates gonadotropin-releasing hormone neuron firing in the mouse. Endocrinology 2009; 150: 2799–2804. [DOI] [PubMed] [Google Scholar]
- Wu M, Dumalska I, Morozova E, van den Pol AN, Alreja M.Gonadotropin inhibitory hormone inhibits basal forebrain vGluT2-gonadotropin-releasing hormone neurons via a direct postsynaptic mechanism. J Physiol 2009; 587: 1401–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojcik-Gladysz A, Misztal T, Wankowska M, Romanowicz K, Polkowska J.Effect of central infusions of neuropeptide Y on GnRH/LH axis in ewes during the early anestrous period. Reprod Biol 2003; 3: 29–46. [PubMed] [Google Scholar]
- Smith JT, Coolen LM, Kriegsfeld LJ, Sari IP, Jaafarzadehshirazi MR, Maltby M, Bateman K, Goodman RL, Tilbrook AJ, Ubuka T, Bentley GE, Clarke IJ, et al. Variation in kisspeptin and RF-amide-related peptide (RFRP) expression and terminal connections to gonadotropin-releasing hormone neurons in the brain: a novel medium for seasonal breeding in the sheep. Endocrinology 2008; 149: 5770–5782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Y, Oldfield BJ, Clarke IJ.Projections of RF-amide-related peptide-3 neurones in the ovine hypothalamus, with special reference to regions regulating energy balance and reproduction. J Neuroendocrinol 2009; 21: 690–697. [DOI] [PubMed] [Google Scholar]
- Jensen JT, Zelinski MB, Stanley JE, Fanton JW, Stouffer RL.The phosphodiesterase 3 inhibitor ORG 9935 inhibits oocyte maturation in the naturally selected dominant follicle in rhesus macaques. Contraception 2008; 77: 303–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backholer K, Smith JT, Rao A, Pereira A, Iqbal J, Ogawa S, Li Q, Clarke IJ.Kisspeptin cells in the ewe brain respond to leptin and communicate with neuropeptide Y and proopiomelanocortin cells. Endocrinology 2010; 151: 2233–2243. [DOI] [PubMed] [Google Scholar]
- BrainInfo, Neuroscience Division National Primate Research Center, University of Washington. World Wide Web. 2007. (URL: http://www.braininfo.org). (March 2010).
- Gu GB, Simerly RB.Projections of the sexually dimorphic anteroventral periventricular nucleus in the female rat. J Comp Neurol 1997; 384: 142–164. [PubMed] [Google Scholar]
- Simerly RB.Organization and regulation of sexually dimorphic neuroendocrine pathways. Behav Brain Res 1998; 92: 195–203. [DOI] [PubMed] [Google Scholar]
- Wintermantel TM, Campbell RE, Porteous R, Bock D, Grone HJ, Todman MG, Korach KS, Greiner E, Perez CA, Schutz G, Herbison AE.Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron 2006; 52: 271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbison AE.Estrogen positive feedback to gonadotropin-releasing hormone (GnRH) neurons in the rodent: the case for the rostral periventricular area of the third ventricle (RP3V). Brain Res Rev 2008; 57: 277–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi S, Yamada S, Takatsu Y, Matsui H, Kinoshita M, Takase K, Sugiura H, Ohtaki T, Matsumoto H, Uenoyama Y, Tsukamura H, Inoue K, et al. Involvement of anteroventral periventricular metastin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. J Reprod Dev 2007; 53: 367–378. [DOI] [PubMed] [Google Scholar]
- Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA.Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology 2005; 146: 3686–3692. [DOI] [PubMed] [Google Scholar]
- Gottsch ML, Navarro VM, Zhao Z, Glidewell-Kenney C, Weiss J, Jameson JL, Clifton DK, Levine JE, Steiner RA.Regulation of Kiss1 and dynorphin gene expression in the murine brain by classical and nonclassical estrogen receptor pathways. J Neurosci 2009; 29: 9390–9395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blache D, Fabre-Nys CJ, Venier G.Ventromedial hypothalamus as a target for estradiol action on proceptivity, receptivity and luteinizing hormone surge of the ewe. Brain Res 1991; 546: 241–249. [DOI] [PubMed] [Google Scholar]
- Caraty A, Fabre-Nys C, Delaleu B, Locatelli A, Bruneau G, Karsch FJ, Herbison A.Evidence that the mediobasal hypothalamus is the primary site of action of estradiol in inducing the preovulatory gonadotropin releasing hormone surge in the ewe. Endocrinology 1998; 139: 1752–1760. [DOI] [PubMed] [Google Scholar]
- Hess DL, Wilkins RH, Moossy J, Chang JL, Plant TM, McCormack JT, Nakai Y, Knobil E.Estrogen-induced gonadotropin surges in decerebrated female rhesus monkeys with medial basal hypothalamic peninsulae. Endocrinology 1977; 101: 1264–1271. [DOI] [PubMed] [Google Scholar]
- Cogen P, Antunes JL, Louis KM, Dyrenfurth I, Ferin M.The effects of anterior hypothalamic disconnection on gonadotropin secretion in the female rhesus monkey. Endocrinology 1980; 107: 677–683. [DOI] [PubMed] [Google Scholar]
- Gibson EM, Humber SA, Jain S, Williams WP, III, Zhao S, Bentley GE, Tsutsui K, Kriegsfeld LJ.Alterations in RF-amide-related peptide expression are coordinated with the preovulatory luteinizing hormone surge. Endocrinology 2008; 149: 4958–4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quennell JH, Rizwan MZ, Relf HL, Anderson GM.Developmental and steroidogenic effects on the gene expression of RFRP and its receptor in the rat brain and pituitary gland. J Neuroendocrinol 2010; 22: 309–316. [DOI] [PubMed] [Google Scholar]
- Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE.Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci 2005; 25: 11349–11356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pielecka-Fortuna J, Chu Z, Moenter SM.Kisspeptin acts directly and indirectly to increase gonadotropin-releasing hormone neuron activity and its effects are modulated by estradiol. Endocrinology 2008; 149: 1979–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson J, Herbison AE.Postnatal development of kisspeptin neurons in mouse hypothalamus: sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology 2006; 147: 5817–5825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita M, Tsukamura H, Adachi S, Matsui H, Uenoyama Y, Iwata K, Yamada S, Inoue K, Ohtaki T, Matsumoto H, Maeda K.Involvement of central metastin in the regulation of preovulatory luteinizing hormone surge and estrous cyclicity in female rats. Endocrinology 2005; 146: 4431–4436. [DOI] [PubMed] [Google Scholar]
- Tachibana T, Sato M, Takahashi H, Ukena K, Tsutsui K, Furuse M.Gonadotropin-inhibiting hormone stimulates feeding behavior in chicks. Brain Res 2005; 1050: 94–100. [DOI] [PubMed] [Google Scholar]
- Dardente H, Birnie M, Lincoln GA, Hazlerigg DG.RF-amide-related peptide and its cognate receptor in the sheep: cDNA cloning, mRNA distribution in the hypothalamus and the effect of photoperiod. J Neuroendocrinol 2008; 20: 1252–1259. [DOI] [PubMed] [Google Scholar]
- Rizwan MZ, Porteous R, Herbison AE, Anderson GM.Cells expressing RF-amide-related peptide-1/3, the mammalian gonadotropin-inhibitory hormone orthologs, are not hypophysiotropic neuroendocrine neurons in the rat. Endocrinology 2009; 150: 1413–1420. [DOI] [PubMed] [Google Scholar]
- Smith JT, Dungan HM, Stoll EA, Gottsch ML, Braun RE, Eacker SM, Clifton DK, Steiner RA.Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology 2005; 146: 2976–2984. [DOI] [PubMed] [Google Scholar]
- Kauffman AS, Gottsch ML, Roa J, Byquist AC, Crown A, Clifton DK, Hoffman GE, Steiner RA, Tena-Sempere M.Sexual differentiation of Kiss1 gene expression in the brain of the rat. Endocrinology 2007; 148: 1774–1783. [DOI] [PubMed] [Google Scholar]
- Kauffman AS, Navarro VM, Kim J, Clifton D, Steiner RA.Sex differences in the regulation of Kiss1/NKB neurons in juvenile mice: implications for the timing of puberty. Am J Physiol Endocrinol Metab 2009; 297: 1212–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng G, Coolen LM, Padmanabhan V, Goodman RL, Lehman MN.The kisspeptin/neurokinin B/dynorphin (KNDy) cell population of the arcuate nucleus: sex differences and effects of prenatal testosterone in sheep. Endocrinology 2010; 151: 301–311. [DOI] [PMC free article] [PubMed] [Google Scholar]